Enhanced Therapeutic Efficacy of Omeprazole Nanosuspension in Ethanol-Induced Gastric Ulcer: A Focus on Oxidative Stress and Inflammatory Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of OMP-NS
2.3. Characterization of OMP-NS
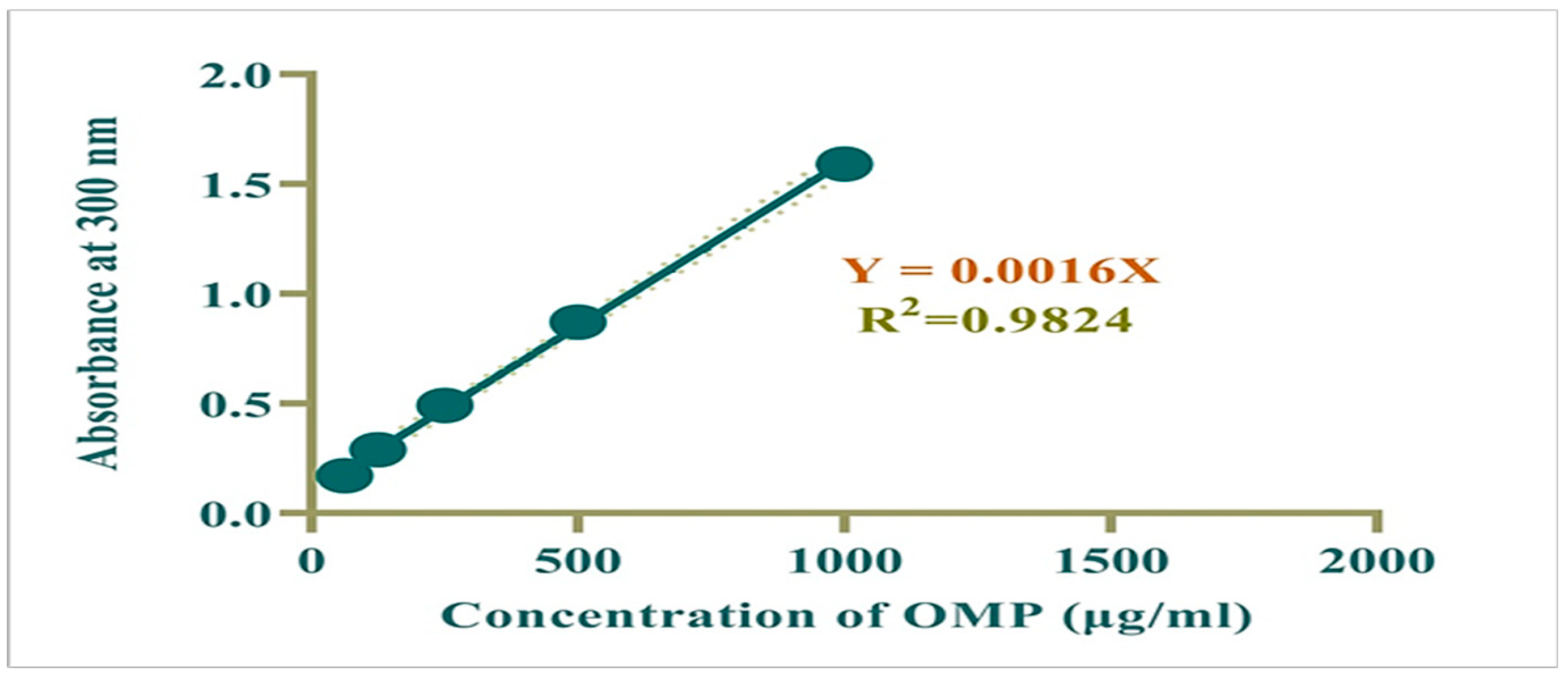
2.4. Preparation of Calibration Curve
2.5. In Vitro Release Study and Entrapment Effectiveness (EE%)
2.6. Gastric Ulcer Induction and Experimental Grouping
2.7. Sample Collection
2.8. Determination of Gastric pH, Assessment of Gastric Mucosal Damage, and Ulcer Index (U.I.)
2.9. Biochemical Analysis
2.9.1. Assessment of Hepatic and Renal Biomarkers
2.9.2. Assessment of Oxidative Stress Biomarkers
2.9.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9.4. RNA Extraction and Quantitative RT-PCR Analysis
2.10. Histopathological Analysis
2.11. Statistical Analysis
3. Results
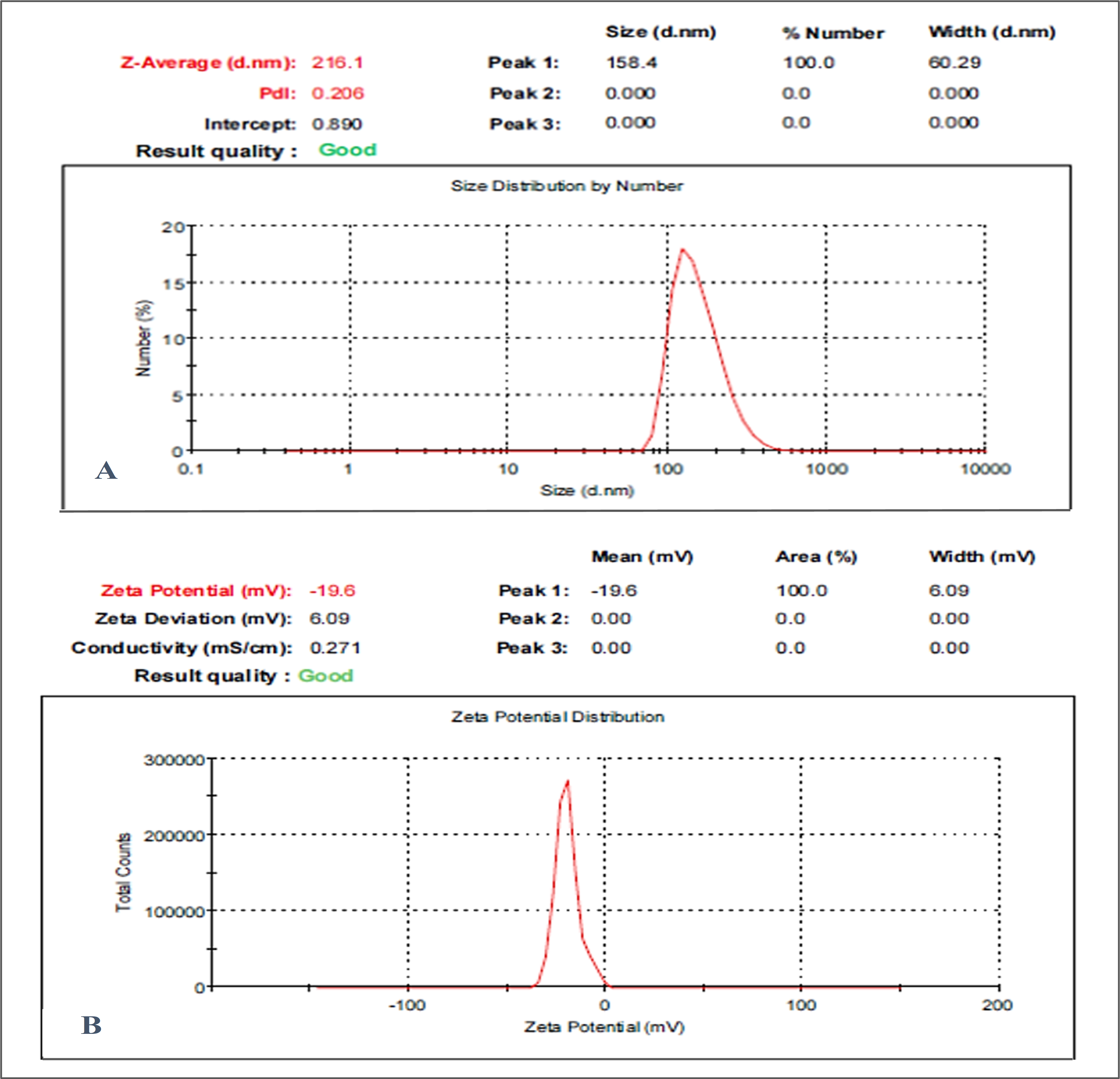
3.1. Particle Size, Zeta Potential, and PDI Analysis
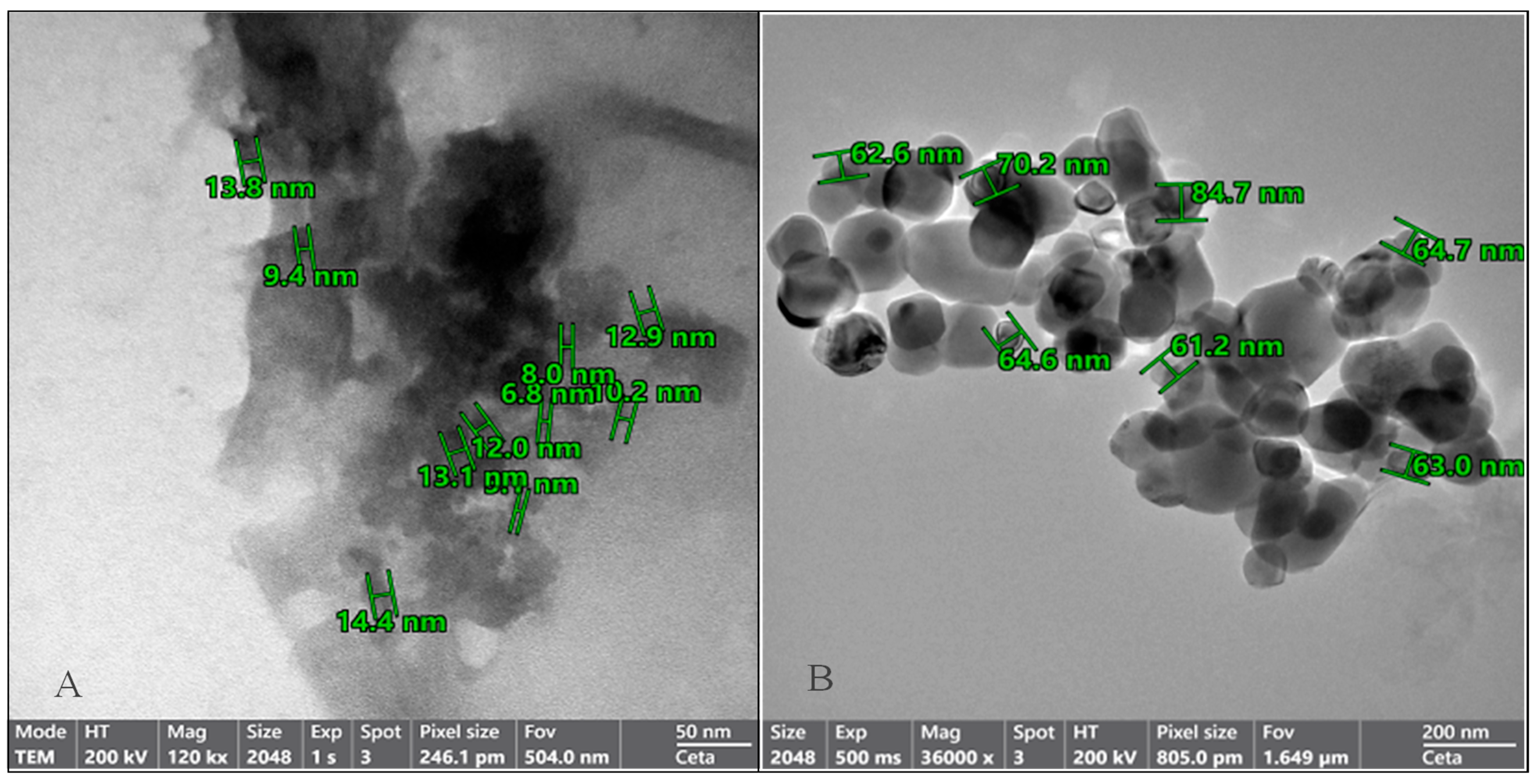
3.2. Structural Characterization of OMP-NS Using TEM Analysis
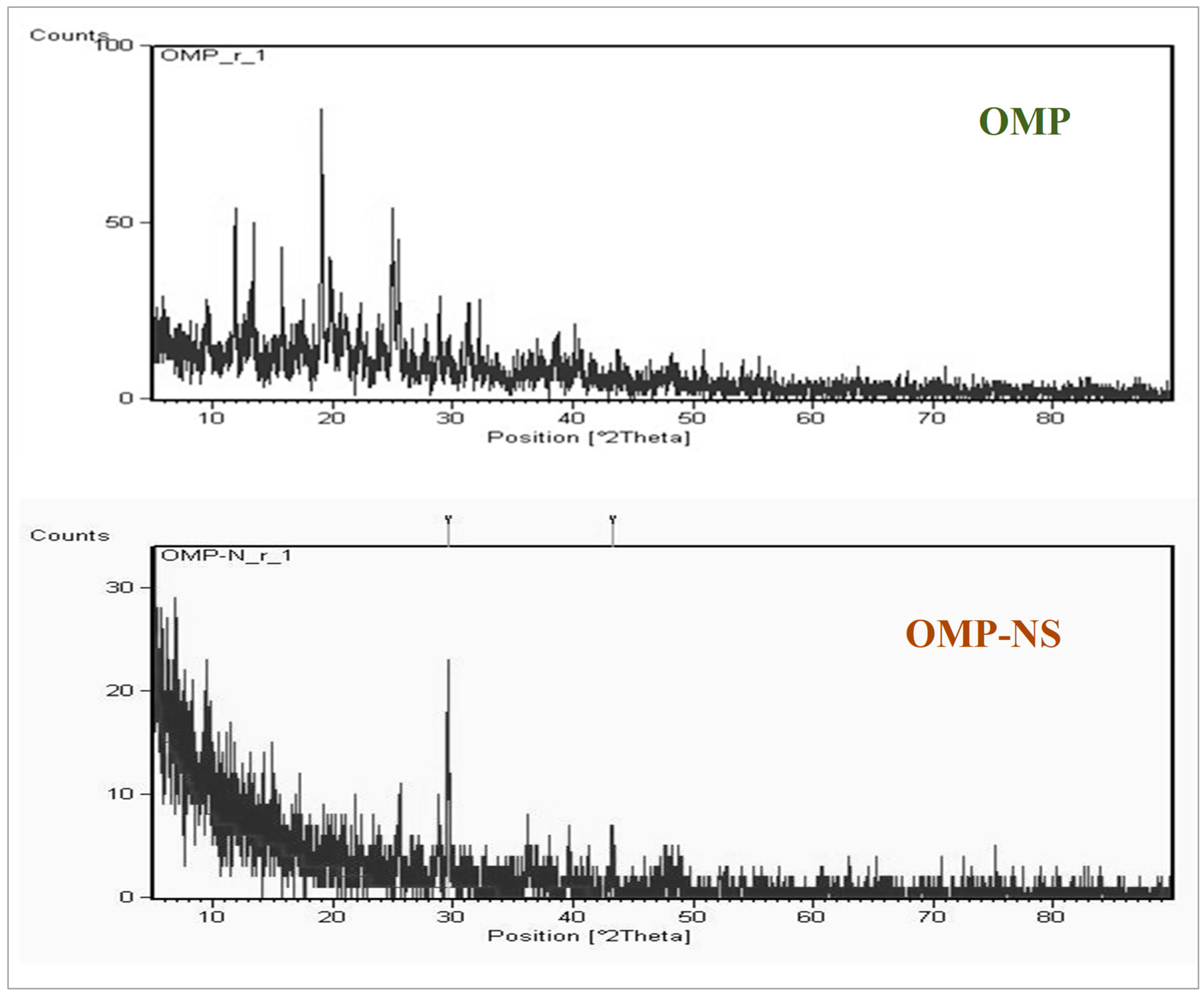
3.3. XRD Analysis of OMP and OMP-NS
3.4. FTIR Spectrum Analysis of OMP and OMP-NS Formulation
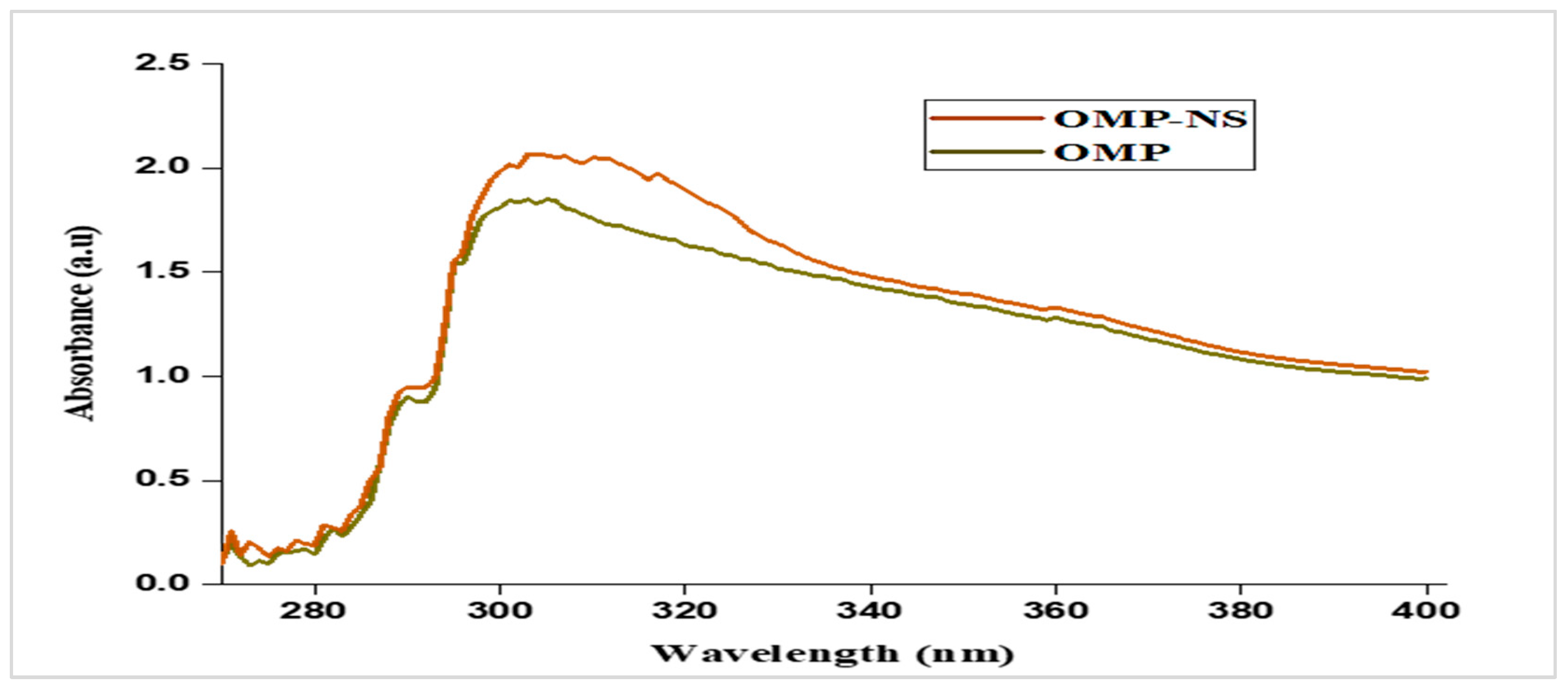
3.5. UV Spectra of OMP and OMP-NS
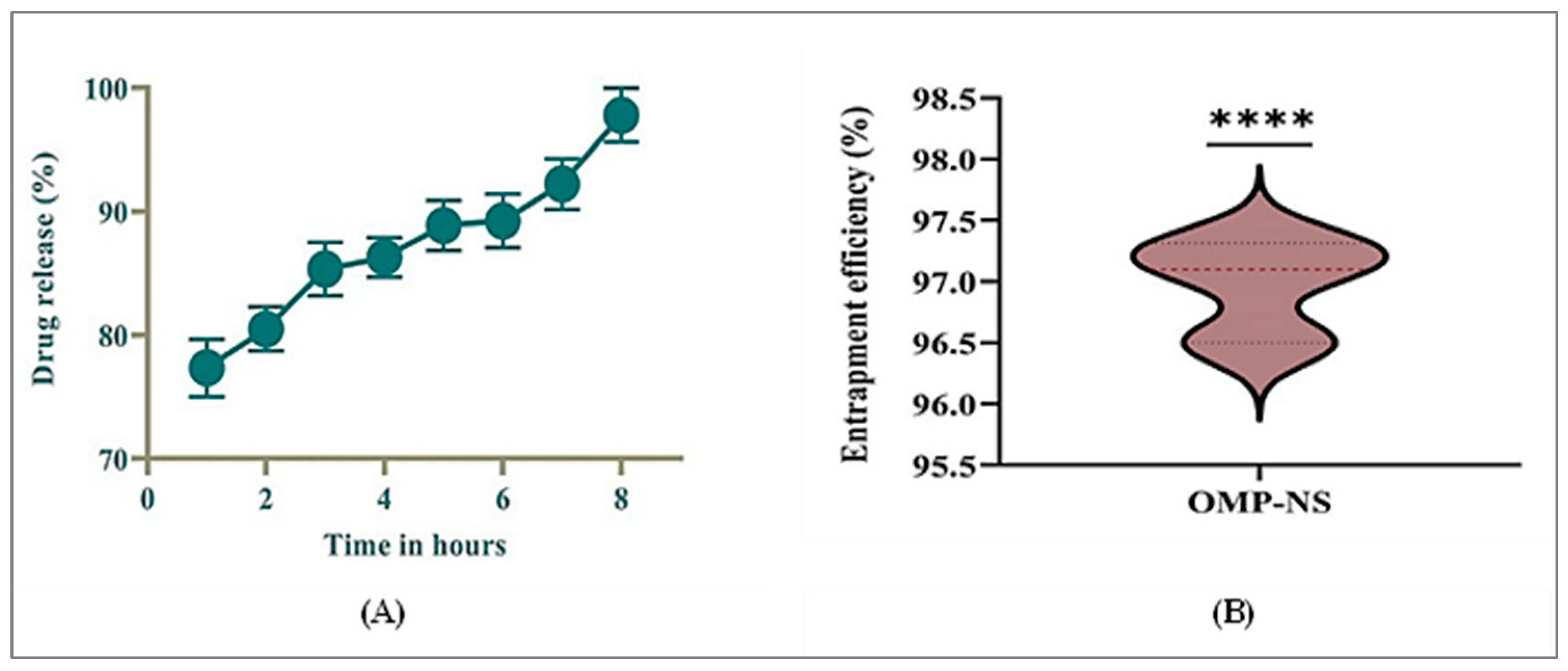
3.6. In Vitro Release Profile and EE% of OMP-NS
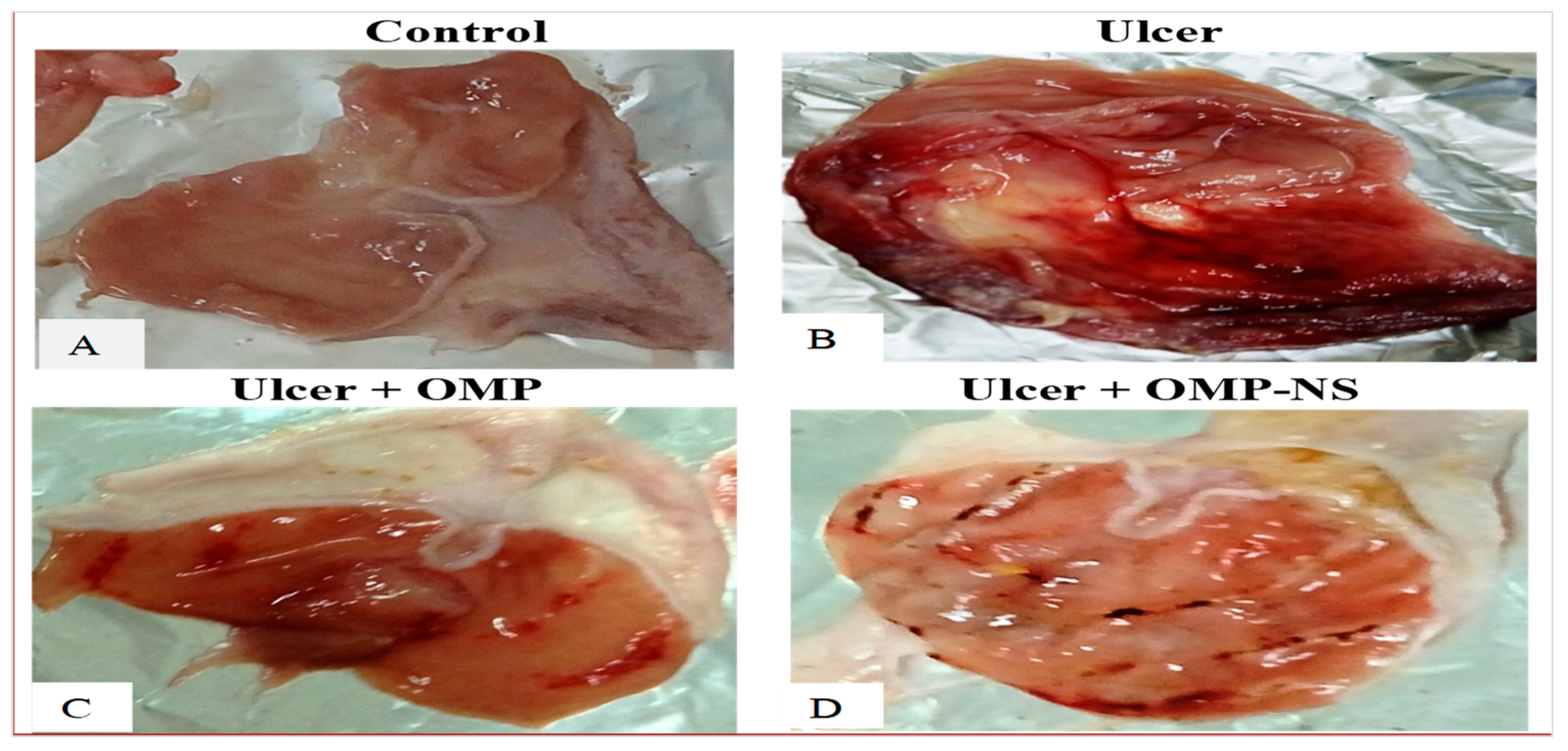
3.7. The Macroscopic Examination of the Gastric Mucosa
3.8. Impact of Treatment on Body Weight, Stomach Weight, Stomach Coefficient, Gastric pH, U.I., and CR in Ethanol-Induced Gastric Ulcer
3.9. Impact of OMP and OMP-NS on Biochemical Markers of Liver and Kidney Function
3.10. Impact of Ulceration and Treatment on Cortisol and Gastrin Levels
3.11. Impact of OMP and OMP-NS on Oxidative Stress Markers
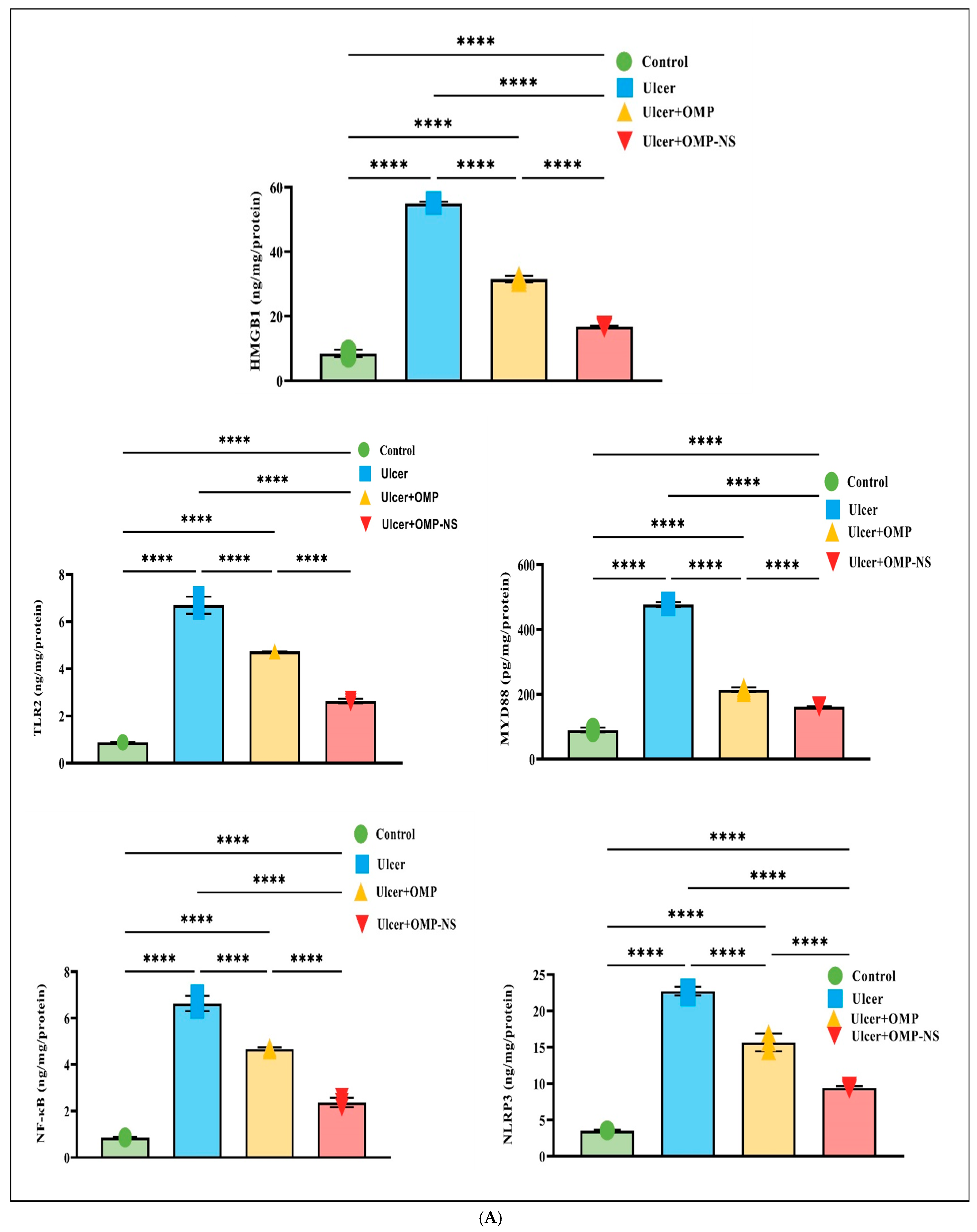
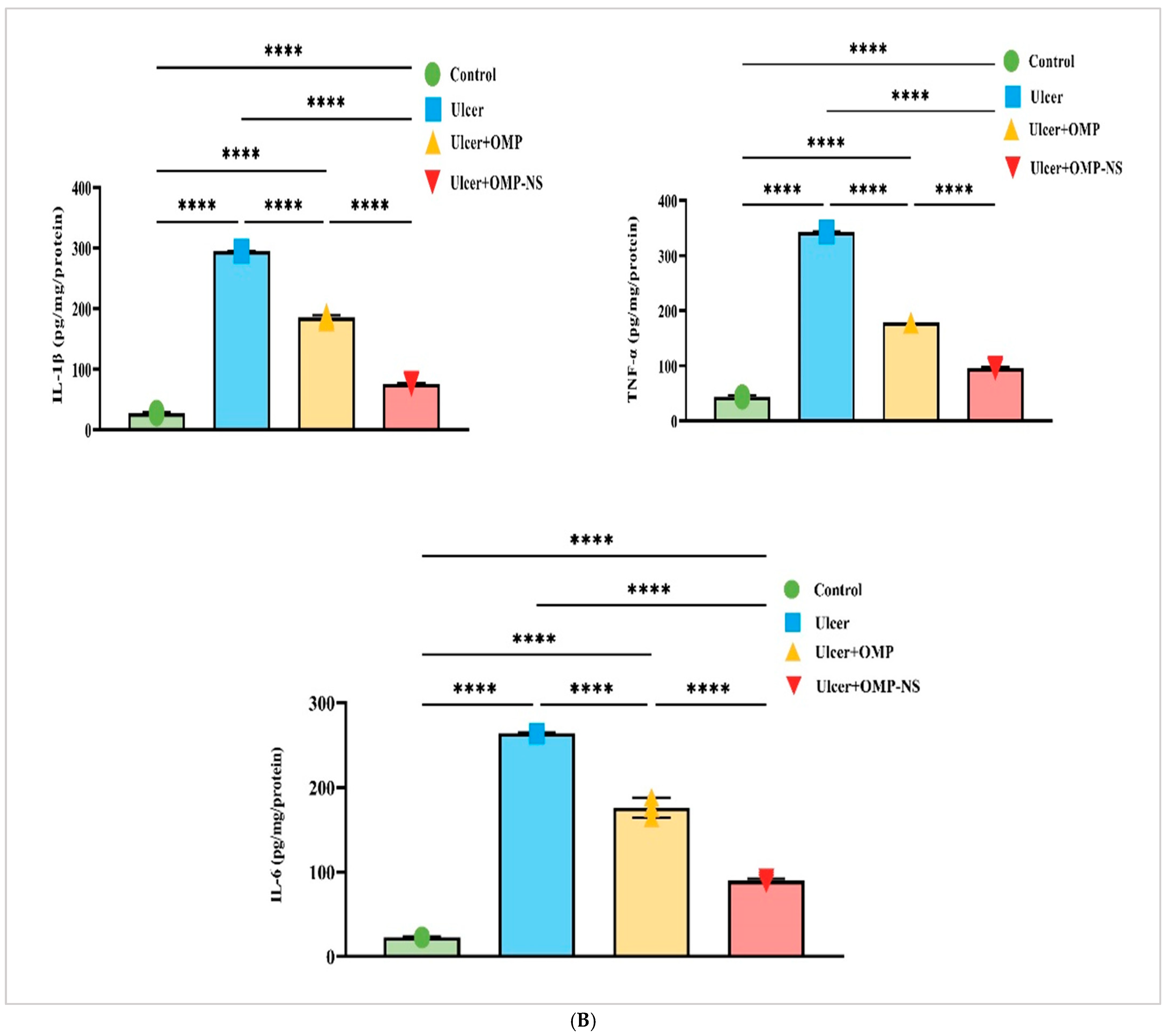
3.12. Therapeutic Modulation of Inflammatory Pathways by OMP and OMP-NS in Gastric Ulceration: Focus on HMGB1/NLRP3/NF-κB, TLR-2/MyD88, and Cytokine Responses
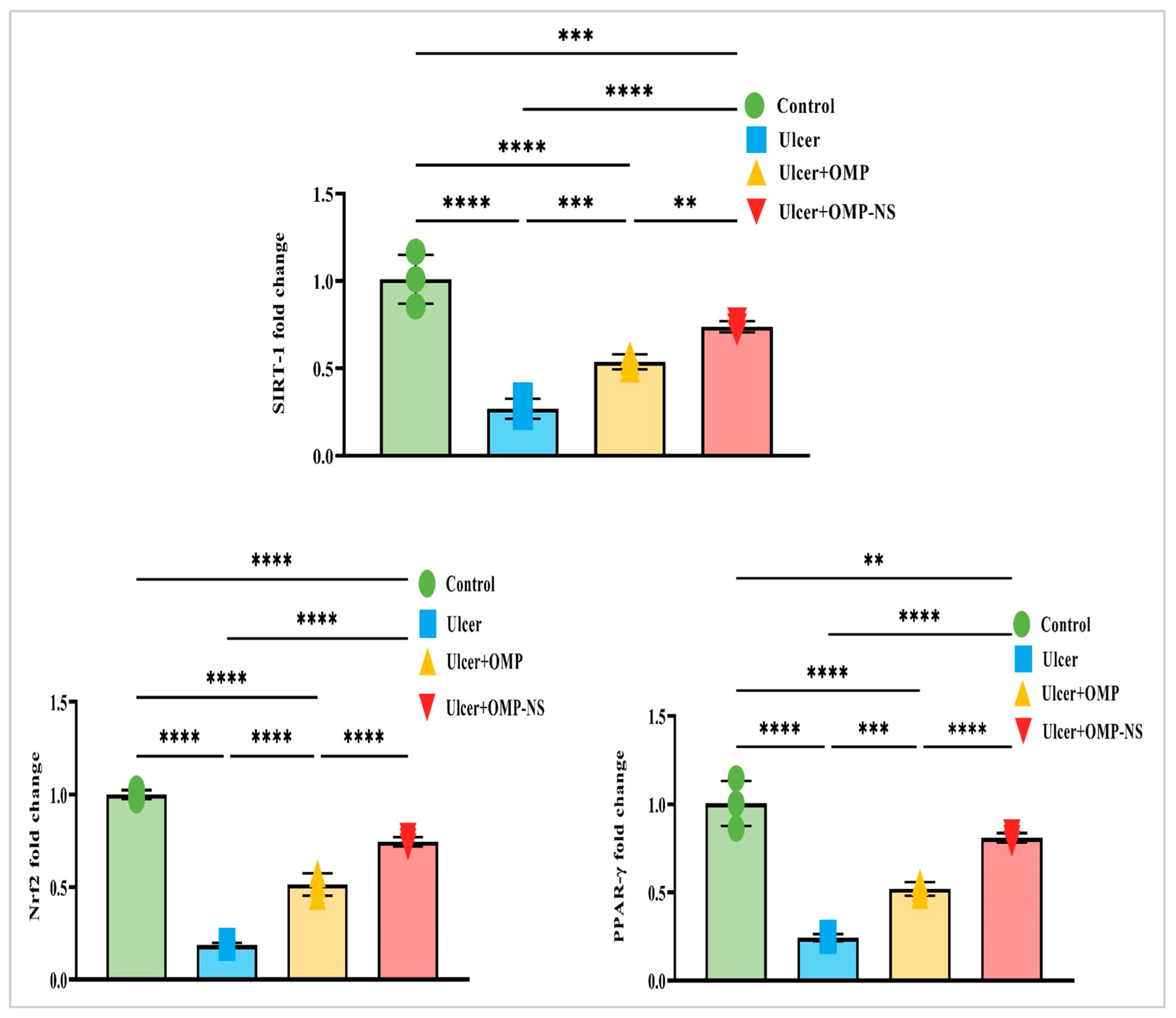
3.13. Impact of OMP and OMP-NS on Nrf2/PPAR-γ/SIRT-1 Pathways in Gastric Ulceration
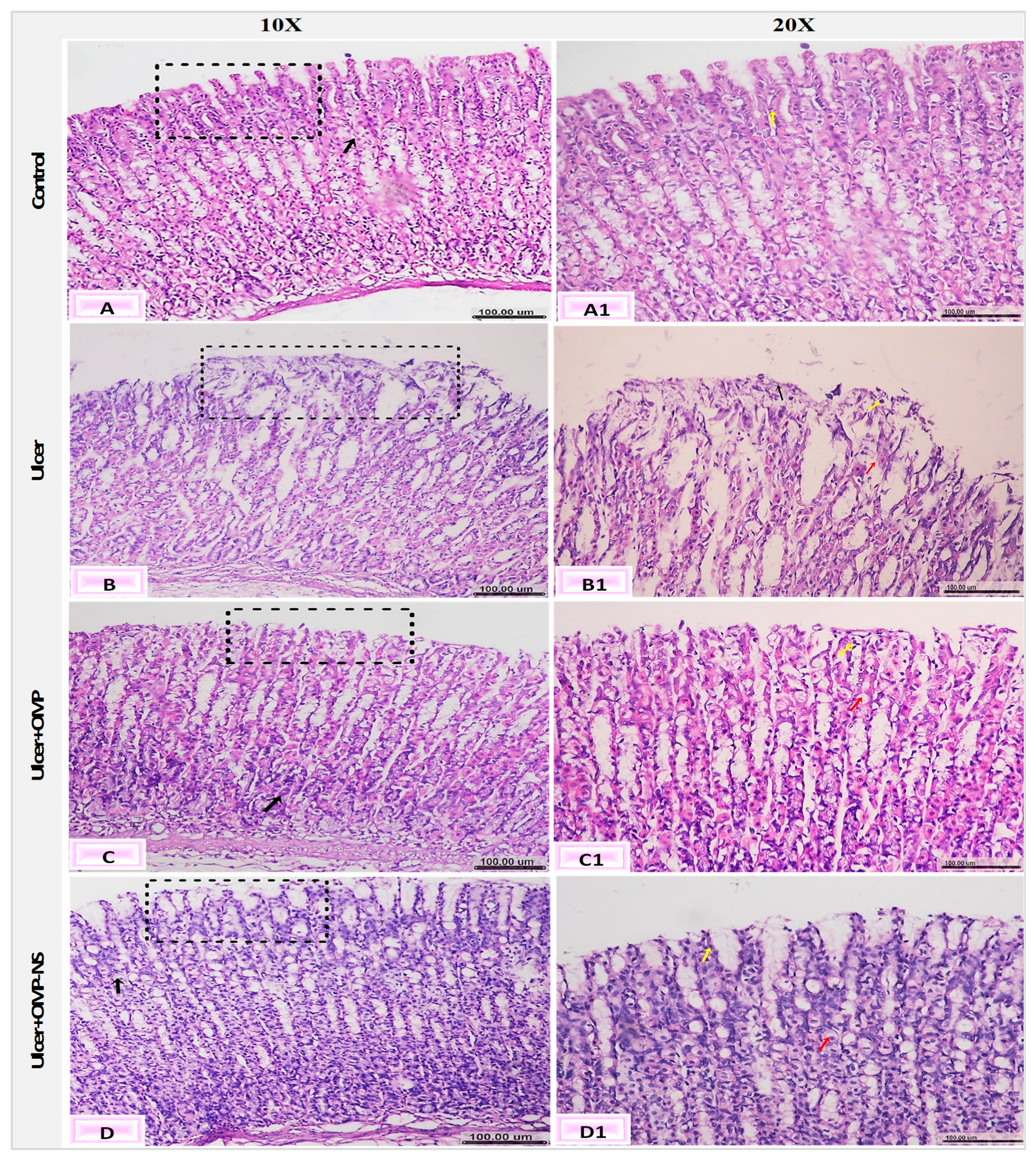
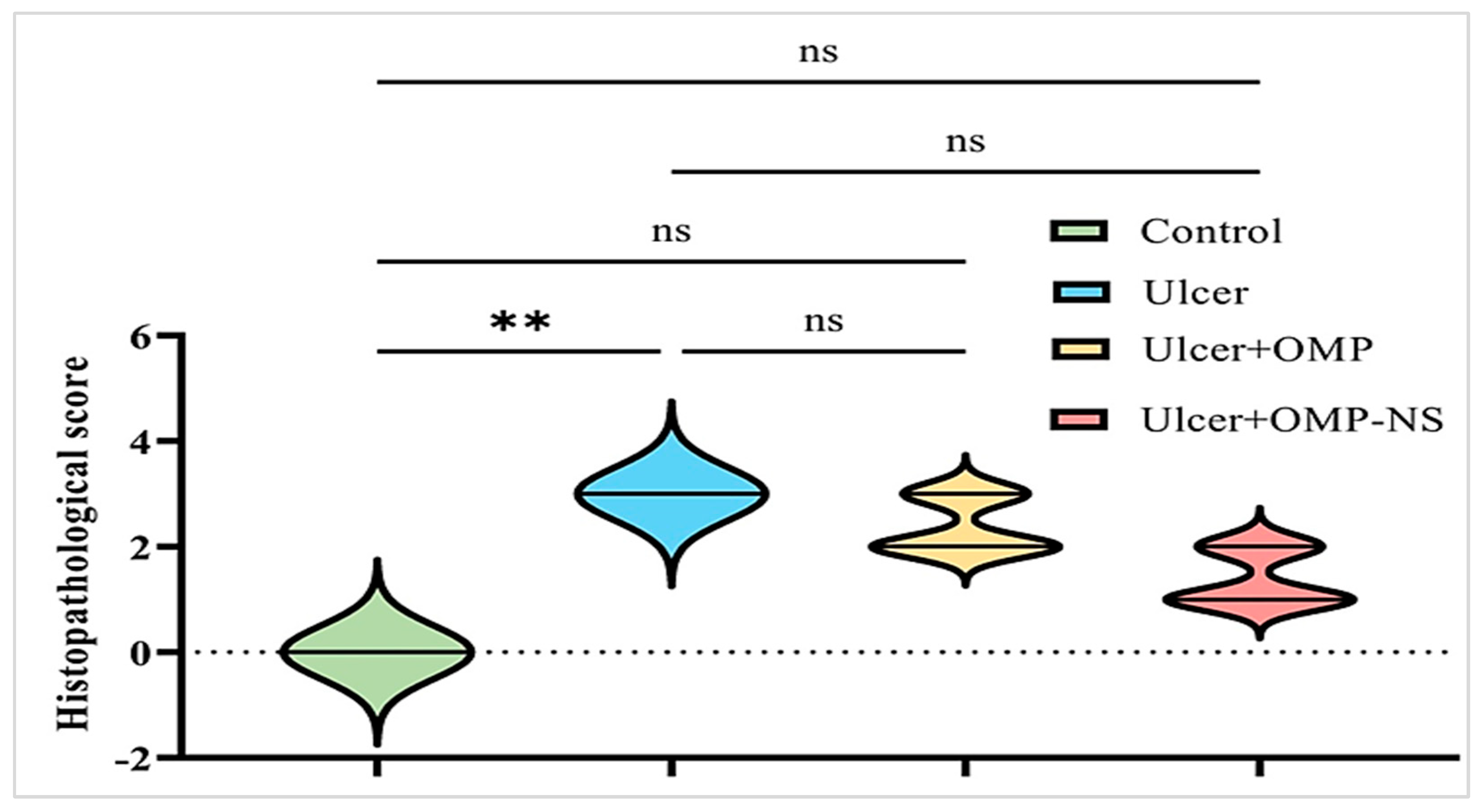
3.14. Impact of OMP and OMP-NS on Histological Alterations in Gastric Mucosa in Ethanol-Induced Ulceration
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari, N.; Darvishi, N.; Shohaimi, S.; Bartina, Y.; Ahmadipanah, M.; Salari, H.R.; Mohammadi, M. The global prevalence of peptic ulcer in the world: A systematic review and meta-analysis. Indian J. Surg. 2022, 84, 913–921. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Nadeem, M.S.; Imam, S.S.; Alshehri, S.; Ahmed, M.M.; Ghoneim, M.M.; Afzal, M.; Alzarea, S.I.; et al. Protective effect of fustin against ethanol-activated gastric ulcer via downregulation of biochemical parameters in rats. ACS Omega 2022, 7, 23245–23254. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective effect of sinapic acid on ethanol-induced gastric ulcers in rats: Involvement of Nrf2/HO-1 and NF-κB signaling and antiapoptotic role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Ali, R.A. The implication of the crosstalk of Nrf2 with NOXs, and HMGB1 in ethanol-induced gastric ulcer: Potential protective effect is afforded by Raspberry Ketone. PLoS ONE 2019, 14, e0220548. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Chen, H.; Li, F.; Zhu, Y.; Yin, W.; Zhuo, Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J. Neuroinflamm. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, J.H.; Kim, S.; Kim, M.Y.; Hong, Y.H.; Kim, H.G.; Cho, J.Y. Gastroprotective effects of the nonsaponin fraction of Korean Red Ginseng through cyclooxygenase-1 upregulation. J. Ginseng Res. 2020, 44, 655–663. [Google Scholar] [CrossRef]
- Song, H.; Hou, X.; Zeng, M.; Chen, X.; Chen, X.; Yang, T.; Xu, F.; Peng, J.; Peng, Q.; Cai, X.; et al. Traditional Chinese Medicine Li-Zhong-Tang accelerates the healing of indomethacin-induced gastric ulcers in rats by affecting TLR-2/MyD88 signaling pathway. J. Ethnopharmacol. 2020, 259, 112979. [Google Scholar] [CrossRef]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Wada, K.; Nakajima, A.; Takahashi, H.; Yoneda, M.; Fujisawa, N.; Ohsawa, E.; Kadowaki, T.; Kubota, N.; Terauchi, Y.; Matsuhashi, N.; et al. Protective effect of endogenous PPARγ against acute gastric mucosal lesions associated with ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G452–G458. [Google Scholar] [CrossRef]
- Ugwu, F.N.; Yu, A.P.; Sin, T.K.; Tam, B.T.; Lai, C.W.; Wong, S.C.; Siu, P.M. Protective effect of unacylated ghrelin on compression-induced skeletal muscle injury mediated by SIRT1-signaling. Front. Physiol. 2017, 8, 962. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, M.Y.; Song, E.K.; Kim, E.K.; Moon, W.S.; Han, M.K.; Park, J.W.; Kwon, K.B.; Park, B.H. Overexpression of SIRT1 protects pancreatic β-cells against cytokine toxicity by suppressing the nuclear factor-κB signaling pathway. Diabetes 2009, 58, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Tavirani, M.R.; Jahani-Sherafat, S.; Tavirani, S.R.; Esmaeili, S.; Ansari, M.; Ahmadzadeh, A. Side effects of omeprazole: A system biology study. Gastroenterol. Hepatol. Bed Bench 2021, 14, 334. [Google Scholar]
- Bendas, E.R.; Abdelbary, A.A. Instantaneous enteric nano-encapsulation of omeprazole: Pharmaceutical and pharmacological evaluation. Int. J. Pharm. 2014, 468, 97–104. [Google Scholar] [CrossRef]
- Mulla, T.; Nemade, M.; Ansari, T.; Parmar, B.; Dave, D. Advancements in solubility enhancement of omeprazole: A comprehensive review. Res. J. Pharm. Dosage Forms Technol. 2024, 16, 163–172. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Z.; Zhang, N.; Zhong, J. Nanosuspension drug delivery system: Preparation, characterization, post-production processing, dosage form, and application. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–443. [Google Scholar]
- Purkayastha, H.D.; Hossian, S.I. Nanosuspension: A modern technology used in drug delivery system. Int. J. Curr. Pharm. Res. 2019, 11, 1–3. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Ma, Y. Advances in nanotechnology for enhancing the solubility and bioavailability of poorly soluble drugs. Drug Des. Devel. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation strategies of nanosuspensions for various administration routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Gupta, M.K.; Sreedharan, S.K.; Sajeeth, C.I. Design development and characterization of omeprazole loaded nanosuspension. J. Pharm. Negat. Results 2022, 13, 1195–1208. [Google Scholar]
- Kathle, P.K.; Gautam, N.; Kesavan, K. Tamoxifen citrate loaded chitosan-gellan nanocapsules for breast cancer therapy: Development, characterisation and in-vitro cell viability study. J. Microencapsul. 2018, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Lopes, R.; Campos, M.C.D.; Marruz, M.J.; da Cruz, M.E.M.; Corvo, L. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Anim. Models Exp. Med. 2019, 2, 121–126. [Google Scholar] [CrossRef]
- Sistani Karampour, N.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef]
- Wellington, D.; Mikaelian, I.; Singer, L. Comparison of ketamine–xylazine and ketamine–dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 481–487. [Google Scholar]
- Elshahat, M.S.; Elshamy, A.I. Gastroprotective actions of hydroethanolic extract of Parapholis incurva on aspirin and ethanol induced gastric ulcer in rats via histological, histochemical, immunohistochemical and biochemical assessments. Egypt. J. Chem. 2024, 67, 647–663. [Google Scholar] [CrossRef]
- Choi, J.; Jang, Y.; Paik, H.G.; Ha, M.H.J.; Kwon, J. Gastroprotective effects of aqueous extracts of broccoli stems on acute injury in rats: A comprehensive evaluation of gastric function and inflammatory responses. Medicina 2025, 61, 89. [Google Scholar] [CrossRef] [PubMed]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Abdulla, M.A. Gastroprophylactic effects of p-cymene in ethanol-induced gastric ulcer in rats. Processes 2022, 10, 1314. [Google Scholar] [CrossRef]
- Ibrahim, I.A.A.; Hussein, A.I.; Muter, M.S.; Mohammed, A.T.; Al-Medhtiy, M.H.; Shareef, S.H.; Abdulla, M.A. Effect of nano silver on gastroprotective activity against ethanol-induced stomach ulcer in rats. Biomed. Pharmacother. 2022, 154, 113550. [Google Scholar] [CrossRef] [PubMed]
- Borra, S.K.; Lagisetty, R.K.; Mallela, G.R. Anti-ulcer effect of Aloe vera in non-steroidal anti-inflammatory drug induced peptic ulcers in rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1867–1871. [Google Scholar]
- Szabo, S.; Hollander, D. Pathways of gastrointestinal protection and repair: Mechanisms of action of sucralfate. Am. J. Med. 1989, 86, 23–31. [Google Scholar] [CrossRef]
- Trajković, S.; Dobrić, S.; Jaćević, V.; Dragojević-Simić, V.; Milovanović, Z.; Đorđević, A. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf. B Biointerfaces 2007, 58, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Al Asmari, A.; Al Shahrani, H.; Al Masri, N.; Al Faraidi, A.; Elfaki, I.; Arshaduddin, M. Vanillin abrogates ethanol-induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2016, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A.; Mothana, R.A.; Abdulla, M.A.; Abdullah, F.O.; Ahmed, K.A.A.; Hussen, R.R.; Hawwal, M.F.; Fantoukh, O.I.; Hasson, S. Mechanisms of anti-ulcer actions of Prangos pabularia (L.) in ethanol-induced gastric ulcer in rats. Saudi Pharm. J. 2023, 31, 101850. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and development of oral fast-dissolving films loaded with nanosuspension to augment paroxetine bioavailability: In vitro characterization, ex vivo permeation, and pharmacokinetic evaluation in healthy human volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid-based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef]
- Leung, D.H. Development of nanosuspension formulations compatible with inkjet printing for the convenient and precise dispensing of poorly soluble drugs. Pharmaceutics 2022, 14, 449. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Hajipour, H.; Ghorbani, M.; Kahroba, H.; Mahmoodzadeh, F.; Emameh, R.Z.; Taheri, R.A. Arginyl-glycyl-aspartic acid (RGD) containing nanostructured lipid carrier co-loaded with doxorubicin and sildenafil citrate enhanced anti-cancer effects and overcomes drug resistance. Process Biochem. 2019, 84, 172–179. [Google Scholar] [CrossRef]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef] [PubMed]
- Diefenthaeler, H.S.; Bianchin, M.D.; Marques, M.S.; Nonnenmacher, J.L.; Bender, E.T.; Bender, J.G.; Nery, S.F.; Cichota, L.C.; Külkamp-Guerreiro, I.C. Omeprazole nanoparticles suspension: Development of a stable liquid formulation with a view to pediatric administration. Int. J. Pharm. 2020, 589, 119818. [Google Scholar] [CrossRef]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The effect of the particle size reduction on the biorelevant solubility and dissolution of poorly soluble drugs with different acid-base character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and evaluation of naringenin nanosuspensions for bioavailability enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.U.; Singh, S.; Sharma, P.; Prajapati, B.G. Amorphization of low soluble drug with amino acids to improve its therapeutic efficacy: A state-of-art review. AAPS PharmSciTech 2023, 24, 253. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; Michel, H.E.; El-Shazly, M.; Singab, A.N.B. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2020, 248, 112297. [Google Scholar] [CrossRef]
- Saremi, K.; Rad, S.K.; Khalilzadeh, M.; Hussaini, J.; Majid, N.A. In vivo acute toxicity and anti-gastric evaluation of a novel dichloro Schiff base: Bax and HSP70 alteration. Acta Biochim. Biophys. Sin. 2020, 52, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A.; Abdullah, F.O.; Abdoulrahman, K.; Galali, Y.; Ibrahim, I.A.A.; Alzahrani, A.R.; Hassan, R.R. Gastroprotective, biochemical and acute toxicity effects of Papaver decaisnei against ethanol-induced gastric ulcers in rats. Processes 2022, 10, 1985. [Google Scholar] [CrossRef]
- Dos Reis Lívero, F.A.; da Silva, L.M.; Ferreira, D.M.; Galuppo, L.F.; Borato, D.G.; Prando, T.B.; Lourenço, E.L.; Strapasson, R.L.; Stefanello, M.É.; Werner, M.F.; et al. Hydroethanolic extract of Baccharis trimera promotes gastroprotection and healing of acute and chronic gastric ulcers induced by ethanol and acetic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 985–998. [Google Scholar] [CrossRef]
- Obied, S.M. Evaluation of prophylactic effect of aqueous liquorice root extract against gastric ulcer in rats induced by ethanol. Egypt. J. Vet. Sci. 2025, 56, 1135–1142. [Google Scholar] [CrossRef]
- Wetchakul, P.; Net-Anong, S.; Goon, J.A.; Sanpinit, S. Anti-oxidative stress and gastroprotective effect of Tri-Tharn-Thip tea against ethanol-induced gastric ulcer in rats. S. Afr. J. Bot. 2024, 170, 130–136. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Mohammed, H.S.; Aboushousha, T.; Lotfy, D.M.; El-Shazly, M.A.; Sobeh, M.; Taha, E.F. Ipomoea carnea mitigates ethanol-induced ulcers in irradiated rats via Nrf2/HO−1 pathway: An in vivo and in silico study. Sci. Rep. 2024, 14, 3469. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.P.; Song, Y.G.; Cheng, W.; Zhao, T.; Yao, Y.L. Gastrin, somatostatin, G and D cells of gastric ulcer in rats. World J. Gastroenterol. 2002, 8, 375. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Q.; Xu, N.; Cai, J.; Luo, D.; Zhang, Q.; Su, Z.; Gao, C.; Liu, Y. Antioxidative and anti-inflammatory effects of water extract of Acrostichum aureum Linn. against ethanol-induced gastric ulcer in rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 3585394. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.; Oh, J.; Lim, J.S.; Lee, J.S.; Kim, J.S. Protective effects of Dioscorea batatas flesh and peel extracts against ethanol-induced gastric ulcer in mice. Nutrients 2018, 10, 1680. [Google Scholar] [CrossRef]
- Mohan, S.; Hobani, Y.H.; Shaheen, E.; Abou-Elhamd, A.S.; Alhazmi, H.A.; Abdelwahab, S.I. Ameliorative effect of Boesenbergin A, a chalcone isolated from Boesenbergia rotunda (Fingerroot) on oxidative stress and inflammation in ethanol-induced gastric ulcer in vivo. J. Ethnopharmacol. 2020, 261, 113104. [Google Scholar] [CrossRef]
- Liu, B.; Feng, X.; Zhang, J.; Wei, Y.; Zhao, X. Preventive effect of Anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in Kunming mice. Biomolecules 2019, 9, 137. [Google Scholar] [CrossRef]
- Biswas, K.; Bandyopadhyay, U.; Chattopadhyay, I.; Varadaraj, A.; Ali, E.; Banerjee, R.K. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem. 2003, 278, 10993–11001. [Google Scholar] [CrossRef]
- Isik, M.; Ozbayer, C.; Donmez, D.B.; Colak, E.; Ustuner, M.C.; Erol, K.; Degirmenci, I. Effects of the probiotic, Lactobacillus rhamnosus GG, on ulcer pathogenesis, HSP70 stress protein and nitric oxide levels in stress induced ulcer. Biotechnic Histochem. 2022, 97, 449–460. [Google Scholar] [CrossRef]
- Hu, T.; Gui, Z.; Gong, J.; Rong, R.; Wang, X.; Tan, W.; Wang, Z.; Xu, X. INOS-mediated acute stomach injury and recovery in mice after oral exposure to halloysite nanotubes. Environ. Pollut. 2020, 258, 113758. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- El-Naga, R.N. Apocynin protects against ethanol-induced gastric ulcer in rats by attenuating the upregulation of NADPH oxidases 1 and 4. Chem.-Biol. Interact. 2015, 242, 317–326. [Google Scholar] [CrossRef]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Gamboni-Robertson, F.; He, Q.; Svetkauskaite, D.; Kim, J.Y.; Strassheim, D.; Sohn, J.W.; Yamada, S.; Maruyama, I.; Banerjee, A.; et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006, 290, C917–C924. [Google Scholar] [CrossRef]
- Nadatani, Y.; Watanabe, T.; Tanigawa, T.; Ohkawa, F.; Takeda, S.; Higashimori, A.; Sogawa, M.; Yamagami, H.; Shiba, M.; Watanabe, K.; et al. High-mobility group box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products. PLoS ONE 2013, 8, e80130. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Wu, J.; Chen, H.; Zhang, Z.; Yang, H.; Chen, L.; Chen, H.; Su, Z.; Li, Y. Transformation of patchouli alcohol to β-patchoulene by gastric juice: β-patchoulene is more effective in preventing ethanol-induced gastric injury. Sci. Rep. 2017, 7, 5591. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κB: A potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid. Med. Cell. Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef]
- Akanda, M.R.; Park, B.Y. Involvement of MAPK/NF-κB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed. Pharmacother. 2017, 95, 1139–1146. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Taher, R.F.; Abd El Ghany, E.M.; El-Gendy, Z.A.; Elghonemy, M.M.; Hassan, H.A.; Abdel Jaleel, G.A.; Elshamy, A.I. In vivo anti-ulceration effect of Pancratium maritimum extract against ethanol-induced rats via NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-κβ signaling pathways and its extract metabolite profile. PLoS ONE 2025, 20, e0321018. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, B.; Zhao, J.; Zhang, M. Ethanol causes non-communicable disease through activation of NLRP3 inflammasome: A review on mechanism of action and potential interventions. Am. J. Drug Alcohol Abuse 2024, 50, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.G.E.; Eissa, R.G. Amelioration of ethanol-induced gastric ulcer in rats by quercetin: Implication of Nrf2/HO1 and HMGB1/TLR4/NF-κB pathways. Heliyon 2022, 8, e11159. [Google Scholar] [CrossRef] [PubMed]
- Alzokaky, A.A.M.; Abdelkader, E.M.; El-Dessouki, A.M.; Khaleel, S.A.; Raslan, N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-κB pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 265–277. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Huang, T.; Huang, D.; Liu, L.; Shen, C.; Jiang, C.; Wang, Z.; Chen, H.; Liang, P.; et al. Licorice flavonoid alleviates gastric ulcers by producing changes in gut microbiota and promoting mucus cell regeneration. Biomed. Pharmacother. 2023, 169, 115868. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, M.; Yang, Y.F. Saccharomyces cerevisiae β-glucan-induced SBD-1 expression in ovine ruminal epithelial cells is mediated through the TLR-2-MyD88-NF-κB/MAPK pathway. Vet. Res. Commun. 2019, 43, 77–89. [Google Scholar] [CrossRef]
- Xiong, H.; Tian, L.; Zhao, Z.; Chen, S.; Zhao, Q.; Hong, J.; Xie, Y.; Zhou, N.; Fu, Y. The sinomenine enteric-coated microspheres suppressed the TLR/NF-κB signaling in DSS-induced experimental colitis. Int. Immunopharmacol. 2017, 50, 251–262. [Google Scholar] [CrossRef]
- Fu, Y.H.; Duan, Y.Z.; Sun, X.Y.; Chen, S.Q. Gastroprotective effect of an active ingredients group of Lindera reflexa Hemsl. on ethanol-induced gastric ulcers in rats: Involvement of VEGFR2/ERK and TLR-2/MyD88 signaling pathway. Int. Immunopharmacol. 2022, 107, 108673. [Google Scholar] [CrossRef]
- Rahman, Z.; Dwivedi, D.K.; Jena, G.B. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: Involvement of Nrf2/HO-1 signalling pathway. Hum. Exp. Toxicol. 2020, 39, 547–562. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Ding, Y.; Ma, Y.; Shang, P.; Liu, T.; Hui, G.; Wang, L.; Wang, M.; Zhu, Z.; et al. Alpha-boswellic acid protects against ethanol-induced gastric injury in rats: Involvement of nuclear factor erythroid-2-related factor 2/heme oxygenase-1 pathway. J. Pharm. Pharmacol. 2016, 68, 514–522. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, E.J.; Park, S.W.; Kim, H.J.; Chang, K.C. A tetrahydroisoquinoline alkaloid THI-28 reduces LPS-induced HMGB1 and diminishes organ injury in septic mice through p38 and PI3K/Nrf2/HO-1 signals. Int. Immunopharmacol. 2013, 17, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, Z.; Zhang, P.; Zheng, C.; Zhou, W.; Cui, W.; Xu, L.; Gao, J. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J. Cell Physiol. 2019, 234, 8862–8872. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Kilic, U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019, 24, 1–10. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronectin and transforming growth factor-β1 in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef]
- Rothgiesser, K.M.; Erener, S.; Waibel, S.; Lüscher, B.; Hottiger, M.O. SIRT2 regulates NF-κB-dependent gene expression through deacetylation of p65 Lys310. J. Cell Sci. 2010, 123, 4251–4258. [Google Scholar] [CrossRef]
- Hah, Y.S.; Cheon, Y.H.; Lim, H.S.; Cho, H.Y.; Park, B.H.; Ka, S.O.; Lee, Y.R.; Jeong, D.W.; Kim, H.O.; Han, M.K.; et al. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS ONE 2014, 9, e87733. [Google Scholar] [CrossRef]
- Arab, H.H.; Eid, A.H.; El-Sheikh, A.A.; Arafa, E.S.A.; Ashour, A.M. Irbesartan reprofiling for the amelioration of ethanol-induced gastric mucosal injury in rats: Role of inflammation, apoptosis, and autophagy. Life Sci. 2022, 308, 120939. [Google Scholar] [CrossRef]
- Su, C.G.; Wen, X.; Bailey, S.T.; Jiang, W.; Rangwala, S.M.; Keilbaugh, S.A.; Flanigan, A.; Murthy, S.; Lazar, M.A.; Wu, G.D. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J. Clin. Investig. 1999, 104, 383–389. [Google Scholar] [CrossRef]
- Kim, T.; Yang, Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J. Cardiol. 2013, 5, 164. [Google Scholar] [CrossRef]
- Gupta, R.A.; Polk, D.B.; Krishna, U.; Israel, D.A.; Yan, F.; DuBois, R.N.; Peek, R.M., Jr. Activation of peroxisome proliferator-activated receptor γ suppresses nuclear factor κB-mediated apoptosis induced by Helicobacter pylori in gastric epithelial cells. J. Biol. Chem. 2001, 276, 31059–31066. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Sen, T.; Palit, G. Involvement of glucocorticoid receptor and peroxisome proliferator activated receptor-γ in pioglitazone mediated chronic gastric ulcer healing in rats. Eur. J. Pharmacol. 2009, 609, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Role of peroxisome proliferator-activated receptors alpha and gamma in gastric ulcer: An overview of experimental evidences. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 120. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Saidy, E.A.; El-Naggar, M.E.; Sokar, S.S. Cilostazol protects against gastric ulcers by regulating PPAR-γ, HO-1, PECAM-1, pErk-1, NF-κB, Bcl-2, and cleaved caspase-3 protein expression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9033–9050. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.C.; Ekinci Akdemir, F.N.; Tanyeli, A.; Eraslan, E.; Bayir, Y. Gastroprotective effects of Fraxin with antioxidant activity on the ethanol-induced gastric ulcer. South. Clin. Istanb. Eurasia 2022, 33, 21–26. [Google Scholar]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.R.; Li, H.; Wang, S.W. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]



| Forward Sequence | Reverse Sequence | |
|---|---|---|
| PPAR-γ | GTACTGCCGCTTCCAGAA | GTGCACGCCATACTTGAG |
| SIRT-1 | TGTTTCCTGTGGGATACCTGA | TGAAGAATGGTCTTGGGTCTTT |
| Nrf2 | CACATCCAGACAGACACCAGT | CTACAAATGGGAATGTCTCTGC |
| GAPDH | TGGATTTGGACGCATTGGTC | TTTGCACTGGTACGTGTTGAT |
| NO | Functional Group | Frequency Range | Observed Frequency OMP | Observed Frequency OMP-NS |
|---|---|---|---|---|
| 1 | C=C aromatic | 1600–1400 cm−1 | 1428.05 cm−1 | 1418.18 cm−1 |
| 2 | C-OH stretch | 1200–1020 cm−1 | 1066.97 cm−1 | 1010.93 cm−1 |
| 5 | C-Br | 750–500 cm−1 | 548.34 cm−1 | 668.57 cm−1 |
| 6 | C-I | ~500 cm−1 | 471.98 cm−1 | 448.62 cm−1 |
| Control | Ulcer | Ulcer + OMP | Ulcer + OMP-NS | |
|---|---|---|---|---|
| Final body weight (g) | 255.7 ± 10.05 | 186 ± 11.2 @ | 213 ± 9.4 @# | 235.5 ± 5.9 @#& |
| Stomach weight (g) | 1.17 ± 0.1 | 1.85 ± 0.07 @ | 1.29± 0.20 # | 1.06 ± 0.12 # |
| Stomach coefficient (%) | 0.46 ± 0.05 | 0.99 ± 0.08 @ | 0.61 ± 0.06 @# | 0.45 ± 0.04 #& |
| Gastric PH | 6.28 ± 0.22 | 4.17 ± 0.25 @ | 4.34 ± 0.31 @ | 5.39 ± 0.45 @#& |
| UI (%) | 0.0 ± 0.0 | 6.05 ± 0.51 @ | 1.37 ± 0.27 @# | 0.55 ± 0.14 @#& |
| CR (%) | - | - | 77.19 ± 5.20 @# | 90.83 ± 2.65 @#& |
| Control | Ulcer | Ulcer + OMP | Ulcer + OMP-NS | |
|---|---|---|---|---|
| ALT (U/mL) | 33.48 ± 1.03 | 91.97 ± 2.68 @ | 69.27 ± 2.31 @# | 48.27 ± 3.42 @#& |
| AST (U/mL) | 44.90 ± 1.35 | 113 ± 1.26 @ | 80.64 ± 3.05 @# | 60.06 ± 1.79 @#& |
| ALP (ng/mL) | 2.04 ± 0.09 | 8.65 ± 0.06 @ | 6.01 ± 0.03 @# | 4.01 ± 0.9 @#& |
| Creatinine (mg/dL) | 2.45 ± 0.09 | 13.42 ± 0.57 @ | 7.48 ± 0.37 @# | 5.07 ± 0.74 @#& |
| Urea (nmol/mL) | 1.77 ± 0.03 | 9.48 ± 0.37 @ | 6.33 ± 0.08 @# | 3.92 ± 0.07 @#& |
| Uric acid (mg/dL) | 0.50 ± 0.03 | 5.48 ± 0.37 @ | 3.28 ± 0.29 @# | 1.75 ± 0.09 @#& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, M.; Khateeb, S. Enhanced Therapeutic Efficacy of Omeprazole Nanosuspension in Ethanol-Induced Gastric Ulcer: A Focus on Oxidative Stress and Inflammatory Pathways. Biomolecules 2025, 15, 902. https://doi.org/10.3390/biom15060902
Albalawi M, Khateeb S. Enhanced Therapeutic Efficacy of Omeprazole Nanosuspension in Ethanol-Induced Gastric Ulcer: A Focus on Oxidative Stress and Inflammatory Pathways. Biomolecules. 2025; 15(6):902. https://doi.org/10.3390/biom15060902
Chicago/Turabian StyleAlbalawi, Mody, and Sahar Khateeb. 2025. "Enhanced Therapeutic Efficacy of Omeprazole Nanosuspension in Ethanol-Induced Gastric Ulcer: A Focus on Oxidative Stress and Inflammatory Pathways" Biomolecules 15, no. 6: 902. https://doi.org/10.3390/biom15060902
APA StyleAlbalawi, M., & Khateeb, S. (2025). Enhanced Therapeutic Efficacy of Omeprazole Nanosuspension in Ethanol-Induced Gastric Ulcer: A Focus on Oxidative Stress and Inflammatory Pathways. Biomolecules, 15(6), 902. https://doi.org/10.3390/biom15060902







