Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease
Abstract
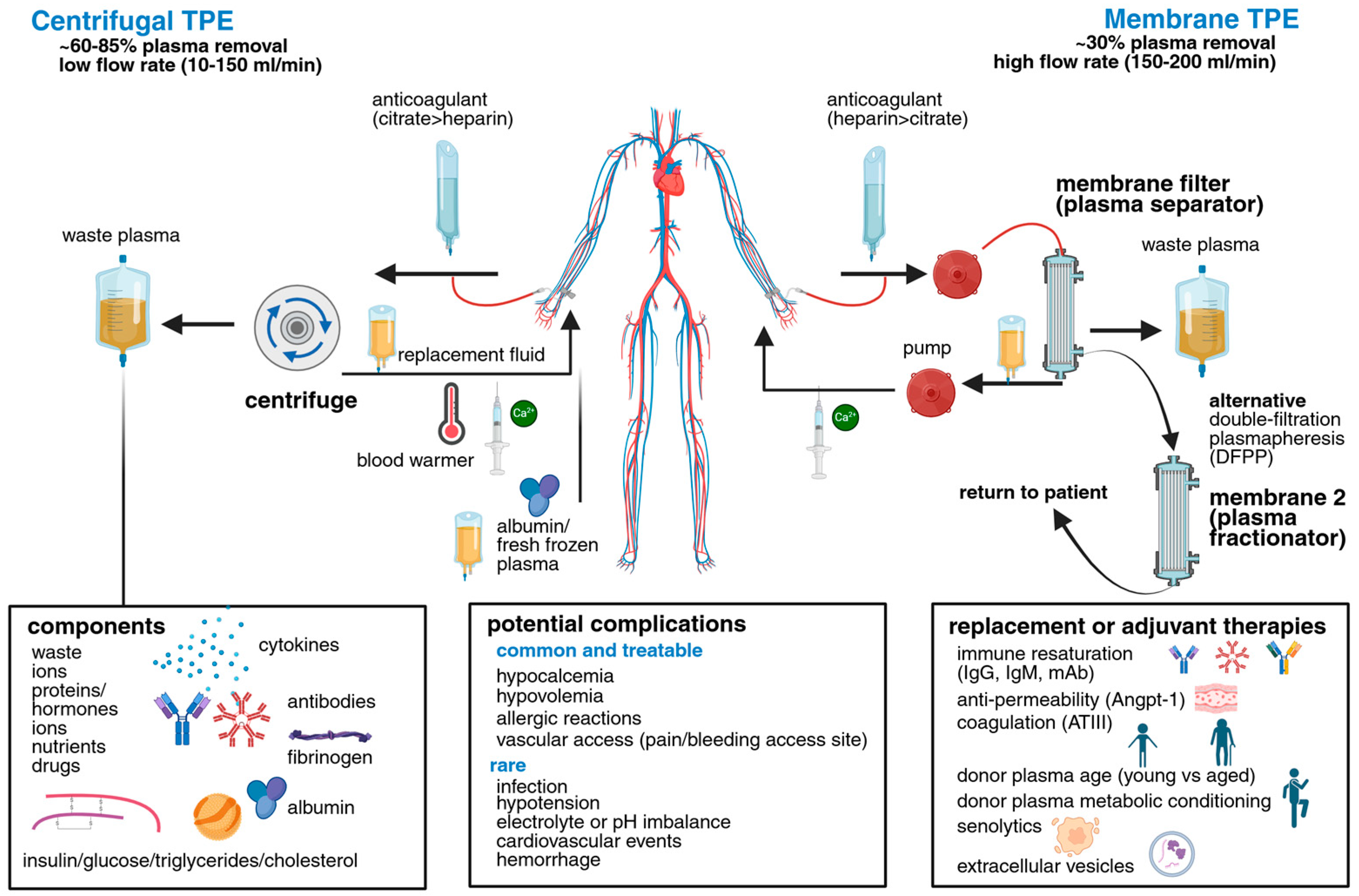
1. An Overview of Plasma and TPE Technologies
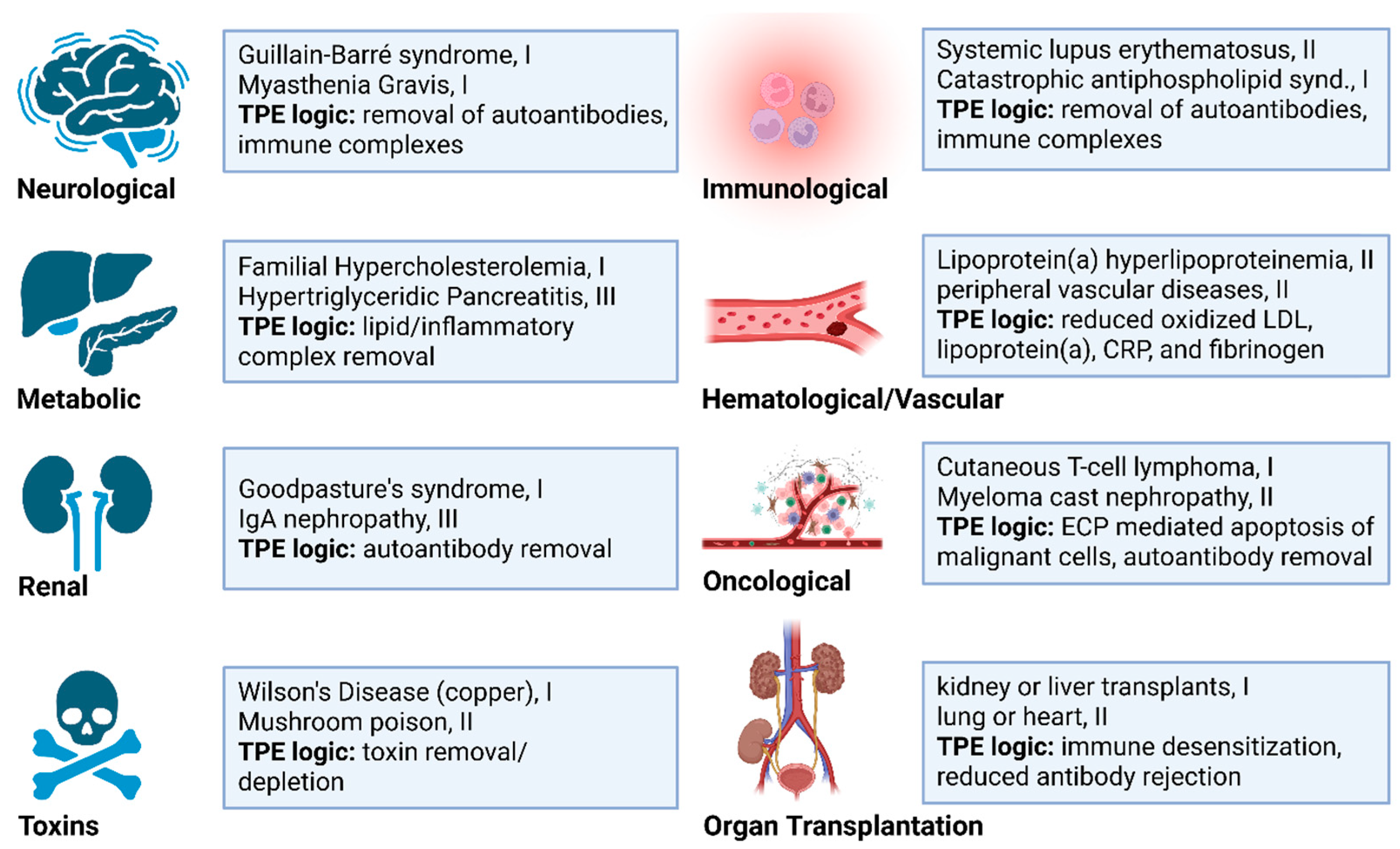
2. Diseases Currently Benefiting from Therapeutic Apheresis
3. New Disease Targets with Potential Therapeutic Responses to TPE
| Indication | ASFA Rank | Frequency | Concurrent Therapies | Benefits | Complications | Ref. |
|---|---|---|---|---|---|---|
| TTP (Thrombotic Thrombocytopenic Purpura) | I | Daily, 1/day, until remission (~1–2 wks) | Plasma replacement, steroids, rituximab | Mortality ↓ from ~90% to ~20%; platelet recovery | Catheter events, FFP reactions | [9,16] |
| GBS (Guillain–Barré syndrome) | I | 3–6 sessions total, typically 1/day | IVIG, steroids | Faster motor recovery; improved outcomes at 1 year | Hypotension, hypocalcemia | [9,17] |
| Myasthenic crisis (MG) | I | 4–6 sessions total, 1/day | Immunosuppressants | Rapid muscle strength/respiratory improvement | Hypotension, catheter-related risks | [9,18,19] |
| Anti-GBM (Goodpasture’s) | I | ~14 sessions, 1/day | Cyclophosphamide, steroids | Autoantibody removal; renal/pulmonary stabilization | Standard TPE risks* | [9,20,21] |
| Catastrophic antiphospholipid syndrome (CAPS) | I | Daily, 1/day for 1–3 weeks | Steroids, anticoagulation | Thrombotic control; antibody removal | Infection, line risks | [9,22] |
| Hyperviscosity syndromes (e.g., Waldenström’s) | I | 5–7 sessions over ~2 weeks, usually 1/day | Rituximab | Viscosity reduction; symptomatic relief | Standard TPE risks * | [9,23] |
| ANCA-associated vasculitis (rapidly progressive glomerulonephritis) | I | ~7 sessions, 1/day | Immunosuppressants (cyclophosphamide, steroids) | Removes pathogenic antibodies; preserves renal function | Standard TPE risks * | [9,24] |
| Neuromyelitis Optica Spectrum Disorder (NMOSD) | I | 5–7 sessions, 1/day | Immunosuppressants | Rapid removal of pathogenic antibodies | Hypotension, line complications | [25] |
| Multiple Sclerosis (acute relapse) | I | 5 sessions over 7–10 days, 1/day | Steroids | Improves symptoms in steroid-refractory relapse | Line infections, hypotension | [9,26] |
| Cryoglobulinemia | I | ~3–8 every 1–3 days, up to ~10 w/DFPP, over 2–4 wks | Antiviral, immunosuppressants | Removal of cryoglobulins | Standard TPE risks * | [27] |
| ABO/HLA desensitization | II | ~3–10+ sessions, generally 1/day | IVIg, anti-CD20 | Enables incompatible transplantation; graft survival | Infection, immunosuppression | [9,28] |
| Hyperlipoproteinemia (familial type) | II | Variable; weekly or biweekly | Lipid-lowering agents | Reduces LDL and triglycerides to prevent cardiovascular events | Vascular access complications | [9,29] |
| HIT/HITT | III | Variable, often 1/day perioperative | Discontinue heparin, alternate anticoagulants | Rapid anti-PF4 Ab removal; thrombosis prevention | Standard TPE risks * | [30,31] |
3.1. Neurodegenerative Disease
3.1.1. Current Neurodegenerative Disease Indications for TPE
3.1.2. Emerging Neuroimmune TPE Indications
3.1.3. Limitations and Opportunities for TPE in CNS Disease
3.2. Metabolic Disease
3.2.1. Current Metabolic Disease Indications for TPE
3.2.2. Emerging TPE Applications in Metabolic Diseases
3.3. Cardiovascular Disease (CVD)
3.3.1. Current CVD Indications for TPE
3.3.2. Emerging TPE Applications in CVD
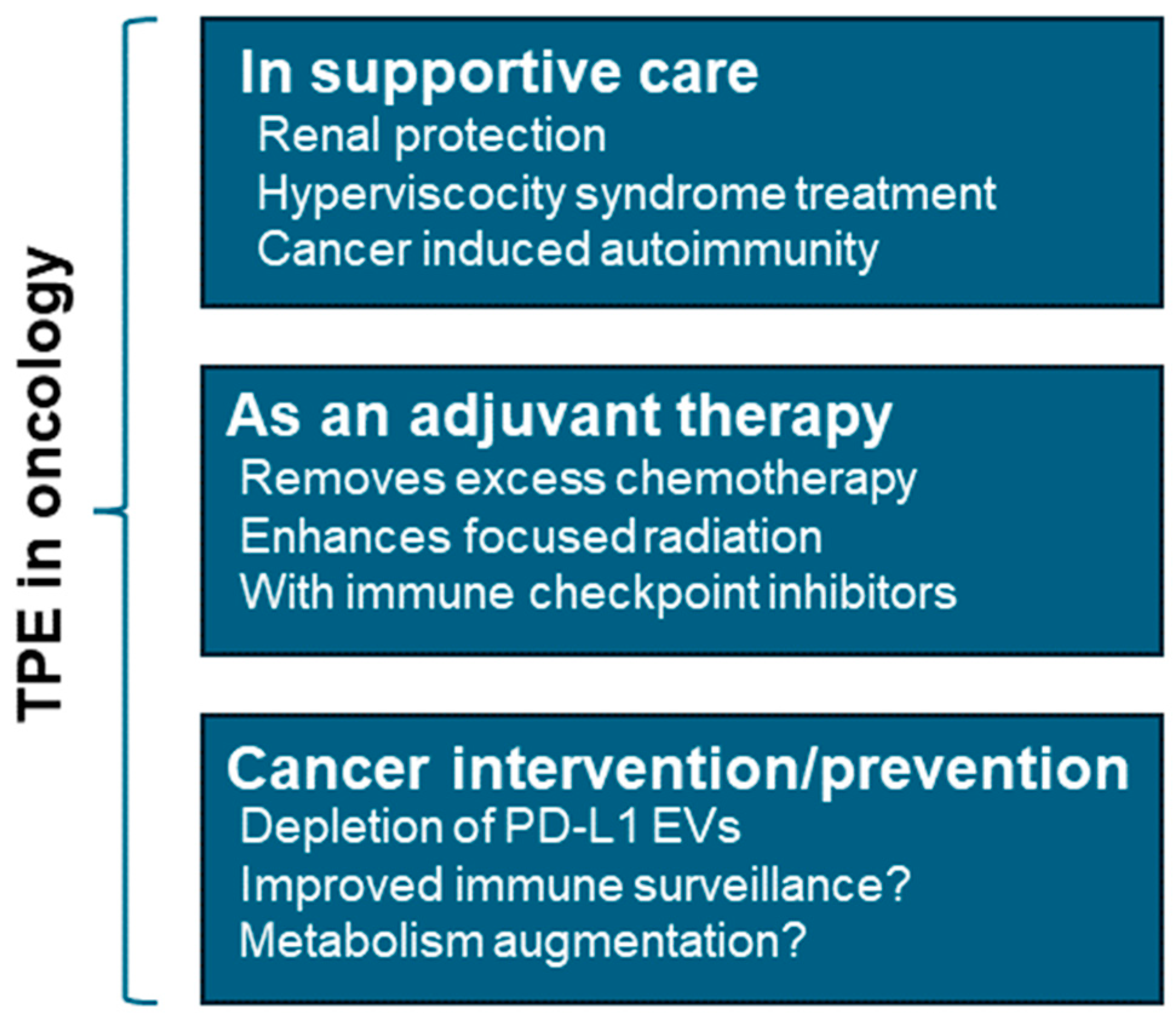
3.4. Cancer
3.4.1. TPE as a Cancer Supportive Therapy
3.4.2. TPE as a Cancer Intervention
3.4.3. Cancer Metabolism and TPE
4. Preclinical Modeling of TPE
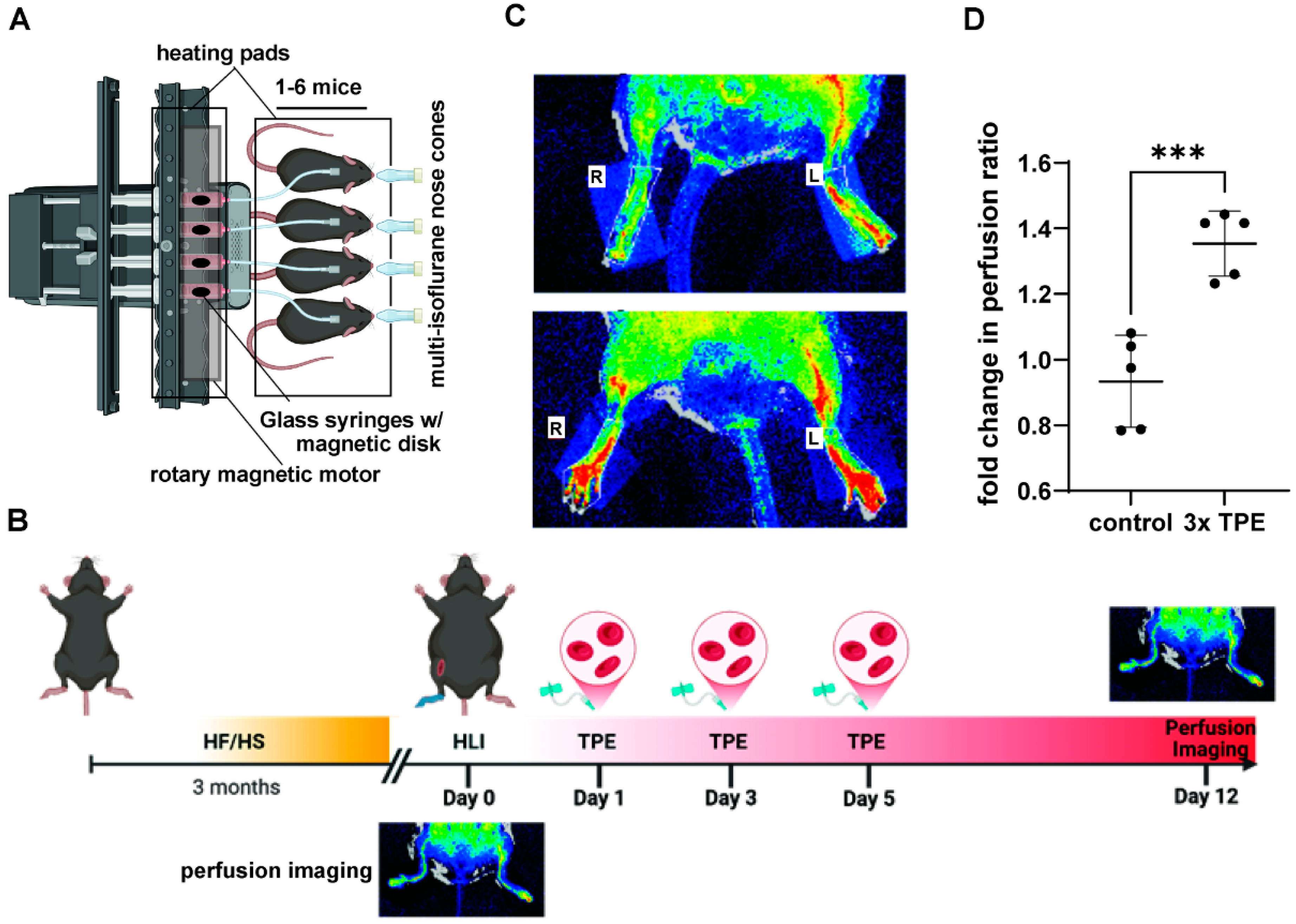
5. Cellular and Molecular Signaling Effects Associated with TPE
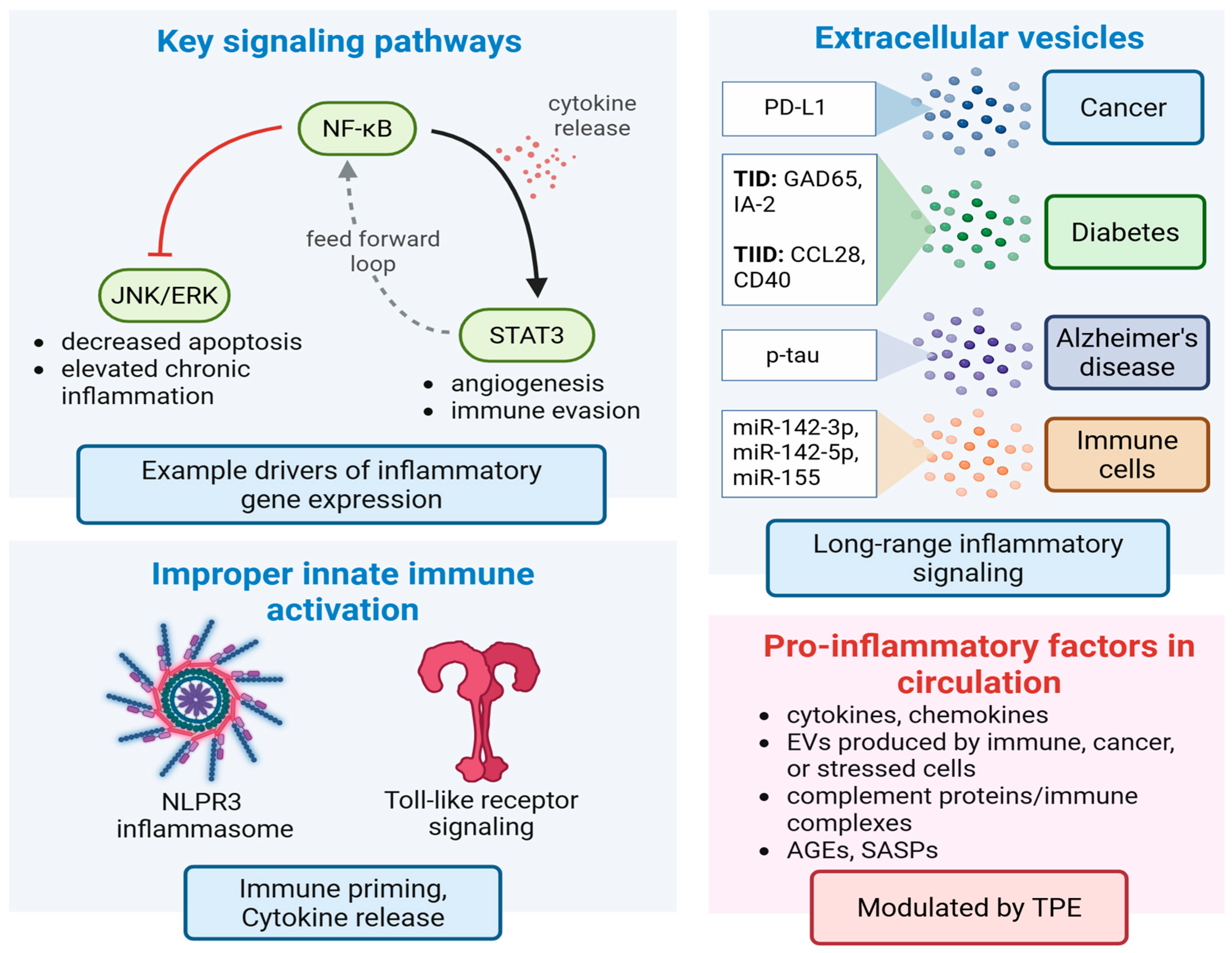
TPE-Mediated Changes to Inflammatory Signaling
6. Evidence for TPE in Tissue Rejuvenation
Effects of TPE on Stem Cell Niches
7. Effect of TPE on Extracellular Vesicle-Based Drivers of Disease
7.1. EVs in Alzheimer’s Disease (AD)
7.2. EVs in Metabolic Disease
7.3. EVs in Tumor Progression
8. Current Limitations and Future Iterations of TPE
8.1. Current Limitations
8.2. Future Iterations
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, J.; Sankar, P.; Varacallo, M.A. Physiology, Blood Plasma; Statpearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mehdipour, M.; Etienne, J.; Liu, C.; Mehdipour, T.; Kato, C.; Conboy, M.; Conboy, I.; Kiprov, D.D. Attenuation of Age-Elevated Blood Factors by Repositioning Plasmapheresis: A Novel Perspective and Approach. Transfus. Apher. Sci. 2021, 60, 103162. [Google Scholar] [CrossRef]
- Cervantes, C.E.; Bloch, E.M.; Sperati, C.J. Therapeutic Plasma Exchange: Core Curriculum 2023. Am. J. Kidney Dis. 2023, 81, 475–492. [Google Scholar] [CrossRef]
- Abel, J.J.; Rowntree, L.G.; Turner, B.B. On the Removal of Diffusible Substances from the Circulating Blood of Living Animals by Dialysis Ii. Some Constituents of the Blood. J. Pharmacol. Exp. Ther. 1914, 5, 611–623. [Google Scholar] [CrossRef]
- Rivera, A.M.; Strauss, K.W.; Van Zundert, A.; Mortier, E. The History of Peripheral Intravenous Catheters: How Little Plastic Tubes Revolutionized Medicine. Acta Anaesthesiol. Belg. 2005, 56, 271–282. [Google Scholar] [PubMed]
- Schwab, P.J.; Fahey, J.L. Treatment of Waldenström’s Macroglobulinemia by Plasmapheresis. N. Engl. J. Med. Fahey. 1960, 263, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J.; Bogen, S.A. Bogen. Role of Plasmapheresis in Waldenström’s Macroglobulinemia. Clin. Lymphoma. Myeloma. Leuk. 2013, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, E.; Parhofer, K.G. Lipoprotein Apheresis to Treat Elevated Lipoprotein (a). J. Lipid. Res. 2016, 57, 1751–1757. [Google Scholar] [CrossRef]
- Connelly-Smith, L.; Alquist, C.R.; Aqui, N.A.; Hofmann, J.C.; Klingel, R.; Onwuemene, O.A.; Patriquin, C.J.; Pham, H.P.; Sanchez, A.P.; Schneiderman, J.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J. Clin. Apher. 2023, 38, 77–278. [Google Scholar] [CrossRef]
- Bereanu, A.-S.; Pisaltu, T.; Bereanu, R.; Vintila, B.; Codru, I.; Chicea, L.; Crisan, O.; Căinap, C.; Cainap, S.; Sava, M. Therapeutic Plasma Exchange in Catastrophic Antiphospholipid Syndrome (Caps): A Rare Case Report and Literature Review. In Vivo 2023, 37, 1914–1919. [Google Scholar] [CrossRef]
- Chandra, T.; Solanki, A.; Singh, A.; Chauhan, A.; Himanshu, D. Therapeutic Plasma Exchange: A Life-Saving Modality in Wegener’s Granulomatosis. Asian J. Transfus. Sci. 2020, 14, 203–205. [Google Scholar] [CrossRef]
- Aguirre-Valencia, D.; Naranjo-Escobar, J.; Posso-Osorio, I.; Macía-Mejía, M.C.; Nieto-Aristizábal, I.; Barrera, T.; Obando, M.A.; Tobón, G.J. Therapeutic Plasma Exchange as Management of Complicated Systemic Lupus Erythematosus and Other Autoimmune Diseases. Autoimmune. Dis. 2019, 2019, 5350960. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.; Bauernhofer, T.; Krippl, P.; Lang-Loidolt, D.; Horn, S.; Goessler, W.; Schippinger, W.; Ploner, F.; Stoeger, H.; Samonigg, H. Plasmapheresis Reverses All Side-Effects of a Cisplatin Overdose—A Case Report and Treatment Recommendation. BMC Cancer 2006, 6, 1. [Google Scholar] [CrossRef]
- Chevret, S.; Hughes, R.A.; Annane, D. Plasma Exchange for Guillain-Barré Syndrome. Cochrane. Database Syst. Rev. 2017, 2, Cd001798. [Google Scholar] [CrossRef] [PubMed]
- Boada, M.; López, O.L.; Olazarán, J.; Núñez, L.; Pfeffer, M.; Paricio, M.; Lorites, J.; Piñol-Ripoll, G.; Gámez, J.E.; Anaya, F.; et al. A Randomized, Controlled Clinical Trial of Plasma Exchange with Albumin Replacement for Alzheimer’s Disease: Primary Results of the Ambar Study. Alzheimer’s Dement. 2020, 16, 1412–1425. [Google Scholar] [CrossRef]
- Coppo, P.; Froissart, A. Treatment of Thrombotic Thrombocytopenic Purpura Beyond Therapeutic Plasma Exchange. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Taylor, R.S. Guillain-Barre Syndrome; Statpearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mandawat, A.; Mandawat, A.; Kaminski, H.J.; Shaker, Z.A.; Alawi, A.A.; Alshekhlee, A. Outcome of Plasmapheresis in Myasthenia Gravis: Delayed Therapy Is Not Favorable. Muscle Nerve. 2011, 43, 578–584. [Google Scholar] [CrossRef]
- Konishi, T. Plasmapheresis in Patients with Myasthenia Gravis. Nihon. Rinsho. 2008, 66, 1165–1171. [Google Scholar]
- Simpson, I.J.; Doak, P.B.; Williams, L.C.; Blacklock, H.A.; Hill, R.S.; Teague, C.A.; Herdson, P.B.; Wilson, C.B. Plasma Exchange in Goodpasture’s Syndrome. Am. J. Nephrol. 1982, 2, 301–311. [Google Scholar] [CrossRef]
- Lockwood, C.M.; Boulton-Jones, J.M.; Lowenthal, R.M.; Simpson, I.J.; Peters, D.K. Recovery from Goodpasture’s Syndrome after Immunosuppressive Treatment and Plasmapheresis. Br. Med. J. 1975, 2, 252–254. [Google Scholar] [CrossRef]
- Rodríguez-Pintó, I.; Lozano, M.; Cid, J.; Espinosa, G.; Cervera, R. Plasma Exchange in Catastrophic Antiphospholipid Syndrome. Presse Med. 2019, 48, 347–353. [Google Scholar] [CrossRef]
- Menke, M.N.; Feke, G.T.; McMeel, J.W.; Treon, S.P. Effect of Plasmapheresis on Hyperviscosity-Related Retinopathy and Retinal Hemodynamics in Patients with Waldenstrom’s Macroglobulinemia. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1157–1160. [Google Scholar] [CrossRef]
- Tsiakas, S.; Marinaki, S.; Lionaki, S.; Boletis, J. Plasma Exchange in Anca-Associated Vasculitis: A Narrative Review. J. Clin. Med. 2021, 10, 5154. [Google Scholar] [CrossRef]
- Siritho, S.; Nopsopon, T.; Pongpirul, K. Therapeutic Plasma Exchange Vs Conventional Treatment with Intravenous High Dose Steroid for Neuromyelitis Optica Spectrum Disorders (Nmosd): A Systematic Review and Meta-Analysis. J. Neurol. 2021, 268, 4549–4562. [Google Scholar] [CrossRef] [PubMed]
- Bunganic, R.; Blahutova, S.; Revendova, K.; Zapletalova, O.; Hradilek, P.; Hrdlickova, R.; Ganesh, A.; Cermakova, Z.; Bar, M.; Volny, O. Therapeutic Plasma Exchange in Multiple Sclerosis Patients with an Aggressive Relapse: An Observational Analysis in a High-Volume Center. Sci. Rep. 2022, 12, 18374. [Google Scholar] [CrossRef] [PubMed]
- Rockx, M.; Clark, W. Plasma Exchange for Treating Cryoglobulinemia: A Descriptive Analysis. Transfus. Apher. Sci. 2010, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Choi, J.; Vo, A. Achieving Incompatible Transplantation through Desensitization: Current Perspectives and Future Directions. Immunotherapy 2015, 7, 377–398. [Google Scholar] [CrossRef]
- Makino, H.; Koezuka, R.; Tamanaha, T.; Ogura, M.; Matsuki, K.; Hosoda, K.; Harada-Shiba, M. Familial Hypercholesterolemia and Lipoprotein Apheresis. J. Atheroscler. Thromb. 2019, 26, 679–687. [Google Scholar] [CrossRef]
- Welsby, I.J.; Um, J.; Milano, C.A.; Ortel, T.L.; Arepally, G. Plasmapheresis and Heparin Reexposure as a Management Strategy for Cardiac Surgical Patients with Heparin-Induced Thrombocytopenia. Anesth. Analg. 2010, 110, 30–35. [Google Scholar] [CrossRef]
- Grazioli, A.; Splinter, N.P.; Plazak, M.E.; Griffith, B.P.; Dahi, S.; Bathula, A.H.; Cheung, N.H.; Padmanabhan, A. Cardiac Surgery in Acute Heparin-Induced Thrombocytopenia Managed with Therapeutic Plasma Exchange and Intravenous Immunoglobulin. Res. Pract. Thromb. Haemost. 2023, 7, 100089. [Google Scholar] [CrossRef]
- Β2-Microglobulin Boosts Β-Amyloid Aggregation and Neurotoxicity in an Alzheimer’s Disease Model. Nat. Neurosci. 2023, 26, 1143–1144. [CrossRef]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in Cns Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef]
- Carpo, M.; Cappellari, A.; Mora, G.; Pedotti, R.; Barbieri, S.; Scarlato, G.; Nobile-Orazio, E. Deterioration of Multifocal Motor Neuropathy after Plasma Exchange. Neurology 1998, 50, 1480–1482. [Google Scholar] [CrossRef]
- Cen, X.; Liu, S.; Cheng, K. The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Front. Pharmacol. 2018, 9, 878. [Google Scholar] [CrossRef]
- Rossi, S.; Farina, A.; Malvaso, A.; Dinoto, A.; Fionda, L.; Cornacchini, S.; Florean, I.; Zuliani, L.; Garibaldi, M.; Lauletta, A.; et al. Clinical Course of Neurologic Adverse Events Associated with Immune Checkpoint Inhibitors: Focus on Chronic Toxicities. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200314. [Google Scholar] [CrossRef]
- Gunning, W.T., III; Stepkowski, S.M.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Inflammatory Biomarkers in Postural Orthostatic Tachycardia Syndrome with Elevated G-Protein-Coupled Receptor Autoantibodies. J. Clin. Med. 2021, 10, 623. [Google Scholar] [CrossRef]
- Guptill, J.T.; Juel, V.C.; Massey, J.M.; Anderson, A.C.; Chopra, M.; Yi, J.S.; Esfandiari, E.; Buchanan, T.; Smith, B.; Atherfold, P.; et al. Effect of Therapeutic Plasma Exchange on Immunoglobulins in Myasthenia Gravis. Autoimmunity 2016, 49, 472–479. [Google Scholar] [CrossRef]
- Thieben, M.J.; Sandroni, P.; Sletten, D.M.; Benrud-Larson, L.M.; Fealey, R.D.; Vernino, S.; Lennon, V.A.; Shen, W.K.; Low, P.A. Postural Orthostatic Tachycardia Syndrome: The Mayo Clinic Experience. Mayo. Clin. Proc. 2007, 82, 308–313. [Google Scholar] [CrossRef]
- Wells, R.; Hissaria, P.; Elliott, A.D.; Sanders, P.; Page, A.; Baumert, M.; Lau, D.H. Plasma Exchange Therapy in Postural Tachycardia Syndrome: A Novel Long-Term Approach? Am. J. Med. 2020, 133, e157–e159. [Google Scholar] [CrossRef]
- López-Ornelas, A.; Jiménez, A.; Pérez-Sánchez, G.; Rodríguez-Pérez, C.E.; Corzo-Cruz, A.; Velasco, I.; Estudillo, E. The Impairment of Blood-Brain Barrier in Alzheimer’s Disease: Challenges and Opportunities with Stem Cells. Int. J. Mol. Sci. 2022, 23, 10136. [Google Scholar] [CrossRef]
- Costa, M.; Ortiz, A.M.; Jorquera, J.I. The Capacity of Albumin to Bind to Beta-Amyloid. Rev. Neurol. 2010, 50, 1–4. [Google Scholar] [CrossRef]
- Stanyon, H.F.; Viles, J.H. Human Serum Albumin Can Regulate Amyloid-Β Peptide Fiber Growth in the Brain Interstitium: Implications for Alzheimer Disease. J. Biol. Chem. 2012, 287, 28163–28168. [Google Scholar] [CrossRef]
- Mehdipour, M.; Mehdipour, T.; Skinner, C.M.; Wong, N.; Liu, C.; Chen, C.-C.; Jeon, O.H.; Zuo, Y.; Conboy, M.J.; Conboy, I.M. Plasma Dilution Improves Cognition and Attenuates Neuroinflammation in Old Mice. Geroscience 2021, 43, 1–18. [Google Scholar] [CrossRef]
- Mehdipour, M.; Skinner, C.; Wong, N.; Lieb, M.; Liu, C.; Etienne, J.; Kato, C.; Kiprov, D.; Conboy, M.J.; Conboy, I.M. Rejuvenation of Three Germ Layers Tissues by Exchanging Old Blood Plasma with Saline-Albumin. Aging 2020, 12, 8790–8819. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Gill, M.G.; Majumdar, A. Metabolic Associated Fatty Liver Disease: Addressing a New Era in Liver Transplantation. World J. Hepatol. 2020, 12, 1168–1181. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and Treatment of Cytokine Storm in Covid-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The Senescence-Associated Secretome as an Indicator of Age and Medical Risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Reeves, H.M.; Winters, J.L. Winters. The Mechanisms of Action of Plasma Exchange. Br. J. Haematol. 2014, 164, 342–351. [Google Scholar] [CrossRef]
- Raghav, A.; Singh, M.; Jeong, G.-B.; Giri, R.; Agarwal, S.; Kala, S.; Gautam, K.A. Extracellular Vesicles in Neurodegenerative Diseases: A Systematic Review. Front. Mol. Neurosci. 2022, 15, 1061076. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused Ultrasound-Mediated Drug Delivery through the Blood-Brain Barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Miao, F.; Braffett, B.H.; Lachin, J.M.; Zhang, L.; Wu, X.; Roshandel, D.; Carless, M.; Li, X.A.; Tompkins, J.D.; et al. DNA Methylation Mediates Development of Hba1c-Associated Complications in Type 1 Diabetes. Nat. Metab. 2020, 2, 744–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Miao, F.; Paterson, A.D.; Lachin, J.M.; Zhang, L.; Schones, D.E.; Wu, X.; Wang, J.; Tompkins, J.D.; Genuth, S.; et al. Epigenomic Profiling Reveals an Association between Persistence of DNA Methylation and Metabolic Memory in the Dcct/Edic Type 1 Diabetes Cohort. Proc. Natl. Acad. Sci. USA 2016, 113, E3002–E3011. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular Mechanisms Underlying Chemical Liver Injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Taylan, C.; Weber, L.T. An Update on Lipid Apheresis for Familial Hypercholesterolemia. Pediatr. Nephrol. 2023, 38, 371–382. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Jahn, J.; Golla, P.; Hafer, C.; Kielstein, J.T.; Kielstein, H. Effect of Therapeutic Plasma Exchange on Plasma Levels and Total Removal of Adipokines and Inflammatory Markers. BMC Obes. 2015, 2, 37. [Google Scholar] [CrossRef]
- Kim, A.S.; Hakeem, R.; Abdullah, A.; Hooper, A.J.; Tchan, M.C.; I Alahakoon, T.; Girgis, C.M. Therapeutic Plasma Exchange for the Management of Severe Gestational Hypertriglyceridaemic Pancreatitis Due to Lipoprotein Lipase Mutation. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 19–0165. [Google Scholar] [CrossRef]
- Liu, R.; Lu, J.; Zhang, D.; Lu, W.; Yu, Z.; Shao, X.; Xie, N.; Duan, L.; Xing, S.; Wang, X.; et al. Timely Application of Plasma Exchange to Correct Acute Pancreatitis Related to Serum Triglyceride Levels: A Report of 6 Cases and a Literature Review. Am. J. Case Rep. 2024, 25, e944763. [Google Scholar] [CrossRef]
- Chris-Olaiya, A.; Kapoor, A.; Ricci, K.S.; Lindenmeyer, C.C. Therapeutic Plasma Exchange in Liver Failure. World J. Hepatol. 2021, 13, 904–915. [Google Scholar] [CrossRef]
- Yadav, M.; Maiwal, R.; Br, V.K.; Tripathi, G.; Sharma, N.; Sharma, N.; Bindal, V.; Mathew, B.; Pandey, S.; Singh, S.P.; et al. Comparative Metabolome Analysis Reveals Higher Potential of Haemoperfusion Adsorption in Providing Favourable Outcome in Aclf Patients. Liver. Int. 2024, 44, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.; Heding, L.; Lieden, G.; Marner, B.; Lernmark, A. Plasmapheresis in the Initial Treatment of Insulin-Dependent Diabetes Mellitus in Children. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Grewal, P.; Baine, I.; Arinsburg, S.A.; Maximos, S.; Shah, N.A. Mycophenolate Mofetil and Plasmapheresis: A Treatment Option for Severe Insulin Resistance Caused by Insulin Antibodies. AACE Clin. Case Rep. 2021, 7, 307–309. [Google Scholar] [CrossRef]
- Sharwood, E.F.; Hughes, I.P.; Pretorius, C.J.; Trnka, P.; Peake, J.; Huynh, T. Therapeutic Plasma Exchange Normalizes Insulin-Mediated Response in a Child with Type 1 Diabetes and Insulin Autoimmune Syndrome. Pediatr. Diabetes. 2018, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.R.; Noser, A.E.; Youngkin, E.M.; Majidi, S.; Clements, M.A. Early Initiation of Diabetes Devices Relates to Improved Glycemic Control in Children with Recent-Onset Type 1 Diabetes Mellitus. Diabetes. Technol. Ther. 2019, 21, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Allegra, A.; Sciarrone, P.; Russo, S.; Cristani, M.; Gerace, D.; Saitta, S.; Alonci, A.; Musolino, C. Effect of Therapeutic Plasma Exchange on Plasma Levels of Oxidative Biomarkers in a Patient with Thrombotic Thrombocytopenic Purpura. Eur. J. Haematol. 2015, 94, 368–373. [Google Scholar] [CrossRef]
- Bolan, C.; Oral, E.A.; Gorden, P.; Taylor, S.; Leitman, S.F. Intensive, Long-Term Plasma Exchange Therapy for Severe Hypertriglyceridemia in Acquired Generalized Lipoatrophy. J. Clin. Endocrinol. Metab. 2002, 87, 380–384. [Google Scholar] [CrossRef]
- Rosa-Bray, M.; Wisdom, C.; Wada, S.; Johnson, B.R.; Grifols-Roura, V.; Grifols-Lucas, V. Prospective Multicentre Study of the Effect of Voluntary Plasmapheresis on Plasma Cholesterol Levels in Donors. Vox. Sang. 2013, 105, 108–115. [Google Scholar] [CrossRef]
- Simons, L.; Gibson, J.; Isbister, J.; Biggs, J. The Effects of Plasma Exchange on Cholesterol Metabolism. Atherosclerosis 1978, 31, 195–204. [Google Scholar] [CrossRef]
- Tompkins, J.D.; Riggs, A.D. An Epigenetic Perspective on the Failing Heart and Pluripotent-Derived-Cardiomyocytes for Cell Replacement Therapy. Front. Biol. 2015, 10, 11–27. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic Vascular Diseases: Molecular Mechanisms and Therapeutic Strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Piedrafita, A.; Ribes, D.; Cointault, O.; Chauveau, D.; Faguer, S.; Huart, A. Plasma Exchange and Thrombotic Microangiopathies: From Pathophysiology to Clinical Practice. Transfus. Apher. Sci. 2020, 59, 102990. [Google Scholar] [CrossRef] [PubMed]
- Winters, J.L. Plasma Exchange in Thrombotic Microangiopathies (Tmas) Other Than Thrombotic Thrombocytopenic Purpura (Ttp). Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 632–638. [Google Scholar] [CrossRef]
- Marchetti, M.; Zermatten, M.G.; Bertaggia Calderara, D.; Aliotta, A.; Alberio, L. Heparin-Induced Thrombocytopenia: A Review of New Concepts in Pathogenesis, Diagnosis, and Management. J. Clin. Med. 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Westbrook, B.; Cuker, A. Heparin-Induced Thrombocytopenia: An Illustrated Review. Res. Pract. Thromb. Haemost. 2023, 7, 100283. [Google Scholar] [CrossRef]
- Arepally, G.M.; Padmanabhan, A. Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arter. Thromb. Vasc. Biol. 2021, 41, 141–152. [Google Scholar] [CrossRef]
- Arepally, G.M. Heparin-Induced Thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef]
- Kajitani, M.; Aguinaga, M.; Johnson, C.E.; Scott, M.A.; Antakli, T. Use of Plasma Exchange and Heparin During Cardiopulmonary Bypass for a Patient with Heparin Induced Thrombocytopenia: A Case Report. J. Card Surg. 2001, 16, 313–318. [Google Scholar] [CrossRef]
- Jaben, E.A.; Torloni, A.S.; Pruthi, R.K.; Winters, J.L. Use of Plasma Exchange in Patients with Heparin-Induced Thrombocytopenia: A Report of Two Cases and a Review of the Literature. J. Clin. Apher. 2011, 26, 219–224. [Google Scholar] [CrossRef]
- Koizumi, S.; Kohno, H.; Watanabe, M.; Iwahana, T.; Maeda, T.; Miyata, S.; Kobayashi, Y.; Matsumiya, G. Left Ventricular Assist Device Implantation after Plasma Exchange for Heparin-Induced Thrombocytopenia. J. Artif. Organs. 2018, 21, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.C.; Klompas, A.M.; Stulak, J.M.; Yalamuri, S.M. The Triple Hit: Perioperative Management of Heparin-Induced Thrombocytopenia Using Plasma Exchange, Intravenous Immunoglobulin, and Protamine Infusion for Left Ventricular Assist Device Implantation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Pham, L.-P.; McNeil, J.; Singh, K.; Tanaka, K.; Eaton, M.; Mazzeffi, M. Efficacy of Therapeutic Plasma Exchange or Cangrelor as an Adjunctive Strategy to Facilitate Cardiopulmonary Bypass in Patients with Heparin-Induced Thrombocytopenia: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2915–2924. [Google Scholar] [CrossRef]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Zheng, S.S.; Rashid, F.N.; Enjeti, A.; Ting, S.B.; Chong, J.J.H.; Chong, B.H. Netosis and Thrombosis in Vaccine-Induced Immune Thrombotic Thrombocytopenia. Nat. Commun. 2022, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Berent, T.; Derfler, K.; Berent, R.; Sinzinger, H. Lipoprotein Apheresis in Austria—Reduction of Cardiovascular Events by Regular Lipoprotein Apheresis Treatment. Atheroscler. Suppl. 2019, 40, 8–11. [Google Scholar] [CrossRef]
- Solignac, J.; Bataille, S.; Touzot, M.; Bruner, F.; Bouchouareb, D.; Brunet, P.; Ridel, C.; Robert, T. Rheopheresis for Severe Peripheral Arterial Disease in Hemodialysis Patients: A Clinical Series. J. Clin. Apher. 2022, 37, 91–99. [Google Scholar] [CrossRef]
- Weiss, N.A. Critical Review on the Use of Lipid Apheresis and Rheopheresis for Treatment of Peripheral Arterial Disease and the Diabetic Foot Syndrome. Semin. Dial. 2012, 25, 220–227. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Aging Insights from Heterochronic Parabiosis Models. npj. Aging 2024, 10, 38. [Google Scholar] [CrossRef]
- Leung, N.; Gertz, M.; Zeldenrust, S.; Rajkumar, S.; Dispenzieri, A.; Fervenza, F.; Kumar, S.; Lacy, M.; Lust, J.; Greipp, P.; et al. Improvement of Cast Nephropathy with Plasma Exchange Depends on the Diagnosis and on Reduction of Serum Free Light Chains. Kidney Int. 2008, 73, 1282–1288. [Google Scholar] [CrossRef]
- Dima, D.; Goel, U.; Sannareddy, A.; Ibeh, N.; Ullah, F.; Afrough, A.; Mazzoni, S.; Mehdi, A.; Rudoni, J.; Raza, S.; et al. Outcomes of Therapeutic Plasma Exchange for the Treatment of Patients with Multiple Myeloma Cast Nephropathy. Hematol. Oncol. 2024, 42, e3293. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, M.; Ferrari, F.; Magistroni, R.; Mariano, M.; Ceccherelli, G.B.; Milanti, G.; De Palma, M.; Albertazzi, A. Plasma Exchange in Acute and Chronic Hyperviscosity Syndrome: A Rheological Approach and Guidelines Study. Ann. Dell’istituto Super. Di Sanità 2007, 43, 171–175. [Google Scholar]
- Gertz, M.A. Acute Hyperviscosity: Syndromes and Management. Blood 2018, 132, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Lv, C.; Hong, D.; Wang, Z.; Mao, W.; Liu, Y.; Zhang, Z.; Li, Y.; Li, G.; et al. Impact of Therapeutic Plasmapheresis on the Duration of Organ Failure in Patients with Hypertriglyceridemia-Associated Acute Pancreatitis. Ann. Intensive Care 2024, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Z.; Liu, Q.; Cao, L.; De-Madaria, E.; Capurso, G.; Stoppe, C.; Wu, D.; Huang, W.; Chen, Y.; et al. Triglyceride-Lowering Therapies in Hypertriglyceridemia-Associated Acute Pancreatitis in China: A Multicentre Prospective Cohort Study. BMC Med. 2024, 22, 535. [Google Scholar] [CrossRef]
- Zieliński, M. Management of Myasthenic Patients with Thymoma. Thorac. Surg. Clin. 2011, 21, 47–57. [Google Scholar] [CrossRef]
- Noridomi, K.; Watanabe, G.; Hansen, M.N.; Han, G.W.; Chen, L. Structural Insights into the Molecular Mechanisms of Myasthenia Gravis and Their Therapeutic Implications. Elife 2017, 6, e23043. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, H.; Fu, S.; Wu, J. Therapeutic Plasma Exchange: For Cancer Patients. Cancer Manag. Res. 2022, 14, 411–425. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Buhler, J.; Chu, E.; Chen, S.A.; Human, T. Drug Dosing in Patients Undergoing Therapeutic Plasma Exchange. Neurocrit. Care 2021, 34, 301–311. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Song, Q.; Yang, S.; Wu, X.; Yang, D.; Marié, I.J.; Qin, H.; Zheng, M.; Nasri, U.; et al. Donor T Cell Stat3 Deficiency Enables Tissue Pd-L1-Dependent Prevention of Graft-Versus-Host Disease While Preserving Graft-Versus-Leukemia Activity. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef] [PubMed]
- Serratì, S.; Guida, M.; Di Fonte, R.; De Summa, S.; Strippoli, S.; Iacobazzi, R.M.; Quarta, A.; De Risi, I.; Guida, G.; Paradiso, A.; et al. Circulating Extracellular Vesicles Expressing Pd1 and Pd-L1 Predict Response and Mediate Resistance to Checkpoint Inhibitors Immunotherapy in Metastatic Melanoma. Mol. Cancer 2022, 21, 20. [Google Scholar] [CrossRef]
- Orme, J.J.; Enninga, E.A.L.; Lucien-Matteoni, F.; Dale, H.; Burgstaler, E.; Harrington, S.M.; Ball, M.K.; Mansfield, A.S.; Park, S.S.; Block, M.S.; et al. Therapeutic Plasma Exchange Clears Circulating Soluble Pd-L1 and Pd-L1-Positive Extracellular Vesicles. J. Immunother. Cancer 2020, 8, e001113. [Google Scholar] [CrossRef] [PubMed]
- Orme, J.J.; Zhang, H.; Lingamaneni, P.; Kim, Y.; Lavoie, R.; Dorr, M.; Dizona, P.; Hirdler, J.; Bering, E.A.; Gicobi, J.K.; et al. Plasma Exchange and Radiation Resensitize Immunotherapy-Refractory Melanoma: A Phase I Trial. Nat. Commun. 2025, 16, 2507. [Google Scholar] [CrossRef] [PubMed]
- Alfred, A.; Taylor, P.C.; Dignan, F.; El-Ghariani, K.; Griffin, J.; Gennery, A.R.; Bonney, D.; Das-Gupta, E.; Lawson, S.; Malladi, R.K.; et al. The Role of Extracorporeal Photopheresis in the Management of Cutaneous T-Cell Lymphoma, Graft-Versus-Host Disease and Organ Transplant Rejection: A Consensus Statement Update from the Uk Photopheresis Society. Br. J. Haematol. 2017, 177, 287–310. [Google Scholar] [CrossRef]
- Zic, J.A. The Treatment of Cutaneous T-Cell Lymphoma with Photopheresis. Dermatol. Ther. 2003, 16, 337–346. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Huang, R.; Kang, T.; Chen, S. The Role of Tumor-Associated Macrophages in Tumor Immune Evasion. J. Cancer Res. Clin. Oncol. 2024, 150, 238. [Google Scholar] [CrossRef]
- Keith, P.D.; Wells, A.H.; Hodges, J.; Fast, S.H.; Adams, A.; Scott, L.K. The Therapeutic Efficacy of Adjunct Therapeutic Plasma Exchange for Septic Shock with Multiple Organ Failure: A Single-Center Experience. Crit. Care 2020, 24, 518. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug. Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Ciorpac, M.; Caratașu, C.; Szilagyi, A.; Mihai, C.; Nistor, I.; Iliescu, R.; Tamba, B.I. A Novel Device and System Concept for Therapeutic Plasma Exchange in Rats. Ther. Apher. Dial. 2023, 27, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, Z.; Xu, X.; Lu, B.; Gu, Y.; Yoon, J.; Xia, J.; Ma, Z.; Upreti, N.; Anwar, I.J.; et al. Acoustofluidic-Based Therapeutic Apheresis System. Nat. Commun. 2024, 15, 6854. [Google Scholar] [CrossRef]
- Rebo, J.; Mehdipour, M.; Gathwala, R.; Causey, K.; Liu, Y.; Conboy, M.J.; Conboy, I.M. A Single Heterochronic Blood Exchange Reveals Rapid Inhibition of Multiple Tissues by Old Blood. Nat. Commun. 2016, 7, 13363. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; Garzotto, F.; Parolin, M.; Manenti, C.; Zanin, A.; Bellettato, M.; Remuzzi, G.; Goldstein, S.L.; Murer, L.; Ronco, C. Therapeutic Plasma Exchange in Neonates and Infants: Successful Use of a Miniaturized Machine. Blood Purif. 2017, 44, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, S.; Wright-Jin, E. Nf-Κb as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The Nlrp3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (Mapkapks) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. Jak-Stat Signaling in Inflammation and Stress-Related Diseases: Implications for Therapeutic Interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef]
- Gehrke, N.; Hövelmeyer, N.; Waisman, A.; Straub, B.K.; Weinmann-Menke, J.; Wörns, M.A.; Galle, P.R.; Schattenberg, J.M. Hepatocyte-Specific Deletion of Il1-Ri Attenuates Liver Injury by Blocking Il-1 Driven Autoinflammation. J. Hepatol. 2018, 68, 986–995. [Google Scholar] [CrossRef]
- Wang, H.; Wei, W.; Zhang, J.-P.; Song, Z.; Li, Y.; Xiao, W.; Liu, Y.; Zeng, M.-S.; Petrus, M.N.; Thomas, C.J.; et al. A Novel Model of Alternative Nf-Κb Pathway Activation in Anaplastic Large Cell Lymphoma. Leukemia 2021, 35, 1976–1989. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. Stats in Cancer Inflammation and Immunity: A Leading Role for Stat3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Steven, M.M.; Tanner, A.R.; Holdstock, G.E.; Cockerell, R.; Smith, J.; Smith, D.S.; Hamblin, T.J.; Wright, R. The Effect of Plasma Exchange on the in Vitro Monocyte Function of Patients with Immune Complex Diseases. Clin. Exp. Immunol. 1981, 45, 240. [Google Scholar]
- Dau, P.C. Immunomodulation During Treatment of Polymyositis with Plasmapheresis and Immunosuppressive Drugs. J. Clin. Apher. 1994, 9, 21–25. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Chien, P.-J.; Hsueh, Y.-M.; Shih, C.-M.; Chiu, H.-C. Changes in the Lymphocyte Subset after Double-Filtration Plasmapheresis. Am. J. Clin. Pathol. 2007, 128, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Baráth, S.; Soltész, P.; Kiss, E.; Aleksza, M.; Zeher, M.; Szegedi, G.; Sipka, S. The Severity of Systemic Lupus Erythematosus Negatively Correlates with the Increasing Number of Cd4+Cd25(High)Foxp3+ Regulatory T Cells During Repeated Plasmapheresis Treatments of Patients. Autoimmunity 2007, 40, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Malvaso, A.; Cerne, D.; Bernini, S.; Bottiroli, S.; Marchioni, E.; Businaro, P.; Masciocchi, S.; Morandi, C.; Scaranzin, S.; Mobilia, E.M. Retrograde Amnesia in Lgi1 and Caspr2 Limbic Encephalitis: Two Case Reports and a Systematic Literature Review. Eur. J. Neurol. 2025, 32, e70113. [Google Scholar] [CrossRef]
- Karpova, D.; Encabo, H.H.; Donato, E.; Calderazzo, S.; Scherer, M.; Llorian-Sopena, M.; Leppä, A.-M.; Würth, R.; Stelmach, P.; Papazoglou, D.; et al. Clonal Hematopoiesis Landscape in Frequent Blood Donors. Blood 2025, 145, 2411–2423. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Q.; Zhang, S.; Chen, Q.; Wang, C.; Zhang, W.; Xiao, C.; Li, Y.; Nian, C.; Li, J.; et al. Fgf15 Activates Hippo Signaling to Suppress Bile Acid Metabolism and Liver Tumorigenesis. Dev. Cell 2019, 48, 460–474.e9. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. A Feedback Loop Controlling Organ Size. Dev. Cell 2019, 48, 425–426. [Google Scholar] [CrossRef]
- Tumaneng, K.; Russell, R.C.; Guan, K.-L. Organ Size Control by Hippo and Tor Pathways. Curr. Biol. 2012, 22, R368–R379. [Google Scholar] [CrossRef]
- Ahmed, A.S.I.; Sheng, M.H.; Wasnik, S.; Baylink, D.J.; Lau, K.-H.W. Effect of Aging on Stem Cells. World J. Exp. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Samiminemati, A.; Aprile, D.; Siniscalco, D.; Di Bernardo, G. Methods to Investigate the Secretome of Senescent Cells. Methods Protoc. 2024, 7, 52. [Google Scholar] [CrossRef]
- Ma, S.; Wang, S.; Ye, Y.; Ren, J.; Chen, R.; Li, W.; Li, J.; Zhao, L.; Zhao, Q.; Sun, G.; et al. Heterochronic Parabiosis Induces Stem Cell Revitalization and Systemic Rejuvenation across Aged Tissues. Cell Stem. Cell 2022, 29, 990–1005.e10. [Google Scholar] [CrossRef]
- Pálovics, R.; Keller, A.; Schaum, N.; Tan, W.; Fehlmann, T.; Borja, M.; Kern, F.; Bonanno, L.; Calcuttawala, K.; Webber, J.; et al. Molecular Hallmarks of Heterochronic Parabiosis at Single-Cell Resolution. Nature 2022, 603, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Yousef, H.; Conboy, M.J.; Morgenthaler, A.; Schlesinger, C.; Bugaj, L.; Paliwal, P.; Greer, C.; Conboy, I.M.; Schaffer, D. Systemic Attenuation of the Tgf-Β Pathway by a Single Drug Simultaneously Rejuvenates Hippocampal Neurogenesis and Myogenesis in the Same Old Mammal. Oncotarget 2015, 6, 11959–11978. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (Misev2023): From Basic to Advanced Approaches. J. Extracell Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Yu, J.; Sane, S.; Kim, J.-E.; Yun, S.; Kim, H.-J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and Delivery of Extracellular Vesicles: Harnessing the Power of Evs for Diagnostics and Therapeutics. Front. Mol. Biosci. 2023, 10, 1330400. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Barnes, M.V.C.; Pantazi, P.; Holder, B. Circulating Extracellular Vesicles in Healthy and Pathological Pregnancies: A Scoping Review of Methodology, Rigour and Results. J. Extracell Vesicles 2023, 12, e12377. [Google Scholar] [CrossRef]
- Jerabkova-Roda, K.; Dupas, A.; Osmani, N.; Hyenne, V.; Goetz, J.G. Circulating Extracellular Vesicles and Tumor Cells: Sticky Partners in Metastasis. Trends Cancer 2022, 8, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Jäger, B.; Piackova, E.; Sztulman, L.; Wegberger, C.; Wojta, J.; Gyöngyösi, M.; Kiss, A.; Podesser, B.K.; Spittler, A.; et al. Changes in Circulating Extracellular Vesicles in Patients with St-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning-a Randomized Controlled Trial. Biomedicines 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Aharon, A.; Spector, P.; Ahmad, R.S.; Horrany, N.; Sabbach, A.; Brenner, B.; Aharon-Peretz, J. Extracellular Vesicles of Alzheimer’s Disease Patients as a Biomarker for Disease Progression. Mol. Neurobiol. 2020, 57, 4156–4169. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Esteban-De-Antonio, E.; Bernuz, M.; Puerta, R.; García-González, P.; de Rojas, I.; Olivé, C.; Pérez-Cordón, A.; Montrreal, L.; Núñez-Llaves, R.; et al. Plasma Extracellular Vesicles Reveal Early Molecular Differences in Amyloid Positive Patients with Early-Onset Mild Cognitive Impairment. J. Nanobiotechnology 2023, 21, 54. [Google Scholar] [CrossRef]
- Ruan, Z.; Pathak, D.; Kalavai, S.V.; Yoshii-Kitahara, A.; Muraoka, S.; Bhatt, N.; Takamatsu-Yukawa, K.; Hu, J.; Wang, Y.; Hersh, S.; et al. Alzheimer’s Disease Brain-Derived Extracellular Vesicles Spread Tau Pathology in Interneurons. Brain 2021, 144, 288–309. [Google Scholar] [CrossRef]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.; et al. Exosome-Associated Tau Is Secreted in Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in Early Alzheimer Disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Zhang, F.-L.; Jin, H.; Yan, X.-L.; Huang, S.; Guo, Z.-N.; Yang, Y. Clinical Potential of Extracellular Vesicles in Type 2 Diabetes. Front. Endocrinol. 2020, 11, 596811. [Google Scholar] [CrossRef]
- Pang, H.; Luo, S.; Xiao, Y.; Xia, Y.; Li, X.; Huang, G.; Xie, Z.; Zhou, Z. Emerging Roles of Exosomes in T1dm. Front. Immunol. 2020, 11, 593348. [Google Scholar] [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef]
- Wu, S.F.; Noren Hooten, N.; Freeman, D.W.; Mode, N.A.; Zonderman, A.B.; Evans, M.K. Extracellular Vesicles in Diabetes Mellitus Induce Alterations in Endothelial Cell Morphology and Migration. J. Transl. Med. 2020, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.; DiStefano, P.V.; Wang, X.F.; Wu, R.; Ghaffari, S.; Ching, C.; Rathnakumar, K.; Alibhai, F.; Syonov, M.; Fitzpatrick, J.; et al. Circulating Small Extracellular Vesicles Mediate Vascular Hyperpermeability in Diabetes. Diabetologia 2024, 67, 1138–1154. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Hamelin, R.; Demurtas, D.; Ahmed, M.A.; Piemonti, L.; Hirosue, S.; Swartz, M.A.; De Palma, M.; et al. Primary Human and Rat Beta-Cells Release the Intracellular Autoantigens Gad65, Ia-2, and Proinsulin in Exosomes Together with Cytokine-Induced Enhancers of Immunity. Diabetes 2017, 66, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, M.C.; Lambooij, J.M.; Pu, X.; Fagundes, R.R.; Enciso-Martinez, A.; Kats, K.; Giepmans, B.N.G.; Guigas, B.; Zaldumbide, A. Extracellular Vesicles Derived from Stressed Beta Cells Mediate Monocyte Activation and Contribute to Islet Inflammation. Front. Immunol. 2024, 15, 1393248. [Google Scholar] [CrossRef]
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-Derived Exosomal Micrornas Promote Pancreatic Beta Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019, 29, 348–361 e6. [Google Scholar] [CrossRef]
- Busatto, S.; Yang, Y.; Iannotta, D.; Davidovich, I.; Talmon, Y.; Wolfram, J. Considerations for Extracellular Vesicle and Lipoprotein Interactions in Cell Culture Assays. J. Extracell Vesicles 2022, 11, e12202. [Google Scholar] [CrossRef]
- Busatto, S.; Yang, Y.; Walker, S.A.; Davidovich, I.; Lin, W.-H.; Lewis-Tuffin, L.; Anastasiadis, P.Z.; Sarkaria, J.; Talmon, Y.; Wurtz, G.; et al. Brain Metastases-Derived Extracellular Vesicles Induce Binding and Aggregation of Low-Density Lipoprotein. J. Nanobiotechnology 2020, 18, 162. [Google Scholar] [CrossRef]
- Clancy, J.W.; D’SOuza-Schorey, C. Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment. Annu. Rev. Pathol. 2023, 18, 205–229. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Zhang, Y.; Tao, Y.; Zhao, L.; Abusalamah, H.; Huffman, C.; Harbison, R.A.; Puram, S.V.; Wang, Y.; et al. Tumor Extracellular Vesicle-Derived Pd-L1 Promotes T Cell Senescence through Lipid Metabolism Reprogramming. Sci. Transl. Med. 2025, 17, eadm7269. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune Evasion Mediated by Pd-L1 on Glioblastoma-Derived Extracellular Vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Liu, J.-Y.; Chen, G. Small Extracellular Vesicle Pd-L1 in Cancer: The Knowns and Unknowns. NPJ Precis. Oncol. 2022, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Perez, D.; Russo, A.; Arrieta, O.; Ak, M.; Barron, F.; Gunasekaran, M.; Mamindla, P.; Lara-Mejia, L.; Peterson, C.B.; Er, M.E.; et al. Extracellular Vesicle Pd-L1 Dynamics Predict Durable Response to Immune-Checkpoint Inhibitors and Survival in Patients with Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 186. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, L.; Menon, R.; Ramakrishnan, N.; Venkatraman, R.; Ramachandran, P. Therapeutic Plasma Exchange Practices in Intensive Care Unit. Indian. J. Crit. Care Med. 2019, 23, 336. [Google Scholar] [PubMed]
- Baldwin, I.; Todd, S. Therapeutic Plasma Exchange in the Intensive Care Unit and with the Critically Ill, a Focus on Clinical Nursing Considerations. J. Clin. Apher. 2022, 37, 397–404. [Google Scholar] [CrossRef]
- Bauer, P.R.; Ostermann, M.; Russell, L.; Robba, C.; David, S.; Ferreyro, B.L.; Cid, J.; Castro, P.; Juffermans, N.P.; Montini, L.; et al. Plasma Exchange in the Intensive Care Unit: A Narrative Review. Intensive Care Med. 2022, 48, 1382–1396. [Google Scholar] [CrossRef]
- Comasòlivas, N.; Dierick, K.; Cordoba, J.A. How Much Can Colombian Hospitals Earn for Each Therapeutic Plasma Exchange (Tpe) Procedure? A Multi-Center Cost Comparison Study between Centrifugal and Membrane Systems. Value Health Reg. Issues 2019, 19, S48. [Google Scholar] [CrossRef]
- Bora, Debashree. Plasmapheresis Machines Market Size, Share & Trends Analysis Report by Collection Type (Plasma Collection System, Multi-Component Collection System), by Technology (Centrifugation-Based Plasmapheresis, Membrane Filtration-Based Plasmapheresis, Others), by Application (Extracorporeal Therapy, Plasma Donation, Others), by End-User (Hospitals and Clinics, Blood Donation Centers, Research Institutes, Home Care Settings, Others) and by Region(North America, Europe, Apac, Middle East and Africa, Latam) Forecasts, 2025-2033. Available online: https://straitsresearch.com/report/plasmapheresis-machines-market (accessed on 6 June 2025).
- Dierick, K.; Comasòlivas, N.; Lee, Y. Pmd21 Cost Benefit Analysis for Centrifugal Versus Membrane-Based Therapeutic Plasma Exchange. Value Health 2019, 22, S219. [Google Scholar] [CrossRef]
- Tabibi, S.; Tabibi, T.; Conic, R.R.Z.; Banisaeed, N.; Streiff, M.B. Therapeutic Plasma Exchange: A Potential Management Strategy for Critically Ill Covid-19 Patients. J. Intensive Care Med. 2020, 35, 827–835. [Google Scholar] [CrossRef]
- Harris, E.S.; Meiselman, H.J.; Moriarty, P.M.; Weiss, J. Successful Long-Term (22 Year) Treatment Of limited Scleroderma Using Therapeutic Plasma Exchange: Is Blood Rheology The key? Clin. Hemorheol. Microcirc. 2017, 65, 131–136. [Google Scholar] [CrossRef]
- Kozul, T.K.; Yudina, A.; Donovan, C.; Pinto, A.; Osman, C. Cost-Minimisation Analysis of Plasma Exchange Versus Ivig in the Treatment of Autoimmune Neurological Conditions. BMC Health Serv. Res. 2022, 22, 904. [Google Scholar]
- Harris, E.S.; Meiselman, H.J.; Moriarty, P.M.; Metzger, A.; Malkovsky, M. Therapeutic Plasma Exchange for the Treatment of Systemic Sclerosis: A Comprehensive Review and Analysis. J. Scleroderma. Relat. Disord. 2018, 3, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Bobati, S.S.; Naik, K.R. Therapeutic Plasma Exchange—An Emerging Treatment Modality in Patients with Neurologic and Non-Neurologic Diseases. J. Clin. Diagn. Res. 2017, 11, Ec35–Ec37. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, M.; Kiprov, D.; Schneider, K.; Mu, W.; Kumaar, P.A.; Kasler, H.; Burton, J.B.; Watson, M.; Halaweh, H.; King, C.D.; et al. Multi-Omics Analysis Reveals Biomarkers That Contribute to Biological Age Rejuvenation in Response to Single-Blinded Randomized Placebo-Controlled Therapeutic Plasma Exchange. Aging Cell 2025, e70103. [Google Scholar] [CrossRef]
- Wu, J.W.; Yaqub, A.; Ma, Y.; Koudstaal, W.; Hofman, A.; Ikram, M.A.; Ghanbari, M.; Goudsmit, J. Biological Age in Healthy Elderly Predicts Aging-Related Diseases Including Dementia. Sci. Rep. 2021, 11, 15929. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, D.; Sun, L. The Comparison of Albumin and 6% Hydroxyethyl Starches (130/0.4) in Cardiac Surgery: A Meta-Analysis of Randomized Controlled Clinical Trials. BMC Surg. 2021, 21, 342. [Google Scholar] [CrossRef]
- Brecher, M.E.; Owen, H.G.; Bandarenko, N. Alternatives to Albumin: Starch Replacement for Plasma Exchange. J. Clin. Apher. 1997, 12, 146–153. [Google Scholar] [CrossRef]
- Khan, F.; Singh, K.; Friedman, M.T. Artificial Blood: The History and Current Perspectives of Blood Substitutes. Discoveries 2020, 8, e104. [Google Scholar] [CrossRef]
- Islam, B.; Islam, Z.; Rahman, S.; Endtz, H.P.; Vos, M.C.; van der Jagt, M.; van Doorn, P.A.; Jacobs, B.C.; Mohammad, Q.D. Small Volume Plasma Exchange for Guillain-Barré Syndrome in Resource Poor Settings: A Safety and Feasibility Study. Pilot Feasibility Stud. 2017, 3, 40. [Google Scholar] [CrossRef]
- Update to Important Information About Young Donor Plasma Infusions Offered for Profit. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/update-important-information-about-young-donor-plasma-infusions-offered-profit (accessed on 7 March 2025).
- Ramirez, S.; Koerich, S.; Astudillo, N.; De Gregorio, N.; Al-Lahham, R.; Allison, T.; Rocha, N.P.; Wang, F.; Soto, C. Plasma Exchange Reduces Aβ Levels in Plasma and Decreases Amyloid Plaques in the Brain in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 17087. [Google Scholar] [CrossRef]
| Neuroimmune Interface | Access Potential * | Key Cell Types | Plasma Factors Implicated | Ref. |
|---|---|---|---|---|
| BBB Intact | Low | Endothelial cells, pericytes, astrocyte endfeet | CCL11, IL-6, TNFα | [46,47,48,49] |
| BBB (aged, dysfunctional) | Moderate | Endothelial cells, microglia, neurons | β2-micoglobulin, oxidized LDL, fibrinogen | [32,44,45] |
| Choroid Plexus (Blood-CSF barrier) | High | Epithelial cells, infiltrating T cells, macrophages | IFN-γ, IL1β, CXCL10 | [41,50] |
| Meninges | High | Dura-resident macrophages, B/T cells, dendritic cells | CXCL12, VEGF, IL-6 | [48,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rony, R.M.I.K.; Shokrani, A.; Malhi, N.K.; Hussey, D.; Mooney, R.; Chen, Z.B.; Scott, T.; Han, H.; Moore, J.; Liu, J.; et al. Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease. Biomolecules 2025, 15, 1000. https://doi.org/10.3390/biom15071000
Rony RMIK, Shokrani A, Malhi NK, Hussey D, Mooney R, Chen ZB, Scott T, Han H, Moore J, Liu J, et al. Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease. Biomolecules. 2025; 15(7):1000. https://doi.org/10.3390/biom15071000
Chicago/Turabian StyleRony, R. M. Imtiaz Karim, Alireza Shokrani, Naseeb Kaur Malhi, Deborah Hussey, Rachael Mooney, Zhen Bouman Chen, Tristan Scott, Haiyong Han, Jaeger Moore, Jiahui Liu, and et al. 2025. "Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease" Biomolecules 15, no. 7: 1000. https://doi.org/10.3390/biom15071000
APA StyleRony, R. M. I. K., Shokrani, A., Malhi, N. K., Hussey, D., Mooney, R., Chen, Z. B., Scott, T., Han, H., Moore, J., Liu, J., Huang, W., Garcia-Ocaña, A., Grant, M. B., Aboody, K., Von Hoff, D., Natarajan, R., & Tompkins, J. D. (2025). Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease. Biomolecules, 15(7), 1000. https://doi.org/10.3390/biom15071000






