Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows
Abstract
1. Introduction
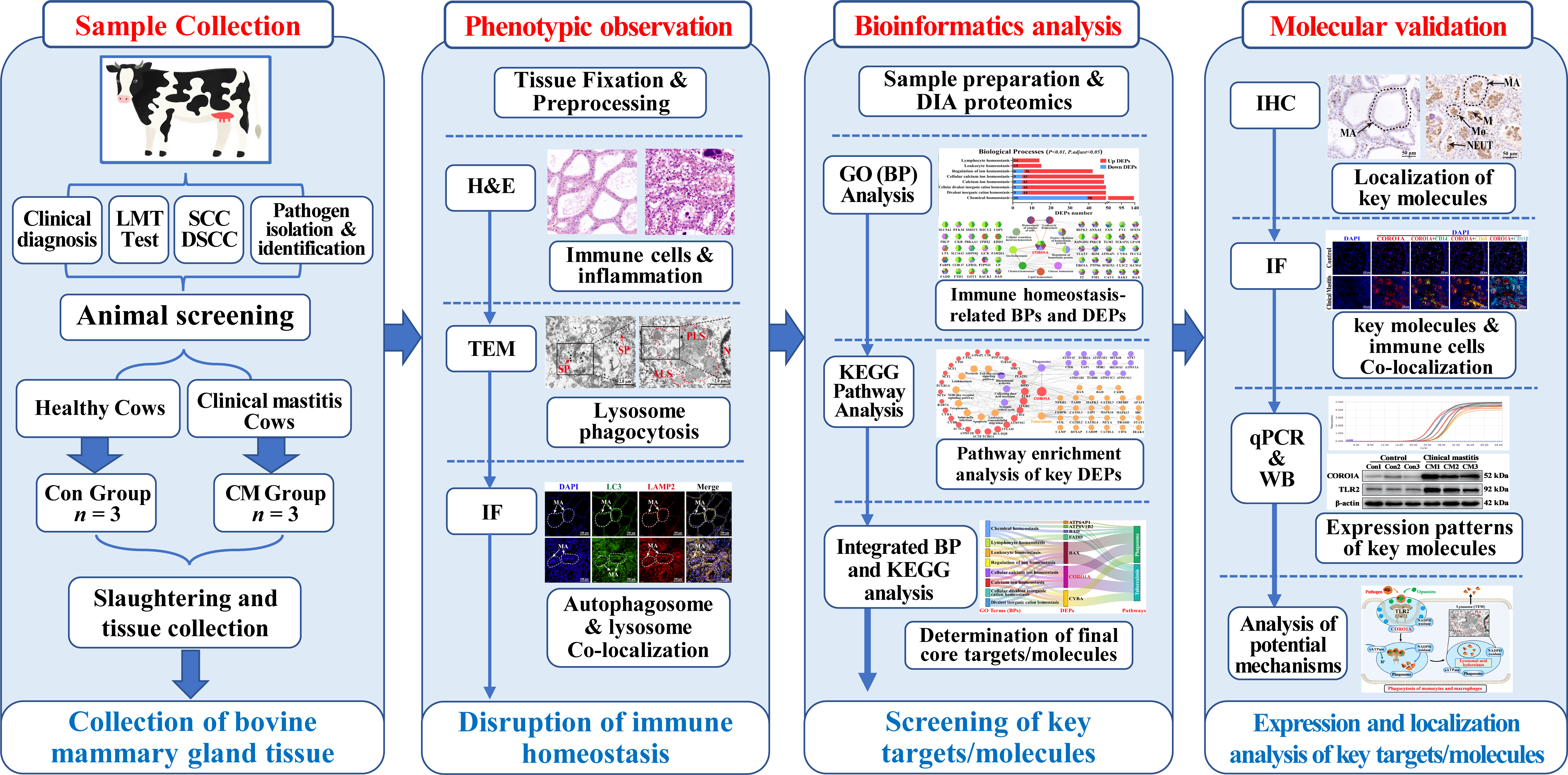
2. Materials and Methods
2.1. Sample Preparation and Collection
2.2. Hematoxylin–Eosin (H&E) Staining
2.3. Transmission Electron Microscopy (TEM)
2.4. Immunofluorescence (IF) Staining
2.5. Bioinformatics Analysis
2.6. Immunohistochemistry (IHC) Staining
2.7. RNA Isolation, cDNA Synthesis, and Quantitative Polymerase Chain Reaction (qPCR) Assays
2.8. Western Blot (WB) Analysis
2.9. Statistical Analysis
3. Results
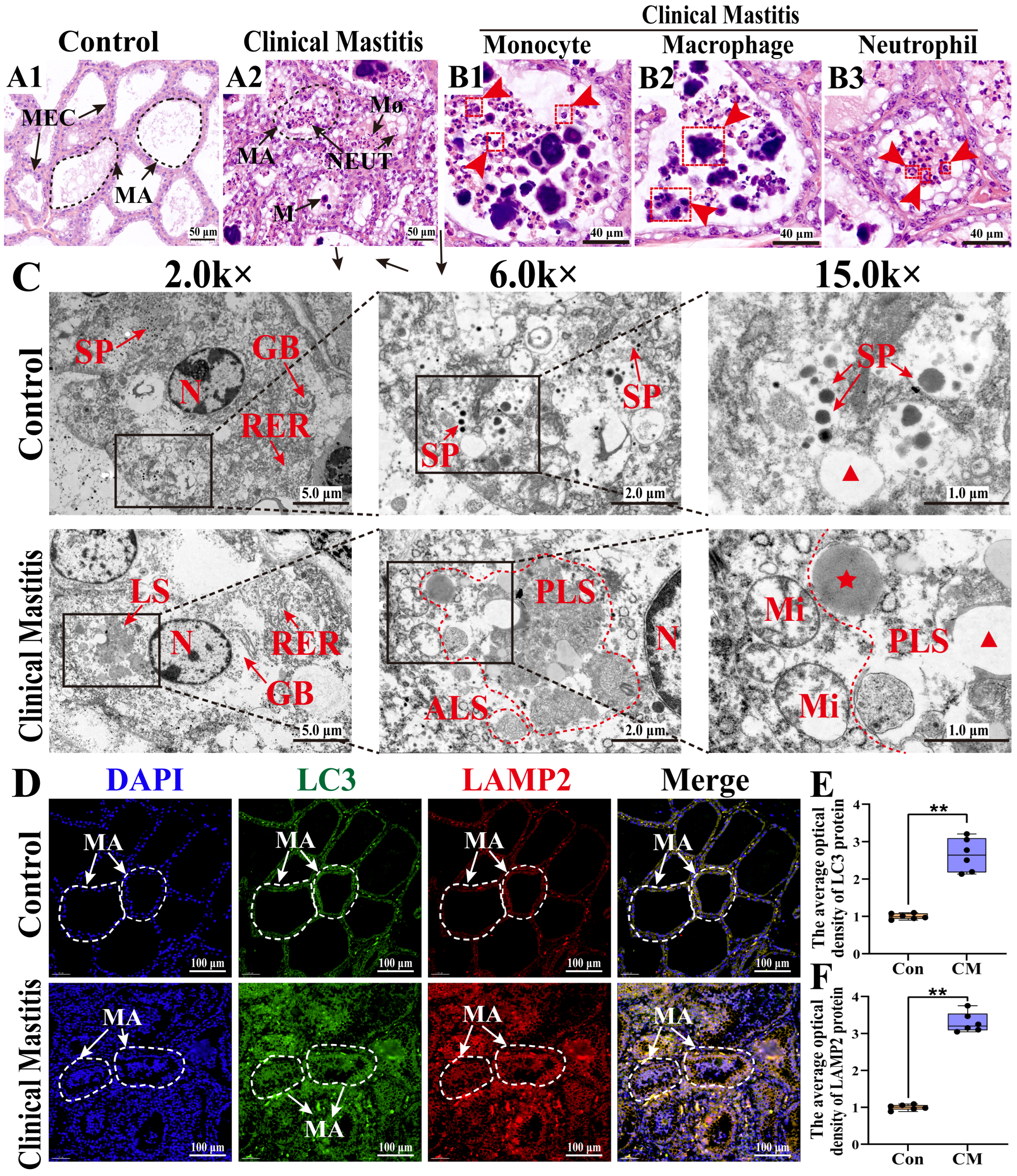
3.1. Histologic and Ultrastructural Pathology Observation of the MGs in Holstein Cows with CM
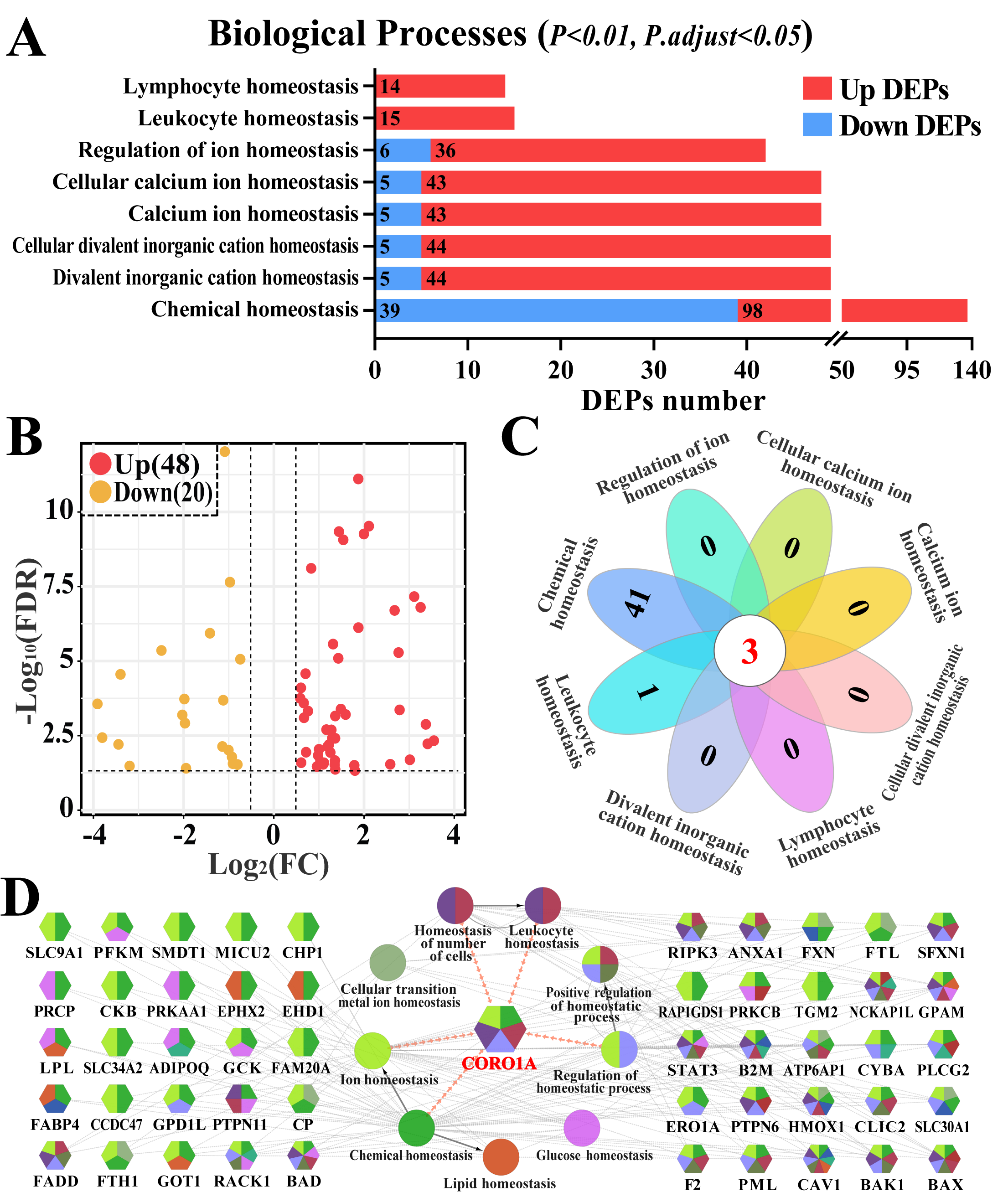
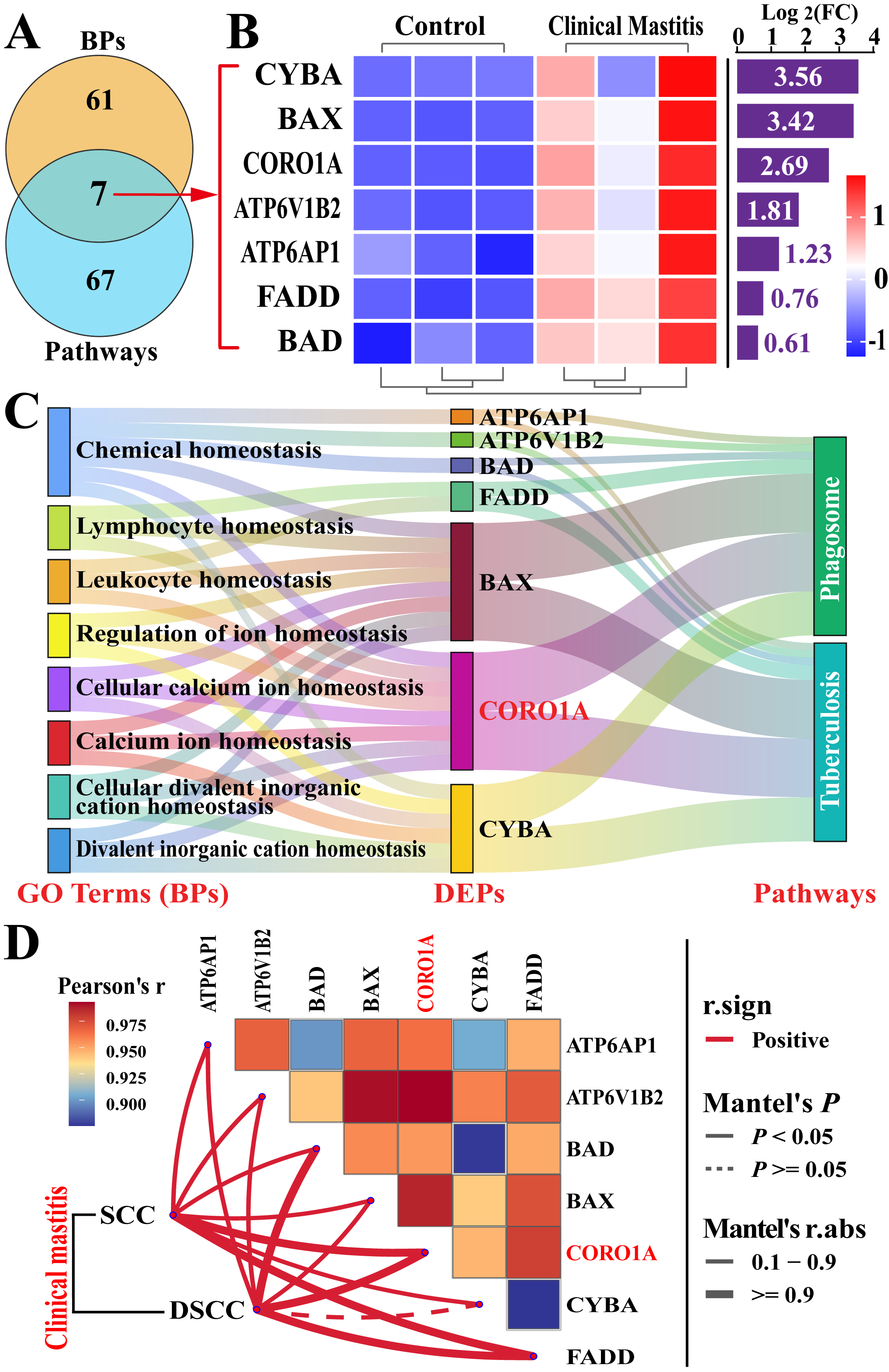
3.2. Screening Immune Homeostasis-Associated GO Terms and DEPs
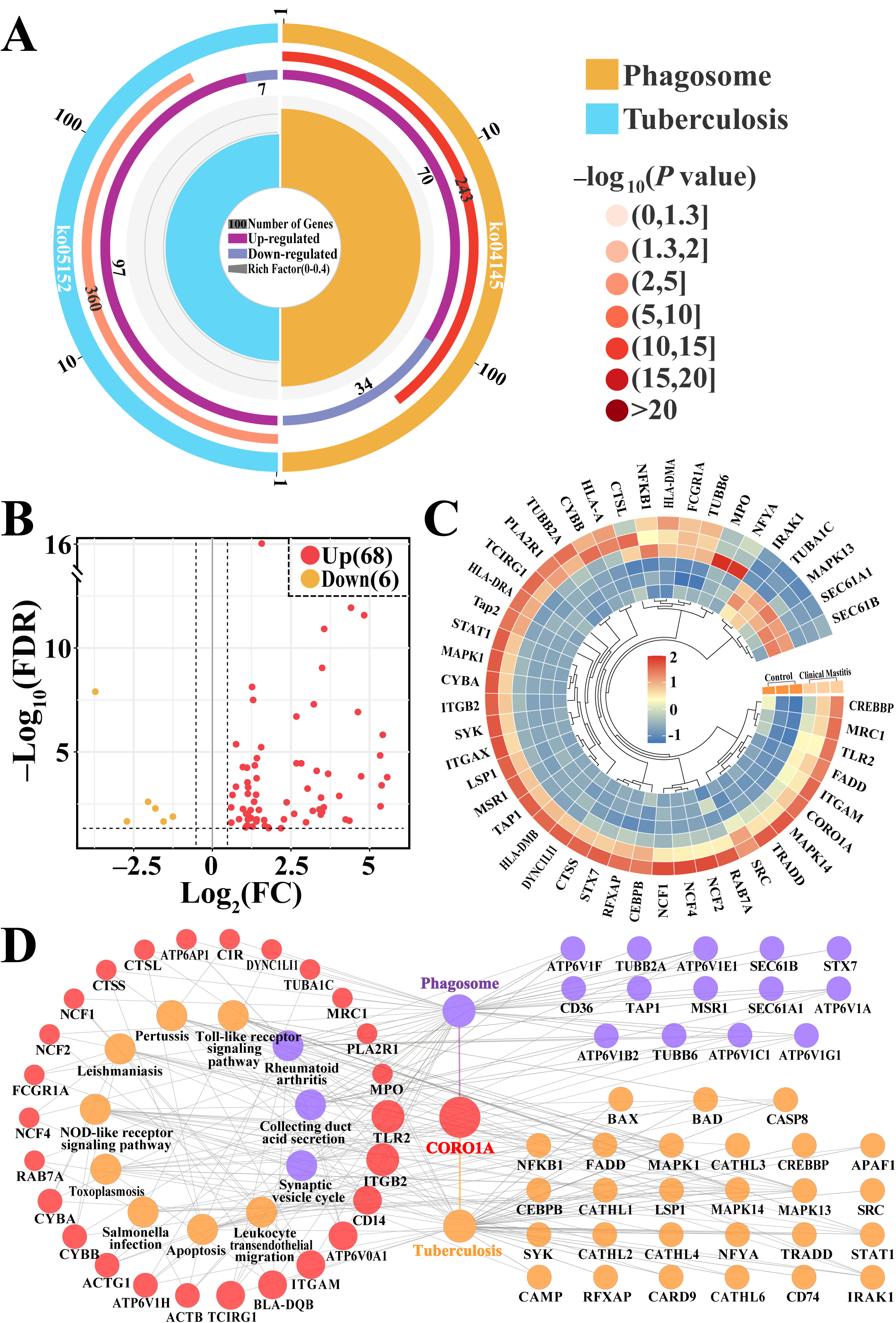
3.3. Screening CORO1A-Associated KEGG Pathways and DEPs
3.4. Conjoint Analysis of Candidate BP Terms and Pathways Associated with CORO1A
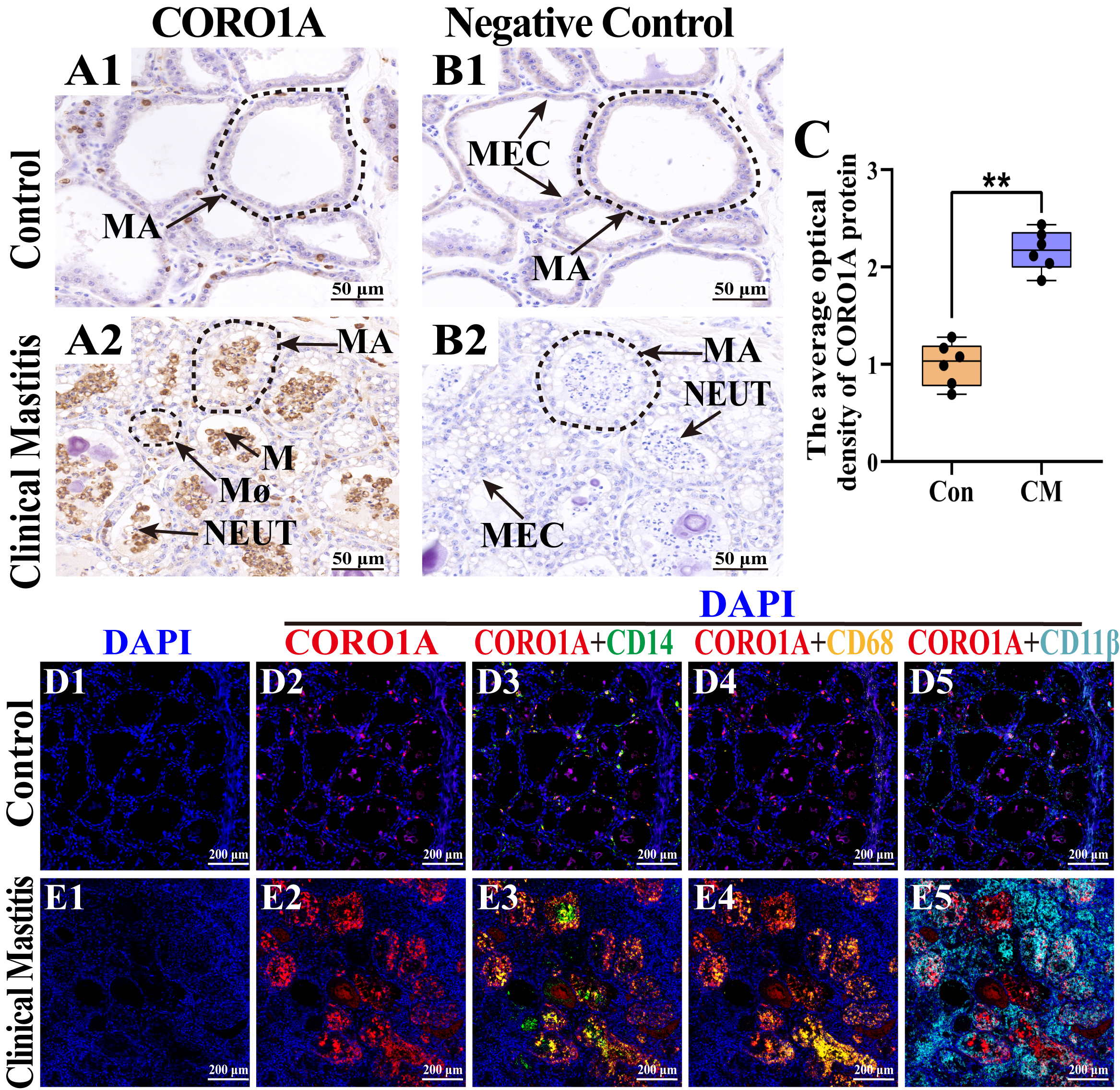
3.5. Distribution and Expression Pattern of CORO1A in MGs of Holstein Cows
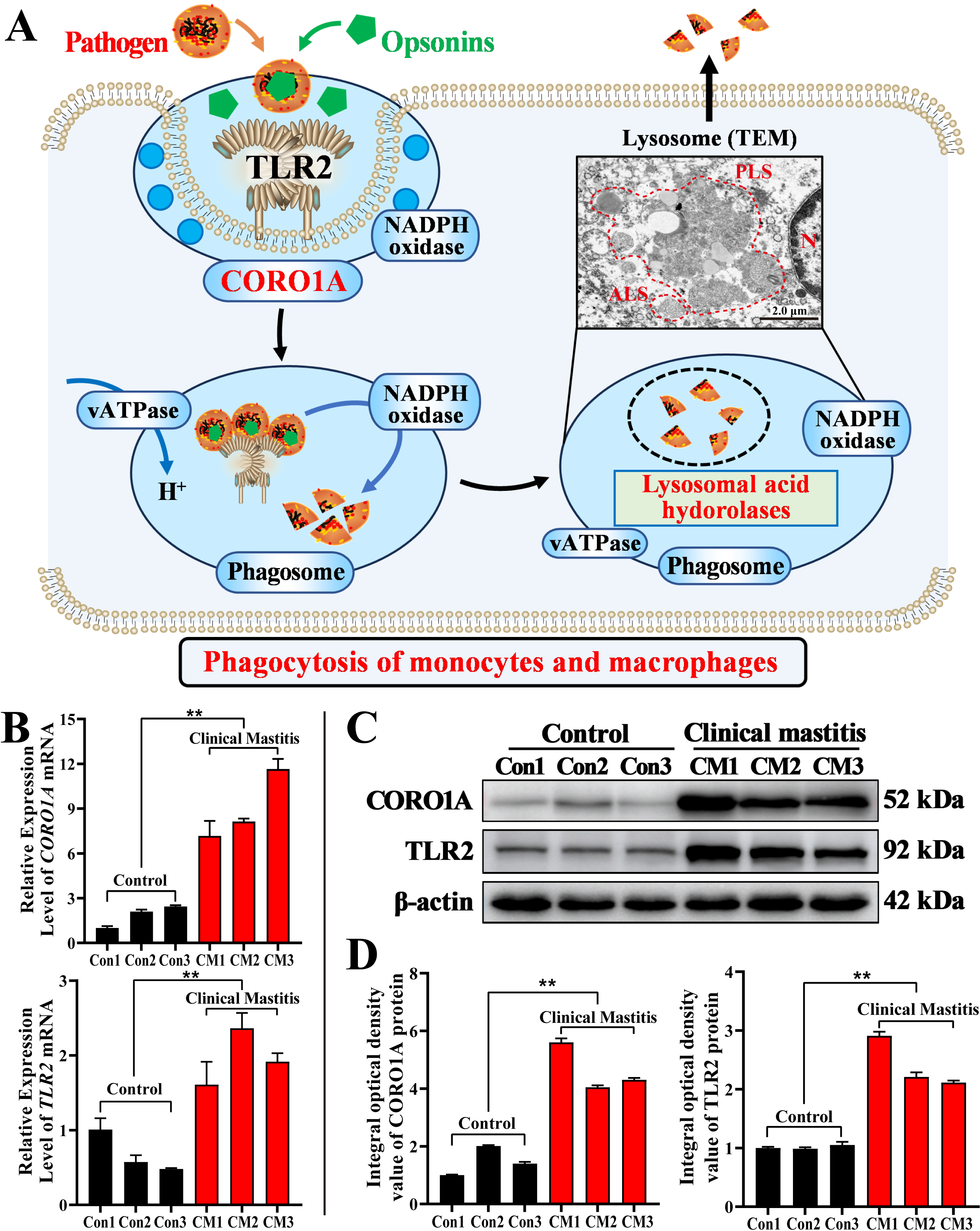
3.6. Molecular Mechanism Prediction and Expression Patterns of CORO1A mRNA and Proteins in MGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jong, E.; McCubbin, K.D.; Speksnijder, D.; Dufour, S.; Middleton, J.R.; Ruegg, P.L.; Lam, T.J.; Kelton, D.F.; McDougall, S.; Godden, S.M.; et al. Invited review: Selective treatment of clinical mastitis in dairy cattle. J. Dairy. Sci. 2023, 106, 3761–3778. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Zhai, J.; Wang, H.; Chen, X.; Qi, Y. Prevalence of clinical mastitis and its associated risk factors among dairy cattle in mainland China during 1982–2022: A systematic review and meta-analysis. J. Front. Vet. Sci. 2023, 10, 1185995. [Google Scholar] [CrossRef] [PubMed]
- Aqeela, A.; Muhammad, I. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar]
- Jóźwik, A.; Krzyzewski, J.; Strzałkowska, N.; Poławska, E.; Horbańczuk, J.O. Relations between the oxidative status, mastitis, milk quality and disorders of reproductive functions in dairy cows—A review. Anim. Sci. Pap. 2012, 30, 297–307. [Google Scholar]
- Saenz-de-Juano, M.D.; Silvestrelli, G.; Buri, S.; Zinsli, L.V.; Schmelcher, M.; Ulbrich, S.E. Mastitis-related Staphylococcus aureus-derived extracellular vesicles induce a pro-inflammatory response in bovine monocyte-derived macrophages. Sci. Rep. 2025, 15, 6059. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Martins, R.P. Adaptive cell-mediated immunity in the mammary gland of dairy ruminants. J. Front. Vet. Sci. 2022, 9, 854890. [Google Scholar] [CrossRef]
- Bhattarai, D.; Worku, T.; Dad, R.; Rehman, Z.U.; Gong, X.; Zhang, S. Mechanism of pattern recognition receptors (PRRs) and host pathogen interplay in bovine mastitis. Microb. Pathog. 2018, 120, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Gilbert, F.B.; Germon, P. Immune defenses of the mammary gland epithelium of dairy ruminants. J. Front. Immunol. 2022, 13, 1031785. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.K.; Kushwah, M.S.; Kaur, M.; Patbandha, T.K.; Mohanty, A.K.; Dang, A.K. Formation of NET, phagocytic activity, surface architecture, apoptosis and expression of toll like receptors 2 and 4 (TLR2 and TLR4) in neutrophils of mastitic cows. Vet. Res. Commun. 2014, 38, 209–219. [Google Scholar] [CrossRef]
- Toyama-Sorimachi, N.; Kobayashi, T. Lysosomal amino acid transporters as key players in inflammatory diseases. Int. Immunol. 2021, 33, 853–858. [Google Scholar] [CrossRef]
- Lauzon, K.; Zhao, X.; Bouetard, A.; Delbecchi, L.; Paquette, B.; Lacasse, P. Antioxidants to Prevent Bovine Neutrophil-Induced Mammary Epithelial Cell Damage. J. Dairy. Sci. 2005, 88, 4295–4303. [Google Scholar] [CrossRef]
- Mordak, R.; Dobrzański, Z.; Popiel, J.; Kupczyński, R. Analysis of correlations between selected blood markers of acid-base balance, blood electrolytes, and milk components in dairy cows during late lactation. Med. Weter. 2023, 79. [Google Scholar] [CrossRef]
- Remigante, A.; Gavazzo, P.; Morabito, R.; Dossena, S. Editorial: Ion transporters and channels in cellular pathophysiology. Front. Cell Dev. Biol. 2022, 10, 1049433. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Dong, W.; Zhao, Y.; Zhao, X.; Zhang, Y.; Zhang, Q. SLC34A2 Targets in Calcium/Phosphorus Homeostasis of Mammary Gland and Involvement in Development of Clinical Mastitis in Dairy Cows. Animals 2024, 14, 1275. [Google Scholar] [CrossRef]
- Kobayashi, K. Culture Models to Investigate Mechanisms of Milk Production and Blood-Milk Barrier in Mammary Epithelial Cells: A Review and a Protocol. J. Mammary Gland. Biol. Neoplasia 2023, 28, 8. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Wang, J.; Ma, Y.; Chen, T.; Ma, M.; Ullah, Q.; Khan, I.M.; Khan, A.; Cao, Z.; Liu, S. Genetic polymorphisms in immune-and inflammation-associated genes and their association with bovine mastitis resistance/susceptibility. Front. Immunol. 2023, 14, 1082144. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.-L.; Li, H.; Shen, Y.-Z.; Pauline, M.S. Aureus Lipoteichoic Acid Failed to Activate NLRP3, but Downregulated the Transduction of NLRP3/MAPK and NF-κB Signaling Pathway in Mouse Mastitis Induced by S. Aureus. 2020. Available online: https://pdfs.semanticscholar.org/d745/b68b98d0f2555c97bcb493d6b50ee73126c6.pdf (accessed on 20 October 2024).
- Zhang, K.; Zhang, R.; Zhang, Y.; Zhang, M.; Su, H.; Zhao, F.; Wang, D.; Cao, G.; Zhang, Y. Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin. Life 2025, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, Y.; Ma, J.; Gao, J.; Cao, Z. Role of the JAK-STAT pathway in bovine mastitis and milk production. Animals 2020, 10, 2107. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, J.; Ma, Y.; Chen, T.; Shao, D.; Cao, Z. Overview of Research Development on the Role of NF-? B Signaling in Mastitis. Animals 2020, 10, 1625. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, H.; Wang, X.; Wang, Y.; Xia, Z. Association of nonpuerperal mastitis with cytokines related to helper T cells TH1/TH2 and TH17/Treg. Altern. Ther. Health Med. 2023, 29, 150–155. [Google Scholar]
- Yu, C.; Zhang, C.; Huai, Y.; Liu, D.; Zhang, M.; Wang, H.; Zhao, X.; Bo, R.; Li, J.; Liu, M. The inhibition effect of caffeic acid on NOX/ROS-dependent macrophages M1-like polarization contributes to relieve the LPS-induced mice mastitis. Cytokine 2024, 174, 156471. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Lin, T.; Wang, X.; Zhang, B.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X. HMOX1 Promotes Ferroptosis in Mammary Epithelial Cells via FTH1 and Is Involved in the Development of Clinical Mastitis in Dairy Cows. Antioxidants 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, X.; Lin, T.; Dong, W.; Gao, Y.; Ji, P.; Zhang, Y.; Zhao, X.; Zhang, Q. Toll-like receptor 2 is associated with the immune response, apoptosis, and angiogenesis in the mammary glands of dairy cows with clinical mastitis. Int. J. Mol. Sci. 2022, 23, 10717. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Gong, J.; Du, J.; Zhang, Y.; Zhao, X. Yak IGF2 Promotes Fibroblast Proliferation Via Suppression of IGF1R and PI3KCG Expression. Genes. 2018, 9, 169. [Google Scholar] [CrossRef]
- Sadat, A.; Farag, A.M.; Elhanafi, D.; Awad, A.; Elmahallawy, E.K.; Alsowayeh, N.; El-Khadragy, M.F.; Elshopakey, G.E. Immunological and oxidative biomarkers in bovine serum from healthy, clinical, and sub-clinical mastitis caused by Escherichia coli and Staphylococcus aureus infection. Animals 2023, 13, 892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, X.; Shi, J.; Wang, X.; Zhang, B.; Dai, L.; Lin, T.; Gao, Y.; Zhang, Y.; Zhao, X. DIA proteomics identified the potential targets associated with angiogenesis in the mammary glands of dairy cows with hemorrhagic mastitis. Front. Vet. Sci. 2022, 9, 980963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lin, T.; Bai, X.; An, X.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X.; Zhang, Q. Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-lyase/H2S in Holstein Cows with Clinical Mastitis. Animals 2022, 12, 1451. [Google Scholar] [CrossRef]
- He, W.; Sun, Z.; Tong, G.; Zeng, L.; He, W.; Chen, X.; Zhen, C.; Chen, P.; Tan, N.; He, P. FUNDC1 alleviates doxorubicin-induced cardiotoxicity by restoring mitochondrial-endoplasmic reticulum contacts and blocked autophagic flux. Theranostics 2024, 14, 3719–3738. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yee, C.S.; Massaad, M.J.; Bainter, W.; Ohsumi, T.K.; Föger, N.; Chan, A.C.; Akarsu, N.A.; Aytekin, C.; Ayvaz, D.Ç.; Tezcan, I.; et al. Recurrent viral infections associated with a homozygous CORO1A mutation that disrupts oligomerization and cytoskeletal association. J. Allergy Clin. Immunol. 2016, 137, 879–888.e872. [Google Scholar] [CrossRef]
- Bagaitkar, J.; Huang, J.; Zeng, M.Y.; Pech, N.K.; Monlish, D.A.; Perez-Zapata, L.J.; Miralda, I.; Schuettpelz, L.G.; Dinauer, M.C. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood 2018, 131, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- El Chemaly, A.; Nunes, P.; Jimaja, W.; Castelbou, C.; Demaurex, N. Hv1 proton channels differentially regulate the pH of neutrophil and macrophage phagosomes by sustaining the production of phagosomal ROS that inhibit the delivery of vacuolar ATPases. J. Leukoc. Biol. 2014, 95, 827–839. [Google Scholar] [CrossRef]
- Paape, M.J.; Shafer-Weaver, K.; Capuco, A.V.; Van Oostveldt, K.; Burvenich, C. Immune surveillance of mammary tissue by phagocytic cells. Biol. Mammary Gland. 2002, 259–277. [Google Scholar]
- Eberl, G. A new vision of immunity: Homeostasis of the superorganism. Mucosal Immunol. 2010, 3, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Swartz, T.H. Review: Following the smoke signals: Inflammatory signaling in metabolic homeostasis and homeorhesis in dairy cattle. Animal 2020, 14, s144–s154. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Koledić, M.; Maćešić, N.; Benić, M.; Dobranić, V.; Đuričić, D.; Cvetnić, L.; Samardžija, M. The role of oxidative stress and inflammatory response in the pathogenesis of mastitis in dairy cows. Mljekarstvo časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2017, 67, 91–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, J.; Yang, D.; Tang, C.; Chen, J. Staphylococcal enterotoxin M induced inflammation and impairment of bovine mammary epithelial cells. J. Dairy. Sci. 2020, 103, 8350–8359. [Google Scholar] [CrossRef]
- Sharma, P.; Vijaykumar, A.; Raghavan, J.V.; Rananaware, S.R.; Alakesh, A.; Bodele, J.; Rehman, J.U.; Shukla, S.; Wagde, V.; Nadig, S.; et al. Particle uptake driven phagocytosis in macrophages and neutrophils enhances bacterial clearance. J. Control. Release 2022, 343, 131–141. [Google Scholar] [CrossRef]
- Eskelinen, E.-L.; Illert, A.L.; Tanaka, Y.; Schwarzmann, G.; Blanz, J.; Von Figura, K.; Saftig, P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 2002, 13, 3355–3368. [Google Scholar] [CrossRef]
- Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Patil, S.; Gewirtz, D.A.; Bhutia, S.K. The lysosome as an imperative regulator of autophagy and cell death. Cell. Mol. Life Sci. 2021, 78, 7435–7449. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Xiao, L.; Zhong, Y.; Liu, Y.; Wu, J.; Tao, H. Intracellular calcium homeostasis and its dysregulation underlying epileptic seizures. Seizure: Eur. J. Epilepsy 2022, 103, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ramírez, C.M.; Aryal, B.; Madrigal-Matute, J.; Liu, X.; Diaz, A.; Torrecilla-Parra, M.; Suárez, Y.; Cuervo, A.M.; Sessa, W.C.; et al. Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, K.; Suzuki, K.; Kimura, H.; Takeshita, F.; Wu, H.; Akama, T.; Kawashima, A.; Ishii, N. Tryptophan aspartate-containing coat protein (CORO1A) suppresses Toll-like receptor signalling in Mycobacterium leprae infection. Clin. Exp. Immunol. 2009, 156, 495–501. [Google Scholar] [CrossRef]
- Hölttä-Vuori, M.; Vainio, S.; Kauppi, M.; Van Eck, M.; Jokitalo, E.; Ikonen, E. Endosomal Actin Remodeling by Coronin-1A Controls Lipoprotein Uptake and Degradation in Macrophages. Circ. Res. 2012, 110, 450–455. [Google Scholar] [CrossRef]
- Mori, M.; Mode, R.; Pieters, J. From Phagocytes to Immune Defense: Roles for Coronin Proteins in Dictyostelium and Mammalian Immunity. Front. Cell. Infect. Microbiol. 2018, 8, 00077. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takeshita, F.; Nakata, N.; Ishii, N.; Makino, M. Localization of CORO1A in the Macrophages Containing Mycobacterium leprae. ACTA Histochem. ET Cytochem. 2006, 39, 107–112. [Google Scholar] [CrossRef]
- Drage, M.G.; Pecora, N.D.; Hise, A.G.; Febbraio, M.; Silverstein, R.L.; Golenbock, D.T.; Boom, W.H.; Harding, C.V. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 2009, 258, 29–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Chen, N.; Yu, X.; Li, J.; Dong, W.; Zhang, Y.; Zhao, X.; Zhang, Q. Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows. Biomolecules 2025, 15, 827. https://doi.org/10.3390/biom15060827
Zhang B, Chen N, Yu X, Li J, Dong W, Zhang Y, Zhao X, Zhang Q. Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows. Biomolecules. 2025; 15(6):827. https://doi.org/10.3390/biom15060827
Chicago/Turabian StyleZhang, Bohao, Na Chen, Xing Yu, Jianfu Li, Weitao Dong, Yong Zhang, Xingxu Zhao, and Quanwei Zhang. 2025. "Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows" Biomolecules 15, no. 6: 827. https://doi.org/10.3390/biom15060827
APA StyleZhang, B., Chen, N., Yu, X., Li, J., Dong, W., Zhang, Y., Zhao, X., & Zhang, Q. (2025). Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows. Biomolecules, 15(6), 827. https://doi.org/10.3390/biom15060827





