Exploring the Antimicrobial and Immunomodulatory Potential of Gecko-Derived Cathelicidin Gj-CATH5
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides and Cells
2.2. Ethics Statement
2.3. Animal Models
2.4. In Vivo Neutrophil and Macrophage Recruitment
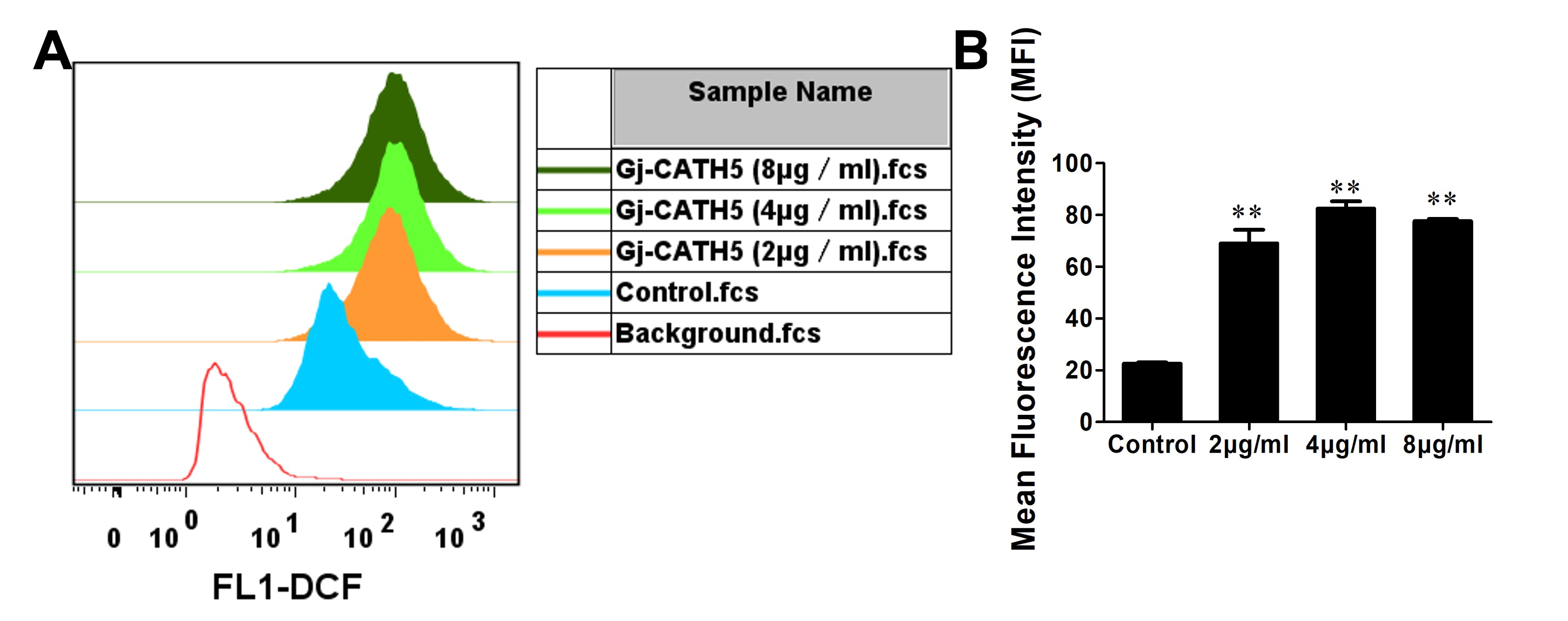
2.5. Measurement of Intracellular Reactive Oxygen Species
2.6. Phagocytosis Assay
2.7. Bacterial Killing Assay
2.8. Myeloperoxidase (MPO) Activity Assay
2.9. NETs Entrapment Assay
2.10. Minimum Bactericidal Concentration (MBC) Assay
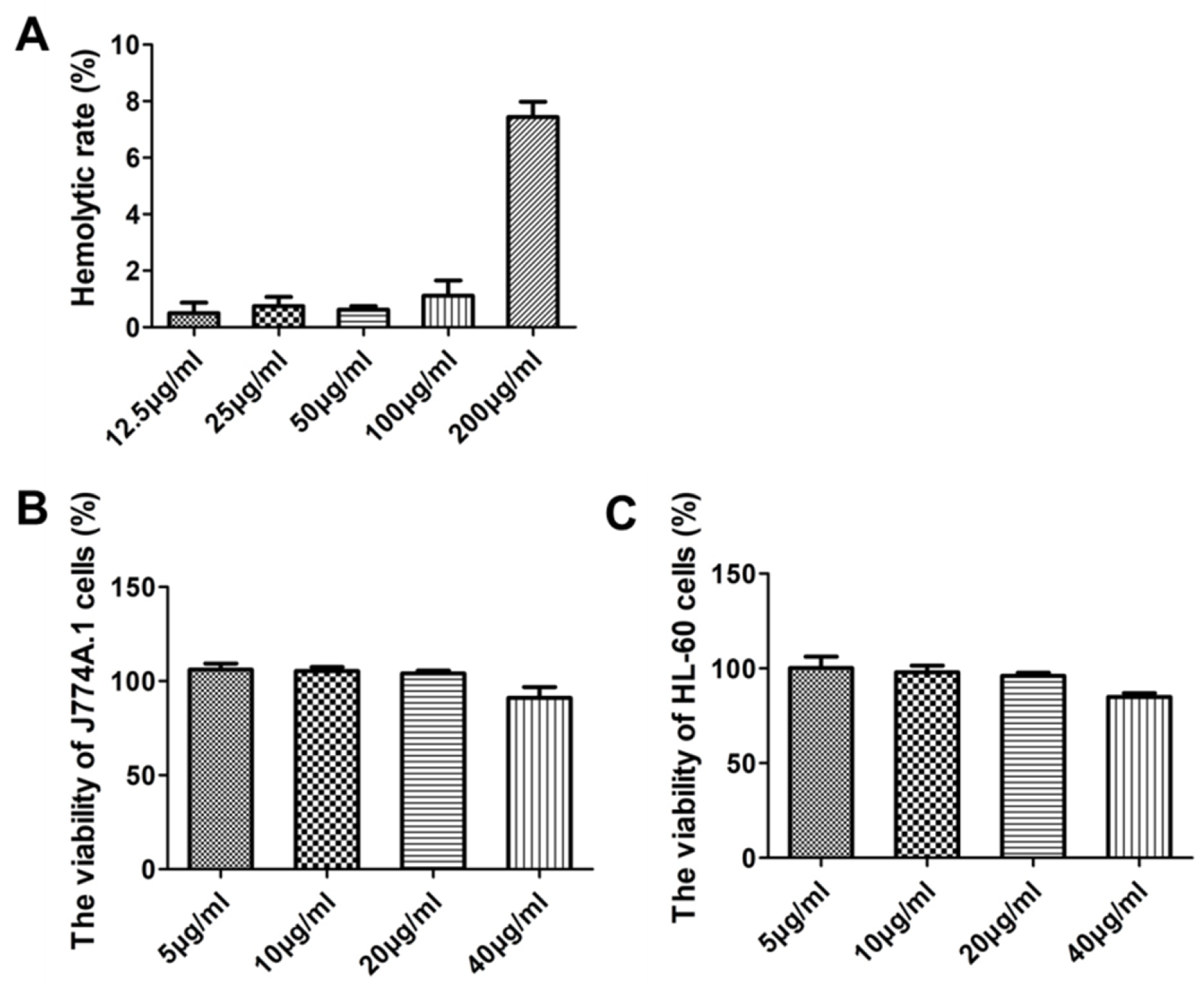
2.11. Cytotoxicity and Hemolysis Assays
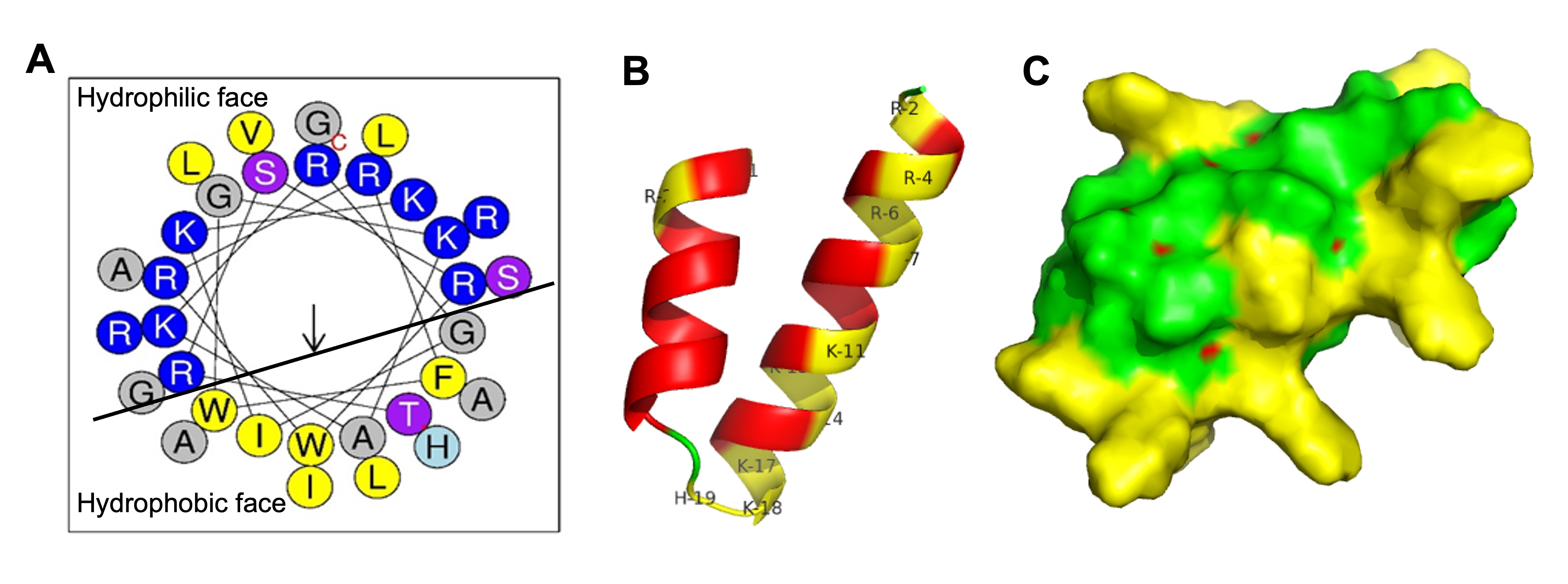
2.12. Structure Analysis
2.13. Statistical Analysis
3. Results
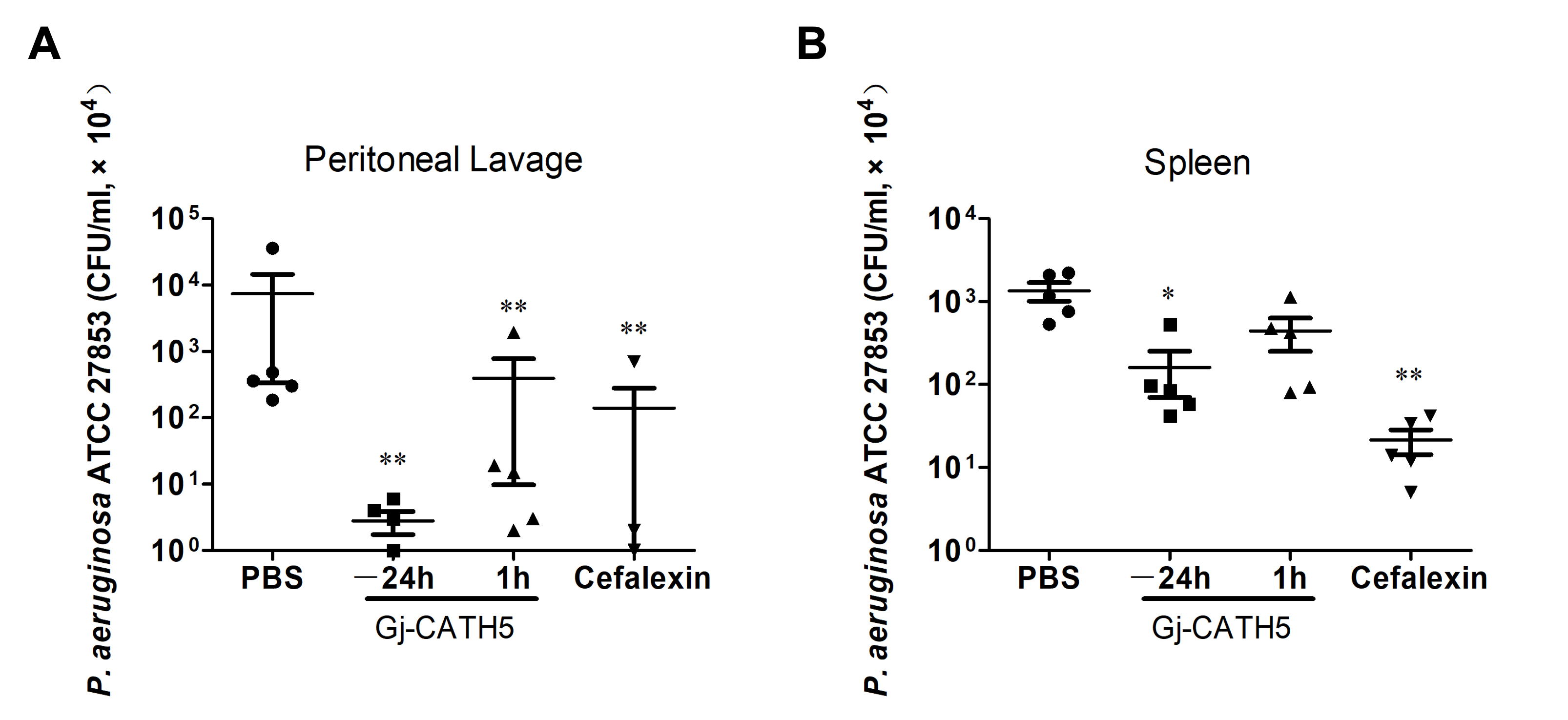
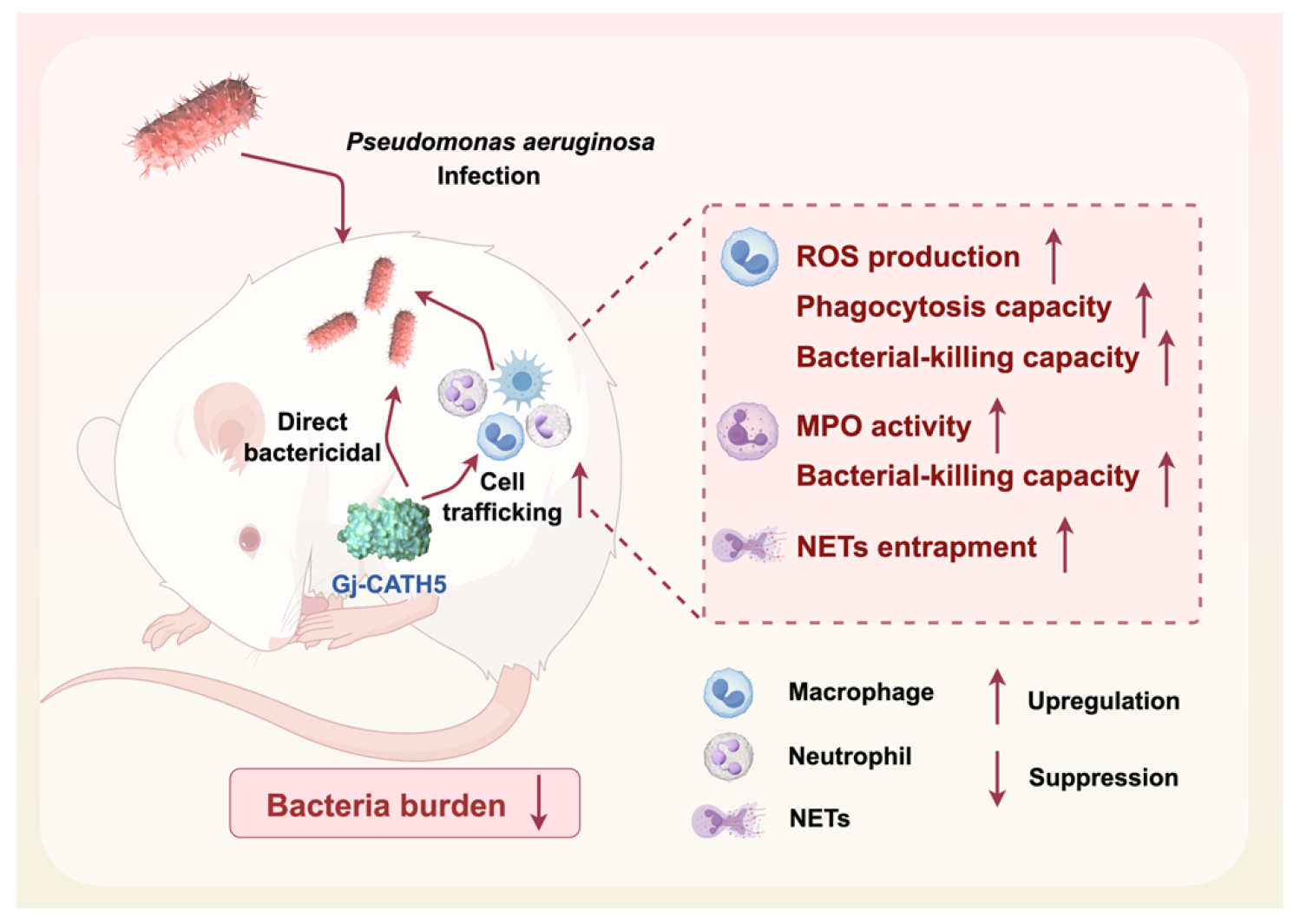
3.1. Efficacy of Gj-CATH5 Against Pseudomonas aeruginosa Infection
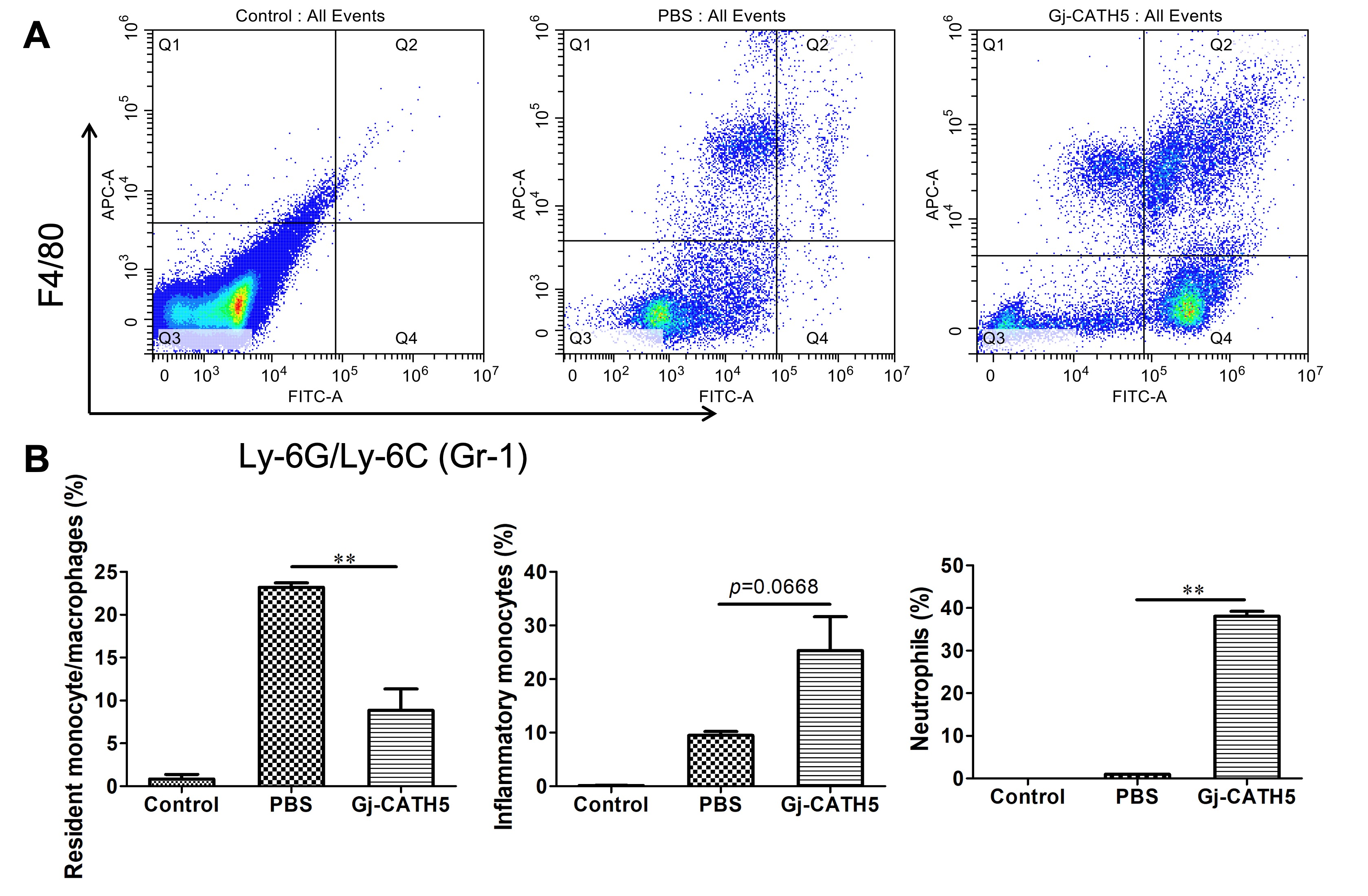
3.2. Gj-CATH5 Exhibited a Significant Chemotactic Effect on Neutrophils and Monocytes/Macrophages
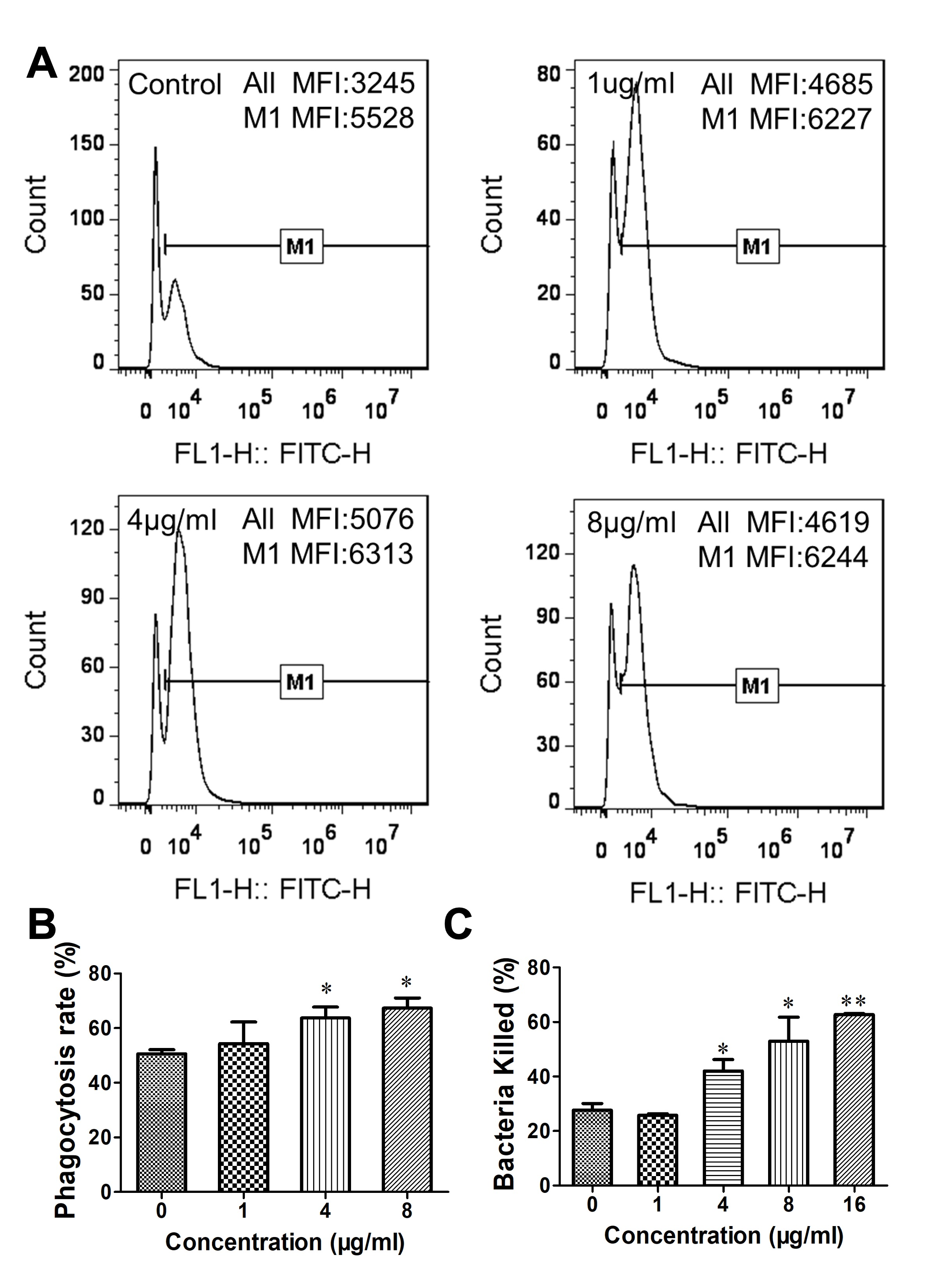
3.3. Gj-CATH5 Has a Regulatory Effect on the Phagocytic Bactericidal Activity of Macrophages
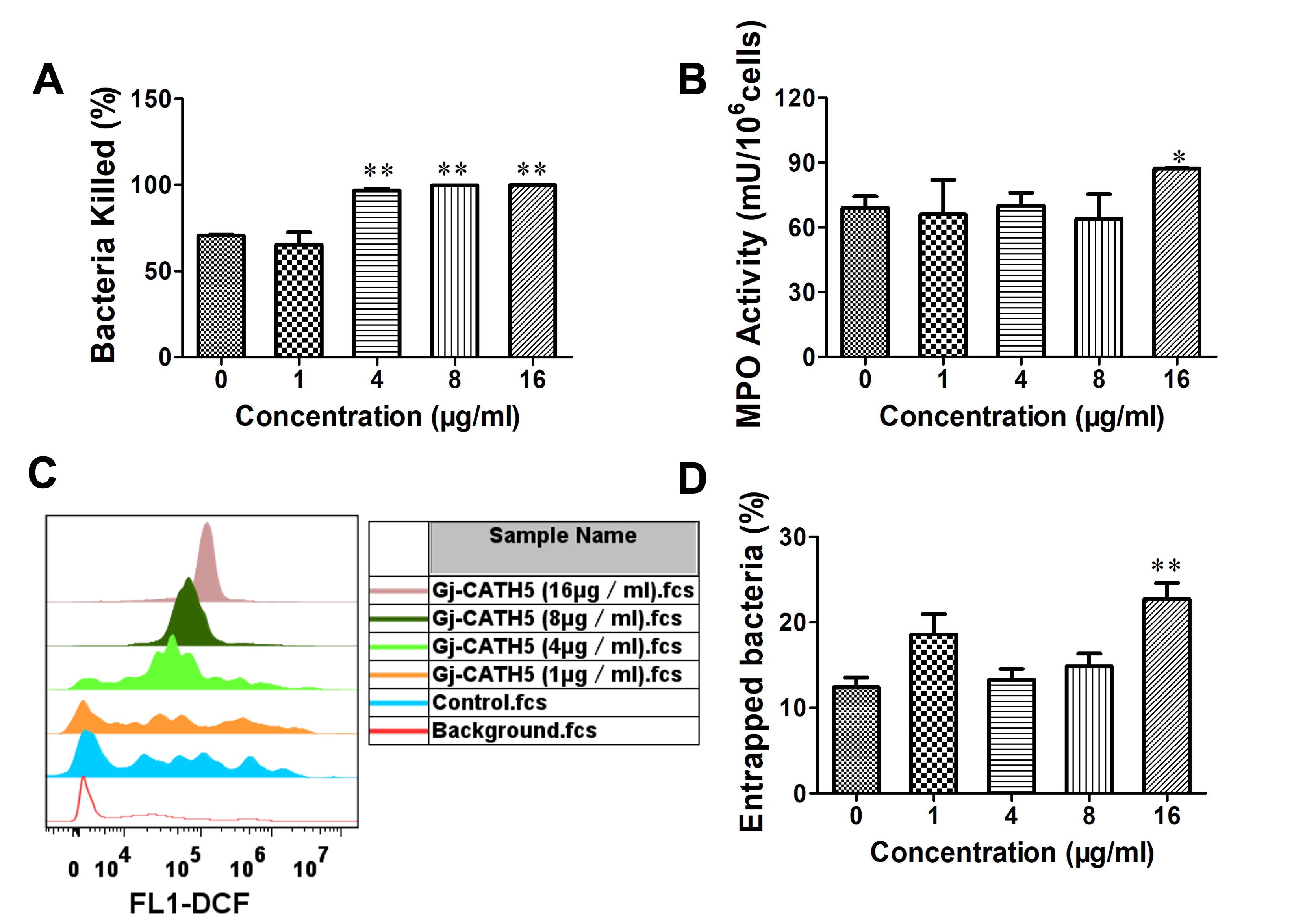
3.4. Gj-CATH5 Regulates the Bactericidal Function of Neutrophil
3.5. The Direct Bactericidal Activity of Gj-CATH5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMN | Primary mouse bone marrow-derived neutrophils |
| CFU | Colony-forming units |
| MFI | Mean fluorescence intensity |
| MOI | Multiplicity of infection |
| MPO | Myeloperoxidase |
| PMA | Phorbol 12-myristate 13-acetate |
| MBC | Minimum bactericidal concentration |
| NETs | Neutrophil Extracellular Traps |
References
- Hancock, R.E.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, J.; Falciani, C.; Bracci, L.; Pini, A. Models of In-Vivo Bacterial Infections for the Development of Antimicrobial Peptide-based Drugs. Curr. Top. Med. Chem. 2017, 17, 613–619. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Kraneburg, U.; Jacobsen, F.; Al-Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 2011, 216, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chow, L.N.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef]
- Cai, S.; Qiao, X.; Feng, L.; Shi, N.; Wang, H.; Yang, H.; Guo, Z.; Wang, M.; Chen, Y.; Wang, Y.; et al. Python Cathelicidin CATHPb1 Protects against Multidrug-Resistant Staphylococcal Infections by Antimicrobial-Immunomodulatory Duality. J. Med. Chem. 2018, 61, 2075–2086. [Google Scholar] [CrossRef]
- Li, S.A.; Lee, W.H.; Zhang, Y. Efficacy of OH-CATH30 and its analogs against drug-resistant bacteria in vitro and in mouse models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef]
- Dalla Valle, L.; Benato, F.; Paccanaro, M.C.; Alibardi, L. Bioinformatic and molecular characterization of cathelicidin-like peptides isolated from the green lizard Anolis carolinensis (Reptilia: Lepidosauria: Iguanidae). Ital. J. Zool. 2013, 80, 177–186. [Google Scholar] [CrossRef]
- Cai, S.; Meng, K.; Liu, P.; Cao, X.; Wang, G. Suppressive effects of gecko cathelicidin on biofilm formation and cariogenic virulence factors of Streptococcus mutans. Arch. Oral. Biol. 2021, 129, 105205. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Lu, C.; Liu, Z.; Wang, W.; Lu, S.; Sun, Z.; Wang, G. Derivatives of gecko cathelicidin-related antioxidant peptide facilitate skin wound healing. Eur. J. Pharmacol. 2021, 890, 173649. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.; Christophersen, L.; Jensen, P.; Bjarnsholt, T.; Moser, C.; HøIby, N. Anti-Pseudomonas aeruginosa IgY antibodies promote bacterial opsonization and augment the phagocytic activity of polymorphonuclear neutrophils. Hum. Vaccines Immunother. 2016, 12, 1690–1699. [Google Scholar]
- Descamps, D.; Le Gars, M.; Balloy, V.; Barbier, D.; Maschalidi, S.; Tohme, M.; Chignard, M.; Ramphal, R.; Manoury, B.; Sallenave, J.M. Toll-like receptor 5 (TLR5), IL-1β secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing—Toll-like receptor 5 (TLR5), IL-1β secretion, and asparagine endopeptidase are critical factors. Proc. Natl. Acad. Sci. USA 2012, 109, 1619–1624. [Google Scholar] [CrossRef]
- Rožman, S.; Yousefi, S.; Oberson, K.; Kaufmann, T.; Benarafa, C.; Simon, H.U. The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Differ. 2015, 22, 445–456. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Mann, E.R.; Menon, M.; Knight, S.B.; Konkel, J.E.; Jagger, C.; Shaw, T.N.; Krishnan, S.; Rattray, M.; Ustianowski, A.; Bakerly, N.D.; et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci. Immunol. 2020, 5, eabd6197. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shan, B.; Qi, J.; Ma, Y. Systemic Responses of Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii Following Exposure to the Antimicrobial Peptide Cathelicidin-BF Imply Multiple Intracellular Targets. Front. Cell Infect. Microbiol. 2017, 7, 466. [Google Scholar] [CrossRef]
- McHugh, B.J.; Wang, R.; Li, H.N.; Beaumont, P.E.; Kells, R.; Stevens, H.; Young, L.; Rossi, A.G.; Gray, R.D.; Dorin, J.R.; et al. Cathelicidin is a “fire alarm”, generating protective NLRP3-dependent airway epithelial cell inflammatory responses during infection with Pseudomonas aeruginosa. PLoS Pathog. 2019, 15, e1007694. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Sun, R.; Gao, X.; Wei, H.; Li, L.J.; Tian, Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat. Commun. 2013, 4, 2106. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, C.; Liu, J.; Yang, S.; Peng, X.; Wang, Q.; Liu, C.; Liu, X.; Luan, J.; Zhao, G. Cathelicidin boosts the antifungal activity of neutrophils and improves prognosis during Aspergillus fumigatus keratitis. Infect. Immun. 2024, 92, e00483-23. [Google Scholar] [CrossRef]
- Thomas, C.J.; Schroder, K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013, 34, 317–328. [Google Scholar] [CrossRef]
- Dahlgren, C.; Lind, S.; Mårtensson, J.; Björkman, L.; Wu, Y.; Sundqvist, M.; Forsman, H. G protein coupled pattern recognition receptors expressed in neutrophils: Recognition, activation/modulation, signaling and receptor regulated functions. Immunol. Rev. 2023, 314, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e524. [Google Scholar] [CrossRef]
- Halverson, T.W.; Wilton, M.; Poon, K.K.; Petri, B.; Lewenza, S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, Y.; Chen, K.; Yang, K.; Huang, X.; Zhang, Y.; Wu, M. Pseudomonas aeruginosa promotes autophagy to suppress macrophage-mediated bacterial eradication. Int. Immunopharmacol. 2016, 38, 214–222. [Google Scholar] [CrossRef]
- Yoon, G.; Puentes, R.; Tran, J.; Multani, A.; Cobo, E.R. The role of cathelicidins in neutrophil biology. J. Leukoc. Biol. 2024, 116, 689–705. [Google Scholar] [CrossRef]
- Runti, G.; Benincasa, M.; Giuffrida, G.; Devescovi, G.; Venturi, V.; Gennaro, R.; Scocchi, M. The Mechanism of Killing by the Proline-Rich Peptide Bac7(1-35) against Clinical Strains of Pseudomonas aeruginosa Differs from That against Other Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2017, 61, e01660-16. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Zhu, J.; Xiao, Y. Antibacterial peptide Reg4 ameliorates Pseudomonas aeruginosa-induced pulmonary inflammation and fibrosis. Microbiol. Spectr. 2024, 12, e0390523. [Google Scholar] [CrossRef] [PubMed]
- Byfield, F.J.; Kowalski, M.; Cruz, K.; Leszczyńska, K.; Namiot, A.; Savage, P.B.; Bucki, R.; Janmey, P.A. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J. Immunol. 2011, 187, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
| Microorganism Strains | MBC 1 (µg/mL) | ||
|---|---|---|---|
| Gj-CATH5 | Cephalexin | Amikacin Sulfate | |
| Gram-negative bacteria | |||
| Escherichia coli ATCC25922 | 8 | 16 | 8 |
| Pseudomonas aeruginosa CMCC10104 | 8 | - | 16 |
| P. aeruginosa ATCC 27853 | 8 | - | 16 |
| Klebsiella pneumonia CMCC46117 | - | - | 16 |
| K. pneumonia IS1368 | 8 | 16 | 32 |
| Gram-positive bacteria | |||
| Staphylococcus aureus CMCC26003 | 8 | 4 | 4 |
| Staphylococcus epidermidis CMCC26069 | 64 | 4 | 8 |
| Streptococcus mutans UA159 | 4 | - | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, S.; Gao, N.; Wang, J.; Li, J. Exploring the Antimicrobial and Immunomodulatory Potential of Gecko-Derived Cathelicidin Gj-CATH5. Biomolecules 2025, 15, 908. https://doi.org/10.3390/biom15070908
Cai S, Gao N, Wang J, Li J. Exploring the Antimicrobial and Immunomodulatory Potential of Gecko-Derived Cathelicidin Gj-CATH5. Biomolecules. 2025; 15(7):908. https://doi.org/10.3390/biom15070908
Chicago/Turabian StyleCai, Shasha, Ningyang Gao, Junhan Wang, and Jing Li. 2025. "Exploring the Antimicrobial and Immunomodulatory Potential of Gecko-Derived Cathelicidin Gj-CATH5" Biomolecules 15, no. 7: 908. https://doi.org/10.3390/biom15070908
APA StyleCai, S., Gao, N., Wang, J., & Li, J. (2025). Exploring the Antimicrobial and Immunomodulatory Potential of Gecko-Derived Cathelicidin Gj-CATH5. Biomolecules, 15(7), 908. https://doi.org/10.3390/biom15070908






