Targeting Glioblastoma Stem Cells: A40s Aptamer-NIR-Dye Conjugate for Glioblastoma Visualization and Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
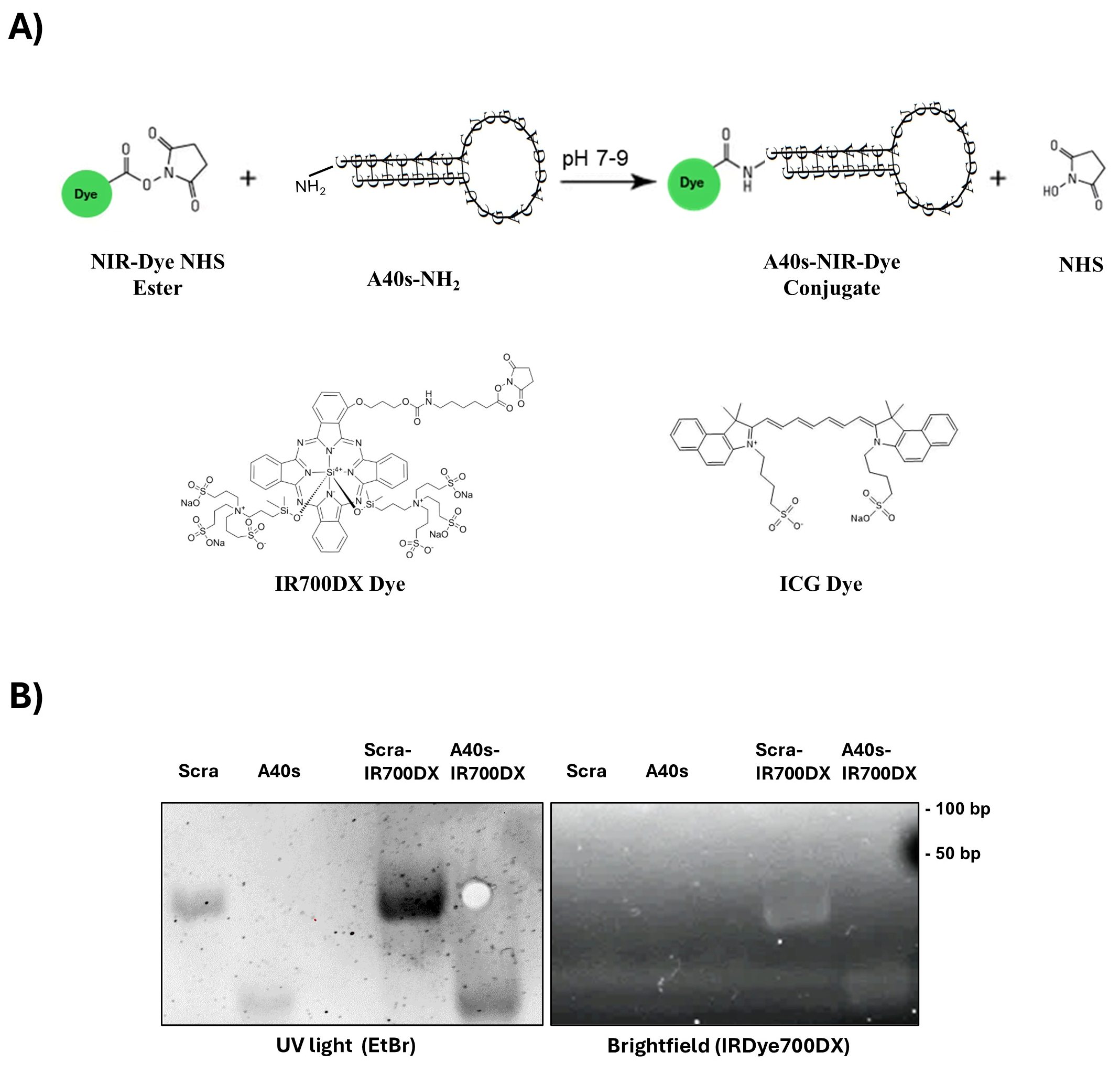
2.2. A40s Conjugation to NIR-Dyes
2.3. Aptamer Binding
2.4. Cell Death and Cell Viability Quantification
2.5. Protein Isolation and Western Blotting
2.6. In Vivo and Ex Vivo Biodistribution
2.7. Statistical Analysis
3. Results
3.1. A40s-Based Chimeras
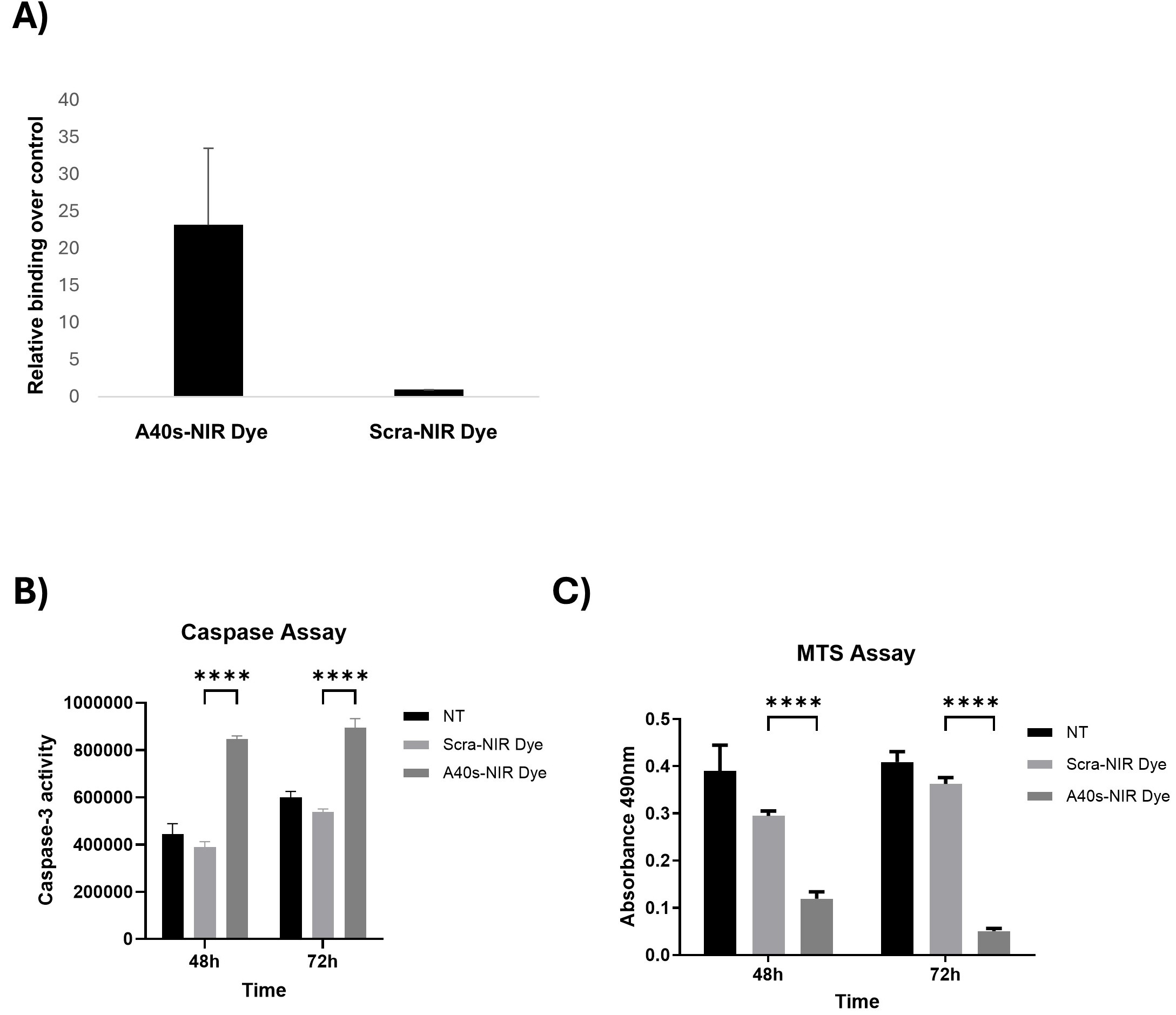
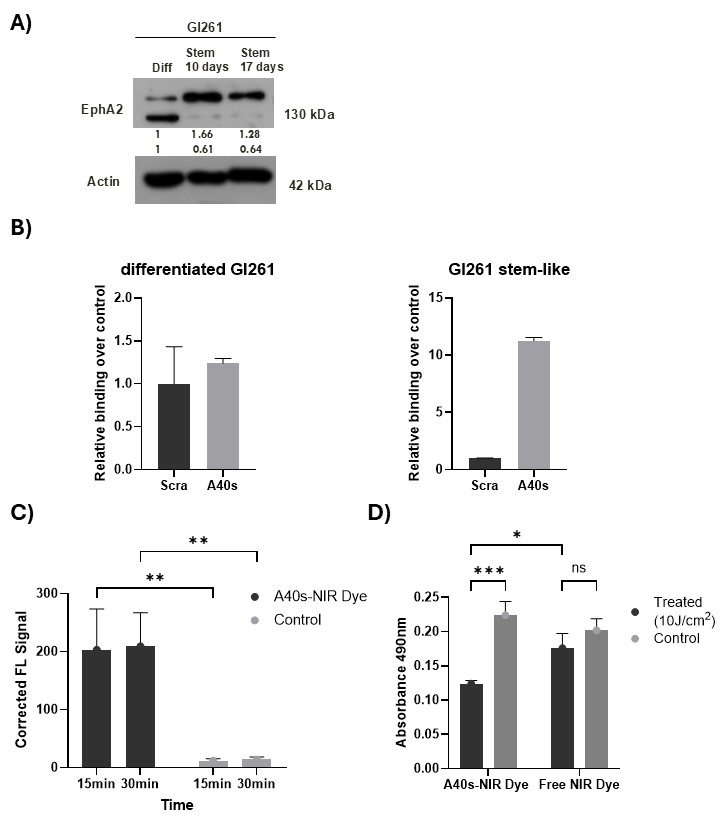
3.2. A40s-NIR-Dye Binding to GSCs
3.3. A40s-NIR-Dye PDT on GSCs
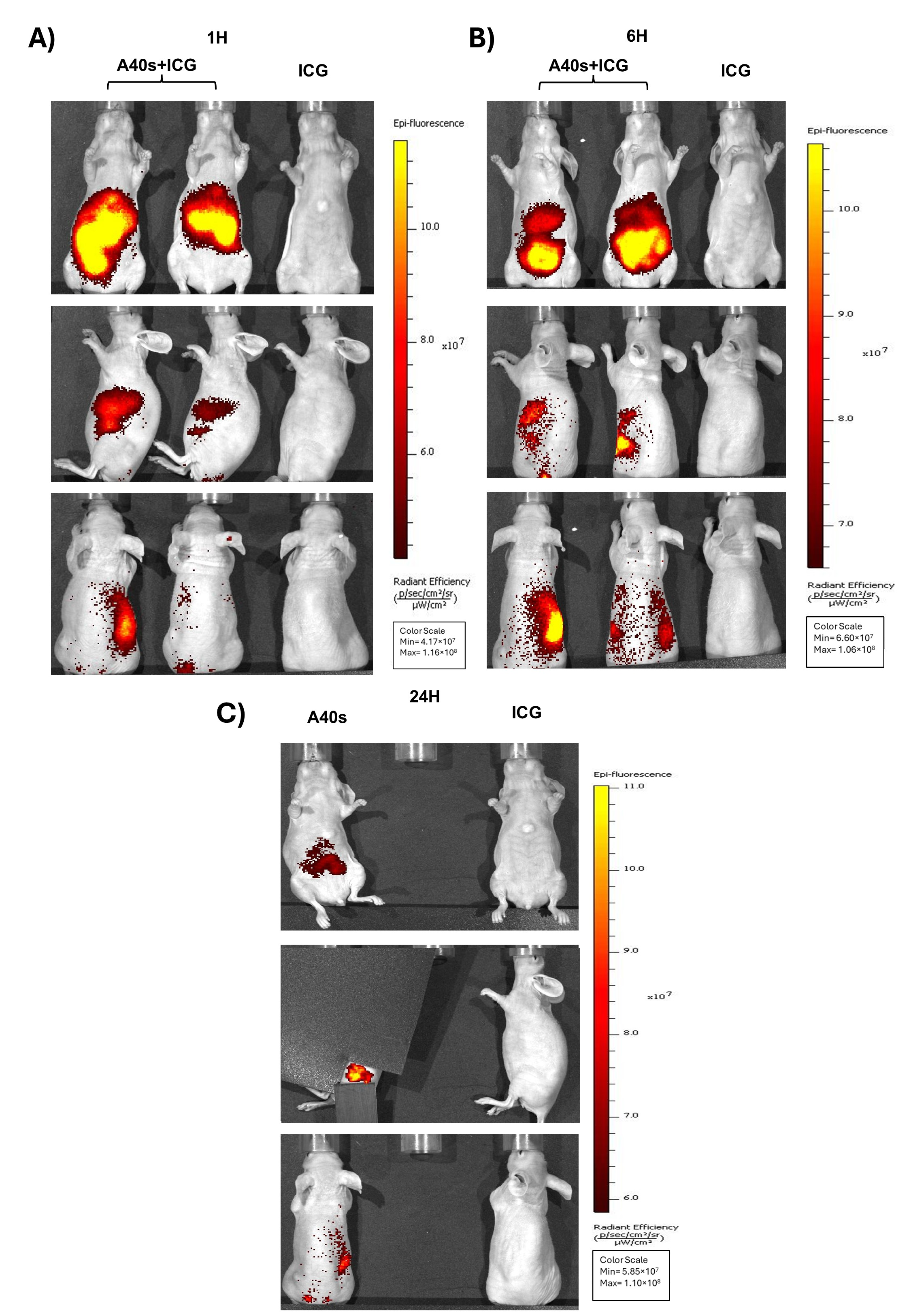
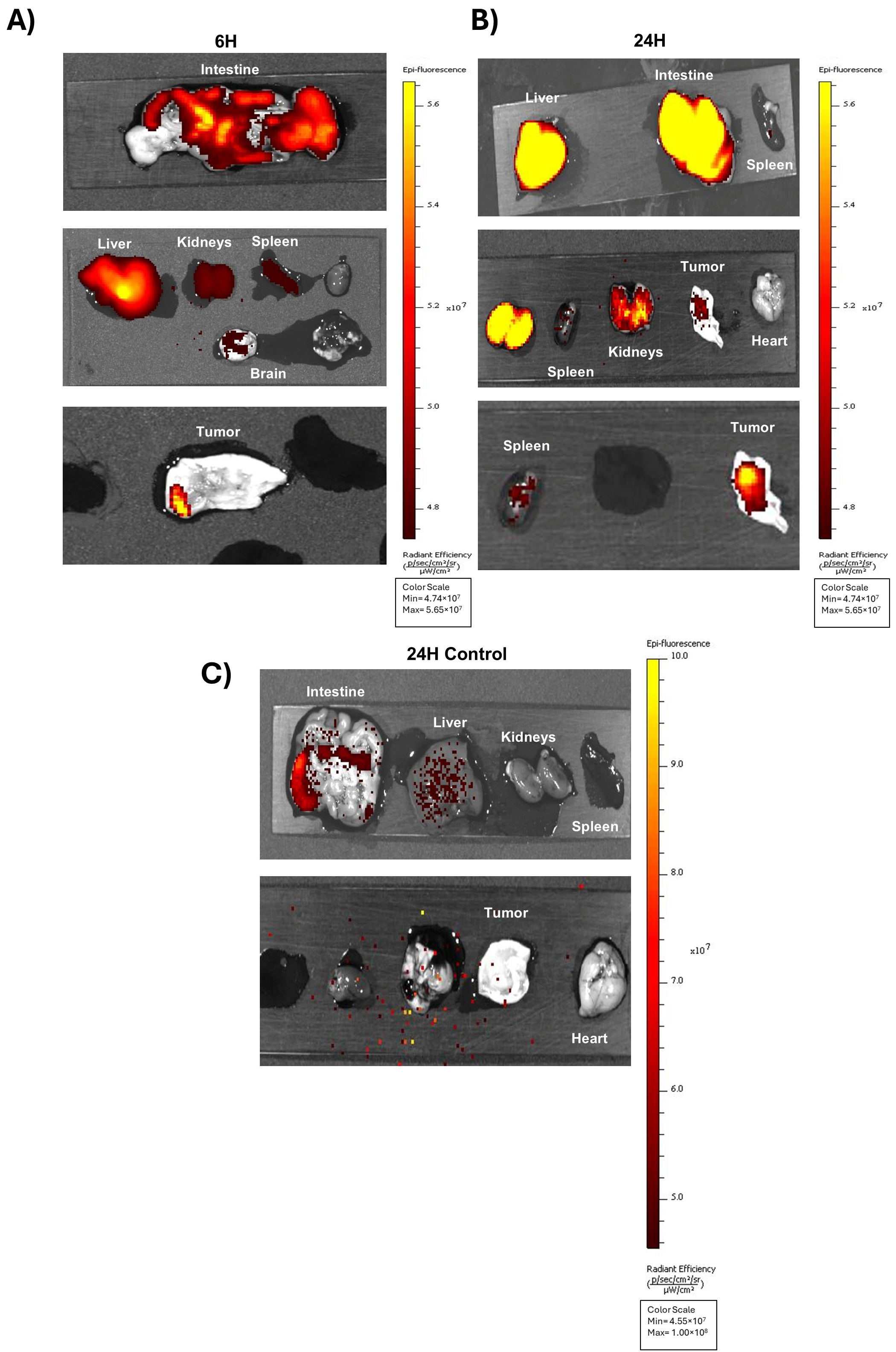
3.4. In Vivo Biodistribution of A40s-NIR-Dye and Ex Vivo Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| GSCs | Glioblastoma Stem Cells |
| NIR | Near-Infrared |
| PDT | Photodynamic Therapy |
| ICG | Indocyanine Green |
| BBB | Blood–Brain Barrier |
References
- Sabouri, M.; Dogonchi, A.F.; Shafiei, M.; Tehrani, D.S. Survival rate of patient with glioblastoma: A population-based study. Egypt. J. Neurosurg. 2024, 39, 42. [Google Scholar] [CrossRef]
- Eckerdt, F.; Platanias, L.C. Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance. Cancers 2023, 15, 3458. [Google Scholar] [CrossRef] [PubMed]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef]
- Miao, H.; Gale, N.W.; Guo, H.; Qian, J.; Petty, A.; Kaspar, J.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.; Hambardzumyan, D.; et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene 2015, 34, 558–567. [Google Scholar] [CrossRef]
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K.L.; Huse, J.T.; Cajola, L.; Zanetti, N.; DiMeco, F.; et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780. [Google Scholar] [CrossRef]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Adams, J.; Singh, M.; Hu, A.; Gorelik, M.; Subapanditha, M.K.; Savage, N.; Yang, J.; et al. Cotargeting Ephrin Receptor Tyrosine Kinases A2 and A3 in Cancer Stem Cells Reduces Growth of Recurrent Glioblastoma. Cancer Res. 2018, 78, 5023–5037. [Google Scholar] [CrossRef]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef]
- Garnier, D.; Meehan, B.; Kislinger, T.; Daniel, P.; Sinha, A.; Abdulkarim, B.; Nakano, I.; Rak, J. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro-Oncology 2018, 20, 236–248. [Google Scholar] [CrossRef]
- Kwiatkowska-Miernik, A.; Mruk, B.; Sklinda, K.; Zaczynski, A.; Walecki, J. Radiomics in the diagnosis of glioblastoma. Pol. J. Radiol. 2023, 88, E461–E466. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Aldave, G.; Tejada, S.; Pay, E.; Marigil, M.; Bejarano, B.; Idoate, M.A.; Diez-Valle, R. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery 2013, 72, 915–920; discussion 920–921. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Kulesza, B.; Stoma, F.; Osuchowski, J.; Mandziuk, S.; Rola, R. Characteristics of Fluorescent Intraoperative Dyes Helpful in Gross Total Resection of High-Grade Gliomas-A Systematic Review. Diagnostics 2020, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Burley, T.A.; Maczynska, J.; Shah, A.; Szopa, W.; Harrington, K.J.; Boult, J.K.R.; Mrozek-Wilczkiewicz, A.; Vinci, M.; Bamber, J.C.; Kaspera, W.; et al. Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment. Int. J. Cancer 2018, 142, 2363–2374. [Google Scholar] [CrossRef]
- Szpunar, M.; Aebisher, D.; Wal, A. Changes in absorption spectra of indocyanine green after visible light exposure and cold dark storage. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 336, 126048. [Google Scholar] [CrossRef]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on and cytotoxicity of photoimmunotherapy using IR-700-antibody conjugates. Free Radic. Bio. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef]
- Kernt, M.; Hirneiss, C.; Wolf, A.; Liegl, R.; Rueping, J.; Neubauer, A.; Alge, C.; Ulbig, M.; Gandorfer, A.; Kampik, A.; et al. Indocyanine green increases light-induced oxidative stress, senescence, and matrix metalloproteinases 1 and 3 in human RPE cells. Acta Ophthalmol. 2012, 90, 571–579. [Google Scholar] [CrossRef]
- Tseng, H.C.; Kuo, C.Y.; Liao, W.T.; Chou, T.S.; Hsiao, J.K. Indocyanine green as a near-infrared theranostic agent for ferroptosis and apoptosis-based, photothermal, and photodynamic cancer therapy. Front. Mol. Biosci. 2022, 9, 1045885. [Google Scholar] [CrossRef]
- Smeets, E.M.M.; Dorst, D.N.; Franssen, G.M.; van Essen, M.S.; Frielink, C.; Stommel, M.W.J.; Trajkovic-Arsic, M.; Cheung, P.F.; Siveke, J.T.; Wilson, I.; et al. Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy. Cells 2023, 12, 1420. [Google Scholar] [CrossRef]
- Huang, Q.L.; Cao, X.; Chai, X.; Wang, X.; Xiao, C.; Wang, J. The Radiological Imaging Features of Easily Misdiagnosed Epithelioid Glioblastoma in Seven Patients. World Neurosurg. 2019, 124, e527–e532. [Google Scholar] [CrossRef] [PubMed]
- Omoto, K.; Matsuda, R.; Nakagawa, I.; Motoyama, Y.; Nakase, H. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: A case report. Surg. Neurol. Int. 2018, 9, 49. [Google Scholar]
- Lakhan, S.E.; Harle, L. Difficult diagnosis of brainstem glioblastoma multiforme in a woman: A case report and review of the literature. J. Med. Case Rep. 2009, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.G.; Zu, Y. Aptamers and Their Applications in Nanomedicine. Small 2015, 11, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, P.; Naghib, S.M.; Kangarshahi, B.M.; Mozafari, M.R. A review of RNA nanoparticles for drug/gene/protein delivery in advanced therapies: Current state and future prospects. Int. J. Biol. Macromol. 2025, 295, 139532. [Google Scholar] [CrossRef]
- Zhou, G.; Latchoumanin, O.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamers as targeting ligands and therapeutic molecules for overcoming drug resistance in cancers. Adv. Drug Deliv. Rev. 2018, 134, 107–121. [Google Scholar] [CrossRef]
- Xiang, D.; Zheng, C.; Zhou, S.F.; Qiao, S.; Tran, P.H.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef]
- Choi, J.W.; Seo, M.; Kim, K.; Kim, A.R.; Lee, H.; Kim, H.S.; Park, C.G.; Cho, S.W.; Kang, J.H.; Joo, J.; et al. Aptamer Nanoconstructs Crossing Human Blood-Brain Barrier Discovered via Microphysiological System-Based SELEX Technology. ACS Nano 2023, 17, 8153–8166. [Google Scholar] [CrossRef]
- Amero, P.; Khatua, S.; Rodriguez-Aguayo, C.; Lopez-Berestein, G. Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology. Cancers 2020, 12, 2889. [Google Scholar] [CrossRef]
- de Almeida, C.E.B.; Alves, L.N.; Rocha, H.F.; Cabral-Neto, J.B.; Missailidis, S. Aptamer delivery of siRNA, radiopharmaceutics and chemotherapy agents in cancer. Int. J. Pharm. 2017, 525, 334–342. [Google Scholar] [CrossRef]
- Chen, G.; Mao, D.; Wang, X.; Chen, J.; Gu, C.; Huang, S.; Yang, Y.; Zhang, F.; Tan, W. Aptamer-based self-assembled nanomicelle enables efficient and targeted drug delivery. J. Nanobiotechnol. 2023, 21, 415. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Sun, W.; Fu, T.; Liu, X.; Chen, P.; Qiu, L.; Qu, F.; Tan, W. Aptamer-Based Targeted Delivery of Functional Nucleic Acids. J. Am. Chem. Soc. 2023, 145, 7677–7691. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, K.; Zhuang, L.; Liu, J.; Zeng, W.; Shi, J.; Zhang, Z. Aptamer/photosensitizer hybridized mesoporous MnO2 based tumor cell activated ROS regulator for precise photodynamic therapy of breast cancer. Colloids Surf. B Biointerfaces 2019, 184, 110536. [Google Scholar] [CrossRef] [PubMed]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Vilardo, C.; Nuzzo, S.; Ricci-Vitiani, L.; De Luca, G.; Pallini, R.; Kichkailo, A.S.; et al. The Discovery of RNA Aptamers that Selectively Bind Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2019, 18, 99–109. [Google Scholar] [CrossRef]
- Pallini, R.; Ricci-Vitiani, L.; Banna, G.L.; Signore, M.; Lombardi, D.; Todaro, M.; Stassi, G.; Martini, M.; Maira, G.; Larocca, L.M.; et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 2008, 14, 8205–8212. [Google Scholar] [CrossRef]
- Esposito, C.L.; Passaro, D.; Longobardo, I.; Condorelli, G.; Marotta, P.; Affuso, A.; de Franciscis, V.; Cerchia, L. A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death. PLoS ONE 2011, 6, e24071. [Google Scholar] [CrossRef]
- Palma, F.; Affinito, A.; Nuzzo, S.; Roscigno, G.; Scognamiglio, I.; Ingenito, F.; Martinez, L.; Franzese, M.; Zanfardino, M.; Soricelli, A.; et al. miR-34c-3p targets CDK1 a synthetic lethality partner of KRAS in non-small cell lung cancer. Cancer Gene Ther. 2021, 28, 413–426. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Chianese, R.V.; Roscigno, G.; Fiore, D.; D’Argenio, V.; Thomas, G.; Savarese, A.; Ingenito, F.; Cocca, L.; et al. MCT4-driven CAF-mediated metabolic reprogramming in breast cancer microenvironment is a vulnerability targetable by miR-425-5p. Cell Death Discov. 2024, 10, 140. [Google Scholar] [CrossRef]
- Roscigno, G.; Cirella, A.; Affinito, A.; Quintavalle, C.; Scognamiglio, I.; Palma, F.; Ingenito, F.; Nuzzo, S.; De Micco, F.; Cuccuru, A.; et al. miR-216a Acts as a Negative Regulator of Breast Cancer by Modulating Stemness Properties and Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2313. [Google Scholar] [CrossRef]
- Pop, C.F.; Veys, I.; Bormans, A.; Larsimont, D.; Liberale, G. Fluorescence imaging for real-time detection of breast cancer tumors using IV injection of indocyanine green with non-conventional imaging: A systematic review of preclinical and clinical studies of perioperative imaging technologies. Breast Cancer Res. Treat. 2024, 204, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Fujimura, D.; Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Real-Time Fluorescence Imaging Using Indocyanine Green to Assess Therapeutic Effects of Near-Infrared Photoimmunotherapy in Tumor Model Mice. Mol. Imaging 2020, 19, 1536012120934965. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.R.; Huang, Y.Y.; Hsiao, J.K. Use of Indocyanine Green (ICG), a Medical Near Infrared Dye, for Enhanced Fluorescent Imaging-Comparison of Organic Anion Transporting Polypeptide 1B3 (OATP1B3) and Sodium-Taurocholate Cotransporting Polypeptide (NTCP) Reporter Genes. Molecules 2019, 24, 2295. [Google Scholar] [CrossRef]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.S.; Qu, C.R.; Diao, S.; Deng, Z.X.; Hu, X.M.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Yu, J.; Jung, B.; Wong, M.S.; Anvari, B. Biodistribution of encapsulated indocyanine green in healthy mice. Mol. Pharm. 2009, 6, 1321–1332. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Rodriguez-Camacho, A.; Flores-Vazquez, J.G.; Moscardini-Martelli, J.; Torres-Rios, J.A.; Olmos-Guzman, A.; Ortiz-Arce, C.S.; Cid-Sanchez, D.R.; Perez, S.R.; Macias-Gonzalez, M.D.S.; Hernandez-Sanchez, L.C.; et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7202. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Bordoloi, B.; Goswami, A.; Roy, D.; Goswami, P.; Das, I. Efficacy of Aminolevulinic Acid Mediated Photodynamic Therapy in the Treatment of Oral Premalignant Lesions: A Systematic Review. Asian Pac. J. Cancer Prev. 2024, 25, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affinito, A.; Ingenito, F.; Verde, S.; Musella, E.; Pattanayak, B.; Fiore, D.; Quintavalle, C.; Fraticelli, A.; Mascolo, M.; Petrillo, G.; et al. Targeting Glioblastoma Stem Cells: A40s Aptamer-NIR-Dye Conjugate for Glioblastoma Visualization and Treatment. Biomolecules 2025, 15, 768. https://doi.org/10.3390/biom15060768
Affinito A, Ingenito F, Verde S, Musella E, Pattanayak B, Fiore D, Quintavalle C, Fraticelli A, Mascolo M, Petrillo G, et al. Targeting Glioblastoma Stem Cells: A40s Aptamer-NIR-Dye Conjugate for Glioblastoma Visualization and Treatment. Biomolecules. 2025; 15(6):768. https://doi.org/10.3390/biom15060768
Chicago/Turabian StyleAffinito, Alessandra, Francesco Ingenito, Sara Verde, Emanuele Musella, Birlipta Pattanayak, Danilo Fiore, Cristina Quintavalle, Aurelia Fraticelli, Martina Mascolo, Gianluca Petrillo, and et al. 2025. "Targeting Glioblastoma Stem Cells: A40s Aptamer-NIR-Dye Conjugate for Glioblastoma Visualization and Treatment" Biomolecules 15, no. 6: 768. https://doi.org/10.3390/biom15060768
APA StyleAffinito, A., Ingenito, F., Verde, S., Musella, E., Pattanayak, B., Fiore, D., Quintavalle, C., Fraticelli, A., Mascolo, M., Petrillo, G., Pignataro, C., De Luca, G., Mezzanotte, L., & Condorelli, G. (2025). Targeting Glioblastoma Stem Cells: A40s Aptamer-NIR-Dye Conjugate for Glioblastoma Visualization and Treatment. Biomolecules, 15(6), 768. https://doi.org/10.3390/biom15060768







