miR-28: A Tiny Player in Cancer Progression and Other Human Diseases
Abstract
1. Introduction
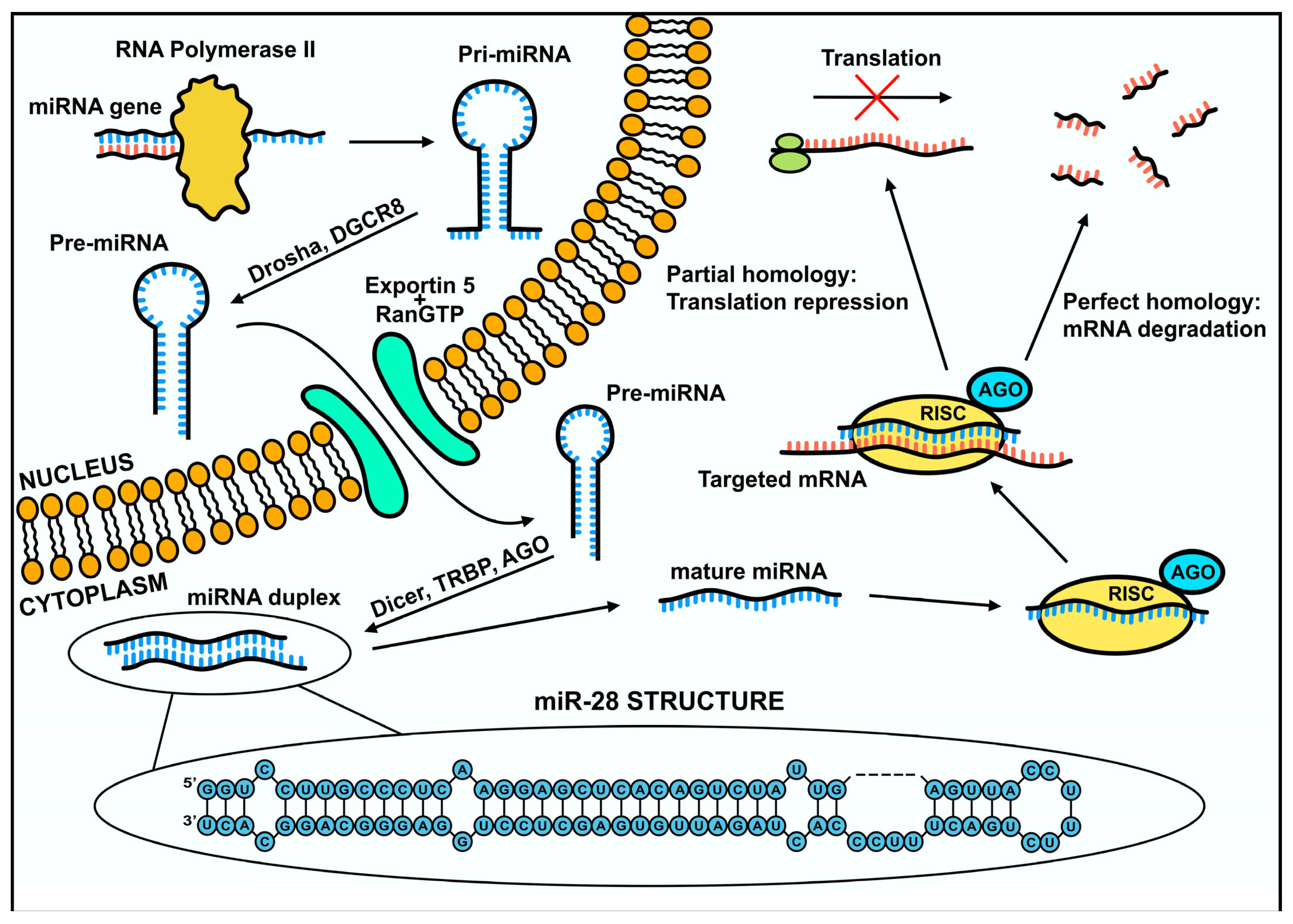
2. MicroRNA Biogenesis
3. Mechanism of Gene Expression Regulation via miR-28
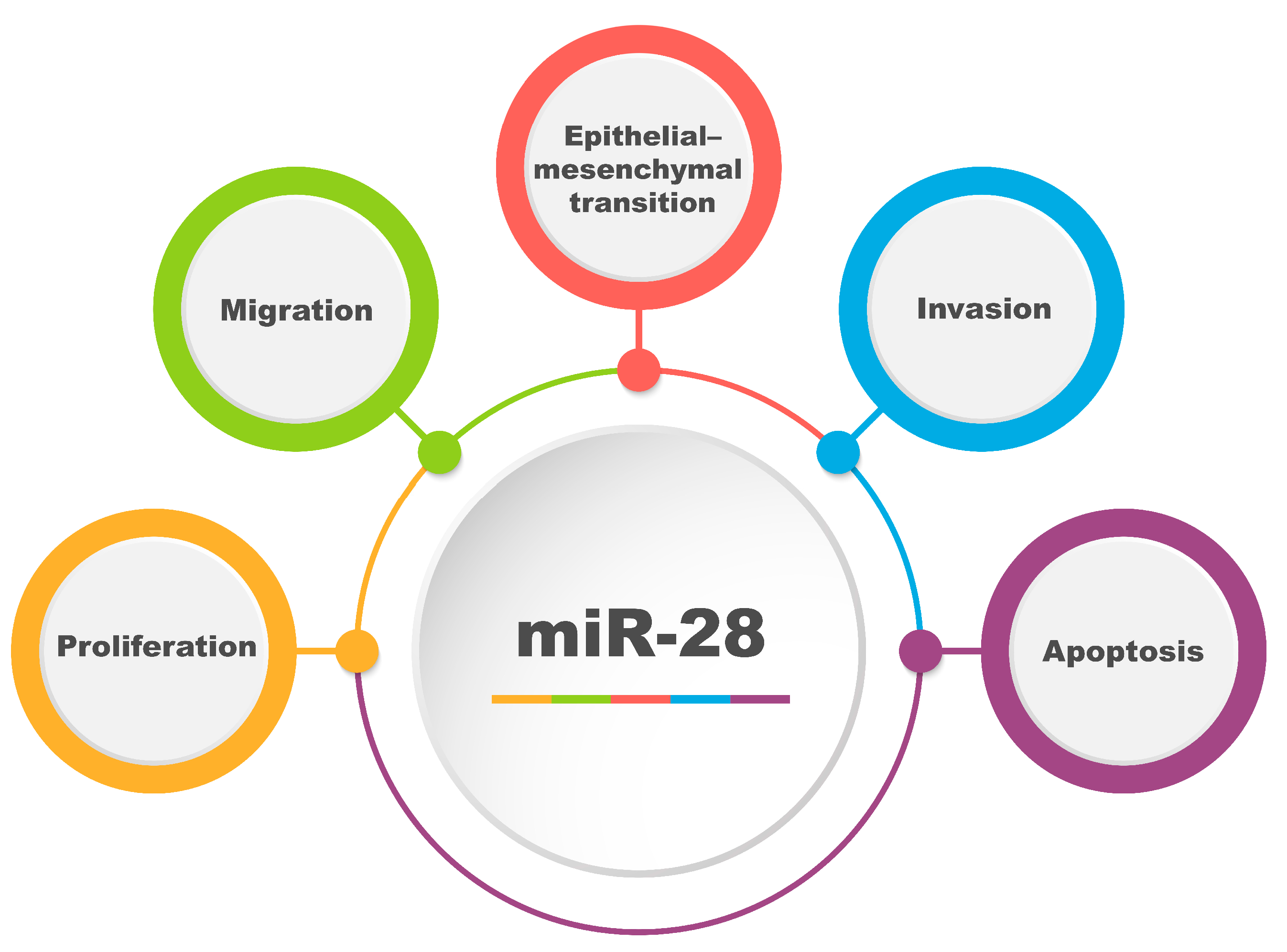
4. Significance of miR-28 in Cancer Progression
4.1. Mechanisms of Cancer Progression
4.2. The Dualistic Role of miRNA in Carcinogenesis
4.3. Regulation of miR-28 in Cancer: lncRNA and circRNA
| lncRNA | Cancer Type | Targets/Pathway | References |
| LOXL1-AS1 | Endometrial cancer | miR-28-5p, RAP1B | [47] |
| Pancreatic cancer | miR-28-5p, SEMA7A/CD44/EGFR | [47] | |
| LINC00514 | Pancreatic cancer | miR-28-5p, RAP1B | [35] |
| LUADT1 | Melanoma | miR-28-5p, RAP1B | [38] |
| UCA1 | Melanoma | miR-28-5p, HOXB3 | [37] |
| Colon cancer | miR-28-5p, HOXB3 | [48] | |
| MCM3AP-AS1 | Breast cancer | miR-28-5p, CENPF | [39] |
| CCAT1 | Prostate cancer | miR-28-5p, DDX5 | [49] |
| CDKN2B-AS1 | Colorectal cancer | miR-28-5p, URGCP | [50] |
| NORAD | Lung cancer | miR-28-3p, E2F2 | [51] |
| CASC9 | Papillary thyroid carcinoma | miR-28-3p, BCL-2/PI3K/AKT | [52] |
| LINC02298 | Hepatocellular Carcinoma | miR-28-5p, CCDC6 | [53] |
| circRNA | Cancer Type | Targets/Pathway | References |
| circ-CSNK1G1 | Triple-negative breast cancer | miR-28-5p, LDHA | [41] |
| circ-002178 | Lung adenocarcinoma | miR-28-5p, PDL1/PD1 | [42] |
| circ-AHNAK | Ovarian cancer | miR-28-5p, EIF2B5/JAK2/STAT3 | [43] |
| circ-0001068 | Ovarian cancer | miR-28-5p, PD1 | [44] |
| circ-MYBL2 | Non-small-cell lung cancer | miR-28-5p | [45] |
| circ-AGFG1 | Non-small-cell lung cancer | miR-28-5p, HIF-1α | [46] |
5. The Role of miRNA-28 in Different Types of Cancer
5.1. Nasopharyngeal Carcinoma (NPC)
5.2. Esophageal Cancer (EC)
5.3. Gastric Cancer (GCa)
5.4. Colorectal Cancer (CRC)
5.5. Hepatocellular Carcinoma (HCC)
5.6. Cholangiocarcinoma (CCA)
5.7. Pancreatic Cancer (PC)
5.8. Breast Cancer
5.9. Ovarian Cancer (OC)
5.10. Prostate Cancer (PCa)
5.11. Bladder Cancer (BCa)
5.12. Non-Small-Cell Lung Cancer (NSCLC)
5.13. Glioma
5.14. Rhabdomyosarcoma (RMS)
5.15. Melanoma
5.16. Non-Hodgkin Lymphoma (NHL)
5.17. Papillary Thyroid Carcinoma (PTC)
5.18. Shared Molecular Mechanisms Regulated by miR-28 Across Cancer Types
6. MicroRNA-28 in Non-Oncological Diseases
6.1. Infectious Diseases (Pathogenic)
6.2. Metabolic and Endocrine
6.3. Neurological and Neurodegenerative Diseases
6.4. Autoimmune and Inflammatory Diseases
6.5. Circulatory Diseases
7. MicroRNA-28 as a Therapeutic Target and Diagnostic Biomarker
8. Challenges, Limitations, and Future Perspectives in miRNA-Based Therapies
9. MicroRNA-28 in Stem Cells
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| AKT | Serine/threonine—protein kinase |
| AMOs | Anti-miRNA antisense inhibitors |
| ARF6 | ADP-ribosylation factor 6 |
| BAG1 | BAG family molecular chaperone regulator 1 |
| BAX | Bcl-2-like protein 4 |
| BM-MSC | Bone marrow mesenchymal stem cells |
| CCND1 | Cycline D1 gene |
| CENPF | Centromere protein F |
| CircRNA | Circulating RNA |
| CSCs | Cancer stem cells |
| E2F6 | E2F Transcription Factor 6 |
| ECM | Extracellular matrix |
| EMT | Epithelial–Mesenchymal Transition |
| ERK | Extracellular-signal-regulated kinase |
| FOXO1 | Forkhead box protein O1 |
| GAGE12I | G antigen 12I |
| GC | Germinal center |
| HOXB3 | Homeobox protein Hox-B3 |
| hREMECs | Human retinal microvascular endothelial cells |
| IGF-1 | Insulin-like growth factor 1 |
| LDHA | Lactate dehydrogenase A |
| LncRNA | Long non-coding RNA |
| MAD2L1 | Mitotic spindle assembly checkpoint protein MAD2A |
| MEF2A | Myocyte-specific enhancer factor 2A |
| miRNA | MicroRNA |
| MRF | Myogenic regulatory factors |
| MSTN | Myostatin |
| MYOD | Myoblast determination protein |
| MYOG | Myogenin |
| N4BP1 | NEDD4 Binding Protein 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PD | Parkinson disease |
| PD1 | Programmed cell death protein 1 |
| PI3K | Phosphoinositide 3-kinases |
| PTEN | Phosphatase and tensin homolog |
| RAP1B | Ras-related protein Rap-1b |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| SEMA7A | Semaphorin 7A |
| SNAIL | Zinc finger protein SNAI1 |
| SREBF2 | Sterol regulatory element-binding protein 2 |
| SSRP1 | Structure specific recognition protein 1 |
| STAT1 | Signal transducer and activator of transcription 1 |
| TRPM7 | Transient receptor potential cation channel subfamily M member 7 |
| WSB2 | WD repeat and SOCS box-containing protein 2 |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional Regulation of the Heterochronic Gene Lin-14 by Lin-4 Mediates Temporal Pattern Formation in C. Elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Tang, G. SiRNA and miRNA: An Insight into RISCs. Trends Biochem. Sci. 2005, 30, 106–114. [Google Scholar] [CrossRef]
- Majorek, K.; Krzyżosiak, W.J. Role of MicroRNA in Pathogenesis, Diagnostics and Therapy of Cancer. Contemp. Oncol./Wspol. Onkol. 2006, 10, 359–366. [Google Scholar]
- Colpaert, R.M.W.; Calore, M. microRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical Significance of miRNAs in Autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Javanshir-Giv, S.; Soleimani, H.; Mollaei, H.; Sadri, F.; Rezaei, Z. The Importance of Hsa-miR-28 in Human Malignancies. Biomed. Pharmacother. 2023, 161, 114453. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of microRNAs in Human Diseases. Avicenna. J. Med. Biotechnol. 2010, 2, 161. [Google Scholar]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Budzynski, M.; Grenda, A.; Filip, A.A. MicroRNA Molecules as a Significant Constituent in Gene Regulation Mechanisms Related to Cancer. Nowotwory. J. Oncol. 2014, 64, 48–60. [Google Scholar] [CrossRef]
- Koralewska, N.; Ciechanowska, K.; Pokornowska, M.; Figlerowicz, M.; Kurzynska-Kokorniak, A. Ludzka Rybonukleaza Dicer a Struktura i Funkcje Biologiczne. Postepy Biochem. 2019, 65, 173–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, A.; Małecki, M. microRNAs role in heart development. Postępy Biol. Kom. 2015, 42, 107–126. [Google Scholar]
- Jonas, S.; Izaurralde, E. Towards a Molecular Understanding of MicroRNA-Mediated Gene Silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-Induced Silencing Complex: A Versatile Gene-Silencing Machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 MicroRNA-Mediated MRNA Deadenylation and Translational Repression in a Mammalian Cell-Free System. Genes Dev. 2007, 21, 1857–1862. [Google Scholar] [CrossRef]
- Liu, J.; Valencia-Sanchez, M.A.; Hannon, G.J.; Parker, R. MicroRNA-Dependent Localization of Targeted MRNAs to Mammalian P-Bodies. Nat. Cell. Biol. 2005, 7, 719–723. [Google Scholar] [CrossRef]
- Weeden, C.E.; Hill, W.; Lim, E.L.; Grönroos, E.; Swanton, C. Impact of Risk Factors on Early Cancer Evolution. Cell 2023, 186, 1541–1563. [Google Scholar] [CrossRef]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental Risk Factors for Cancer—Review Paper. Ann. Agric. Environ. Med. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Garutti, M.; Foffano, L.; Mazzeo, R.; Michelotti, A.; Da Ros, L.; Viel, A.; Miolo, G.; Zambelli, A.; Puglisi, F. Hereditary Cancer Syndromes: A Comprehensive Review with a Visual Tool. Genes 2023, 14, 1025. [Google Scholar] [CrossRef]
- Giatagana, E.M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Willmott, L.J.; Monk, B.J. Cervical Cancer Therapy: Current, Future and Anti-Angiogensis Targeted Treatment. Expert. Rev. Anticancer Ther. 2009, 9, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of microRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24, 7268. [Google Scholar] [CrossRef]
- Otmani, K.; Lewalle, P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021, 11, 708765. [Google Scholar] [CrossRef]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of microRNAs in Cancer. Cancer. Res. 2016, 76, 3666. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as Oncogenes and Tumor Suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of microRNAs in Human Cancer. Signal Transduct. Target Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Non-Coding RNA (LncRNA) and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Majka, M. Interplay among SNAIL Transcription Factor, microRNAs, Long Non-Coding RNAs, and Circular RNAs in the Regulation of Tumor Growth and Metastasis. Cancers 2020, 12, 209. [Google Scholar] [CrossRef]
- Han, Q.; Li, J.; Xiong, J.; Song, Z. Long Noncoding RNA LINC00514 Accelerates Pancreatic Cancer Progression by Acting as a CeRNA of miR-28-5p to Upregulate Rap1b Expression. J. Exp. Clin. Cancer Res. 2020, 39, 151. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Li, F.; Wu, L. LncRNA LOXL1-AS1/miR-28-5p/SEMA7A Axis Facilitates Pancreatic Cancer Progression. Cell Biochem. Funct. 2020, 38, 58–65. [Google Scholar] [CrossRef]
- Han, C.; Tang, F.; Chen, J.; Xu, D.; Li, X.; Xu, Y.; Wang, S.; Zhou, J. Knockdown of LncRNA UCA1 Inhibits the Proliferation and Migration of Melanoma Cells through Modulating the miR 28 5p/HOXB3 Axis. Exp. Ther. Med. 2019, 17, 4294–4302. [Google Scholar] [CrossRef]
- Xu, J.H.; Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Zhang, D.D.; Hu, Y.Y.; Zheng, B.; Tan, W.Q. Long Noncoding RNA LUADT1 Is Upregulated in Melanoma and May Sponge miR-28-5p to Upregulate RAP1B. Cancer Biother. Radiopharm. 2020, 35, 307–312. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, H.; Zhu, J.; Feng, K.; Hu, C. LncRNA MCM3AP-AS1 Promotes Breast Cancer Progression via Modulating miR-28-5p/CENPF Axis. Biomed. Pharmacother. 2020, 128, 110289. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Li, W.; Wang, G.; Yuan, J.; Ai, Y.; Huang, J.; Li, Z. Circ-CSNK1G1 Promotes Cell Proliferation, Migration, Invasion and Glycolysis Metabolism during Triple-Negative Breast Cancer Progression by Modulating the miR-28-5p/LDHA Pathway. Reprod. Biol. Endocrinol. 2022, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Zhao, X.H.; Wang, Y.B.; Ren, F.H.; Sun, D.W.; Yan, Y.B.; Kong, X.L.; Bu, J.L.; Liu, M.F.; Xu, S.D. CircRNA-002178 Act as a CeRNA to Promote PDL1/PD1 Expression in Lung Adenocarcinoma. Cell Death Dis. 2020, 11, 32. [Google Scholar] [CrossRef]
- He, S.L.; Zhao, X.; Yi, S.J. CircAHNAK Upregulates EIF2B5 Expression to Inhibit the Progression of Ovarian Cancer by Modulating the JAK2/STAT3 Signaling Pathway. Carcinogenesis 2022, 43, 941–955. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Jin, M. Circ-0001068 Is a Novel Biomarker for Ovarian Cancer and Inducer of PD1 Expression in T Cells. Aging 2020, 12, 19095–19106. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, C. A Cytoplasm-Enriched CircRNA Circ-MYBL2 Is Downregulated in Non-Small Cell Lung Cancer and Sponges Oncogenic miR-28 to Regulate Cancer Cell Proliferation and Apoptosis. Cancer Manag. Res. 2021, 13, 6499–6506. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, C.; Chen, J.; Wei, D.; Yu, F.; Sun, J. CircAGFG1 Sponges miR-28-5p to Promote Non-Small-Cell Lung Cancer Progression through Modulating HIF-1α Level. Open Med. 2021, 16, 703–717. [Google Scholar] [CrossRef]
- Tang, M.; Rong, Y.; Liu, S.; Wu, Z.; Ma, G.; Li, X.; Cai, H. Potential Role of LncRNA LOXL1-AS1 in Human Cancer Development: A Narrative Review. Transl. Cancer. Res. 2024, 13, 1997–2011. [Google Scholar] [CrossRef]
- Cui, M.; Chen, M.; Shen, Z.; Wang, R.; Fang, X.; Song, B. LncRNA-UCA1 Modulates Progression of Colon Cancer through Regulating the miR-28-5p/HOXB3 Axis. J. Cell Biochem. 2019, 120, 6926–6936. [Google Scholar] [CrossRef]
- You, Z.; Liu, C.; Wang, C.; Ling, Z.; Wang, Y.; Wang, Y.; Zhang, M.; Chen, S.; Xu, B.; Guan, H.; et al. LncRNA CCAT1 Promotes Prostate Cancer Cell Proliferation by Interacting with DDX5 and MIR-28-5P. Mol. Cancer Ther. 2019, 18, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.L.; Zhang, H.Y.; Zhang, S.Y.; Yi, X.L. LncRNA CDKN2B-AS1 Sponges miR-28-5p to Regulate Proliferation and Inhibit Apoptosis in Colorectal Cancer. Oncol. Rep. 2021, 46, 213. [Google Scholar] [CrossRef]
- Mao, W.; Wang, S.; Chen, R.; He, Y.; Lu, R.; Zheng, M. LncRNA NORAD Promotes Lung Cancer Progression by Competitively Binding to miR-28-3p with E2F2. Open Med. 2022, 17, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, C.; Peng, Y.; Wen, Y.; Wang, J. LncRNA CASC9 Facilitates Papillary Thyroid Cancer Development and Doxorubicin Resistance via miR-28-3p/BCL-2 Axis and PI3K/AKT Signaling Pathway. J. Cardiothorac. Surg. 2024, 19, 629. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Liang, L.; Chen, Q. Long Non-Coding RNA 02298 Promotes the Malignancy of HCC by Targeting the miR-28-5p/CCDC6 Pathway. Biochem. Genet. 2024, 62, 4967–4986. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. The Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, H.; Ma, X.; Wu, G. Strand-Specific miR-28-3p and miR-28-5p Have Differential Effects on Nasopharyngeal Cancer Cells Proliferation, Apoptosis, Migration and Invasion. Cancer Cell Int. 2019, 19, 187. [Google Scholar] [CrossRef]
- Stenzel, A.; Żuryń, A.; Grzanka, A.A.; Grzanka, A. Cykliny Jako Markery Chorób Nowotworowych. Nowotwory. J. Oncol. 2012, 62, 115–122. [Google Scholar]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Huang, F.L.; Yu, S.J. Esophageal Cancer: Risk Factors, Genetic Association, and Treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Liu, X.; Lv, M.; Shen, E.; Zhu, G.; Sun, Z. miR-28-5p Targets MTSS1 to Regulate Cell Proliferation and Apoptosis in Esophageal Cancer. Acta Biochim. Biophys. Sin. 2020, 52, 842–852. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Cheng, Z.; Wang, P.; Gong, B.; Huang, H.; Xing, Y.; Liu, F. MicroRNA-28-5p Inhibits the Migration and Invasion of Gastric Cancer Cells by Suppressing AKT Phosphorylation. Oncol. Lett. 2018, 15, 9777–9785. [Google Scholar] [CrossRef]
- Yuan, D.; Xia, H.; Zhang, Y.; Chen, L.; Leng, W.; Chen, T.; Chen, Q.; Tang, Q.; Mo, X.; Liu, M.; et al. P-Akt/miR 200 Signaling Regulates Epithelial-Mesenchymal Transition, Migration and Invasion in Circulating Gastric Tumor Cells. Int. J. Oncol. 2014, 45, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.F.; Li, L.S.; Ai, L.; Deng, J.K.; Guo, Y.M. SMicroRNA-28-5p Acts as a Metastasis Suppressor in Gastric Cancer by Targeting Nrf2. Exp. Cell Res. 2021, 402, 112553. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, X.; Shou, T.; Yang, L.; Cheng, X.; Wang, J.; Deng, L.; Zheng, Y. MicroRNA-28 Promotes Cell Proliferation and Invasion in Gastric Cancer via the Pten/Pi3k/Akt Signalling Pathway. Mol. Med. Rep. 2018, 17, 4003–4010. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Jia, S.; Guo, B.; Wang, L.; Peng, L.; Zhang, L. The Current Status of SSRP1 in Cancer: Tribulation and Road Ahead. J. Healthc. Eng. 2022, 2022, 3528786. [Google Scholar] [CrossRef]
- Wu, W.; He, K.; Guo, Q.; Chen, J.; Zhang, M.; Huang, K.; Yang, D.; Wu, L.; Deng, Y.; Luo, X.; et al. SSRP1 Promotes Colorectal Cancer Progression and Is Negatively Regulated by miR-28-5p. J. Cell Mol. Med. 2019, 23, 3118–3129. [Google Scholar] [CrossRef]
- Luan, X.F.; Wang, L.; Gai, X.F. The miR-28-5p-CAMTA2 Axis Regulates Colon Cancer Progression via Wnt/β-Catenin Signaling. J. Cell Biochem. 2021, 122, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.; Nicoloso, M.S.; Zeng, L.; Ivan, C.; Spizzo, R.; Gafà, R.; Xiao, L.; Zhang, X.; Vannini, I.; Fanini, F.; et al. Strand-Specific miR-28-5p and miR-28-3p Have Distinct Effects in Colorectal Cancer Cells. Gastroenterology 2012, 142, 886. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, Z.; Zhang, Z.; Zhang, H.; Li, S.; Wu, T.; Chen, X.; Guo, J.; Wang, A.; Tian, H.; et al. HOXB3 Drives WNT-Activation Associated Progression in Castration-Resistant Prostate Cancer. Cell Death Dis. 2023, 14, 215. [Google Scholar] [CrossRef]
- Mátyási, B.; Farkas, Z.; Kopper, L.; Sebestyén, A.; Boissan, M.; Mehta, A.; Takács-Vellai, K. The Function of NM23-H1/NME1 and Its Homologs in Major Processes Linked to Metastasis. Pathol. Oncol. Res. 2020, 26, 49. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or Feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Zhu, T.P.; Gui, Y.C.; Tan, Z.B.; Wei, R.Q.; Hu, B.L.; Xu, J.W. miR-28-5p Inhibits Carcinogenesis in Colon Cancer Cells and Is Necessary for Erastin-Induced Ferroptosis. Transl. Cancer Res. 2020, 9, 2931. [Google Scholar] [CrossRef]
- Taniguchi, H. Liver Cancer 2.0. Int. J. Mol. Sci. 2023, 24, 17275. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Han, T.; Yang, P.; Wang, R.; Li, H.; Zhang, J.; Zhou, X. MicroRNA-28-5p Regulates Liver Cancer Stem Cell Expansion via IGF-1 Pathway. Stem Cells Int. 2019, 2019, 8734362. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168. [Google Scholar] [CrossRef]
- Chen, T.; Wang, H.; Yan, H. miR-28-5p Inhibits Cholangiocarcinoma Progression and Predicts Good Prognosis of Patients. Cell Cycle 2022, 21, 2079–2090. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer. J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Y.; Hu, F. miR-28-5p Inhibits the Migration of Breast Cancer by Regulating WSB2. Int. J. Mol. Med. 2020, 46, 1562–1570. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhao, W.; Hu, H.; Zhao, L.; Zhu, Y.; Yang, X.; Gao, B.; Yang, H.; Huang, Y.; et al. WD Repeat and SOCS Box Containing Protein 2 in the Proliferation, Cycle Progression, and Migration of Melanoma Cells. Biomed. Pharmacother. 2019, 116, 108974. [Google Scholar] [CrossRef]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. miR-28 Regulates Nrf2 Expression through a Keap1-Independent Mechanism. Breast Cancer Res. Treat. 2011, 129, 983. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in Cancers: A Double-edged Sword. Cancer Med. 2019, 8, 2252. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhang, T.; Chang, C.Y.; Wang, J.; Bazile, L.; Zhang, L.; Haffty, B.G.; Hu, W.; Feng, Z. Metabolic Enzyme LDHA Activates Rac1 GTPase as a Noncanonical Mechanism to Promote Cancer. Nat. Metab. 2022, 4, 1830. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, X.; Ye, F.; Chen, B.; Song, C.; Wen, J.; Zhang, Z.; Zheng, G.; Tang, H.; Xie, X. The miR-34a-LDHA Axis Regulates Glucose Metabolism and Tumor Growth in Breast Cancer. Sci. Rep. 2016, 6, 21735. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, N.; Shi, H.; Zhao, S.; Yao, S.; Shen, H. miR-28-5p Promotes the Development and Progression of Ovarian Cancer through Inhibition of N4BP1. Int. J. Oncol. 2017, 50, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Murillas, R.; Simms, K.S.; Hatakeyama, S.; Weissman, A.M.; Kuehn, M.R. Identification of Developmentally Expressed Proteins That Functionally Interact with Nedd4 Ubiquitin Ligase. J. Biol. Chem. 2002, 277, 2897–2907. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Li, H.; Ye, S. miR-28-3p Inhibits Prostate Cancer Cell Proliferation, Migration and Invasion, and Promotes Apoptosis by Targeting ARF6. Exp. Ther. Med. 2021, 22, 1205. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Berti, G.; Russo, F.; Evangelista, M.; Pellegrini, M.; Rainaldi, G. The miRNA Pull Out Assay as a Method to Validate the miR-28-5p Targets Identified in Other Tumor Contexts in Prostate Cancer. Int. J. Genom. 2017, 2017, 5214806. [Google Scholar] [CrossRef]
- Fazio, S.; Berti, G.; Russo, F.; Evangelista, M.; D’Aurizio, R.; Mercatanti, A.; Pellegrini, M.; Rizzo, M. The miR-28-5p Targetome Discovery Identified SREBF2 as One of the Mediators of the miR-28-5p Tumor Suppressor Activity in Prostate Cancer Cells. Cells 2020, 9, 354. [Google Scholar] [CrossRef]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef]
- Piao, X.M.; Cha, E.J.; Yun, S.J.; Kim, W.J. Role of Exosomal miRNA in Bladder Cancer: A Promising Liquid Biopsy Biomarker. Int. J. Mol. Sci. 2021, 22, 1713. [Google Scholar] [CrossRef]
- Yasui, T.; Yanagida, T.; Ito, S.; Konakade, Y.; Takeshita, D.; Naganawa, T.; Nagashima, K.; Shimada, T.; Kaji, N.; Nakamura, Y.; et al. Unveiling Massive Numbers of Cancer-Related Urinary-MicroRNA Candidates via Nanowires. Sci. Adv. 2017, 3, e1701133. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, J.Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhou, Q.; Xiao, K.; Qian, H. MicroRNA-28 Promotes the Proliferation of Non-Small-Cell Lung Cancer Cells by Targeting PTEN. Mol. Med. Rep. 2020, 21, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Parsons, R.E. Molecular Pathways: Intercellular PTEN and the Potential of PTEN Restoration Therapy. Clin. Cancer Res. 2014, 20, 5379. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284. [Google Scholar] [CrossRef]
- Wan, J.; Guo, A.A.; Chowdhury, I.; Guo, S.; Hibbert, J.; Wang, G.; Liu, M. TRPM7 Induces Mechanistic Target of Rap1b Through the Downregulation of miR-28-5p in Glioma Proliferation and Invasion. Front. Oncol. 2019, 9, 492527. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, M.; Song, L.; Zhang, M.; Zhangd, J. Function, Significance, and Regulation of Rap1b in Malignancy. Crit Rev Eukaryot. Gene Expr. 2019, 29, 151–160. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Z.; Mijiti, M.; Du, G.; Li, Y.; Dangmurenjiafu, G. miR-28-5p Promotes Human Glioblastoma Cell Growth through Inactivation of FOXO1. Int. J. Clin. Exp. Pathol. 2019, 12, 2972. [Google Scholar]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers. 2019, 5, 1. [Google Scholar] [CrossRef]
- Skrzypek, K.; Nieszporek, A.; Badyra, B.; Lasota, M.; Majka, M. Enhancement of Myogenic Differentiation and Inhibition of Rhabdomyosarcoma Progression by miR-28-3p and miR-193a-5p Regulated by SNAIL. Mol. Ther. Nucleic Acids 2021, 24, 888–904. [Google Scholar] [CrossRef]
- Skrzypek, K.; Kot, M.; Konieczny, P.; Nieszporek, A.; Kusienicka, A.; Lasota, M.; Bobela, W.; Jankowska, U.; Kędracka-Krok, S.; Majka, M. SNAIL Promotes Metastatic Behavior of Rhabdomyosarcoma by Increasing EZRIN and AKT Expression and Regulating MicroRNA Networks. Cancers 2020, 12, 1870. [Google Scholar] [CrossRef]
- Skrzypek, K.; Kusienicka, A.; Trzyna, E.; Szewczyk, B.; Ulman, A.; Konieczny, P.; Adamus, T.; Badyra, B.; Kortylewski, M.; Majka, M. SNAIL Is a Key Regulator of Alveolar Rhabdomyosarcoma Tumor Growth and Differentiation through Repression of MYF5 and MYOD Function. Cell Death Dis. 2018, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.Y.; Hsu, S.K.; Liu, T.Y.; Wu, C.Y.; Chiu, C.C. Melanoma Biology and Treatment: A Review of Novel Regulated Cell Death-Based Approaches. Cancer Cell Int. 2024, 24, 63. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Johnston, N.; Zheng, X.; Wang, H.; Zhang, X.; Gao, D.; Min, W. miR-28 Modulates Exhaustive Differentiation of T Cells through Silencing Programmed Cell Death-1 and Regulating Cytokine Secretion. Oncotarget 2016, 7, 53735–53750. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef]
- Schneider, C.; Setty, M.; Holmes, A.B.; Maute, R.L.; Leslie, C.S.; Mussolin, L.; Rosolen, A.; Dalla-Favera, R.; Bassoa, K. MicroRNA 28 Controls Cell Proliferation and Is Down-Regulated in B-Cell Lymphomas. Proc. Natl. Acad. Sci. USA 2014, 111, 8185–8190. [Google Scholar] [CrossRef]
- Bartolome-Izquierdo, N.; De Yebenes, V.G.; Alvarez-Prado, A.F.; Mur, S.M.; Del Olmo, J.A.L.; Roa, S.; Vazquez, J.; Ramiro, A.R. miR-28 Regulates the Germinal Center Reaction and Blocks Tumor Growth in Preclinical Models of Non-Hodgkin Lymphoma. Blood 2017, 129, 2408–2419. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112. [Google Scholar] [CrossRef]
- Selvam, S.; Ayyavoo, V.; Sharma, V.K. Biomarkers in Neurodegenerative Diseases: A Broad Overview. Open Explor. 2024, 4, 119–147. [Google Scholar] [CrossRef]
- Prats-Puig, A.; Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Bonet, N.; Ricart, W.; López-Bermejo, A.; Fernández-Real, J.M. Changes in Circulating microRNAs Are Associated with Childhood Obesity. J. Clin. Endocrinol. Metab. 2013, 98, E1655–E1660. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.T.; Nicot, C. miR-28-3p Is a Cellular Restriction Factor That Inhibits Human T Cell Leukemia Virus, Type 1 (HTLV-1) Replication and Virus Infection. J. Biol. Chem. 2015, 290, 5381–5390. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Argyris, E.; Chen, K.; Liang, Z.; Tian, H.; Huang, W.; Squires, K.; Verlinghieri, G.; Zhang, H. Cellular microRNAs Contribute to HIV-1 Latency in Resting Primary CD4+ T Lymphocytes. Nat. Med. 2007, 13, 1241–1247. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Q.; Zhang, X.; Zhu, M. Circulating MicroRNA Signatures Serve as Potential Diagnostic Biomarkers for Helicobacter Pylori Infection. J. Cell Biochem. 2019, 120, 1735–1741. [Google Scholar] [CrossRef]
- COVID-19 Deaths|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 26 February 2025).
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=o (accessed on 26 February 2025).
- Das, R.; Sinnarasan, V.S.P.; Paul, D.; Venkatesan, A. A Machine Learning Approach to Identify Potential miRNA-Gene Regulatory Network Contributing to the Pathogenesis of SARS-CoV-2 Infection. Biochem. Genet. 2024, 62, 987–1006. [Google Scholar] [CrossRef]
- XU, Y.; LI, Y. MicroRNA-28-3p Inhibits Angiotensin-Converting Enzyme 2 Ectodomain Shedding in 293T Cells Treated with the Spike Protein of Severe Acute Respiratory Syndrome Coronavirus 2 by Targeting A Disintegrin and Metalloproteinase 17. Int. J. Mol. Med. 2021, 48, 189. [Google Scholar] [CrossRef] [PubMed]
- Mahjoob, G.; Ahmadi, Y.; Fatima rajani, H.; Khanbabaei, N.; Abolhasani, S. Circulating microRNAs as Predictive Biomarkers of Coronary Artery Diseases in Type 2 Diabetes Patients. J. Clin. Lab. Anal. 2022, 36, e24380. [Google Scholar] [CrossRef]
- Milluzzo, A.; Maugeri, A.; Barchitta, M.; Sciacca, L.; Agodi, A. Epigenetic Mechanisms in Type 2 Diabetes Retinopathy: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10502. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, K.P.; Wang, Y.X.; Liu, Z.C.; Tian, L.; Yang, X.Z.; Ding, J.Y.; Wu, W.T.; Yang, W.H.; Li, Y.L.; et al. RNA Sequencing Identified Specific Circulating miRNA Biomarkers for Early Detection of Diabetes Retinopathy. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E374–E385. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use When? Endocrinol. Metab. Clin. N. Am. 2021, 50, 11. [Google Scholar] [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Meng, L.; Yang, H.; Jin, C.; Quan, S. miR-28-5p Suppresses Cell Proliferation and Weakens the Progression of Polycystic Ovary Syndrome by Targeting Prokineticin-1. Mol. Med. Rep. 2019, 20, 2468–2475. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; Ibáñez-Cabellos, J.S.; Pallardo, F.V.; García-Giménez, J.L.; Aulinas, A.; Martel-Duguech, L.; Webb, S.M.; Valassi, E. Circulating miR-28-5p Is Overexpressed in Patients with Sarcopenia despite Long-Term Remission of Cushing’s Syndrome: A Pilot Study. Front. Endocrinol. 2024, 15, 1410080. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Gilson, H.; Thissen, J.P. Mechanisms of Glucocorticoid-Induced Myopathy. J. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Huang, L.; Shao, C.; Nie, T.; Xia, L.; Cui, B.; Lu, F.; Zhu, L.; Chen, B.; Yang, Q. Several miRNAs Derived from Serum Extracellular Vesicles Are Potential Biomarkers for Early Diagnosis and Progression of Parkinson’s Disease. Transl. Neurodegener. 2021, 10, 25. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, S.; Xie, Y.; Jin, K.; Bai, Y.; Shan, S. miR-28-5p Relieves Neuropathic Pain by Targeting Zeb1 in CCI Rat Models. J. Cell Biochem. 2018, 119, 8555–8563. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front Immunol 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Feng, Y.; Xu, J.; Liang, J. T-Cell Exhaustion in Immune-Mediated Inflammatory Diseases: New Implications for Immunotherapy. Front Immunol 2022, 13, 977394. [Google Scholar] [CrossRef]
- Hemmatzadeh, M.; Parvin, E.A.; Ghanavatinejad, A.; Rostami, N.; Hajaliloo, M.; Shomali, N.; Mohammadi, H.; Jadidi-Niaragh, F. microRNAs Targeting Programmed Cell Death Protein 1 (PD-1) Promote Natural Killer Cell Exhaustion in Rheumatoid Arthritis. Iran J. Allergy Asthma Immunol. 2022, 21, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Machanahalli Balakrishna, A.; Reddi, V.; Belford, P.M.; Alvarez, M.; Jaber, W.A.; Zhao, D.X.; Vallabhajosyula, S. Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management. Medicina 2022, 58, 1186. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Qian, J.; Li, H.; Jiang, T.; Wang, W.; Cheng, W.; Wang, F.; Qi, L.; et al. miR-28-3p as a Potential Plasma Marker in Diagnosis of Pulmonary Embolism. Thromb. Res. 2016, 138, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Xu, M.; Zhou, F.; Yan, J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and Their Target B7-H3 May Serve as Novel Biomarkers for Colorectal Cancer. J. BUON 2019, 24, 1120–1127. [Google Scholar]
- Jeddi, F.; Alipour, S.; Najafzadeh, N.; Dadashpour, M.; Pouremamali, F.; Sadeghi, M.R.; Samadi, N.; Soozangar, N.; Khamaneh, A.M. Reduced Levels of miR–28 and miR–200a Act as Predictor Biomarkers of Aggressive Clinicopathological Characteristics in Gastric Cancer Patients. Galen Med. J. 2019, 8, e1329. [Google Scholar] [CrossRef]
- Silva, C.M.S.; Barros-Filho, M.C.; Wong, D.V.T.; Mello, J.B.H.; Nobre, L.M.S.; Wanderley, C.W.S.; Lucetti, L.T.; Muniz, H.A.; Paiva, I.K.D.; Kuasne, H.; et al. Circulating Let-7e-5p, miR-106a-5p, miR-28-3p, and miR-542-5p as a Promising MicroRNA Signature for the Detection of Colorectal Cancer. Cancers 2021, 13, 1493. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, X.; Zhang, Y.; Ma, X.; Ren, A.; Huang, H. Diagnostic and Prognostic Value of Serum miR-296-5p and miR-28-3p in Human Gastric Cancer. Cancer Biother. Radiopharm. 2023, 38, 95–101. [Google Scholar] [CrossRef]
- Sun, R.; Zheng, Z.; Wang, L.; Cheng, S.; Shi, Q.; Qu, B.; Fu, D.; Leboeuf, C.; Zhao, Y.; Ye, J.; et al. A Novel Prognostic Model Based on Four Circulating miRNA in Diffuse Large B-cell Lymphoma: Implications for the Roles of MDSC and Th17 Cells in Lymphoma Progression. Mol. Oncol. 2020, 15, 246. [Google Scholar] [CrossRef]
- Wang, C.; Hu, J.; Lu, M.; Gu, H.; Zhou, X.; Chen, X.; Zen, K.; Zhang, C.Y.; Zhang, T.; Ge, J.; et al. A Panel of Five Serum miRNAs as a Potential Diagnostic Tool for Early-Stage Renal Cell Carcinoma. Sci. Rep. 2015, 5, 7610. [Google Scholar] [CrossRef]
- Fuertes, T.; Álvarez-Corrales, E.; Gómez-Escolar, C.; Ubieto-Capella, P.; Serrano-Navarro, Á.; de Molina, A.; Méndez, J.; Ramiro, A.R.; de Yébenes, V.G. miR-28-Based Combination Therapy Impairs Aggressive B Cell Lymphoma Growth by Rewiring DNA Replication. Cell Death Dis. 2023, 14, 687. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shang, Q.; Zhu, H.; Chen, K.; Li, X.; Gao, H.; Liu, B.; Feng, M.; Gao, L. Effects of Hsa-miR-28-5p on Adriamycin Sensitivity in Diffuse Large B-Cell Lymphoma. Evid. Based Complement. Alternat. Med. 2022, 2022, 4290994. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Sun, W.L.; Lu, X.F.; Wang, X.L.; Jiang, L. miR-28-5p Mediates the Anti-Proliferative and pro-Apoptotic Effects of Curcumin on Human Diffuse Large B-Cell Lymphoma Cells. J. Int. Med. Res. 2020, 48, 300060520943792. [Google Scholar] [CrossRef]
- Shah, M.Y.; Calin, G.A. microRNAs as Therapeutic Targets in Human Cancers. Wiley Interdiscip. Rev. RNA 2014, 5, 537. [Google Scholar] [CrossRef]
- Huang, P.; Jia, L. MicroRNA-28-5p as a Potential Diagnostic Biomarker for Chronic Periodontitis and Its Role in Cell Proliferation and Inflammatory Response. J. Dent. Sci. 2022, 17, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.V.; Rai, P.; Kabekkodu, S.P.; Karunasagar, I.; Kumar, B.K. Identification of Circulating miRNA Biomarkers in Leptospirosis for Early Detection: A Promising Diagnostic Approach. Microb. Pathog. 2024, 193, 106781. [Google Scholar] [CrossRef]
- Peronace, C.; Cione, E.; Abrego-Guandique, D.M.; De Fazio, M.; Panduri, G.; Caroleo, M.C.; Cannataro, R.; Minchella, P. FAM19A4 and Hsa-miR124-2 Double Methylation as Screening for ASC-H- and CIN1 HPV-Positive Women. Pathogens 2024, 13, 312. [Google Scholar] [CrossRef]
- Starlinger, P.; Hackl, H.; Pereyra, D.; Skalicky, S.; Geiger, E.; Finsterbusch, M.; Tamandl, D.; Brostjan, C.; Grünberger, T.; Hackl, M.; et al. Predicting Postoperative Liver Dysfunction Based on Blood-Derived MicroRNA Signatures. Hepatology 2019, 69, 2636. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of miRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Telkoparan-Akillilar, P.; Chichiarelli, S.; Tucci, P.; Saso, L. Integration of microRNAs with Nanomedicine: Tumor Targeting and Therapeutic Approaches. Front. Cell Dev. Biol. 2025, 13, 1569101. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Matsuzaki, J.; Ochiya, T. Circulating microRNAs: Challenges with Their Use as Liquid Biopsy Biomarkers. Cancer Biomarkers 2022, 35, 1–9. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A Synthetic MicroRNA-92a Inhibitor (MRG-110) Accelerates Angiogenesis and Wound Healing in Diabetic and Nondiabetic Wounds. Wound Repair and Regeneration 2018, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. miRNA Nanotherapeutics for Cancer. Drug Discov. Today 2016, 22, 424. [Google Scholar] [CrossRef]
- Wang, M.; Dai, T.; Meng, Q.; Wang, W.; Li, S. Regulatory Effects of miR-28 on Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Bioengineered 2022, 13, 684. [Google Scholar] [CrossRef]

| Cancer Type | miRNA | Regulated Factors | Role | Function | References |
|---|---|---|---|---|---|
| Nasopharyngeal carcinoma | miR-28-5p | CCND1 | Tumor suppressor | Cell cycle, apoptosis | [55] |
| miR-28-3p | NM23-H1 | OncomiR | EMT, migration, invasion | [55] | |
| Esophageal cancer | miR-28-5p | MTSS1 | OncomiR | Cancer progression, proliferation, apoptosis | [59] |
| Gastric cancer | miR-28-5p | AKT | Tumor suppressor | Migration, invasion | [61] |
| NRF2 | Tumor suppressor | Migration, invasion | [63] | ||
| miR-28 | PTEN | Tumor suppressor | Proliferation, invasion | [64] | |
| Colorectal cancer | miR-28-5p | SSRP1 | Tumor suppressor | Proliferation, migration, EMT | [67] |
| CCND1 | Tumor suppressor | Cell cycle, proliferation | [69] | ||
| HOXB3 | Tumor suppressor | Cancer progression | [69] | ||
| CAMTA2 | Tumor suppressor | Proliferation, metastasis | [68] | ||
| miR-28-3p | NM23-H1 | OncomiR | Migration, invasion | [69] | |
| Hepatocellular carcinoma | miR-28-5p | IGF-1 | Tumor suppressor | Cancer stem cell expansion | [76] |
| Cholangiocarcinoma | miR-28-5p | CD44 | Tumor suppressor | Cancer progression, Cell cycle arrest, proliferation, metastasis | [78] |
| Pancreatic cancer | miR-28-5p | SEMA7A | Tumor suppressor | Proliferation, migration, and cancer progression | [36] |
| RAP1B | Tumor suppressor | Tumor growth, metastasis | [35] | ||
| Breast cancer | miR-28-5p | WSB2 | Tumor suppressor | Migration | [82] |
| NRF2 | Tumor suppressor | Cancer progression | [84] | ||
| CENPF | Tumor suppressor | Migration, invasion, proliferation | [39] | ||
| LDHA | Tumor suppressor | Proliferation, migration, apoptosis | [41] | ||
| Ovarian cancer | miR-28-5p | N4BP1 | OncomiR | Proliferation, migration, invasion, EMT | [89] |
| Prostate cancer | miR-28-5p | E2F6 | Tumor suppressor | Apoptosis | [93] |
| SREBF2 | Tumor suppressor | Proliferation, cell survival, migration, invasion | [94] | ||
| miR-28-3p | ARF6 | Tumor suppressor | Migration, invasion | [92] | |
| BCL2, BAX | Tumor suppressor | Apoptosis | [92] | ||
| Non-small-cellular lung cancer | miR-28 | PTEN | OncomiR | Proliferation, cell survival, tumor microenvironment | [99] |
| Glioma | miR-28-5p | RAP1B | Tumor suppressor | Proliferation, migration | [102] |
| FOXO1 | OncomiR | Tumor spheres formation, proliferation, viability | [104] | ||
| Rhabdomyosarcoma | miR-28-3p | MYOG, MYOD, MEF2A, MSTN | Tumor suppressor | Cell differentiation, cell cycle arrest, migration, proliferation | [106] |
| Melanoma | miR-28-5p | RAP1B | Tumor suppressor | Proliferation | [38] |
| HOXB3 | Tumor suppressor | Cancer progression | [37] | ||
| miR-28 | PD1 | Tumor suppressor | Inhibiting overexpression of inhibitory receptors (IR) on exhausted T-cells | [111] | |
| Non-Hodgkin lymphoma | miR-28 | MAD2L1 | Tumor suppressor | Proliferation | [115] |
| BAG1 | Tumor suppressor | Proliferation, cell cycle | [115] | ||
| Papillary thyroid carcinoma | miR-28-3p | BCL2 | Tumor suppressor | Apoptosis | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotarski, K.; Kot, M.; Skrzypek, K. miR-28: A Tiny Player in Cancer Progression and Other Human Diseases. Biomolecules 2025, 15, 757. https://doi.org/10.3390/biom15060757
Kotarski K, Kot M, Skrzypek K. miR-28: A Tiny Player in Cancer Progression and Other Human Diseases. Biomolecules. 2025; 15(6):757. https://doi.org/10.3390/biom15060757
Chicago/Turabian StyleKotarski, Karol, Marta Kot, and Klaudia Skrzypek. 2025. "miR-28: A Tiny Player in Cancer Progression and Other Human Diseases" Biomolecules 15, no. 6: 757. https://doi.org/10.3390/biom15060757
APA StyleKotarski, K., Kot, M., & Skrzypek, K. (2025). miR-28: A Tiny Player in Cancer Progression and Other Human Diseases. Biomolecules, 15(6), 757. https://doi.org/10.3390/biom15060757








