Preparation of Nanoparticle-Immobilized Gold Surfaces for the Reversible Conjugation of Neurotensin Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
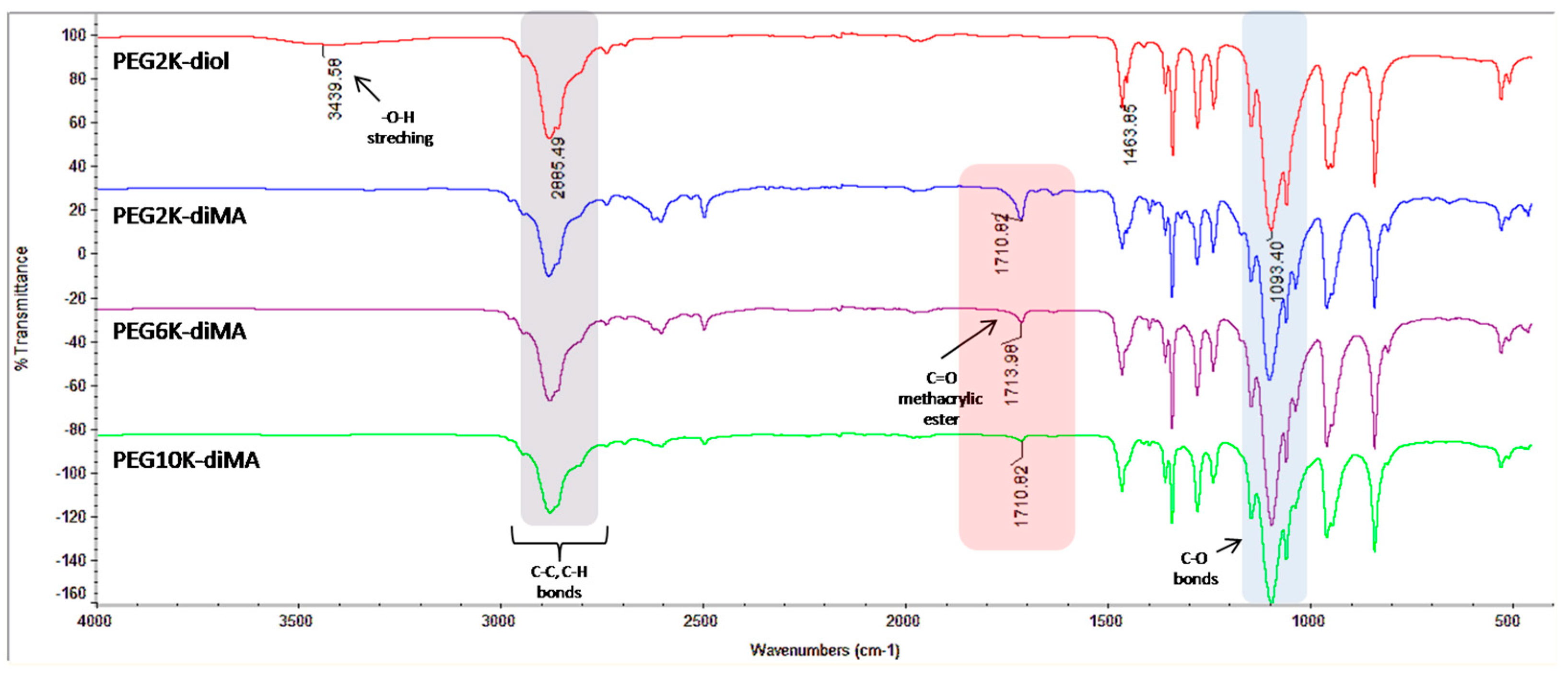
2.2. Synthesis of Methacrylated PEGs
2.3. Preparation of PEG-Based NPs
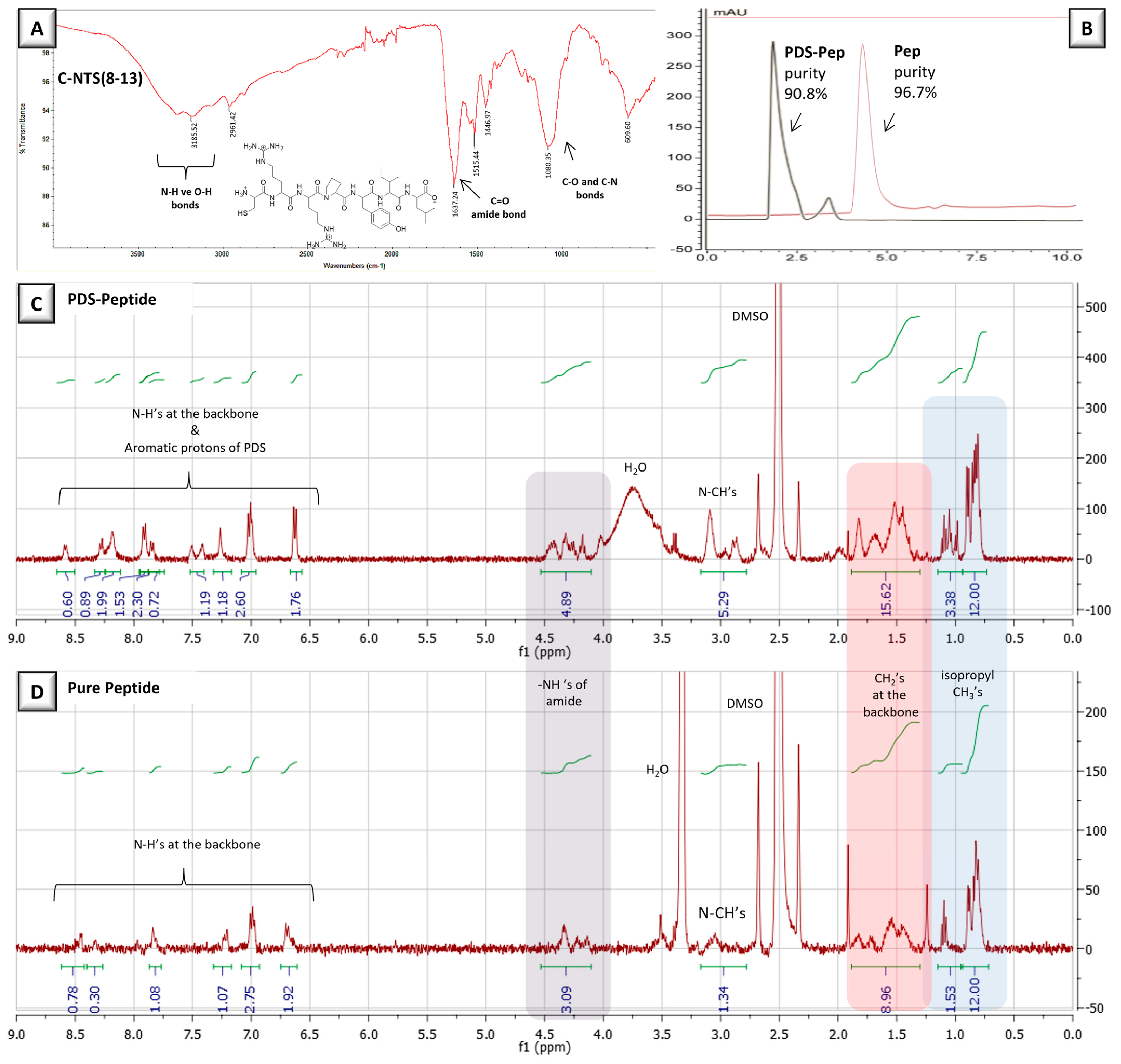
2.4. Synthesis of Functionalized Peptide, (PDS-NTS(8–13))
2.5. Preparation of Peptide-Conjugated NPs (Pep-NP)
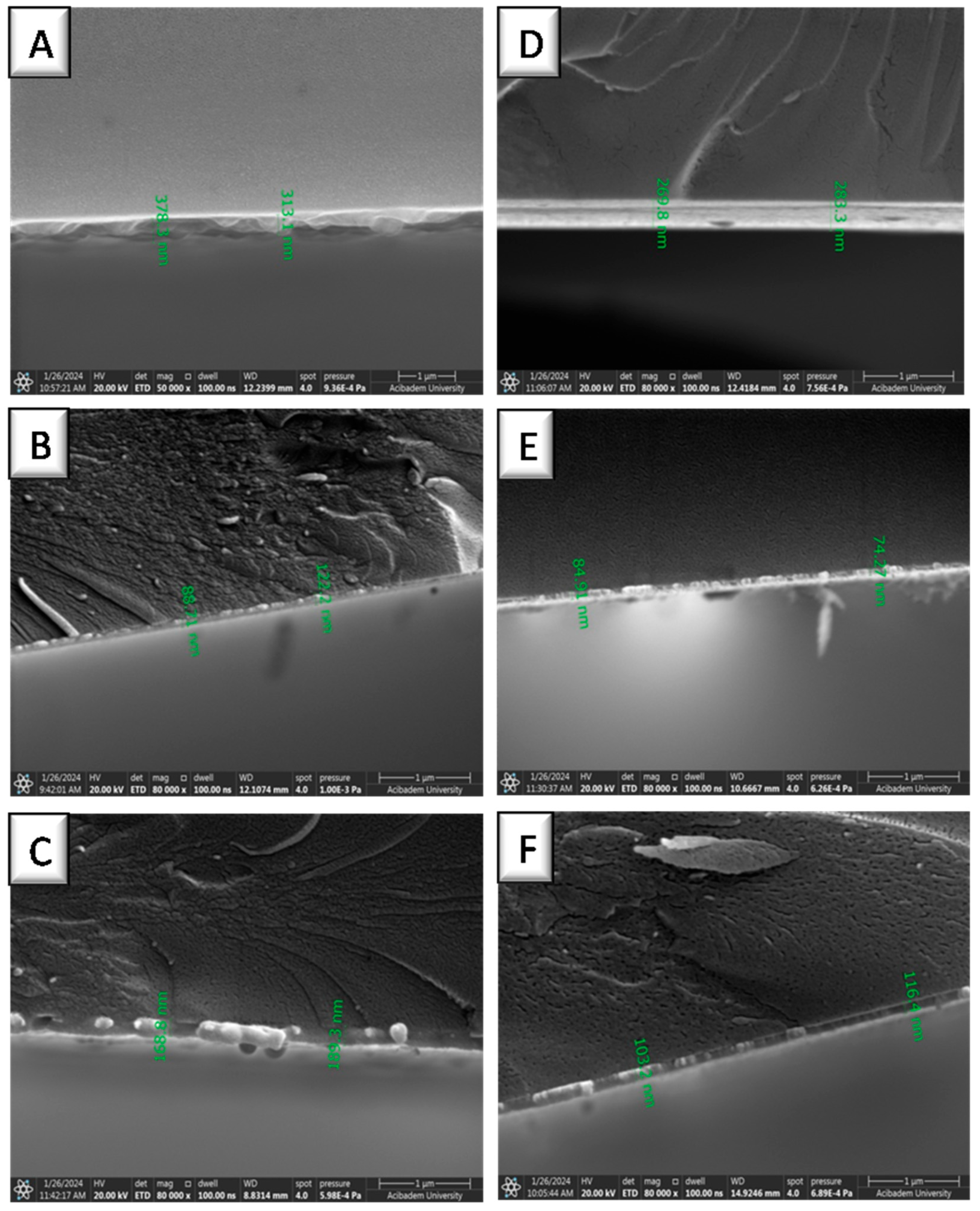
2.6. Surface Coating with NPs
2.7. Reversible Peptide Immobilization
2.8. Binding Experiments
2.8.1. Affinity Study
2.8.2. Surface Attachment/Detachment Study
3. Results and Discussion
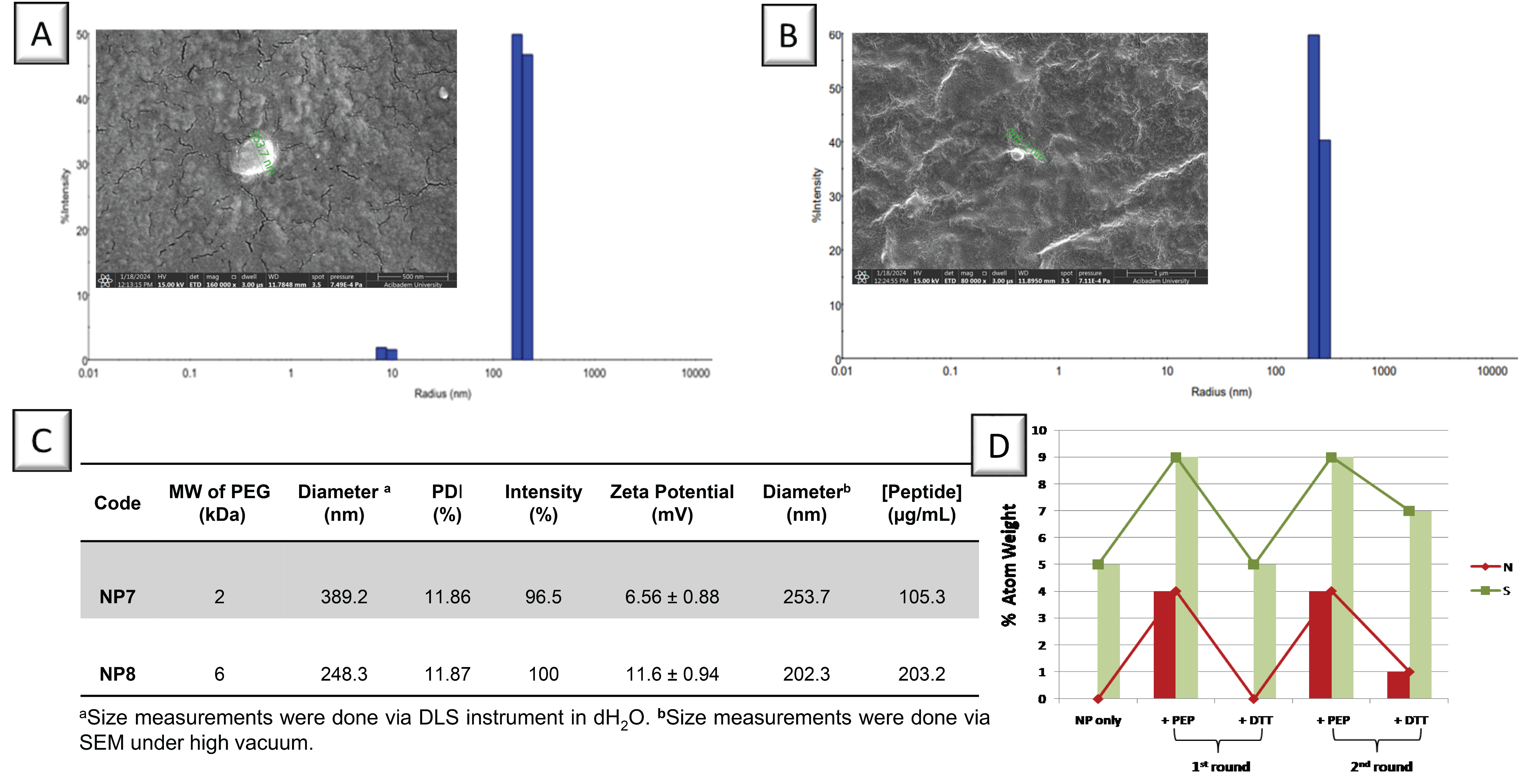
3.1. Preparation of PEG-Based NPs
3.2. Coating of NPs on Gold Surfaces
3.3. Peptide-Modified NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| DLS | Dynamic light scattering |
| DTT | Dithiothreitol |
| EDS | Energy-dispersive X-Ray spectroscopy |
| FT-IR | Fourier-transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| NTS | Neurotensin |
| NTSR | Neurotensin receptor |
| PEG | Poly(ethylene glycol) |
| PDS | Pyridyldisulfide |
| NMR | Nuclear magnetic resonance spectroscopy |
| MA | Methacrylate |
References
- Azzazy, H.M.E.; Mansour, M.M.H. In Vitro Diagnostic Prospects of Nanoparticles. Clin. Chim. Acta 2009, 403, 1–8. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Khanna, V.K. Nanoparticle-Based Sensors. Def. Sci. J. 2008, 58, 608–616. [Google Scholar] [CrossRef]
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-Invasive Electrochemical Immunosensor for Sweat Cortisol Based on L-Cys/AuNPs/ MXene Modified Thread Electrode. Biosens. Bioelectron. 2022, 203, 114039. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Mazzaracchio, V.; Cinti, S.; Colozza, N.; Di Natale, C.; Netti, P.A.; Saraji, M.; Roggero, S.; Moscone, D.; Arduini, F. Electroanalytical Sensor Based on Gold-Nanoparticle-Decorated Paper for Sensitive Detection of Copper Ions in Sweat and Serum. Anal. Chem. 2021, 93, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Amer, L.; Thompson, E.; Clark, A.E.; Garretson, A.F.; Carlin, A.F.; Chen, C.; Retout, M.; Jokerst, J.V. Matrix-Insensitive Sensor Arrays via Peptide-Coated Nanoparticles: Rapid Saliva Screening for Pathogens in Oral and Respiratory Diseases. ACS Appl. Mater. Interfaces 2024, 16, 67362–67372. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.C.; Deka, N.; Dash, A.; Khan, S.A.; Misra, T.K.; Mondal, S.P. Noninvasive Electrochemical Nitric Oxide Detection in Human Saliva Using Pt and TiO2 Nanoparticle Composite Electrodes. ACS Appl. Electron. Mater. 2023, 5, 832–845. [Google Scholar] [CrossRef]
- Uludag, Y.; Tothill, I.E. Cancer Biomarker Detection in Serum Samples Using Surface Plasmon Resonance and Quartz Crystal Microbalance Sensors with Nanoparticle Signal Amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A Point-of-Care Selenium Nanoparticle-Based Test for the Combined Detection of Anti-SARS-CoV-2 IgM and IgG in Human Serum and Blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef]
- Loock, H.P.; Wentzell, P.D. Detection Limits of Chemical Sensors: Applications and Misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Yu, Q.; Peng, W. Self-Referencing SPR Biosensing with an Ultralow Limit-of-Detection Using Long-Wavelength Excitation. Sens. Actuators B Chem. 2021, 327, 128935. [Google Scholar] [CrossRef]
- Woythe, L.; Tito, N.B.; Albertazzi, L. A Quantitative View on Multivalent Nanomedicine Targeting. Adv. Drug. Deliv. Rev. 2021, 169, 1–21. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V.V. Maleimide-Functionalised PLGA-PEG Nanoparticles as Mucoadhesive Carriers for Intravesical Drug Delivery. Eur. J. Pharm. Biopharm. 2019, 143, 24–34. [Google Scholar] [CrossRef]
- Szaloki, M.; Skribanek, R.; Dudas, Z.; Hartmann, J.F.; Hegedus, C.; Borbely, J. Preparation of Reactive Polymeric Nanoparticles (RPNPs). Colloid Polym. Sci. 2008, 286, 435–444. [Google Scholar] [CrossRef]
- Neves, A.R.; van der Putten, L.; Queiroz, J.F.; Pinheiro, M.; Reis, S. Transferrin-Functionalized Lipid Nanoparticles for Curcumin Brain Delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef]
- Goutayer, M.; Dufort, S.; Josserand, V.; Royère, A.; Heinrich, E.; Vinet, F.; Bibette, J.; Coll, J.L.; Texier, I. Tumor Targeting of Functionalized Lipid Nanoparticles: Assessment by in Vivo Fluorescence Imaging. Eur. J. Pharm. Biopharm. 2010, 75, 137–147. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, J.; Thayumanavan, S. Functionalizable Amine-Based Polymer Nanoparticles. ACS Macro Lett. 2013, 2, 948–951. [Google Scholar] [CrossRef]
- Su, P.G.; Chang, Y.P. Low-Humidity Sensor Based on a Quartz-Crystal Microbalance Coated with Polypyrrole/Ag/TiO2 Nanoparticles Composite Thin Films. Sens. Actuators B Chem. 2008, 129, 915–920. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.E.; Goicoechea, J.; Rivero, P.J.; Arregui, F.J. In-Situ Synthesis of Gold Nanoparticles in Layer-by-Layer Polymeric Coatings for the Fabrication of Optical Fiber Sensors. Polymers 2022, 14, 776. [Google Scholar] [CrossRef] [PubMed]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Haidar, L.L.; Bilek, M.; Akhavan, B. Surface Bio-Engineered Polymeric Nanoparticles. Small 2024, 20, 2310876. [Google Scholar] [CrossRef]
- Wang, X.; Mei, Z.; Wang, Y.; Tang, L. Gold Nanorod Biochip Functionalization by Antibody Thiolation. Talanta 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.T.; Shear, J.B. Enzyme-Nanoparticle Functionalization of Three-Dimensional Protein Scaffolds. Anal. Chem. 2006, 78, 7022–7026. [Google Scholar] [CrossRef]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef]
- Jo, H.J.; Robby, A.I.; Kim, S.G.; Lee, G.; Lee, B.C.; Park, S.Y. Reusable Biosensor-Based Polymer Dot-Coated Electrode Surface for Wireless Detection of Bacterial Contamination. Sens. Actuators B Chem. 2021, 346, 130503. [Google Scholar] [CrossRef]
- Forinová, M.; Pilipenco, A.; Lynn, N.S.; Obořilová, R.; Šimečková, H.; Vrabcová, M.; Spasovová, M.; Jack, R.; Horák, P.; Houska, M.; et al. A Reusable QCM Biosensor with Stable Antifouling Nano-Coating for on-Site Reagent-Free Rapid Detection of E. Coli O157:H7 in Food Products. Food Control 2024, 165, 110695. [Google Scholar] [CrossRef]
- Mosley, R.J.; Talarico, M.V.; Byrne, M.E. Recent Applications of QCM-D for the Design, Synthesis, and Characterization of Bioactive Materials. J. Bioact. Compat. Polym. 2021, 36, 261–275. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz Crystal Microbalance-Based Biosensors as Rapid Diagnostic Devices for Infectious Diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, A.; Singh, O.P.; Sharan, P. A Review of Surface Plasmon Resonance (SPR) Technology In Biosensing: Innovations, Applications And Future Trends. J. Opt. 2024. [Google Scholar] [CrossRef]
- Ren, H.; Cao, X.; Zhang, Y.; Chehelamirani, M.; Salahub, D.R. Theoretical Investigation of 6-Mercaptopurine Isomers’ Adsorption on the Au(001) Surface: Revealing the Fate of Different Isomers. ACS Omega 2020, 5, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Triyana, K.; Sembiring, A.; Rianjanu, A.; Hidayat, S.N.; Riowirawan, R.; Julian, T.; Kusumaatmaja, A.; Santoso, I.; Roto, R. Chitosan-Based Quartz Crystal Microbalance for Alcohol Sensing. Electronics 2018, 7, 181. [Google Scholar] [CrossRef]
- Wu, C.; Li, X.; Song, S.; Pei, Y.; Guo, L.; Pei, Z. QCM Biosensor Based on Polydopamine Surface for Real-Time Analysis of the Binding Kinetics of Protein-Protein Interactions. Polymers 2017, 9, 482. [Google Scholar] [CrossRef]
- Yang, Y.; Poss, G.; Weng, Y.; Qi, R.; Zheng, H.; Nianias, N.; Kay, E.R.; Guldin, S. Probing the Interaction of Nanoparticles with Small Molecules in Real Time: Via Quartz Crystal Microbalance Monitoring. Nanoscale 2019, 11, 11107–11113. [Google Scholar] [CrossRef] [PubMed]
- Diltemiz, S.E.; Ecevit, K. High-Performance Formaldehyde Adsorption on CuO/ZnO Composite Nanofiber Coated QCM Sensors. J. Alloys Compd. 2019, 783, 608–616. [Google Scholar] [CrossRef]
- Güler, K.Ç.; Göktürk, I.; Yılmaz, F.; Araz, A.; Denizli, A. Plasmonic Nanosensors for Environmental Pollutants Sensing: Recent Advances and Perspectives. Essent. Chem. 2024, 1, 1–19. [Google Scholar] [CrossRef]
- Cai, J.; Yan, Y.; Wang, W.; Ma, Y.; Cai, L.; Wu, L.; Zhou, H. Detection of Formic Acid and Acetic Acid Gases by a QCM Sensor Coated with an Acidified Multi-Walled Carbon Nanotube Membrane. Environ. Technol. 2023, 44, 751–761. [Google Scholar] [CrossRef]
- Su, T.; He, R. Methods in Determination of Formaldehyde. In Formaldehyde and Cognition; Springer: Dordrecht, The Netherlands, 2017; pp. 195–271. [Google Scholar] [CrossRef]
- Islam, M.A.; Masson, J.F. Plasmonic Biosensors for Health Monitoring: Inflammation Biomarker Detection. ACS Sens. 2025, 10, 577–601. [Google Scholar] [CrossRef]
- Yi, X.; Chen, H.; He, Y.; Wang, J. Assay of Biomarkers for Alzheimer’s Disease by Surface Plasmon Resonance. J. Anal. Test. 2024, 8, 251–261. [Google Scholar] [CrossRef]
- Atha, D.H.; Reipa, V. Quantitative Measurement of P53-P53 Antibody Interactions by Quartz Crystal Microbalance: A Model system for Immunochemical Calibration. J. Biophys. Chem. 2012, 3, 211–220. [Google Scholar] [CrossRef]
- Kim, Y.J.; Rahman, M.M.; Lee, J.J. Ultrasensitive and Label-Free Detection of Annexin A3 Based on Quartz Crystal Microbalance. Sens. Actuators B Chem. 2013, 177, 172–177. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F. Design of Surface Modifications for Nanoscale Sensor Applications. Sensors 2015, 15, 1635–1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, X.; Zhu, X.; Yang, C.; Liu, L.; Shi, H. Isoelectric Bovine Serum Albumin: Robust Blocking Agent for Enhanced Performance in Optical-Fiber Based DNA Sensing. ACS Sens. 2017, 2, 257–262. [Google Scholar] [CrossRef]
- Mao, X.; Yang, L.; Su, X.L.; Li, Y. A Nanoparticle Amplification Based Quartz Crystal Microbalance DNA Sensor for Detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2006, 21, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, S.; Park, M.-K.; Zhang, Z.; Cheng, Z.; Petrenko, V.A.; Chin, B.A. Blocking Agent Optimization for Nonspecific Binding on Phage Based Magnetoelastic Biosensors. J. Electrochem. Soc. 2012, 159, B818–B823. [Google Scholar] [CrossRef]
- Topor, C.V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors 2023, 13, 465. [Google Scholar] [CrossRef]
- Reimhult, K.; Petersson, K.; Krozer, A. QCM-D Analysis of the Performance of Blocking Agents on Gold and Polystyrene Surfaces. Langmuir 2008, 24, 8695–8700. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the Art in PEGylation: The Great Versatility Achieved after Forty Years of Research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. PEG Conjugates in Clinical Development or Use as Anticancer Agents: An Overview. Adv. Drug Deliv. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef]
- Bi, X.; Xu, H.; Lai, S.L.; Yang, K.L. Bifunctional oligo(ethylene glycol) decorated surfaces which permit covalent protein immobilization and resist protein adsorption. Biofouling 2009, 25, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wyszynski, B.; Somboon, P.; Nakamoto, T. Pegylated Lipids as Coatings for QCM Odor-Sensors. Sens. Actuators B Chem. 2007, 121, 538–544. [Google Scholar] [CrossRef]
- Perrino, C.; Lee, S.; Spencer, N.D. Quantitative Comparison of the Hydration Capacity of Surface-Bound Dextran and Polyethylene Glycol. Langmuir 2024, 40, 14130–14140. [Google Scholar] [CrossRef]
- Mehne, J.; Markovic, G.; Pröll, F.; Schweizer, N.; Zorn, S.; Schreiber, F.; Gauglitz, G. Characterisation of Morphology of Self-Assembled PEG Monolayers: A Comparison of Mixed and Pure Coatings Optimised for Biosensor Applications. Anal. Bioanal. Chem. 2008, 391, 1783–1791. [Google Scholar] [CrossRef]
- Ma, C.; Yang, H.; Zhou, X.; Wu, B.; Zhang, G. Polymeric Material for Anti-Biofouling. Colloids Surf. B Biointerfaces 2012, 100, 31–35. [Google Scholar] [CrossRef]
- Jin, J.; Han, Y.; Zhang, C.; Liu, J.; Jiang, W.; Yin, J.; Liang, H. Effect of Grafted PEG Chain Conformation on Albumin and Lysozyme Adsorption: A Combined Study Using QCM-D and DPI. Colloids Surf. B Biointerfaces 2015, 136, 838–844. [Google Scholar] [CrossRef]
- Hamami, M.; Mars, A.; Raouafi, N. Biosensor Based on Antifouling PEG/Gold Nanoparticles Composite for Sensitive Detection of Aflatoxin M1 in Milk. Microchem. J. 2021, 165, 106102. [Google Scholar] [CrossRef]
- Teramura, Y.; Kuroyama, K.; Takai, M. Influence of Molecular Weight of PEG Chain on Interaction between Streptavidin and Biotin-PEG-Conjugated Phospholipids Studied with QCM-D. Acta Biomater. 2016, 30, 135–143. [Google Scholar] [CrossRef]
- Villalonga, R.; Villalonga, M.L.; Díez, P.; Pingarrón, J.M. Decorating Carbon Nanotubes with Polyethylene Glycol-Coated Magnetic Nanoparticles for Implementing Highly Sensitive Enzyme Biosensors. J. Mater. Chem. 2011, 21, 12858–12864. [Google Scholar] [CrossRef]
- Antarnusa, G.; Suharyadi, E. A synthesis of polyethylene glycol (PEG)-coated magnetite Fe3O4 nanoparticles and their characteristics for enhancement of biosensor. Mater. Res. Express 2020, 7, 056103. [Google Scholar] [CrossRef]
- BenHaddada, M.; Huebner, M.; Casale, S.; Knopp, D.; Niessner, R.; Salmain, M.; Boujday, S. Gold Nanoparticles Assembly on Silicon and Gold Surfaces: Mechanism, Stability, and Efficiency in Diclofenac Biosensing. J. Phys. Chem. C 2016, 120, 29302–29311. [Google Scholar] [CrossRef]
- Kerr, J.; Schlosser, J.L.; Griffin, D.R.; Wong, D.Y.; Kasko, A.M. Steric Effects in Peptide and Protein Exchange with Activated Disulfides. Biomacromolecules 2013, 14, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Sahsuvar, S.; Kocagoz, T.; Gok, O.; Can, O. In Vitro Efficacy of Different PEGylation Designs on Cathelicidin-like Peptide with High Antibacterial and Antifungal Activity. Sci. Rep. 2023, 13, 11213. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zaidi, J.; Cusack, B.; Richelson, E. Synthesis and Biological Studies of Novel Neurotensin(8–13) Mimetics. Bioorganic Med. Chem. 2002, 10, 3849–3858. [Google Scholar] [CrossRef]
- Pacchioni, G. A Not-so-Strong Bond. Nat. Rev. Mater. 2019, 4, 226. [Google Scholar] [CrossRef]
- Kan Yeung, A.W.; Georgieva, M.G.; Kirilov, K.; Balacheva, A.A.; Peeva, M.I.; Horbańczuk, O.K.; Horbańczuk, J.O.; Lucarini, M.; Durazzo, A.; Santini, A.; et al. Neurotensins and Their Therapeutic Potential: Research Field Study. Future Med. Chem. 2020, 12, 1779–1803. [Google Scholar] [CrossRef]
- Askari Rizvi, S.F.; Zhang, H. Emerging Trends of Receptor-Mediated Tumor Targeting Peptides: A Review with Perspective from Molecular Imaging Modalities. Eur. J. Med. Chem. 2021, 221, 113538. [Google Scholar] [CrossRef]
- Christou, N.; Blondy, S.; David, V.; Verdier, M.; Lalloué, F.; Jauberteau, M.O.; Mathonnet, M.; Perraud, A. Neurotensin Pathway in Digestive Cancers and Clinical Applications: An Overview. Cell Death Dis. 2020, 11, 1027. [Google Scholar] [CrossRef]
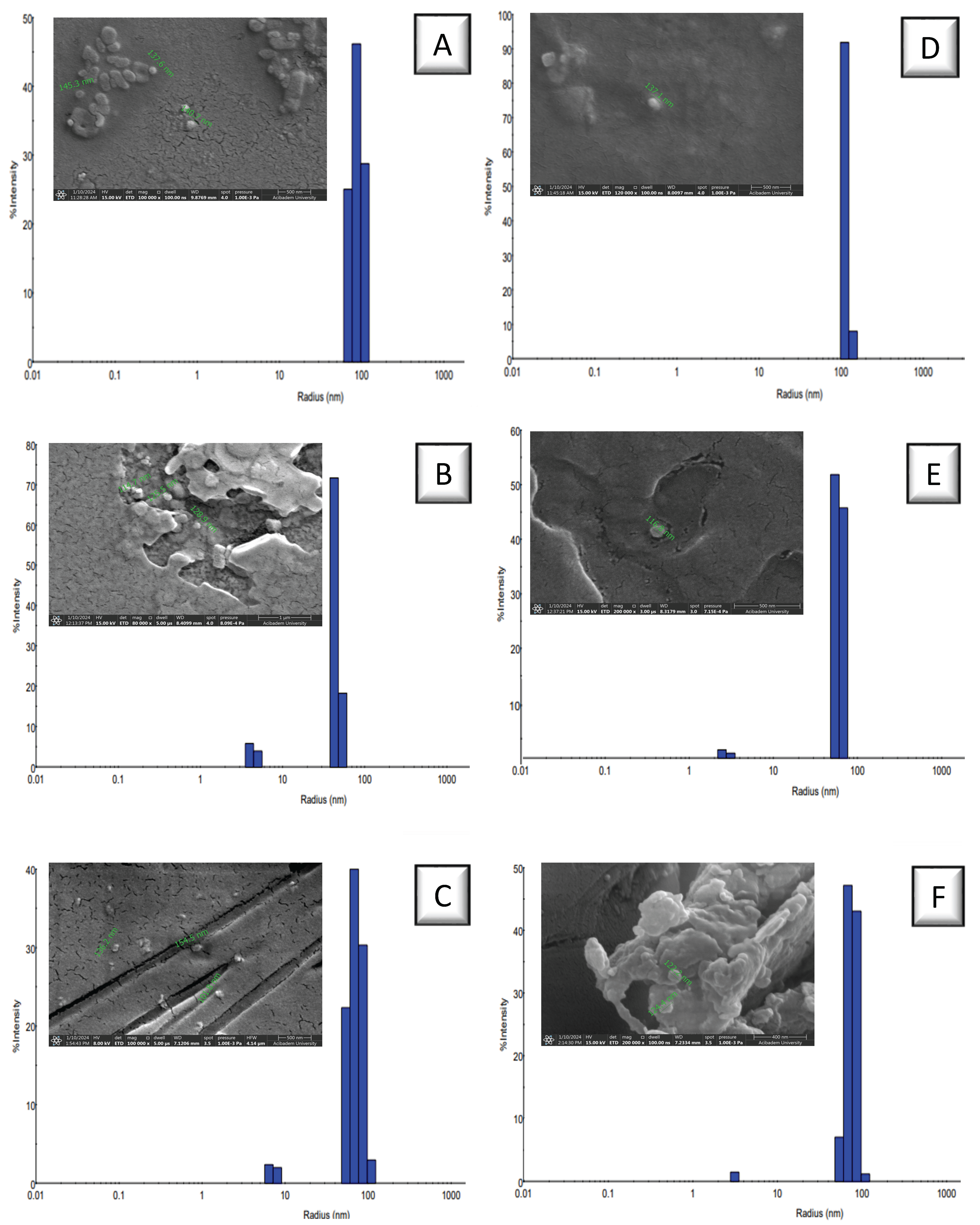

| Code | MW of PEG a (kDa) | Thiol Amount b (mM) | Diameter c (nm) | PDI (%) | Intensity (%) | Diameter d (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| NP1 | 2 | - | 177.5 | 17.28 | 100 | 137.6 | −5.77 ± 6.97 |
| NP2 | 6 | - | 89.60 | 10.23 | 90.1 | 114.7 | −4.67 ± 7.92 |
| NP3 | 10 | - | 144.1 | 19.37 | 95.6 | 101.8 | −12.0 ± 5.53 |
| NP4 | 2 | 1.350 | 224.5 | 7.13 | 100.0 | 137.1 | −11.7 ± 6.79 |
| NP5 | 6 | 1.615 | 121.3 | 11.89 | 97.6 | 116.8 | −14.9 ± 3.38 |
| NP6 | 10 | 0.434 | 151.6 | 14.87 | 98.5 | 122.2 | −21.1 ± 4.25 |
| No | Coating Material | MW of PEG a (kDa) | Water Contact Angle a (°) | Thickness b (nm) |
|---|---|---|---|---|
| 1 | none | - | 24.07 | - |
| 2 | NP1 | 2 | 9.75 | 378.3 |
| 3 | NP2 | 6 | 20.35 | 122.1 |
| 4 | NP3 | 10 | 12.42 | 189.3 |
| 5 | NP4 | 2 | 26.28 | 283.3 |
| 6 | NP5 | 6 | 18.67 | 84.9 |
| 7 | NP6 | 10 | 12.64 | 116.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gok, H.; Gol, D.; Temur, B.Z.; Turkan, N.; Can, O.; Kirimli, C.E.; Ozgun, G.; Gok, O. Preparation of Nanoparticle-Immobilized Gold Surfaces for the Reversible Conjugation of Neurotensin Peptide. Biomolecules 2025, 15, 767. https://doi.org/10.3390/biom15060767
Gok H, Gol D, Temur BZ, Turkan N, Can O, Kirimli CE, Ozgun G, Gok O. Preparation of Nanoparticle-Immobilized Gold Surfaces for the Reversible Conjugation of Neurotensin Peptide. Biomolecules. 2025; 15(6):767. https://doi.org/10.3390/biom15060767
Chicago/Turabian StyleGok, Hidayet, Deniz Gol, Betul Zehra Temur, Nureddin Turkan, Ozge Can, Ceyhun Ekrem Kirimli, Gokcen Ozgun, and Ozgul Gok. 2025. "Preparation of Nanoparticle-Immobilized Gold Surfaces for the Reversible Conjugation of Neurotensin Peptide" Biomolecules 15, no. 6: 767. https://doi.org/10.3390/biom15060767
APA StyleGok, H., Gol, D., Temur, B. Z., Turkan, N., Can, O., Kirimli, C. E., Ozgun, G., & Gok, O. (2025). Preparation of Nanoparticle-Immobilized Gold Surfaces for the Reversible Conjugation of Neurotensin Peptide. Biomolecules, 15(6), 767. https://doi.org/10.3390/biom15060767





