Conditional Deletion of Translin/Trax in Dopaminergic Neurons Reveals No Impact on Psychostimulant Behaviors or Adiposity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunostaining
2.3. Open-Field Locomotor Activity
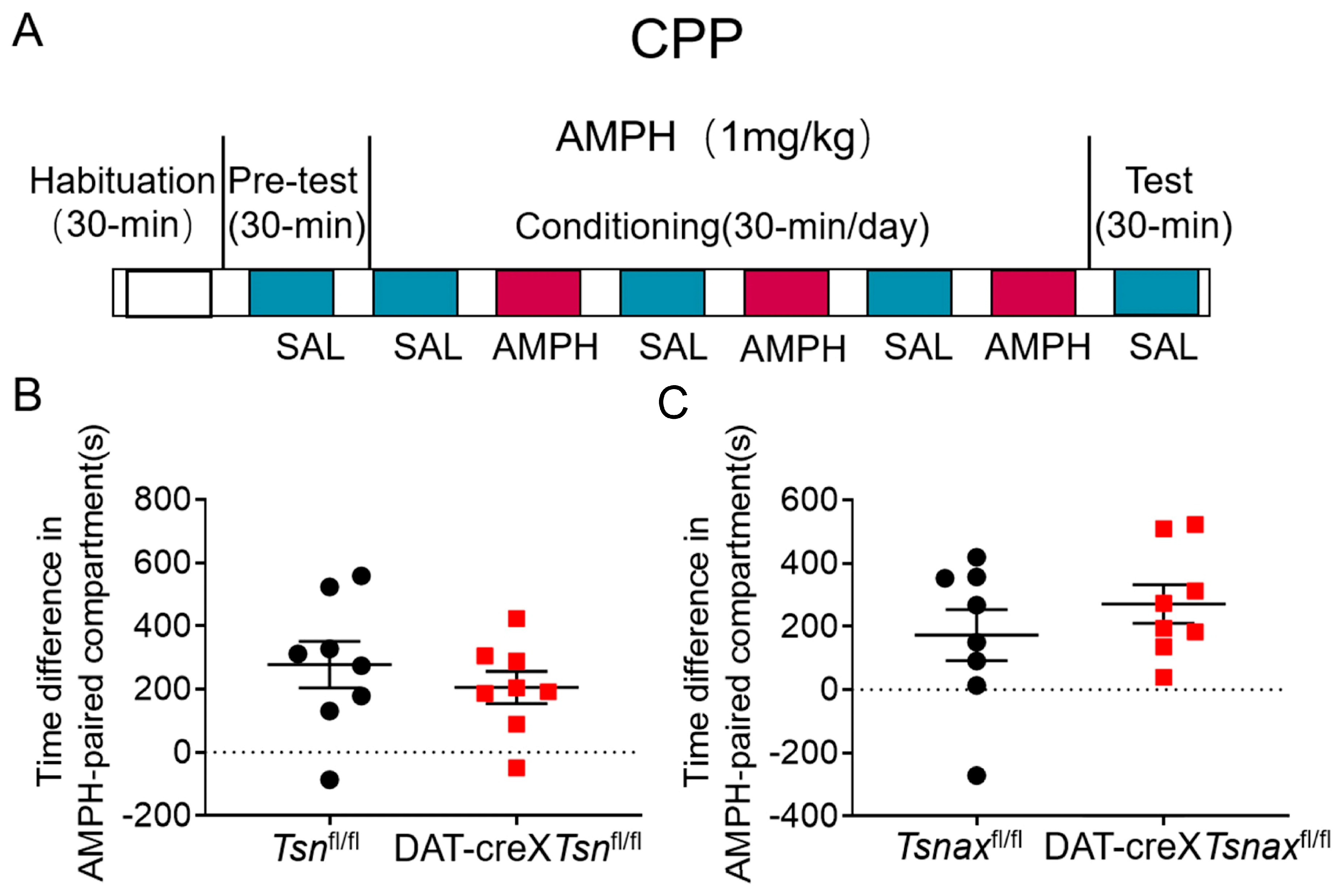
2.4. Conditioned Place Preference (CPP)
2.5. Body Composition
2.6. Statistical Analysis
3. Results
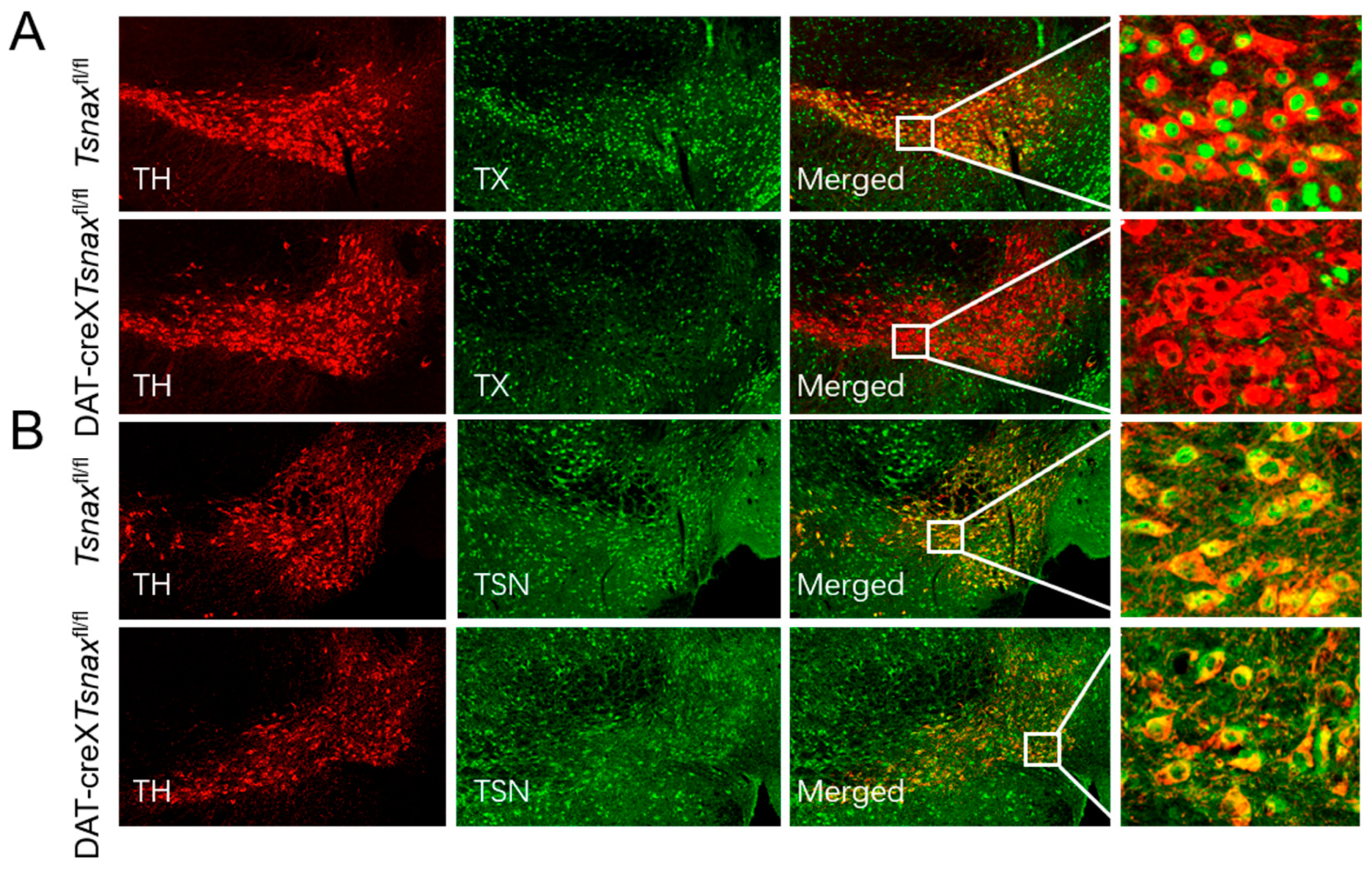
3.1. Conditional Deletion of Tsnax from DA Neurons
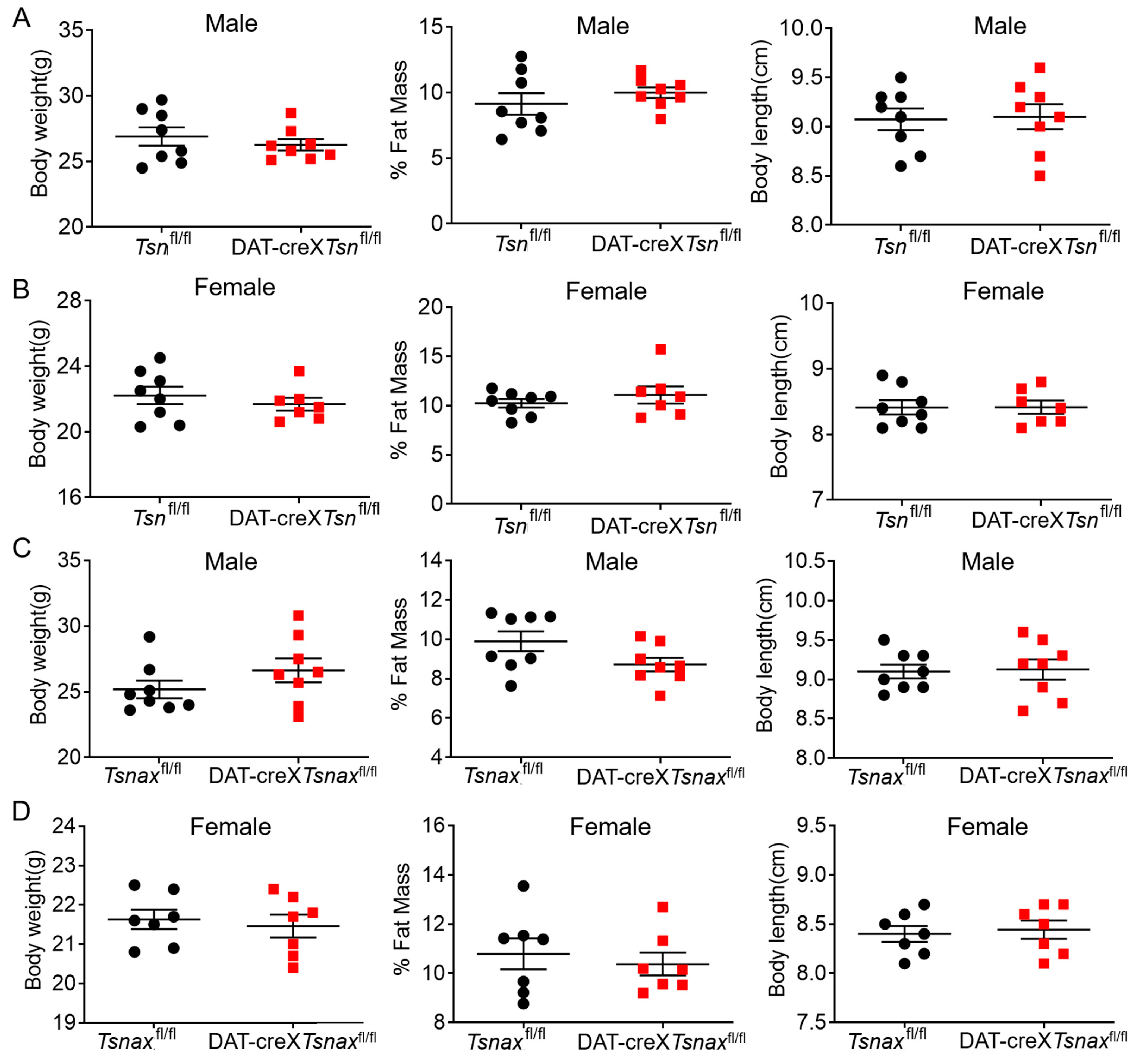
3.2. Effect of Conditional Deletion of Tsn or Tsnax from DA Neurons on Adiposity
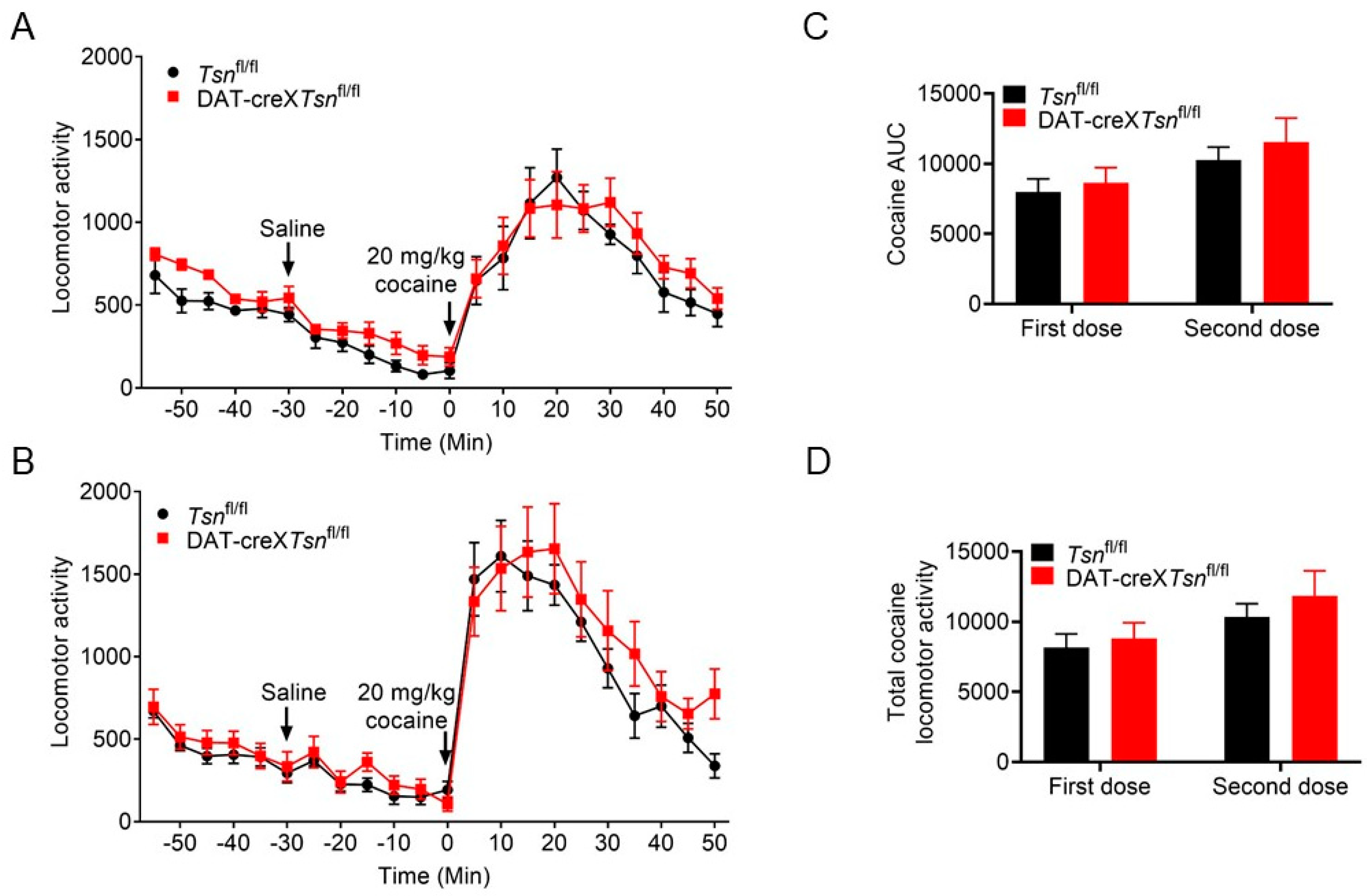
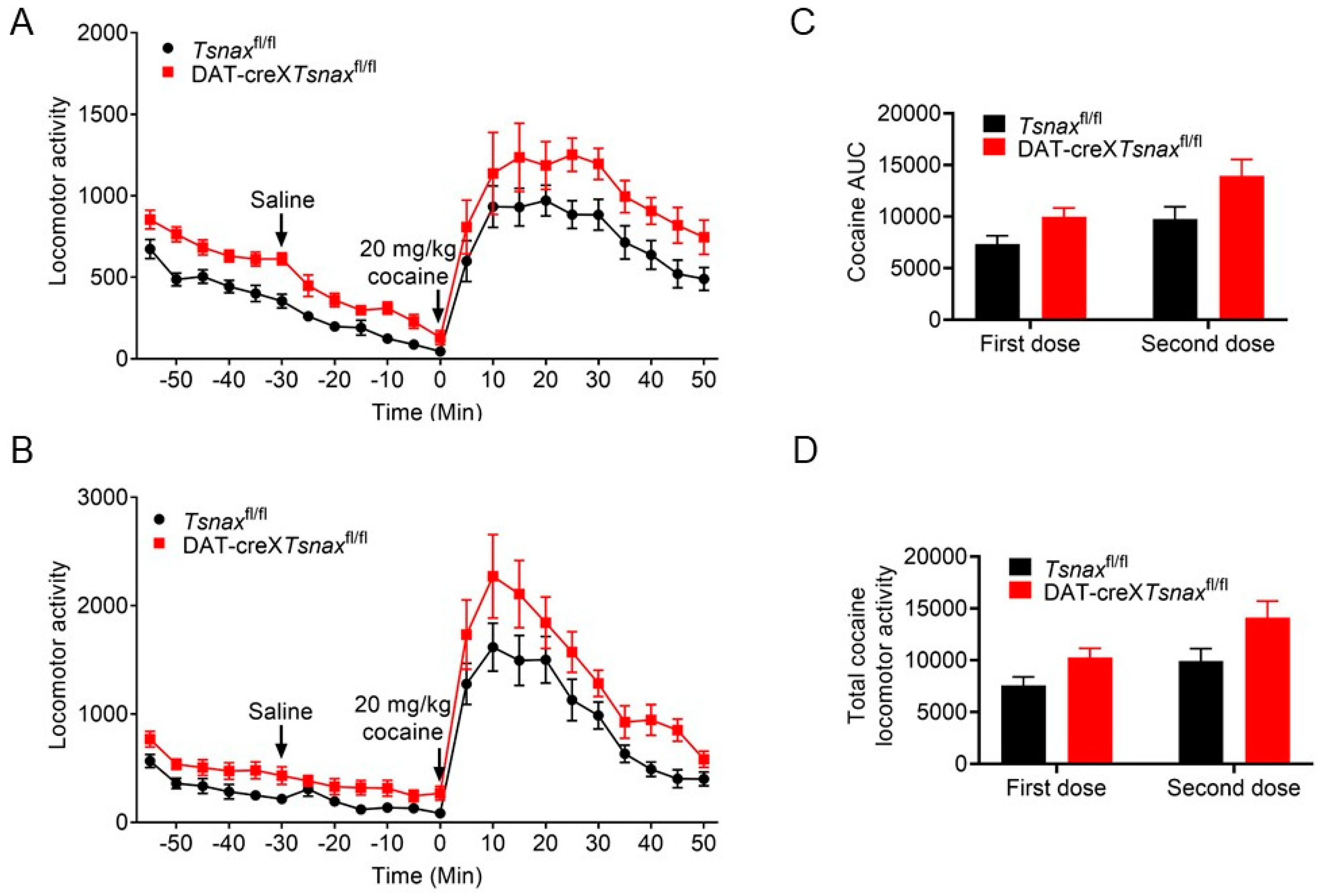
3.3. Effect of Conditional Deletion of Tsn or Tsnax from DA Neurons on Locomotor Response to Cocaine
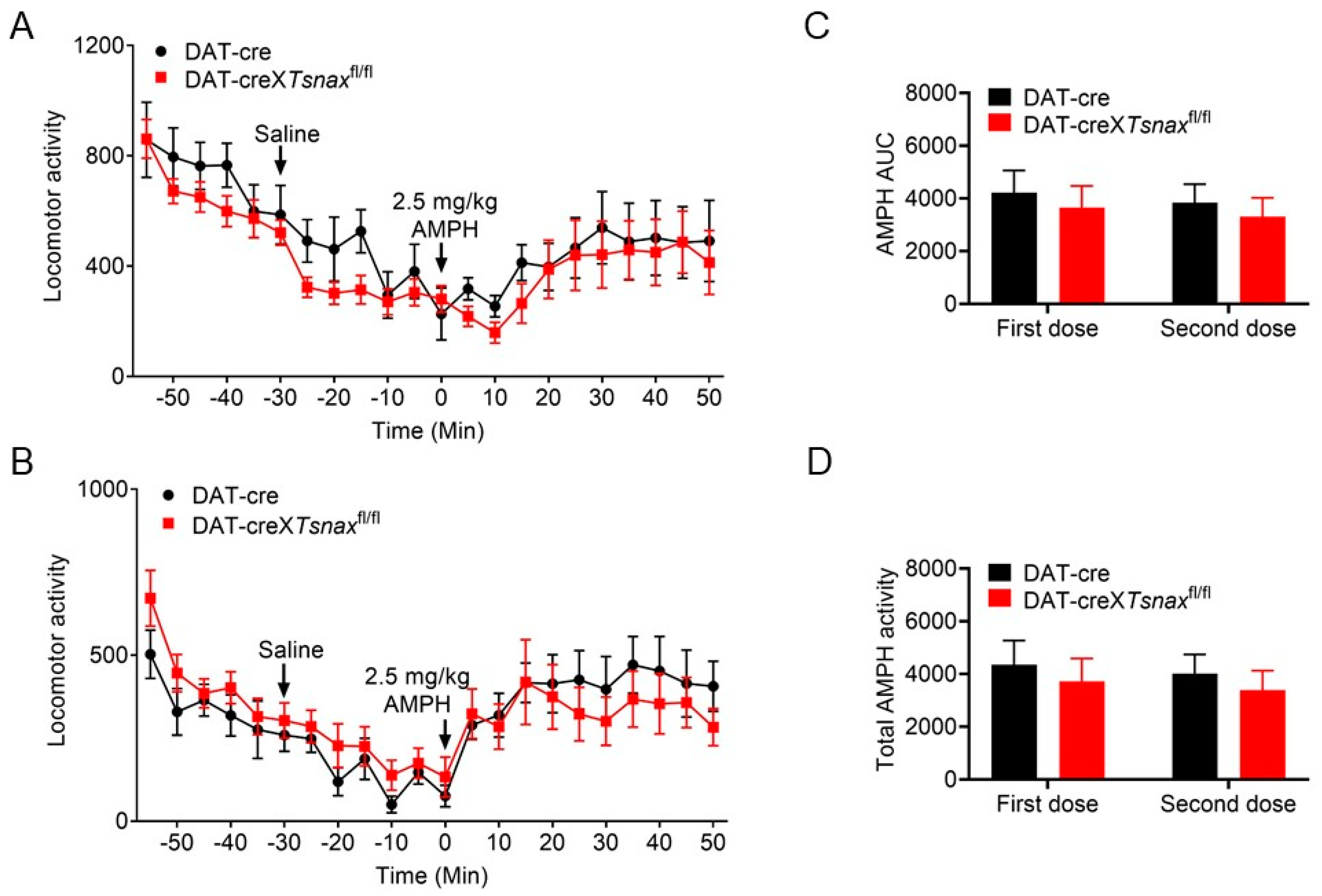
3.4. Effect of Conditional Deletion of Tsn or Tsnax from DA Neurons on Locomotor Response to Amphetamine

3.5. Effect of Conditional Deletion of Tsn or Tsnax from DA Neurons on Amphetamine-Induced CPP
4. Discussion
4.1. Role of TN and TX in Cocaine or Amphetamine-Induced Behaviors
4.2. Role of TN and TX in Adiposity
4.3. Functional Independence of TN and TX
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascale, E.; Divisato, G.; Palladino, R.; Auriemma, M.; Ngalya, E.F.; Caiazzo, M. Noncoding RNAs and Midbrain DA Neurons: Novel Molecular Mechanisms and Therapeutic Targets in Health and Disease. Biomolecules 2020, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Barajas, C.; Coronel, I.; Florán, B. Dopamine Receptors and Neurodegeneration. Aging Dis. 2015, 6, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yeo, G.; Muotri, A.R.; Kuwabara, T.; Gage, F.H. Noncoding RNAs in the mammalian central nervous system. Annu. Rev. Neurosci. 2006, 29, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Fiore, R.; Siegel, G.; Schratt, G. MicroRNA function in neuronal development, plasticity and disease. Biochim. Biophys. Acta 2008, 1779, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kwon, E.J.; Tsai, L.H. MicroRNAs in learning, memory, and neurological diseases. Learn. Mem. 2012, 19, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Kolshus, E.; Dalton, V.S.; Ryan, K.M.; McLoughlin, D.M. When less is more--microRNAs and psychiatric disorders. Acta Psychiatr. Scand. 2014, 129, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Lewis Luján, L.M.; McCarty, M.F.; Di Nicolantonio, J.J.; Gálvez Ruiz, J.C.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Iloki Assanga, S.B. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.B.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Konovalova, J.; Najam, S.S.; Alter, H.; Piepponen, T.P.; Erfle, H.; Sonntag, K.C.; Schütz, G.; Vinnikov, I.A.; Domanskyi, A. Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis. 2017, 8, e2813. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Ishida, R.; Nakahara, K.; Okumura, K.; Aoki, K. Mesenchymal cell differentiation and diseases: Involvement of translin/TRAX complexes and associated proteins. Ann. N. Y. Acad. Sci. 2018, 1421, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tuday, E.; Nomura, Y.; Ruhela, D.; Nakano, M.; Fu, X.; Shah, A.; Roman, B.; Yamaguchi, A.; An, S.S.; Steenbergen, C.; et al. Deletion of the microRNA-degrading nuclease, translin/trax, prevents pathogenic vascular stiffness. Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H1116–H1124. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shah, A.P.; Keighron, J.; Mou, T.M.; Ladenheim, B.; Alt, J.; Fukudome, D.; Niwa, M.; Tamashiro, K.L.; Tanda, G.; et al. Elevated body fat increases amphetamine accumulation in brain: Evidence from genetic and diet-induced forms of adiposity. Transl. Psychiatry 2021, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Kahlig, K.M.; Galli, A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 2003, 479, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.H.; Seviour, P.W.; Iversen, S.D. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975, 94, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Im, H.I.; Venø, M.T.; Fowler, C.D.; Min, A.; Intrator, A.; Kjems, J.; Kenny, P.J.; O’Carroll, D.; Greengard, P. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J. Exp. Med. 2010, 207, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Bastle, R.M.; Oliver, R.J.; Gardiner, A.S.; Pentkowski, N.S.; Bolognani, F.; Allan, A.M.; Chaudhury, T.; St Peter, M.; Galles, N.; Smith, C.; et al. In silico identification and in vivo validation of miR-495 as a novel regulator of motivation for cocaine that targets multiple addiction-related networks in the nucleus accumbens. Mol. Psychiatry 2018, 23, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Plotkin, J.L.; Venø, M.T.; von Schimmelmann, M.; Feinberg, P.; Mann, S.; Handler, A.; Kjems, J.; Surmeier, D.J.; O’Carroll, D.; et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 2013, 342, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.W.; Kenny, P.J. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 2018, 17, e12424. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A.; Bozarth, M.A. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987, 94, 469–492. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shah, A.P.; Li, Z.; Li, M.; Tamashiro, K.L.; Baraban, J.M. Genetic inactivation of the TN/TX microRNA-degrading enzyme phenocopies the robust adiposity induced by Translin (Tsn) deletion. Mol. Metab. 2020, 40, 101013. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.P.; Wu, R.K.; Shah, A.P.; Ladenheim, B.; Alt, J.; Cadet, J.L.; Rais, R.; Chandra, R.; Cen, X.B.; Baraban, J.M. Translin deletion impairs cocaine-induced locomotor sensitization and RGS8 expression in the nucleus accumbens. Acta Pharmacol. Sin. 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Shetty, M.S.; Baraban, J.M.; Abel, T. Selective role of the TN/TX RNase complex in hippocampal synaptic plasticity. Mol. Brain 2020, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Finkenstadt, P.M.; Jeon, M.; Baraban, J.M. Trax is a component of the Translin-containing RNA binding complex. J. Neurochem. 2002, 83, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.; Weng, Y.T.; Chang, S.Y.; Lai, H.L.; Chiu, F.L.; Kuo, H.C.; Chuang, D.M.; Chern, Y. GSK3β negatively regulates TRAX, a scaffold protein implicated in mental disorders, for NHEJ-mediated DNA repair in neurons. Mol. Psychiatry 2018, 23, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Chen, S.Y.; Sun, C.N.; Chien, T.; Chern, Y. A central role of TRAX in the ATM-mediated DNA repair. Oncogene 2016, 35, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.J. Reward mechanisms in obesity: New insights and future directions. Neuron 2011, 69, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.M.; Castro, D.C.; Difeliceantonio, A.G.; Robinson, M.J.; Berridge, K.C. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci. Biobehav. Rev. 2013, 37 Pt A, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Esses, S.; Haft, J.; Ahmari, S.E.; Veenstra-VanderWeele, J. Altered baseline and amphetamine-mediated behavioral profiles in dopamine transporter Cre (DAT-Ires-Cre) mice compared to tyrosine hydroxylase Cre (TH-Cre) mice. Psychopharmacology 2020, 237, 3553–3568. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 2000, 95 (Suppl. S2), S91–S117. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Licata, S.C.; Pierce, R.C. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J. Neurochem. 2003, 85, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Volkow, N.D. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Pontieri, F.E.; Di Chiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 1997, 276, 2048–2050. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Gatley, S.J.; Logan, J.; Wang, G.J.; Ding, Y.S.; Dewey, S. PET evaluation of the dopamine system of the human brain. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1996, 37, 1242–1256. [Google Scholar]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 1991, 48, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Delis, F.; Thanos, P.K.; Rombola, C.; Rosko, L.; Grandy, D.; Wang, G.J.; Volkow, N.D. Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav. Neurosci. 2013, 127, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Dagher, A. Genetic variation in dopaminergic reward in humans. Forum Nutr. 2010, 63, 176–185. [Google Scholar] [PubMed]
- Shah, A.P.; Johnson, M.D.; Fu, X.; Boersma, G.J.; Shah, M.; Wolfgang, M.J.; Tamashiro, K.L.; Baraban, J.M. Deletion of translin (Tsn) induces robust adiposity and hepatic steatosis without impairing glucose tolerance. Int. J. Obes. 2020, 44, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Simanshu, D.K.; Ascano, M.; Diaz-Avalos, R.; Park, A.Y.; Juranek, S.A.; Rice, W.J.; Yin, Q.; Robinson, C.V.; Tuschl, T.; et al. Multimeric assembly and biochemical characterization of the Trax-translin endonuclease complex. Nat. Struct. Mol. Biol. 2011, 18, 658–664. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, R.J.; Wakeman, J.A. Translin-Trax: Considerations for Oncological Therapeutic Targeting. Trends Cancer 2020, 6, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, T.; Bilican, B.; Oncel, D.; Goding, C.R.; Yavuzer, U. DNA damage-dependent interaction of the nuclear matrix protein C1D with Translin-associated factor X (TRAX). J. Cell Sci. 2002, 115 Pt 1, 207–216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, R.; Geng, G.; Yang, H.; Wang, C.; Ren, M.; Fu, X. Conditional Deletion of Translin/Trax in Dopaminergic Neurons Reveals No Impact on Psychostimulant Behaviors or Adiposity. Biomolecules 2025, 15, 1040. https://doi.org/10.3390/biom15071040
Liu Y, Wu R, Geng G, Yang H, Wang C, Ren M, Fu X. Conditional Deletion of Translin/Trax in Dopaminergic Neurons Reveals No Impact on Psychostimulant Behaviors or Adiposity. Biomolecules. 2025; 15(7):1040. https://doi.org/10.3390/biom15071040
Chicago/Turabian StyleLiu, Yunlong, Renkun Wu, Gaiyuan Geng, Helian Yang, Chunmiao Wang, Mengtian Ren, and Xiuping Fu. 2025. "Conditional Deletion of Translin/Trax in Dopaminergic Neurons Reveals No Impact on Psychostimulant Behaviors or Adiposity" Biomolecules 15, no. 7: 1040. https://doi.org/10.3390/biom15071040
APA StyleLiu, Y., Wu, R., Geng, G., Yang, H., Wang, C., Ren, M., & Fu, X. (2025). Conditional Deletion of Translin/Trax in Dopaminergic Neurons Reveals No Impact on Psychostimulant Behaviors or Adiposity. Biomolecules, 15(7), 1040. https://doi.org/10.3390/biom15071040





