3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Expression of 3-O Sulfotransferase-3 (3-OST-3)
2.3. C. muridarum Infectivity Assay
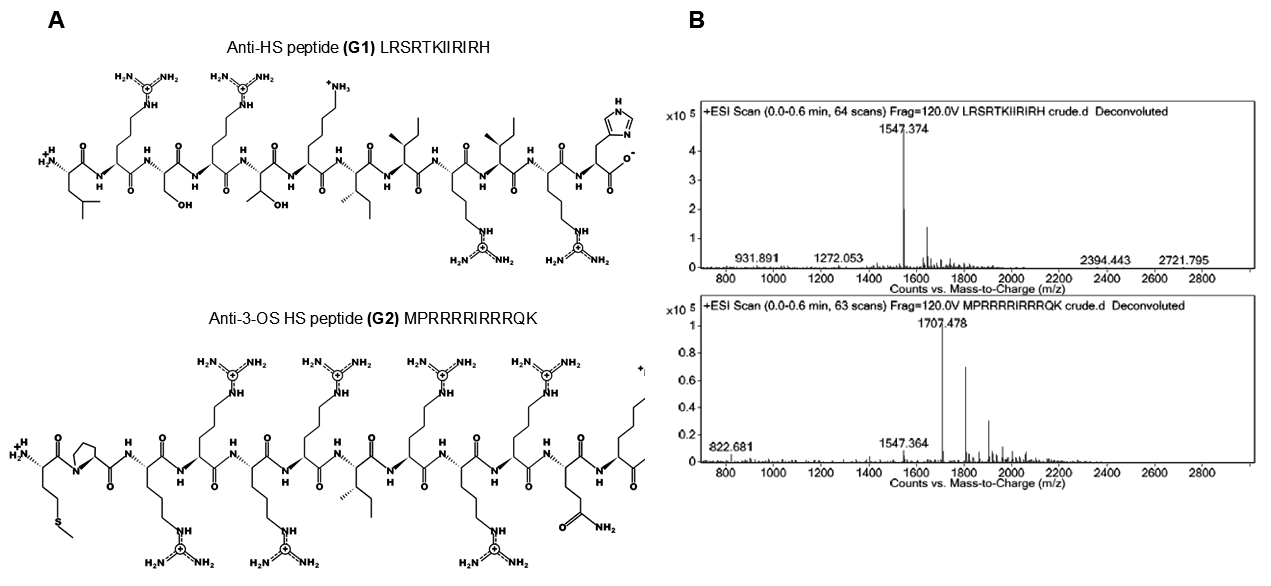
2.4. Chemical Structure of G1 and G2 Peptide Ligands
2.5. Pretreatment of C. muridarum with G1 and or G2 Peptide Ligands
2.6. Target Cell Pretreatment with G1 and or G2 Peptide Ligands
2.7. Exploring the Binding Mode and Affinity of G1 and G2 Peptides with the Lipid Bilayer Membrane Complex of C. muridarum Using Molecular Dynamics Simulation
2.7.1. G1 and G2 Peptide Modeling
2.7.2. C. muridarum Membrane Generation and MD Simulation
2.7.3. Binding Energy Calculations
2.8. Statistical Analysis
3. Results
3.1. Expression of 3-O Sulfotransferase-3 (3-OST-3) and C. muridarum Entry
3.2. Preincubation of G1 and G2 Peptide Effects on Elementary Body (EB) Infectivity
3.3. Investigating the Interaction of G1 and G2 Peptides with the Chlamydia Lipid Bilayer
3.4. Binding Energy Calculations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bachmann, N.L.; Polkinghorne, A.; Timms, P. Chlamydia genomics: Providing novel insights into chlamydial biology. Trends Microbiol. 2014, 22, 464–472. [Google Scholar] [CrossRef]
- Mandell, G.L.; Bennett, J.E.; Dolin, R. (Eds.) Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Churchill Livingstone/Elsevier: London, UK, 2010; Volume 2. [Google Scholar]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Porritt, R.A.; Crother, T.R. Chlamydia pneumoniae Infection and Inflammatory Diseases. For. Immunopathol. Dis. Therap. 2016, 7, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Dockterman, J.; Coers, J. Immunopathogenesis of genital Chlamydia infection: Insights from mouse models. Pathog. Dis. 2021, 79, ftab012. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Raymond, L.; Rockey, D.D.; Fischer, E.; Hackstadt, T.; Caldwell, H.D. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 11143–11148. [Google Scholar] [CrossRef] [PubMed]
- Taraktchoglou, M.; Pacey, A.A.; Turnbull, J.E.; Eley, A. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect. Immun. 2001, 69, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Wuppermann, F.N.; Hegemann, J.H.; Jantos, C.A. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J. Infect. Dis. 2001, 184, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jiang, S.; Elwell, C.A.; Engel, J.N. Chlamydia trachomatis co-opts the FGF2 signaling pathway to enhance infection. PLoS Pathog. 2011, 7, e1002285. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Arnold, K.; Liu, J. Using structurally defined oligosaccharides to understand the interactions between proteins and heparan sulfate. Curr. Opin. Struct. Biol. 2018, 50, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thacker, B.E.; Xu, D.; Lawrence, R.; Esko, J.D. Heparan sulfate 3-O-sulfation: A rare modification in search of a function. Matrix Biol. 2014, 35, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Maus, E.; Sigar, I.M.; Ramsey, K.H.; Shukla, D. Role of heparan sulfate in sexually transmitted infections. Glycobiology 2012, 22, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Tarbutton, M.S.; Shukla, D. Diversity of heparan sulfate and HSV entry: Basic understanding and treatment strategies. Molecules 2015, 20, 2707. [Google Scholar] [CrossRef]
- Darville, T.; Yedgar, S.; Krimsky, M.; Andrews, C.W., Jr.; Jungas, T.; Ojcius, D.M. Protection against Chlamydia trachomatis infection in vitro and modulation of inflammatory response in vivo by membrane-bound glycosaminoglycans. Microbes Infect. 2004, 6, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Inic-Kanada, A.; Stein, E.; Stojanovic, M.; Schuerer, N.; Ghasemian, E.; Filipovic, A.; Marinkovic, E.; Kosanovic, D.; Barisani-Asenbauer, T. Effects of iota-carrageenan on ocular Chlamydia trachomatis infection in vitro and in vivo. J. Appl. Phycol. 2018, 30, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Zaretzky, F.R.; Pearce-Pratt, R.; Phillips, D.M. Sulfated polyanions block Chlamydia trachomatis infection of cervix-derived human epithelia. Infect. Immun. 1995, 63, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Zhang, J.P.; Stephens, R.S. Structural requirements of heparin binding to Chlamydia trachomatis. J. Biol. Chem. 1996, 271, 11134–11140. [Google Scholar] [PubMed]
- Hou, S.; Lei, L.; Yang, Z.; Qi, M.; Liu, Q.; Zhong, G. Chlamydia trachomatis outer membrane complex protein B (OmcB) is processed by the protease CPAF. J. Bacteriol. 2013, 195, 951–957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.H.; Chan, C.; Elwell, C.; Singer, M.S.; Dierks, T.; Lemjabbar-Alaoui, H.; Rosen, S.D.; Engel, J.N. Endosulfatases SULF1 and SULF2 limit Chlamydia muridarum infection. Cell Microbiol. 2013, 15, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J. Biol. Chem. 2011, 286, 25406–25415. [Google Scholar] [CrossRef] [PubMed]
- Lourtet-Hascoet, J.; Mine, L.; Spindler, L.; Pilmis, B.; Aubert, M.; El Mituialy, A.; Vieillefond, V.; de Parades, V.; Le Monnier, A. Epidemiology of symptomatic infective anoproctitis in a population of men having sex with men (MSM). Infection 2022, 50, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Larew, M.S.; Myers, M.G. Recovery of cytomegalovirus and Chlamydia trachomatis from vaginal tampons. J. Med. Virol. 1982, 9, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Maïza, A.; Sidahmed-Adrar, N.; Michel, P.P.; Carpentier, G.; Habert, D.; Dalle, C.; Redouane, W.; Hamza, M.; van Kuppevelt, T.H.; Ouidja, M.O.; et al. 3-O-sulfated heparan sulfate interactors target synaptic adhesion molecules from neonatal mouse brain and inhibit neural activity and synaptogenesis in vitro. Sci. Rep. 2020, 10, 19114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Patel, V.N.; Song, X.; Xu, Y.; Kaminski, A.M.; Doan, V.U.; Su, G.; Liao, Y.; Mah, D.; Zhang, F.; et al. Increased 3-O-sulfated heparan sulfate in Alzheimer’s disease brain is associated with genetic risk gene HS3ST1. Sci Adv. 2023, 9, eadf6232. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, M.D.P.H.; Osborn, B.K.; Lowak, K.D.; McDonald, M.M.; Wang, Y.W.; Pa, V.; Richter, J.R.; Xu, Y.; Arnold, K.; Liu, J.; et al. A 3-O-sulfated heparan sulfate dodecasaccharide (12-mer) suppresses thromboinflammation and attenuates early organ injury following trauma and hemorrhagic shock. Front. Immunol. 2023, 14, 1158457. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Sato, H.; Mizumoto, S.; Wakai, K.; Yoneda, K.; Yamamoto, K.; Nakanishi, H.; Ikeda, J.I.; Sakamoto, S.; Ichikawa, T.; et al. Switching mechanism from AR to EGFR signaling via 3-O-sulfated heparan sulfate in castration-resistant prostate cancer. Sci. Rep. 2023, 13, 11618. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gauche, C.; Coughtrie, M.W.; Bui, C.; Gulberti, S.; Merhi-Soussi, F.; Ramalanjaona, N.; Bertin-Jung, I.; Diot, A.; Dumas, D.; et al. The heparan sulfate sulfotransferase 3-OST3A (HS3ST3A) is a novel tumor regulator and a prognostic marker in breast cancer. Oncogene 2016, 35, 5043–5055. [Google Scholar] [CrossRef] [PubMed]
- Tátrai, P.; Egedi, K.; Somorácz, A.; van Kuppevelt, T.H.; Ten Dam, G.; Lyon, M.; Deakin, J.A.; Kiss, A.; Schaff, Z.; Kovalszky, I. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J. Histochem. Cytochem. 2010, 58, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Karasneh, G.A.; Jarding, M.J.; Tiwari, V.; Shukla, D. A 3-O-sulfated heparan sulfate binding peptide preferentially targets herpes simplex virus 2-infected cells. J. Virol. 2012, 86, 6434–6443. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Liu, J.; Blaiklock, P.; Shworak, N.W.; Bai, X.; Esko, J.D.; Cohen, G.H.; Eisenberg, R.J.; Rosenberg, R.D.; Spear, P.G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 1999, 99, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1501–1518. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D350. [Google Scholar] [CrossRef]
- Kosma, P. Chlamydial lipopolysaccharide. Biochim. Biophys. Acta 1999, 1455, 385–402. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ranadive, S.; Fastman, J.; Almond, D.E.; York, D.M.; MacKerell, A.D., Jr. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Biomolecules 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2005, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Bhandare, V.V.; Kumbhar, B.V.; Kunwar, A. Differential binding affinity of tau repeat region R2 with neuronal-specific β-tubulin isotypes. Sci. Rep. 2019, 9, 10795. [Google Scholar] [CrossRef]
- Tiwari, V.; Clement, C.; Duncan, M.B.; Chen, J.; Liu, J.; Shukla, D. A role for 3-O-sulfated heparan sulfate in cell fusion induced by herpes simplex virus type 1. J. Gen. Virol. 2004, 85 Pt 4, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Lathrop, S.J.; Koshiyama, K.; Phillips, N.; Stephens, R.S. Chlamydia-dependent biosynthesis of a heparan sulphate-like compound in eukaryotic cells. Cell Microbiol. 2000, 2, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S. Molecular mimicry and Chlamydia trachomatis infection of eukaryotic cells. Trends Microbiol. 1994, 2, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Stephens, R.S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 1992, 69, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Moelleken, K.; Hegemann, J.H. The Chlamydia outer membrane protein OmcB is required for adhesion and exhibits biovar-specific differences in glycosaminoglycan binding. Mol. Microbiol. 2008, 67, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Rosmarin, D.M.; Carette, J.E.; Olive, A.J.; Starnbach, M.N.; Brummelkamp, T.R.; Ploegh, H.L. Attachment of Chlamydia trachomatis L2 to host cells requires sulfation. Proc. Natl. Acad. Sci. USA 2012, 109, 10059–10064. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, S.; Muller, J.; Drees, F.; Spona, D.; Bonda, L.; Hartmann, L.; Hegemann , J.H.; Schmidt, S. Sulfated Glycosaminoglycans as Inhibitors for Chlamydia Infections. Macromol. Biosci. 2025, 25, 2400443. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Schripsema, J.H.; Imtiaz, M.T.; Sigar, I.M.; Kasimos, J.; Matos, P.G.; Inouye, S.; Ramsey, K.H. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 2005, 32, 49–56. [Google Scholar] [CrossRef]
- Hackstadt, T. Chapter 6—Initial Interactions of Chlamydiae with the Host Cell. In Intracellular Pathogens 1: Chlamydiales; Tan, M., Bavoil, P.M., Eds.; ASM Press: Washington, DC, USA, 2012; Volume 1, pp. 126–148. [Google Scholar]
- Mehlitz, A.; Rudel, T. Modulation of host signaling and cellular responses by Chlamydia. Cell Commun. Signal. 2013, 11, 90. [Google Scholar] [CrossRef]
- Ajonuma, L.C.; Fok, K.L.; Ho, L.S.; Chan, P.K.; Chow, P.H.; Tsang, L.L.; Wong, C.H.; Chen, J.; Li, S.; Rowlands, D.K.; et al. CFTR is required for cellular entry and internalization of Chlamydia trachomatis. Cell Biol. Int. 2010, 34, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Puolakkainen, M.; Kuo, C.C.; Campbell, L.A. Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect. Immun. 2005, 73, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, S.; Hegemann, J.H. The Chlamydia trachomatis Ctad1 invasin exploits the human integrin beta1 receptor for host cell entry. Cell Microbiol. 2016, 18, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Igietseme, J.U.; Partin, J.; George, Z.; Omosun, Y.; Goldstein, J.; Joseph, K.; Ellerson, D.; Eko, F.O.; Pohl, J.; Bandea, C.; et al. Epidermal Growth Factor Receptor and Transforming Growth Factor beta Signaling Pathways Cooperate To Mediate Chlamydia Pathogenesis. Infect. Immun. 2020, 88, e00819-19. [Google Scholar] [CrossRef] [PubMed]
- Birkelund, S.; Johnsen, H.; Christiansen, G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect. Immun. 1994, 62, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Johnston, S.C.; Chou, J.; Dean, D. A systemic network for Chlamydia pneumoniae entry into human cells. J. Bacteriol. 2010, 192, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Abromaitis, S.; Stephens, R.S. Attachment and entry of Chlamydia have distinct requirements for host protein disulfide isomerase. PLoS Pathog. 2009, 5, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Conant, C.G.; Stephens, R.S. Chlamydia attachment to mammalian cells requires protein disulfide isomerase. Cell Microbiol. 2007, 9, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Mariotton, J.; Rozenberg, F.; Sams, A.; van Kuppevelt, T.H.; Barry Delongchamps, N.; Zerbib, M.; Bomsel, M.; Ganor, Y. CGRP inhibits human Langerhans cells infection with HSV by differentially modulating specific HSV-1 and HSV-2 entry mechanisms. Mucosal Immunol. 2022, 15, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille, C.; Deligny, A.; Delehedde, M.; Denys, A.; Melchior, A.; Liénard, X.; Lyon, M.; Mazurier, J.; Fernig, D.G.; Allain, F. The heparin/heparan sulfate sequence that interacts with cyclophilin B contains a 3-O-sulfated N-unsubstituted glucosamine residue. J. Biol. Chem. 2007, 282, 24416–24429. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Khan, A.; Denkewitz, M.; Maccarana, M.; Lundkvist, Å.; Li, J.P.; Li, J. Dual roles of exostosin glycosyltransferase 1 in Zika virus infection. Virulence 2025, 16, 2458681. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Barria, E.; Boothroyd, J.C. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 1999, 274, 1267–1276. [Google Scholar] [PubMed]
- Azzouz, N.; Kamena, F.; Laurino, P.; Kikkeri, R.; Mercier, C.; Cesbron-Delauw, M.F.; Dubremetz, J.F.; De Cola, L.; Seeberger, P.H. Toxoplasma gondii secretory proteins bind to sulfated heparin structures. Glycobiology 2013, 23, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Bour, J.B.; Lidholt, K.; Gauthray, C.; Pothier, P. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J. Virol. 1998, 72, 7221–7227. [Google Scholar] [PubMed]
- Suryawanshi, R.K.; Patil, C.D.; Koganti, R.; Singh, S.K.; Ames, J.M.; Shukla, D. Heparan Sulfate Binding Cationic Peptides Restrict SARS-CoV-2 Entry. Pathogens 2021, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Antoine, T.E.; Farooq, A.V.; Valyi-Nagy, T.; Shukla, D. An investigative peptide-acyclovir combination to control herpes simplex virus type 1 ocular infection. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6373–6381. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, T.; Stallmann, S.; Moelleken, K.; Meyer, K.L.; Hegemann, J.H. Characterization of the interaction between the chlamydial adhesin OmcB and the human host cell. J. Bacteriol. 2013, 195, 5323–5333. [Google Scholar] [CrossRef] [PubMed]

| Parameters | G1 Peptide | G2 Peptide |
|---|---|---|
| Confidence score (C-score) | −1.02 | 0.12 |
| Template modelling score (TM score) | 0.59 ± 0.14 | 0.73 ± 0.11 |
| Root mean square deviation (RMSD) | 1.9 ± 1.6 Å | 0.5 ± 0.5 Å |
| Peptide–Membrane Complexes | ΔEVDW | ΔEELE | ΔEGB | ΔESURF | ΔGGAS | ΔGSOLV | ΔTotal |
|---|---|---|---|---|---|---|---|
| G1 membrane | −44.66 | −20,879.97 | 20,893.80 | −9.78 | −20,924.63 | 20,884.02 | −40.61 |
| G2 membrane | −26.62 | −36,077.70 | 36,001.98 | −9.42 | −36,104.32 | 35,992.55 | −111.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanusiak, W.; Khodke, P.; Mayen, J.; Van, K.; Sigar, I.; Plotkin, B.J.; Kaminski, A.; Elste, J.; Kumbhar, B.V.; Tiwari, V. 3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum. Biomolecules 2025, 15, 999. https://doi.org/10.3390/biom15070999
Hanusiak W, Khodke P, Mayen J, Van K, Sigar I, Plotkin BJ, Kaminski A, Elste J, Kumbhar BV, Tiwari V. 3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum. Biomolecules. 2025; 15(7):999. https://doi.org/10.3390/biom15070999
Chicago/Turabian StyleHanusiak, Weronika, Purva Khodke, Jocelyn Mayen, Kennedy Van, Ira Sigar, Balbina J. Plotkin, Amber Kaminski, James Elste, Bajarang Vasant Kumbhar, and Vaibhav Tiwari. 2025. "3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum" Biomolecules 15, no. 7: 999. https://doi.org/10.3390/biom15070999
APA StyleHanusiak, W., Khodke, P., Mayen, J., Van, K., Sigar, I., Plotkin, B. J., Kaminski, A., Elste, J., Kumbhar, B. V., & Tiwari, V. (2025). 3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum. Biomolecules, 15(7), 999. https://doi.org/10.3390/biom15070999







