Hostile Environments: Modifying Surfaces to Block Microbial Adhesion and Biofilm Formation
Abstract
1. Introduction
1.1. Biofilms: Discovery, Importance and Formation
1.2. Biofilms in Medicine and Industry
2. How to Combat Biofilms
2.1. When to Combat Biofilms
2.2. How to Prevent Cell Adhesion, the First Step in Biofilm Formation
3. Physical Modifications of Surfaces
3.1. Laser Treatment
3.2. Lithography
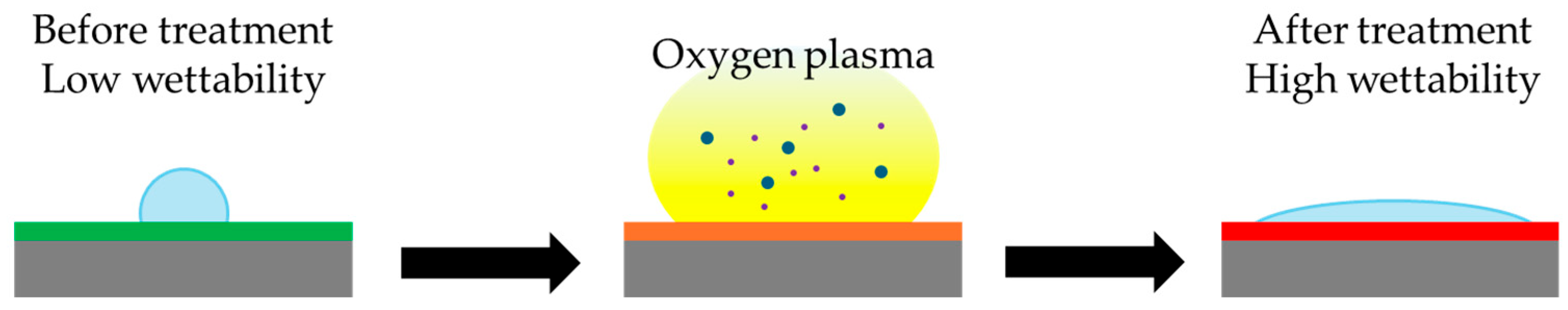
3.3. Plasma Treatment
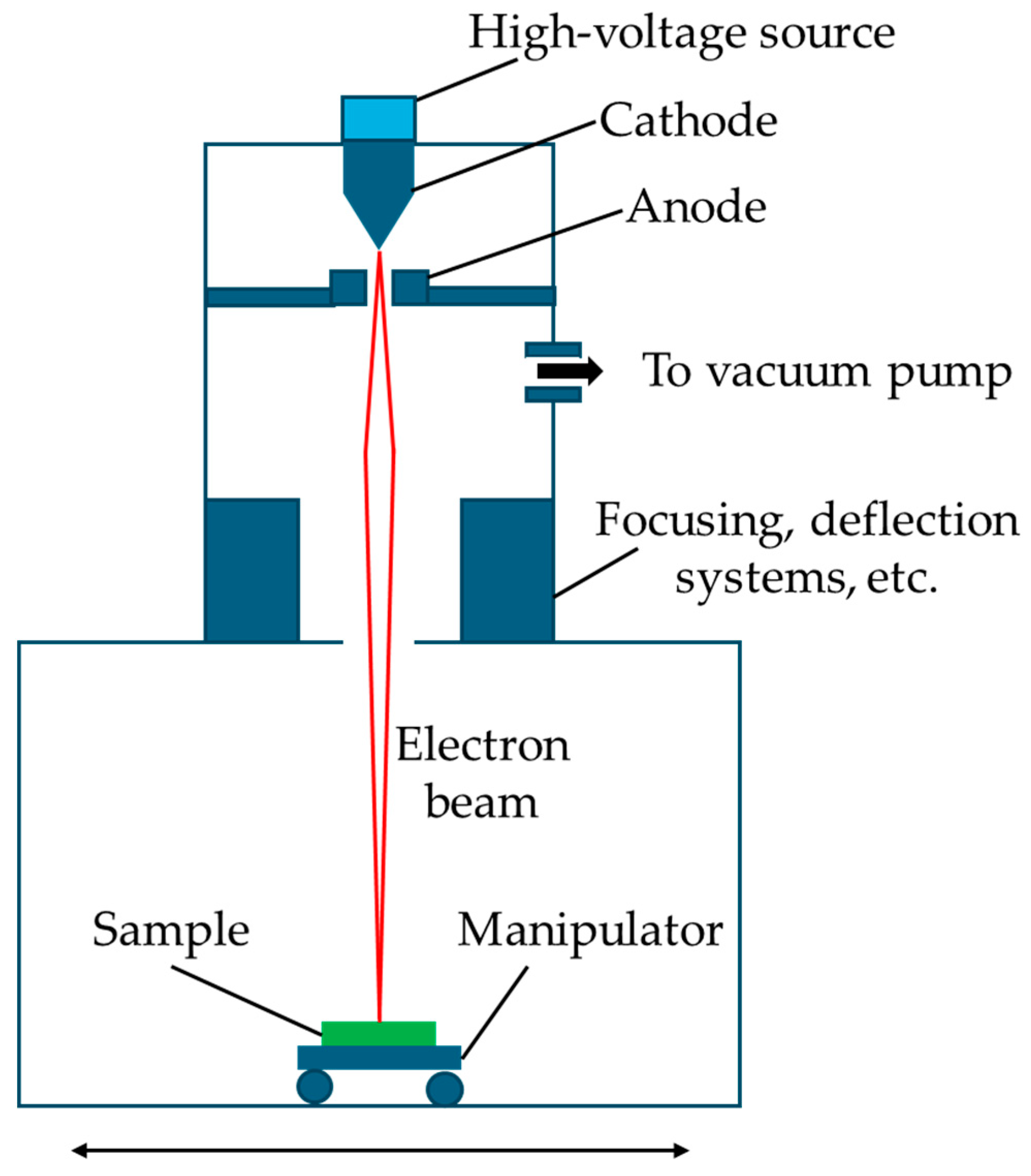
3.4. Electron Beam
3.5. Physical Vapor Deposition
4. Chemical Modifications of Surfaces
4.1. Passive Chemical Modification
4.1.1. Polyethylene Glycol (PEG)
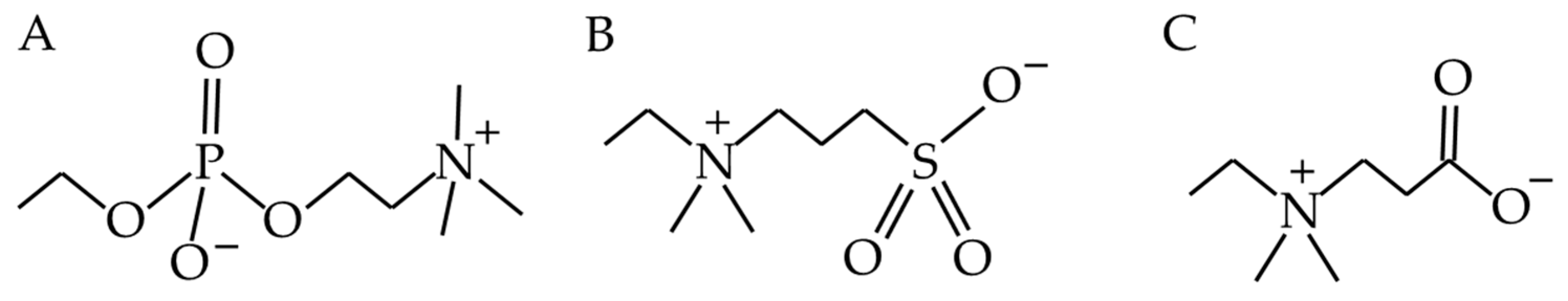
4.1.2. Zwitterions
4.1.3. Other Modifications of Polymers to Change Hydrophobicity or Charge
4.1.4. Electrical Conductivity
4.1.5. Superhydrophobic and Superhydrophilic Surfaces
4.1.6. Adding Charge to a Surface
4.1.7. Poly Sodium Sulfonate
4.2. Active Chemical Modification
4.2.1. Quaternary Ammonium Compounds (QACs)
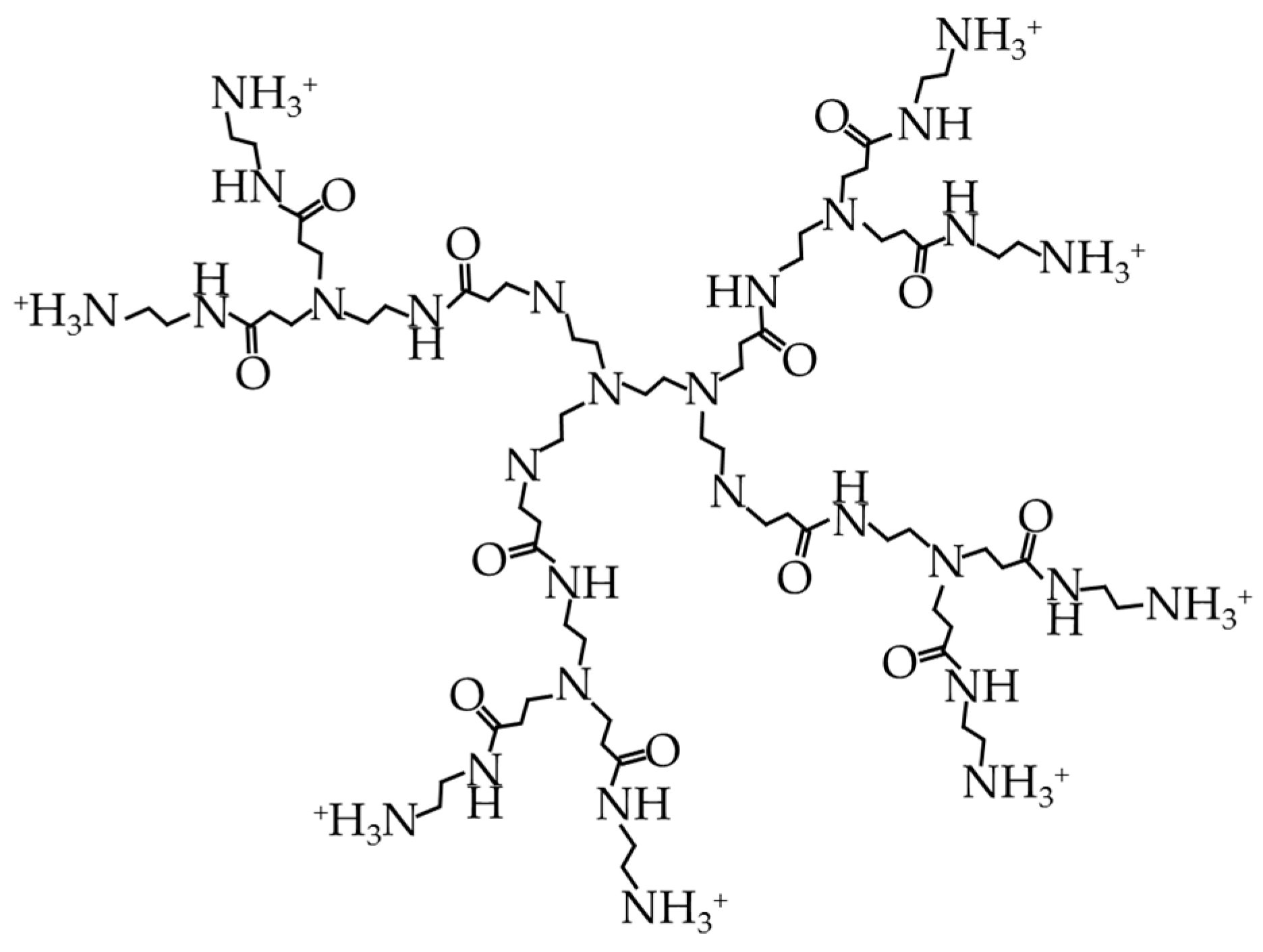
4.2.2. Cationic Dendrimers
4.2.3. Nanoparticles
4.2.4. Calcium Phosphate Coatings
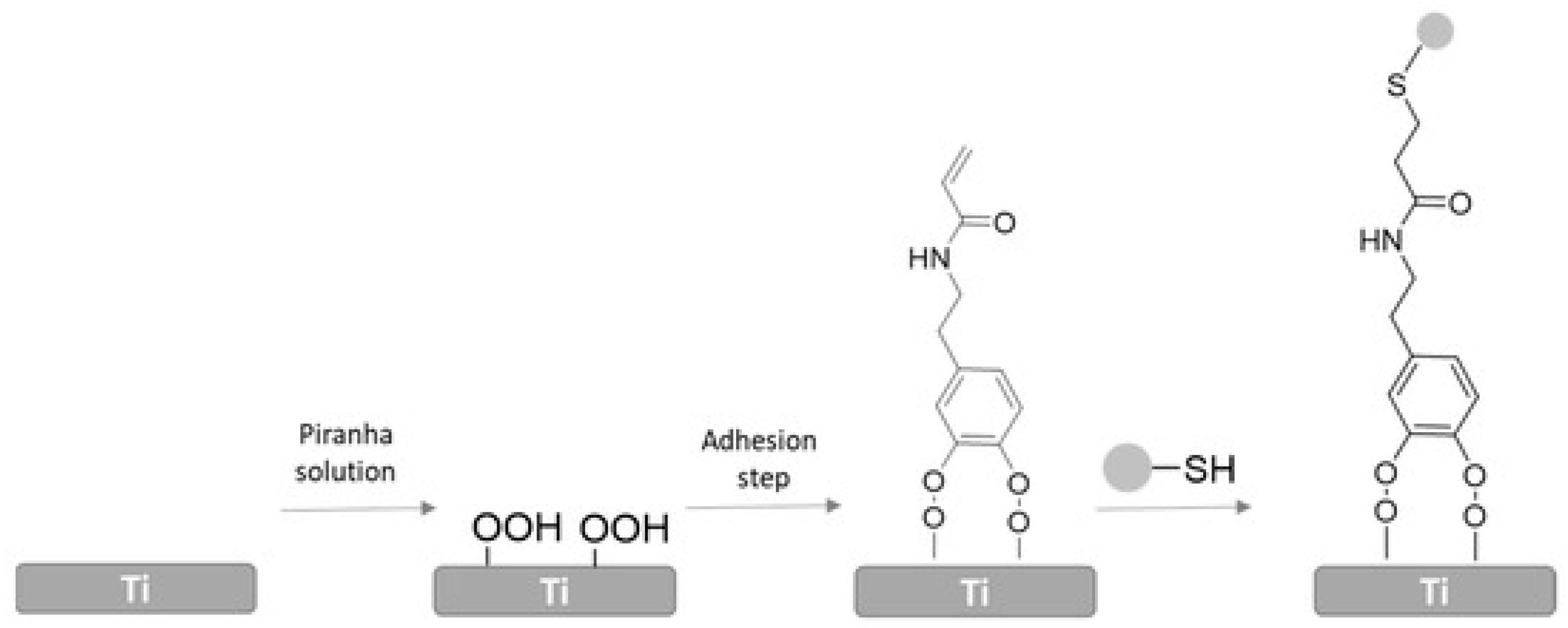
4.2.5. Toremifene
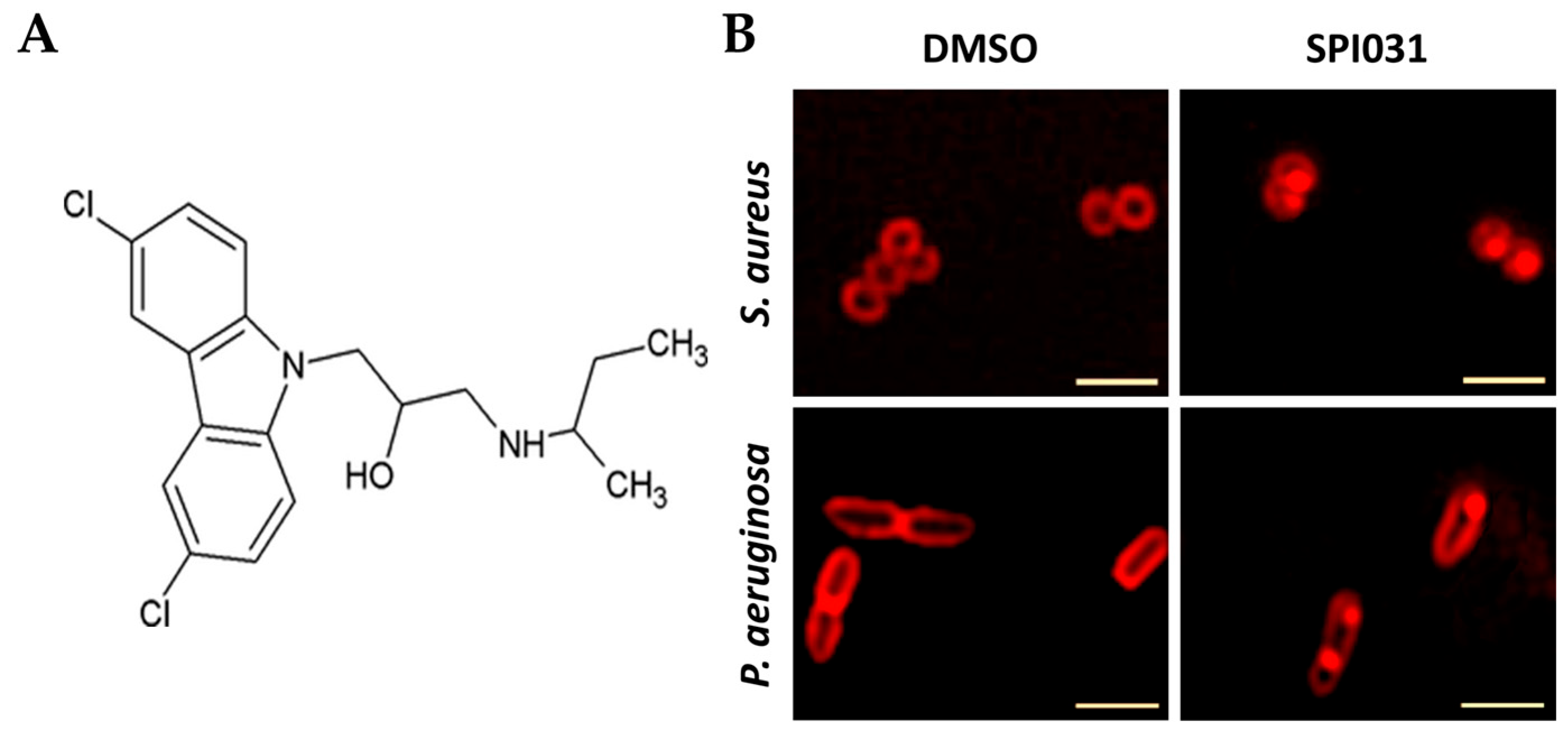
4.2.6. SPI031
5. Biological Modifications of Surfaces
5.1. Antimicrobial Peptides (AMPs)
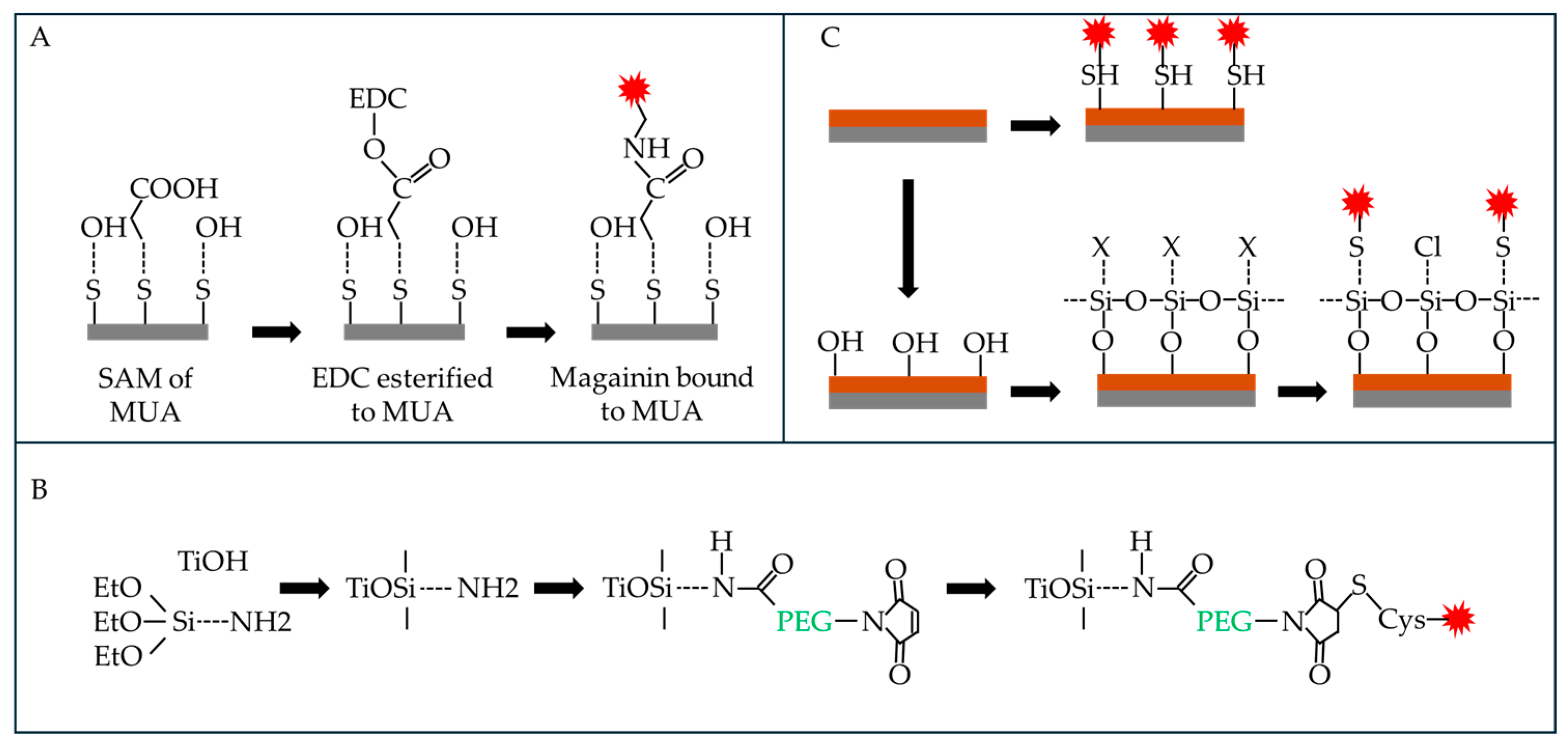
5.1.1. Magainins (MAGs)
5.1.2. Human Cathelicidin, LL-37
5.1.3. hLF1-11
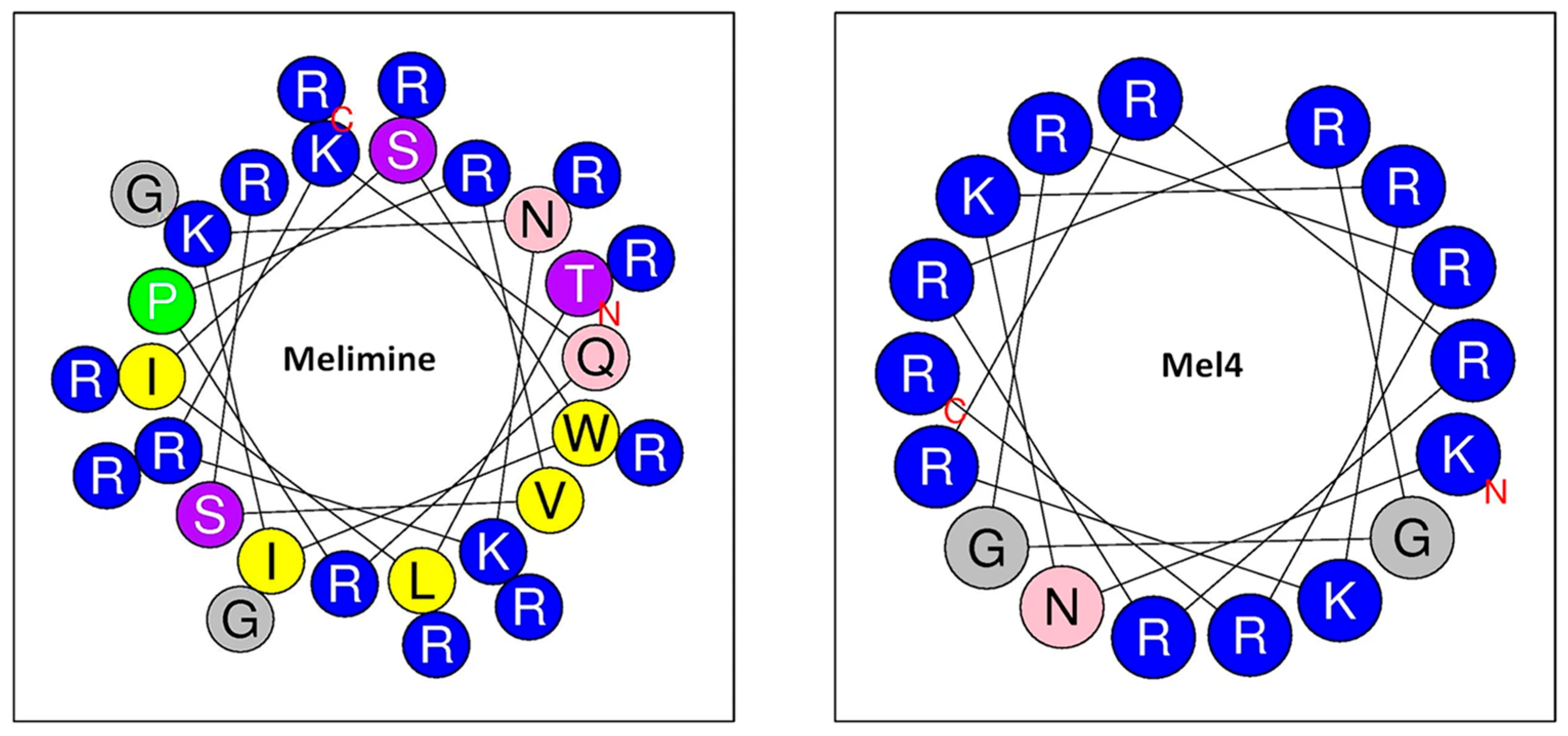
5.1.4. Melimine
5.1.5. Cateslytin
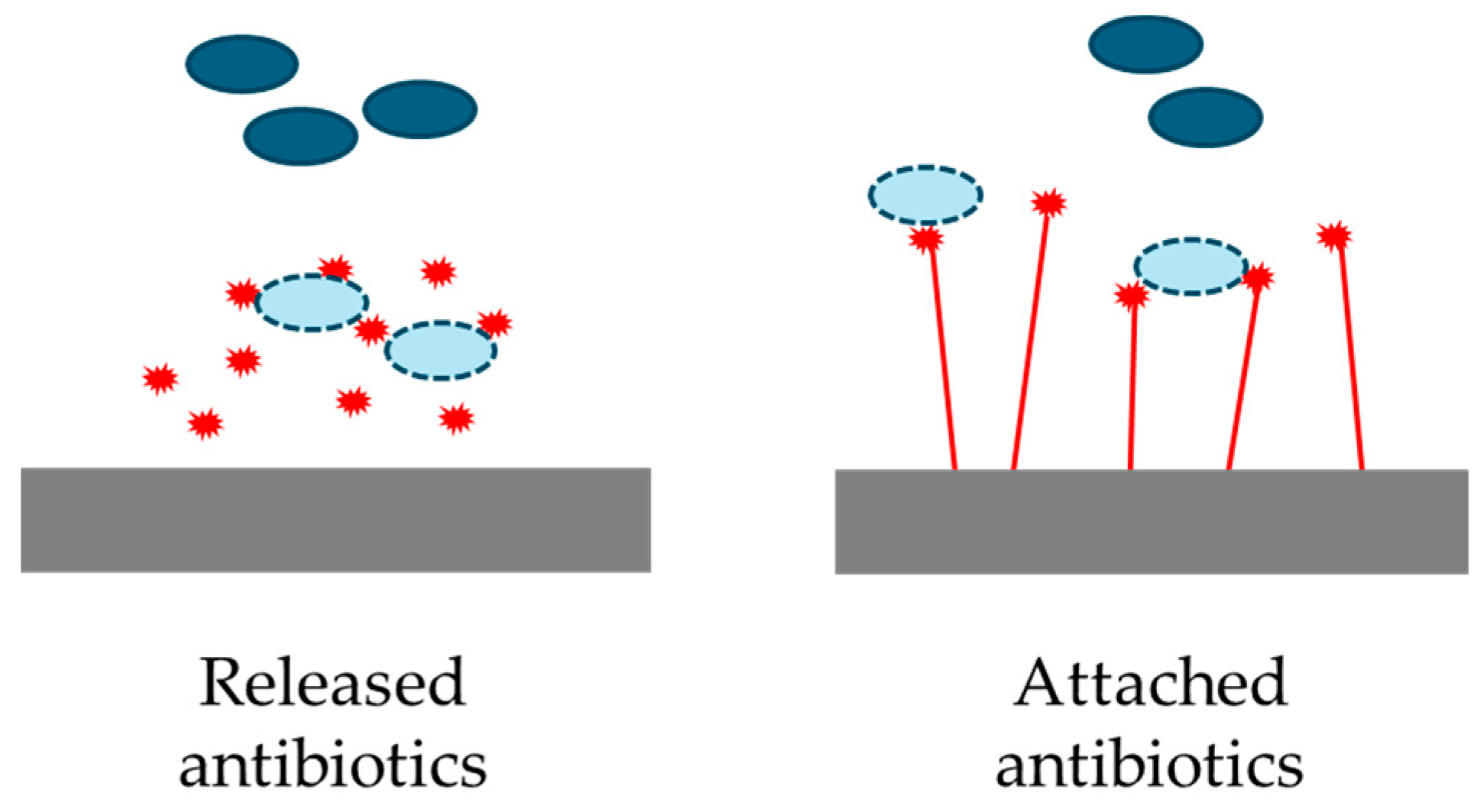
5.2. Antibiotics
Gentamicin and Other Antibiotics on Hydroxyapatite Coatings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Cheng, K.; Akin, D.; Costerton, J. Rumen bacteria: Interaction with particulate dietary components and response to dietary variation. Fed. Proc. 1977, 36, 193–197. [Google Scholar] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.-J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- McCowan, R.; Cheng, K.; Bailey, C.; Costerton, J. Adhesion of bacteria to epithelial cell surfaces within the reticulo-rumen of cattle. Appl. Environ. Microbiol. 1978, 35, 149–155. [Google Scholar] [CrossRef]
- Lappin-Scott, H.; Burton, S.; Stoodley, P. Revealing a world of biofilms—The pioneering research of Bill Costerton. Nat. Rev. Microbiol. 2014, 12, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Geesey, G.; Richardson, W.; Yeomans, H.; Irvin, R.; Costerton, J. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can. J. Microbiol. 1977, 23, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Geesey, G.; Mutch, R.; Costerton, J.; Green, R. Sessile bacteria: An important component of the microbial population in small mountain streams 1. Limnol. Oceanogr. 1978, 23, 1214–1223. [Google Scholar] [CrossRef]
- Costerton, J.; Gessey, G. Microbial contamination of surfaces. In Surface Contamination: Genesis, Detection, and Control; Springer: Berlin/Heidelberg, Germany, 1979; pp. 211–221. [Google Scholar]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar] [CrossRef]
- Marrie, T.J.; Lam, J.; Costerton, J.W. Bacterial adhesion to uroepithelial cells: A morphologic study. J. Infect. Dis. 1980, 142, 239–246. [Google Scholar] [CrossRef]
- Costerton, J.W.; Irvin, R.T.; Cheng, K. The bacterial glycocalyx in nature and disease. Ann. Rev. Microbiol. 1981, 35, 299–324. [Google Scholar] [CrossRef]
- Gristina, A.G.; Costerton, J. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. JBJS 1985, 67, 264–273. [Google Scholar] [CrossRef]
- Costerton, J.W.; Cheng, K.-J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Ann. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.A. Bacteria as multicellular organisms. Sci. Am. 1988, 258, 82–89. [Google Scholar] [CrossRef]
- Palková, Z. Multicellular microorganisms: Laboratory versus nature. EMBO Rep. 2004, 5, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef]
- Robertson, S.R.; McLean, R.J. Beneficial biofilms. AIMS Bioeng. 2015, 2, 437–448. [Google Scholar] [CrossRef]
- Ünal Turhan, E.; Erginkaya, Z.; Korukluoğlu, M.; Konuray, G. Beneficial biofilm applications in food and agricultural industry. In Health and Safety Aspects of Food Processing Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 445–469. [Google Scholar]
- Ghiasian, M. Microbial biofilms: Beneficial applications for sustainable agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–155. [Google Scholar]
- Clarke, R.; Bauchop, T. Microbial Ecology of the Gut; Academic Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Rastelli, M.; Knauf, C.; Cani, P.D. Gut microbes and health: A focus on the mechanisms linking microbes, obesity, and related disorders. Obesity 2018, 26, 792–800. [Google Scholar] [CrossRef]
- Álvarez, J.; Real, J.M.F.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; de Pipaon, M.S.; Sanz, Y. Gut microbes and health. Gastroenterol. Hepatol. (Engl. Ed.) 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Pasmore, M.; Costerton, J.W. Biofilms, bacterial signaling, and their ties to marine biology. J. Ind. Microbiol. Biotechnol. 2003, 30, 407–413. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells and the riddle of biofilm survival. Biochemistry 2005, 70, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Lee, J.-H. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Bu, F.; Liu, M.; Xie, Z.; Chen, X.; Li, G.; Wang, X. Targeted anti-biofilm therapy: Dissecting targets in the biofilm life cycle. Pharmaceuticals 2022, 15, 1253. [Google Scholar] [CrossRef]
- Yu, J.-L.; Andersson, R.; Ljungh, Å. Protein adsorption and bacterial adhesion to biliary stent materials. J. Surg. Res. 1996, 62, 69–73. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Y.; Wu, S.; Zhang, H.; Guo, B.; Zhang, B.; Qin, X.-J.; Li, H. Preadsorption of serum proteins regulates bacterial infections and subsequent macrophage phagocytosis on biomaterial surfaces. ACS Appl. Bio Mater. 2019, 2, 5957–5964. [Google Scholar] [CrossRef]
- Zegans, M.E.; Wozniak, D.; Griffin, E.; Toutain-Kidd, C.M.; Hammond, J.H.; Garfoot, A.; Lam, J.S. Pseudomonas aeruginosa exopolysaccharide Psl promotes resistance to the biofilm inhibitor polysorbate 80. Antimicrob. Agents Chemother. 2012, 56, 4112–4122. [Google Scholar] [CrossRef]
- Pierce, C.G.; Vila, T.; Romo, J.A.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J.L. The Candida albicans biofilm matrix: Composition, structure and function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.H.; Elbanna, K.; Osman, G.E.; Abulreesh, H.H. Candida diagnostic techniques: A review. J. Umm Al-Qura Uni Appl. Sci. 2023, 9, 360–377. [Google Scholar] [CrossRef]
- Bruchmann, J.; Pini, I.; Gill, T.S.; Schwartz, T.; Levkin, P.A. Patterned SLIPS for the formation of arrays of biofilm microclusters with defined geometries. Adv. Healthc. Mater. 2017, 6, 1601082. [Google Scholar] [CrossRef]
- Grande, R.; Puca, V.; Muraro, R. Antibiotic resistance and bacterial biofilm. Expert. Opin. Ther. Pat. 2020, 30, 897–900. [Google Scholar] [CrossRef]
- Jahagirdar, V.L.; Davane, M.S.; Aradhye, S.C.; Nagoba, B.S. Candida species as potential nosocomial pathogens—A review. Electron. J. Gen. Med. 2018, 15. [Google Scholar]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef]
- Váchová, L.; Šťovíček, V.; Hlaváček, O.; Chernyavskiy, O.; Štěpánek, L.; Kubínová, L.; Palková, Z. Flo11p, drug efflux pumps, and the extracellular matrix cooperate to form biofilm yeast colonies. J. Cell Biol. 2011, 194, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Eboigbodin, K.E. Biophysical Investigation of Bacterial Aggregation; University of Sheffield: Sheffield, UK, 2008. [Google Scholar]
- Speth, C.; Rambach, G.; Lass-Flörl, C.; Howell, P.L.; Sheppard, D.C. Galactosaminogalactan (GAG) and its multiple roles in Aspergillus pathogenesis. Virulence 2019, 10, 976–983. [Google Scholar] [CrossRef]
- Kernien, J.F.; Johnson, C.J.; Bayless, M.L.; Chovanec, J.F.; Nett, J.E. Neutrophils from patients with invasive candidiasis are inhibited by Candida Albicans biofilms. Front. Immunol. 2020, 11, 587956. [Google Scholar] [CrossRef]
- Mannan, M.; Nabeela, S.; Mishra, R.; Uppuluri, P. Host immune response against fungal biofilms. Curr. Opin. Microbiol. 2024, 81, 102520. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Al-Madboly, L.A.; Aboulmagd, A.; El-Salam, M.A.; Kushkevych, I.; El-Morsi, R.M. Microbial enzymes as powerful natural anti-biofilm candidates. Microb. Cell Fact. 2024, 23, 343. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-derived depolymerases against bacterial biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against pathogenic bacterial biofilms and biofilm-based infections: A review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.; Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora’s box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef]
- Kingshott, P.; Griesser, H.J. Surfaces that resist bioadhesion. Curr. Opin. Solid. State Mater. Sci. 1999, 4, 403–412. [Google Scholar] [CrossRef]
- Chandra, J.; Patel, J.D.; Li, J.; Zhou, G.; Mukherjee, P.K.; McCormick, T.S.; Anderson, J.M.; Ghannoum, M.A. Modification of surface properties of biomaterials influences the ability of Candida albicans to form biofilms. Appl. Environ. Microbiol. 2005, 71, 8795–8801. [Google Scholar] [CrossRef]
- Balazs, D.; Triandafillu, K.; Chevolot, Y.; Aronsson, B.O.; Harms, H.; Descouts, P.; Mathieu, H. Surface modification of PVC endotracheal tubes by oxygen glow discharge to reduce bacterial adhesion. Surf. Interface Anal. 2003, 35, 301–309. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Introduction to surface coating and modification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–60. [Google Scholar]
- Waugh, D.G.; Toccaceli, C.; Gillett, A.R.; Ng, C.-H.; Hodgson, S.D.; Lawrence, J. Surface Treatments to Modulate Bioadhesion: A Critical Review; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.-U.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Ghilini, F.; Pissinis, D.E.; Minan, A.; Schilardi, P.L.; Diaz, C. How functionalized surfaces can inhibit bacterial adhesion and viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef] [PubMed]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid. Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Hauschwitz, P.; Palkova, Z.; Vachova, L.; Bicistova, R.; Prochazka, M.; Plocek, V.; Tarant, I.; Pathak, S.; Brajer, J.; Muzik, J. Rapid laser-induced nanostructuring for yeast adhesion-reducing surfaces using beam shaping with SLM. J. Mater. Res. Technol. 2025, 35, 193–198. [Google Scholar] [CrossRef]
- Schwibbert, K.; Menzel, F.; Epperlein, N.; Bonse, J.; Krüger, J. Bacterial adhesion on femtosecond laser-modified polyethylene. Materials 2019, 12, 3107. [Google Scholar] [CrossRef]
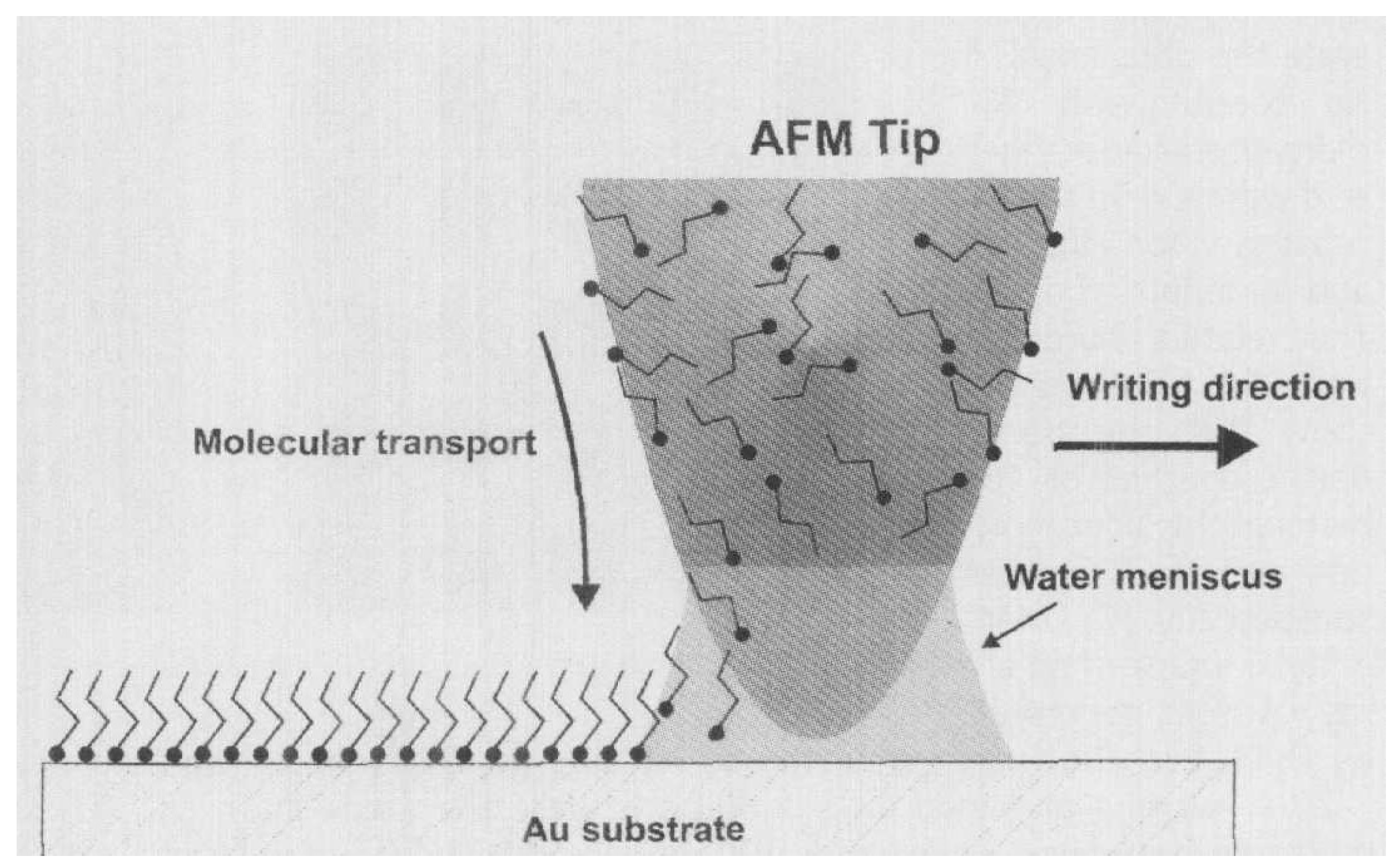
- Arango-Santander, S.; Pelaez-Vargas, A.; Freitas, S.C.; García, C. Surface Modification by Combination of Dip-Pen Nanolithography and Soft Lithography for Reduction of Bacterial Adhesion. J. Nanotechnol. 2018, 2018, 8624735. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Hoppe, V.; Rusińska, M.; Gąsiorek, J.; Ziółkowski, G.; Dydak, K.; Czajkowska, J.; Junka, A. The impact of EBM-manufactured Ti6Al4V ELI alloy surface modifications on cytotoxicity toward eukaryotic cells and microbial biofilm formation. Materials 2020, 13, 2822. [Google Scholar] [CrossRef]
- Diaz, C.; Salvarezza, R.C.; Fernandez Lorenzo de Mele, M.A.; Schilardi, P.L. Organization of Pseudomonas fluorescens on chemically different nano/microstructured surfaces. ACS Appl. Mater. Interfaces 2010, 2, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Roosjen, A.; Kaper, H.J.; Van Der Mei, H.C.; Norde, W.; Busscher, H.J. Inhibition of adhesion of yeasts and bacteria by poly (ethylene oxide)-brushes on glass in a parallel plate flow chamber. Microbiology 2003, 149, 3239–3246. [Google Scholar] [CrossRef]
- Ziemba, C.; Khavkin, M.; Priftis, D.; Acar, H.; Mao, J.; Benami, M.; Gottlieb, M.; Tirrell, M.; Kaufman, Y.; Herzberg, M. Antifouling properties of a self-assembling glutamic acid-lysine zwitterionic polymer surface coating. Langmuir 2018, 35, 1699–1713. [Google Scholar] [CrossRef]
- Masotti, E.; Poma, N.; Guazzelli, E.; Fiaschi, I.; Glisenti, A.; Vivaldi, F.; Bonini, A.; Di Francesco, F.; Tavanti, A.; Galli, G. Fluorinated vs. zwitterionic-polymer grafted surfaces for adhesion prevention of the fungal pathogen Candida albicans. Polymers 2020, 12, 398. [Google Scholar] [CrossRef]
- Holban, A.-M.; Farcasiu, C.; Andrei, O.-C.; Grumezescu, A.M.; Farcasiu, A.-T. Surface modification to modulate microbial biofilms—Applications in dental medicine. Materials 2021, 14, 6994. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Glauser, S.; Fischer, J.; Schmidli, F.; Waltimo, T.; Rohr, N. Biofilm formation on restorative materials and resin composite cements. Dent. Mater. 2018, 34, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.; Kohnen, W. Prevention of biofilm formation by polymer modification. J. Ind. Microbiol. Biotechnol. 1995, 15, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Alcheikh, A.; Pavon-Djavid, G.; Helary, G.; Petite, H.; Migonney, V.; Anagnostou, F. PolyNaSS grafting on titanium surfaces enhances osteoblast differentiation and inhibits Staphylococcus aureus adhesion. J. Mater. Sci. Mater. Med. 2013, 24, 1745–1754. [Google Scholar] [CrossRef]
- Majumdar, P.; Lee, E.; Patel, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Development of environmentally friendly, antifouling coatings based on tethered quaternary ammonium salts in a crosslinked polydimethylsiloxane matrix. J. Coat. Technol. Res. 2008, 5, 405–417. [Google Scholar] [CrossRef]
- Schaer, T.P.; Stewart, S.; Hsu, B.B.; Klibanov, A.M. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials 2012, 33, 1245–1254. [Google Scholar] [CrossRef]
- Tarabal, V.S.; Abud, Y.K.; da Silva, F.G.; da Cruz, L.F.; Fontes, G.N.; da Silva, J.A.; Filho, C.B.; Sinisterra, R.D.; Granjeiro, J.M.; Granjeiro, P.A. Effect of DMPEI coating against biofilm formation on PVC catheter surface. World J. Microbiol. Biotechnol. 2024, 40, 6. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Junkar, I.; Humpolíček, P.; Capáková, Z.; Radaszkiewicz, K.A.; Mikušová, N.; Pacherník, J.; Lehocký, M.; Iglič, A.; Hanáčková, M. Interaction of nanostructured TiO2 biointerfaces with stem cells and biofilm-forming bacteria. Mater. Sci. Eng C 2017, 77, 500–507. [Google Scholar] [CrossRef]
- Braem, A.; De Cremer, K.; Delattin, N.; De Brucker, K.; Neirinck, B.; Vandamme, K.; Martens, J.A.; Michiels, J.; Vleugels, J.; Cammue, B.P. Novel anti-infective implant substrates: Controlled release of antibiofilm compounds from mesoporous silica-containing macroporous titanium. Colloids Surf. B Biointerfaces 2015, 126, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Kucharíková, S.; Van Dijck, P.; Erdtmann, M.; Krona, A.; Lövenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res.® 2016, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin–hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.-M.; Pradier, C.-M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Gabriel, M.; Nazmi, K.; Veerman, E.C.; Nieuw Amerongen, A.V.; Zentner, A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjugate Chem. 2006, 17, 548–550. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Cado, G.; Aslam, R.; Séon, L.; Garnier, T.; Fabre, R.; Parat, A.; Chassepot, A.; Voegel, J.C.; Senger, B.; Schneider, F. Self-defensive biomaterial coating against bacteria and yeasts: Polysaccharide multilayer film with embedded antimicrobial peptide. Adv. Funct. Mater. 2013, 23, 4801–4809. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, D.; Wolfrum, B.; de Silva, J.P. Fabrication, properties and applications of gold nanopillars. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 317–354. [Google Scholar]
- McFadden, R.; Quinn, J.; Buchanan, F.; Carson, L.; Acheson, J.G.; McKillop, S.; Chan, C.-W. An effective laser surface treatment method to reduce biofilm coverage of multiple bacterial species associated with medical device infection. Surf. Coat. Tech. 2023, 453, 129092. [Google Scholar] [CrossRef]
- Fu, C.C.; Grimes, A.; Long, M.; Ferri, C.G.; Rich, B.D.; Ghosh, S.; Ghosh, S.; Lee, L.P.; Gopinathan, A.; Khine, M. Tunable nanowrinkles on shape memory polymer sheets. Adv. Mater. 2009, 21, 4472–4476. [Google Scholar] [CrossRef]
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-induced superhydrophobic and antibacterial surfaces in consumer plastics. PLos ONE 2012, 7, e40987. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.a.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S.a. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Peterdi, G.F. Printmaking; Chapter: Lithography. In Encyclopedia Britannica; Encyclopedia Britannica: Edinburgh, UK, 2021. [Google Scholar]
- Ginger, D.S.; Zhang, H.; Mirkin, C.A. The evolution of dip-pen nanolithography. Angew. Chem. Int. Ed. 2004, 43, 30–45. [Google Scholar] [CrossRef]
- Piner, R.D.; Zhu, J.; Xu, F.; Hong, S.; Mirkin, C.A. “Dip-pen” nanolithography. Science 1999, 283, 661–663. [Google Scholar] [CrossRef]
- Benčina, M.; Rawat, N.; Paul, D.; Kovač, J.; Iglič, A.; Junkar, I. Surface Modification of Stainless Steel for Enhanced Antibacterial Activity. ACS Omega 2025, 10, 13361–13369. [Google Scholar] [CrossRef]
- Paiwand, S.; Schäfer, S.; Kopp, A.; Beikler, T.; Fiedler, I.; Gosau, M.; Fuest, S.; Smeets, R. Antibacterial potential of silver and zinc loaded plasma-electrolytic oxidation coatings for dental titanium implants. Int. J. Implant. Dent. 2025, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Mrsic, I.; Baeuerle, T.; Ulitzsch, S.; Lorenz, G.; Rebner, K.; Kandelbauer, A.; Chasse, T. Oxygen plasma surface treatment of polymer films—Pellethane 55DE and EPR-g-VTMS. Appl. Surf. Sci. 2021, 536, 147782. [Google Scholar] [CrossRef]
- Valkov, S.; Ormanova, M.; Petrov, P. Electron-beam surface treatment of metals and alloys: Techniques and trends. Metals 2020, 10, 1219. [Google Scholar] [CrossRef]
- Díaz, C.; Schilardi, P.L.; Salvarezza, R.C.; Fernández Lorenzo de Mele, M. Nano/microscale order affects the early stages of biofilm formation on metal surfaces. Langmuir 2007, 23, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Gruttadauria, M.; D’Anna, F. Quaternary ammonium salts-based materials: A review on environmental toxicity, anti-fouling mechanisms and applications in marine and water treatment industries. Biomolecules 2024, 14, 957. [Google Scholar] [CrossRef]
- Chiang, W.-C.; Schroll, C.; Hilbert, L.R.; Møller, P.; Tolker-Nielsen, T. Silver-palladium surfaces inhibit biofilm formation. Appl. Environ. Microbiol. 2009, 75, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Assenza, B.; Piattelli, M.; Iezzi, G.; Leghissa, G.C.; Quaranta, A.; Tortora, P.; Piattelli, A. A 16–year study of the microgap between 272 human titanium implants and their abutments. J. Oral. Implantol. 2005, 31, 269–275. [Google Scholar] [CrossRef]
- Coad, B.R.; Kidd, S.E.; Ellis, D.H.; Griesser, H.J. Biomaterials surfaces capable of resisting fungal attachment and biofilm formation. Biotechnol. Adv. 2014, 32, 296–307. [Google Scholar] [CrossRef]
- Hazen, K.C.; Hazen, B.W. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol. Lett. 1993, 107, 83–87. [Google Scholar] [CrossRef]
- Goswami, R.R.; Pohare, S.D.; Raut, J.S.; Karuppayil, S.M. Cell surface hydrophobicity as a virulence factor in Candida albicans. Biosci. Biotechnol. Res. Asia 2017, 14, 1503. [Google Scholar] [CrossRef]
- Desai, N.P.; Hubbell, J.A. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials 1991, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Zhu, Y.-H.; Wang, X.-Y.; Shen, C.; Wei, X.-W.; Xu, T.; He, Z.-Y. Novel zwitterionic vectors: Multi-functional delivery systems for therapeutic genes and drugs. Comput. Struct. Biotechnol. J. 2020, 18, 1980–1999. [Google Scholar] [CrossRef] [PubMed]
- Camagay, A.V.; Kendall, N.; Connolly, M.K. Quaternary Ammonium Compound Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Y.; Ge-Zhang, S.; Mu, P.; Wang, X.; Li, S.; Qiao, L.; Mu, H. Advances in sol-gel-based superhydrophobic coatings for wood: A review. Int. J. Mol. Sci. 2023, 24, 9675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Y.; Chen, Y.; Han, T.; Chen, Y.; Wang, C. A preliminary study on surface bioactivation of polyaryletherketone by UV-grafting with PolyNaSS: Influence on osteogenic and antibacterial activities. J. Biomater. Sci. Polym. Ed. 2022, 33, 1845–1865. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From nanobiotechnology, positively charged biomimetic dendrimers as novel antibacterial agents: A review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Paul, S.; Verma, S.; Chen, Y.-C. Peptide dendrimer-based antibacterial agents: Synthesis and applications. ACS Infect. Dis. 2024, 10, 1034–1055. [Google Scholar] [CrossRef]
- Stallard, C.P.; McDonnell, K.; Onayemi, O.; O’gara, J.; Dowling, D. Evaluation of protein adsorption on atmospheric plasma deposited coatings exhibiting superhydrophilic to superhydrophobic properties. Biointerphases 2012, 7, 31. [Google Scholar] [CrossRef]
- Lam, M.; Moris, V.; Humblot, V.; Migonney, V.; Falentin-Daudre, C. A simple way to graft a bioactive polymer–Polystyrene sodium sulfonate on silicone surfaces. Eur. Polym. J. 2020, 128, 109608. [Google Scholar] [CrossRef]
- Hélary, G.; Noirclère, F.; Mayingi, J.; Migonney, V. A new approach to graft bioactive polymer on titanium implants: Improvement of MG 63 cell differentiation onto this coating. Acta Biomater. 2009, 5, 124–133. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Aissa, I.B.; Evans, M.D.; Migonney, V. Contributions of adhesive proteins to the cellular and bacterial response to surfaces treated with bioactive polymers: Case of poly (sodium styrene sulfonate) grafted titanium surfaces. J. Mater. Sci. Mater. Med. 2015, 26, 261. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bodón, J.; Andrade del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive coatings on titanium: A review on hydroxylation, self-assembled monolayers (SAMs) and surface modification strategies. Polymers 2021, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.D.; Martins, R.; Quintas-Nunes, F.; Reynolds-Brandão, P.; Crespo, M.T.B.; Nascimento, F.X. Exploring the functional and genomic features of Cellulophaga lytica NFXS1, a zeaxanthin and lytic enzyme-producing marine bacterium that promotes microalgae growth. Microbe 2024, 4, 100142. [Google Scholar] [CrossRef]
- Smith, S.A.; Choi, S.H.; Collins, J.N.; Travers, R.J.; Cooley, B.C.; Morrissey, J.H. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood J. Am. Soc. Hematol. 2012, 120, 5103–5110. [Google Scholar] [CrossRef]
- Fail, C.; Evenson, S.; Ward, L.; Schofield, W.; Badyal, J. Controlled attachment of PAMAM dendrimers to solid surfaces. Langmuir 2002, 18, 264–268. [Google Scholar] [CrossRef]
- Lai, Z.; Jian, Q.; Li, G.; Shao, C.; Zhu, Y.; Yuan, X.; Chen, H.; Shan, A. Self-assembling peptide dendron nanoparticles with high stability and a multimodal antimicrobial mechanism of action. ACS Nano 2021, 15, 15824–15840. [Google Scholar] [CrossRef]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Carmo, P.H.F.d.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.d.; Junqueira, J.C. Metal nanoparticles to combat Candida albicans infections: An update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An overview of stimuli-responsive intelligent antibacterial nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef]
- Mwangi, V.N.; Madivoli, E.S.; Kangogo, M.; Wanakai, S.I.; Waudo, W.; Nzilu, D.M. Antimicrobial surface coating as a pathway to curb resistance: Preparation, mode of action and future perspective. J. Coat. Technol. Res. 2024, 21, 799–810. [Google Scholar] [CrossRef]
- Gerits, E.; Defraine, V.; Vandamme, K.; De Cremer, K.; De Brucker, K.; Thevissen, K.; Cammue, B.P.; Beullens, S.; Fauvart, M.; Verstraeten, N. Repurposing toremifene for treatment of oral bacterial infections. Antimicrob. Agents Chemother. 2017, 61, e01846-16. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Blommaert, E.; Lippell, A.; O’Neill, A.J.; Weytjens, B.; De Maeyer, D.; Fierro, A.C.; Marchal, K.; Marchand, A.; Chaltin, P. Elucidation of the mode of action of a new antibacterial compound active against Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE 2016, 11, e0155139. [Google Scholar] [CrossRef] [PubMed]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm peptides and peptidomimetics with focus on surface immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial peptides therapy: An emerging alternative for treating drug-resistant bacteria. Yale J. Biol. Med. 2022, 95, 445. [Google Scholar]
- Baharin, N.H.Z.; Mokhtar, N.F.K.; Desa, M.N.M.; Gopalsamy, B.; Zaki, N.N.M.; Yuswan, M.H.; Muthanna, A.; Dzaraly, N.D.; Abbasiliasi, S.; Hashim, A.M. The characteristics and roles of antimicrobial peptides as potential treatment for antibiotic-resistant pathogens: A review. PeerJ 2021, 9, e12193. [Google Scholar] [CrossRef]
- Weledji, E.P.; Weledji, E.K.; Assob, J.C.; Nsagha, D.S. Pros, cons and future of antibiotics. New Horiz. Transl. Med. 2017, 4, 9–14. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Nicolas, M.; Beito, B.; Oliveira, M.; Tudela Martins, M.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for antimicrobial peptides immobilization on surfaces to prevent biofilm growth on biomedical devices. Antibiotics 2021, 11, 13. [Google Scholar] [CrossRef]
- Swidergall, M.; Ernst, J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell 2014, 13, 950–957. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.A.; Coombs, M.R.P. Examining the natural role of amphibian antimicrobial peptide magainin. Molecules 2020, 25, 5436. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Willcox, M.; Hume, E.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D. Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar]
- Etienne, O.; Gasnier, C.; Taddei, C.; Voegel, J.-C.; Aunis, D.; Schaaf, P.; Metz-Boutigue, M.-H.; Bolcato-Bellemin, A.-L.; Egles, C. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials 2005, 26, 6704–6712. [Google Scholar] [CrossRef]
- Patil, R.; Ajagunde, J.; Khan, S.; Kannuri, S.; Gandham, N.; Mukhida, S. Rhino-orbital cerebral mycosis: A case series of non-mucorales in COVID patients. Access Microbiol. 2023, 5, 000575.v4. [Google Scholar] [CrossRef]
- Lu, Y.; Mo, Y.; Weng, Y.; Li, X. Fungal keratitis caused by Neurospora: A case report. Front. Med. 2024, 11, 1496010. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Ristić, T.; Zemljič, L.F.; Novak, M.; Kunčič, M.K.; Sonjak, S.; Cimerman, N.G.; Strnad, S. Antimicrobial efficiency of functionalized cellulose fibres as potential medical textiles. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 6, 36–51. [Google Scholar]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Stigter, M.; Bezemer, J.; De Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control Release 2004, 99, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Raman, N.; Welsh, M.A.; Zayas-Gonzalez, Y.M.; Blackwell, H.E.; Palecek, S.P.; Lynn, D.M. Slippery liquid-infused porous surfaces that prevent microbial surface fouling and kill non-adherent pathogens in surrounding media: A controlled release approach. Adv. Funct. Mater. 2016, 26, 3599–3611. [Google Scholar] [CrossRef]
- Huo, D.; Wang, F.; Yang, F.; Lin, T.; Zhong, Q.; Deng, S.-P.; Zhang, J.; Tan, S.; Huang, L. Medical titanium surface-modified coatings with antibacterial and anti-adhesive properties for the prevention of implant-associated infections. J. Mater. Sci. Technol. 2024, 179, 208–223. [Google Scholar] [CrossRef]
- Chang, Y.; Yandi, W.; Chen, W.-Y.; Shih, Y.-J.; Yang, C.-C.; Chang, Y.; Ling, Q.-D.; Higuchi, A. Tunable bioadhesive copolymer hydrogels of thermoresponsive poly (N-isopropyl acrylamide) containing zwitterionic polysulfobetaine. Biomacromolecules 2010, 11, 1101–1110. [Google Scholar] [CrossRef]
- Chow, J.Y.; Yang, Y.; Tay, S.B.; Chua, K.L.; Yew, W.S. Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrob. Agents Chemother. 2014, 58, 1802–1805. [Google Scholar] [CrossRef]
- Üstükarcı, H.; Ozyilmaz, G.; Ozyilmaz, A.T. Marine antifouling properties of enzyme modified polyaniline coated stainless steel surface. Enzyme Microb. Technol. 2024, 172, 110340. [Google Scholar] [CrossRef]





| Technique | Species Tested | References |
|---|---|---|
| Physical | ||
| Laser treatment | S. cerevisiae | [62] |
| S. aureus | [63] | |
| E. coli | [63] | |
| Dip pen nanolithography | S. mutans | [64] |
| Plasma treatment | P. aeruginosa | [57] |
| S. aureus | [57] | |
| E. coli | [57] | |
| S. epidermidis | [57] | |
| Reactive ion etching | B. subtilis | [65] |
| S. aureus | [65] | |
| Electron beam | S. aureus | [66] |
| S. epidermidis | [66] | |
| P. aeruginosa | [66] | |
| Electron beam melting | C. albicans | [67] |
| P. aeruginosa | [67] | |
| S. aureus | [67] | |
| Physical vapor deposition | P. fluorescens | [68] |
| Chemical | ||
| Polyethylene glycol | S. epidermidis | [69] |
| S. aureus | [69] | |
| S. salivarius | [69] | |
| E. coli | [69] | |
| P. aeruginosa | [69] | |
| C. albicans | [69] | |
| C. tropicalis | [69] | |
| Zwitterions | C. albicans | [70] |
| P. aeruginosa | [71] | |
| Add polyethylene oxide to polyurethane | C. albicans | [37] |
| Add acrylic polymer to zirconia | S. sanguinis | [72] |
| P. gingivalis | [72] | |
| F. nucleatum | [72] | |
| C. albicans | [73] | |
| Fluorosiloxane coating | S. aureus | [58] |
| Add organic compounds to polyurethane | S. epidermidis | [74] |
| Graft polyNaSS onto the surface of titanium | S. aureus | [75] |
| Tether QACs to polysiloxane to coat aluminum | C. lytica | [76] |
| N. incerta | [76] | |
| Paint QACs on titanium or stainless steel | S. aureus | [77] |
| DMPEI on PVC | E. coli | [78] |
| S. aureus | [78] | |
| C. albicans | [78] | |
| Embed silver nanoparticles in titanium | S. aureus | [79] |
| Calcium phosphate coatings | P. gingivalis | [80] |
| Diffusion of Toremifenefrom sol-filled pores in Ti | C. albicans | [81] |
| Attach to roughened titanium via a silane anchor | S. aureus | [82] |
| P. aeruginosa | [82] | |
| Attach gentamicin to hydroxyapatite coating | S. aureus | [83] |
| Biological | ||
| Attach magainin to SAM of MUA on gold | L. ivanovii | [84] |
| S. aureus | [84] | |
| E. faecalis | [84] | |
| Attach LL-37 to titanium via silanized PEG | E. coli | [85] |
| Attach hLf1-11 to surface via silane/copolymer brush | S. sanguinis | [86] |
| L. sailvarius | [86] | |
| Attach melamine to surface via silane | S. aureus | [87] |
| P. aeruginosa | [87] | |
| Embed cateslytin between hyaluronic acid and chitosan | C. albicans | [88] |
| S. aureus | [88] | |
| Advantages | Refs | Disadvantages | Refs |
|---|---|---|---|
| Surface roughening and altered wettability can be effective against a broad spectrum of microbes | [57,63,66] | In some experiments, the surface roughening effect is species-specific | [67] |
| Molds can be made of modified surfaces to cast surface patterns in cheaper plastics | [64] | Requires expensive equipment, though cheaper laser techniques can be effective | [91] |
| Nanopillars and other structures are microbiocidal | [95] | ||
| Nanopillars appear to be biocompatible | [90] | ||
| No need for toxic chemicals | [61] |
| Advantages | Refs | Disadvantages | Refs |
|---|---|---|---|
| PEG is highly effective and safe to use in vivo | [61] | Ether link can be oxidized in vivo, and attaching other molecules can trigger an immune response | [61] |
| Easy to manipulate characteristics of hydrogel via altering chemical structure and easy to attach | [61] | Some hydrogels are toxic. First-generation chitosan hydrogels are toxic, but later generations are not | [61] |
| Zwitterions become highly hydrated, which is effective at reducing microbial adherence. They have good biocompatibility, low toxicity, cause almost no immune reaction, are stable and persist in the body for a long time | [111] | Some zwitterionic polymers are not very biocompatible | [61] |
| QACs are stable, have low toxicity and are effective at low concentrations. They kill microbes on contact, and some also reduce microbial adhesion | [61] | QACs persist in the environment and can trigger allergic reactions, respiratory problems, reproductive issues and endocrine dysfunction | [112] |
| Superhydrophobic surfaces are self-cleaning since liquid rolls off the surface | [61] | Some production methods require expensive equipment, cause pollution or require high amounts of energy, are toxic or lead to poor performance (sol–gel) | [113] |
| Grafting polyNaSS onto polyaryletherketone reduces microbial adhesion and enhances hydrophilicity, protein adsorption, bone repair and biocompatibility | [114] | ||
| A hydrogel containing PAMAM and ketoconazole (KET) had a greater effect on C. albicans viability than one containing only KET, possibly because PAMAM enhanced the solubility of KET | [115] | Later-generation cationic dendrimers take a long time to synthesize, are cytotoxic and are quickly cleared from the body | [116] |
| Dendrimer degradation by proteases is low, resulting in high bioavailability. Effective at low concentrations. Peptide dendrimers are more effective and exhibit higher biodegradability | [117] | Dendrimers are cytotoxic and trigger an immune response when the density of dendrimers is high. Synthesis and purification are difficult, with a high percentage of impurities | [117] |
| Advantages | Refs | Disadvantages | Refs |
|---|---|---|---|
| AMPs are broad-spectrum, effective at low dosages, combat microbes that are resistant to antibiotics, do not trigger an immune response, and exhibit low toxicity | [135] | AMP degradation by proteases in vivo leads to low bioavailability. Synthetic AMPs are more resistant to degradation but are expensive to produce | [136] |
| Antibiotics are critical for treating infection during the early stages to prevent sepsis and for prophylaxis in high-risk patients | [137] | Some antibiotics have serious side-effects, including allergic reactions, toxicity and interaction with other drugs, damage to the patient’s microbiome and increasing microbial antibiotic resistance | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, D.; Váchová, L.; Palková, Z. Hostile Environments: Modifying Surfaces to Block Microbial Adhesion and Biofilm Formation. Biomolecules 2025, 15, 754. https://doi.org/10.3390/biom15060754
Wilkinson D, Váchová L, Palková Z. Hostile Environments: Modifying Surfaces to Block Microbial Adhesion and Biofilm Formation. Biomolecules. 2025; 15(6):754. https://doi.org/10.3390/biom15060754
Chicago/Turabian StyleWilkinson, Derek, Libuše Váchová, and Zdena Palková. 2025. "Hostile Environments: Modifying Surfaces to Block Microbial Adhesion and Biofilm Formation" Biomolecules 15, no. 6: 754. https://doi.org/10.3390/biom15060754
APA StyleWilkinson, D., Váchová, L., & Palková, Z. (2025). Hostile Environments: Modifying Surfaces to Block Microbial Adhesion and Biofilm Formation. Biomolecules, 15(6), 754. https://doi.org/10.3390/biom15060754






