A Convenient Fluorogenic Detection Strategy for Phosphorothioate Modification of DNA Through Photocatalytic Oligonucleotide-Templated Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Modified Method of PT-Modified DNA Sample (Figure S3a)
2.3. Synthesis of Fluorogenic Nucleic Acid Probes (Figure S3b)
2.4. The Method of Fluorescence Detection
3. Results and Discussion
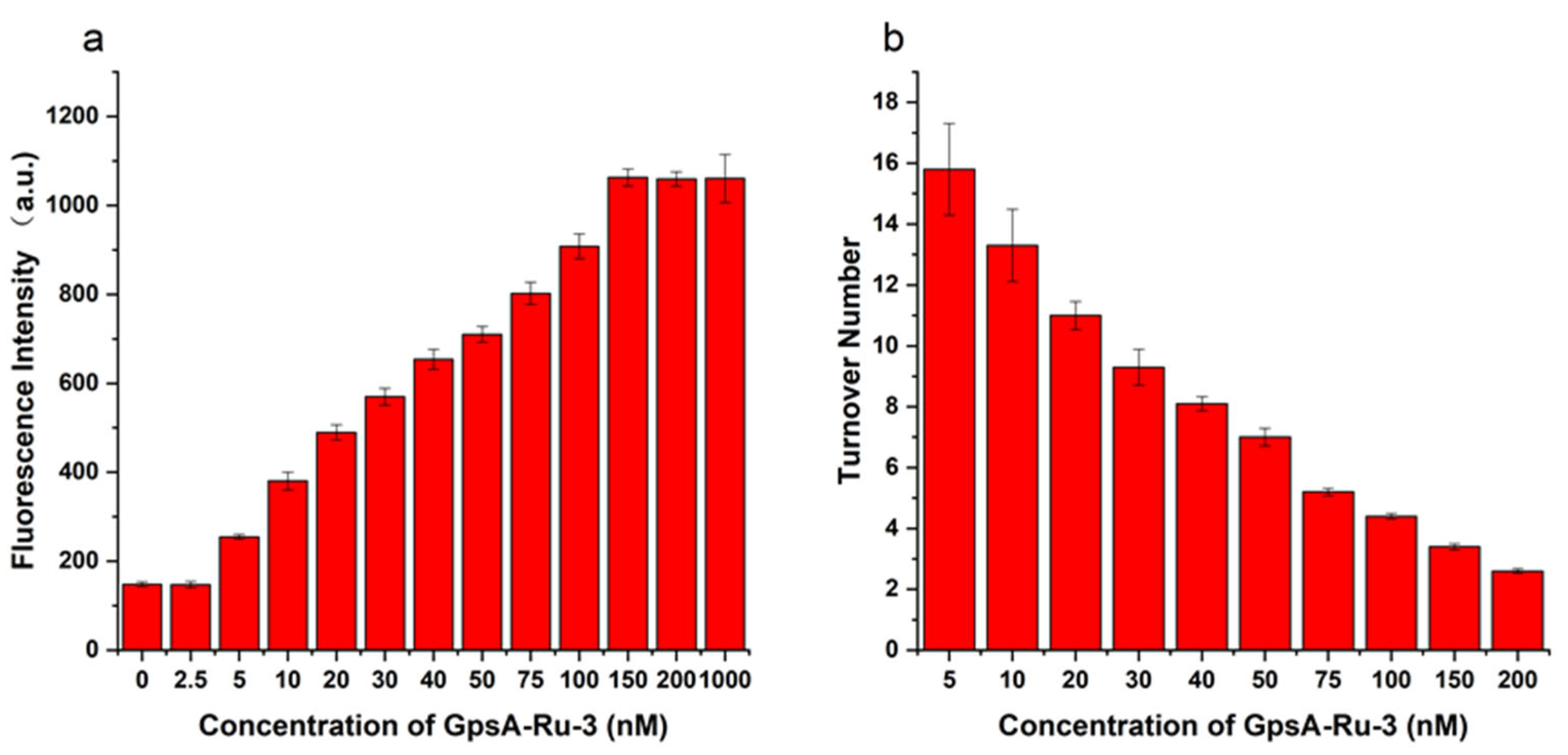
3.1. Rational Design of Fluorogenic Nucleic Acid Probes in PT Modification Quantification
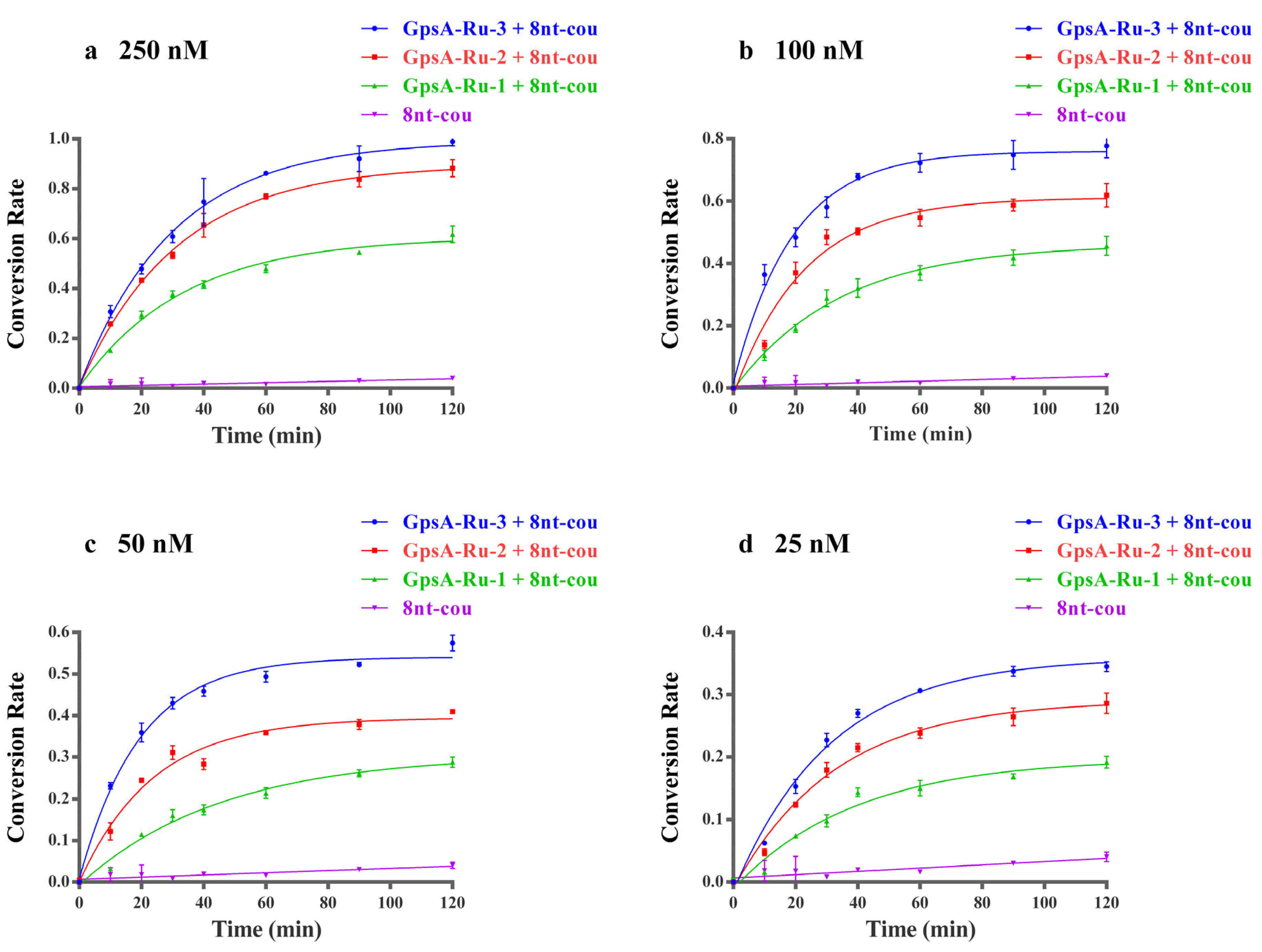
3.2. Optimization of Relative Distance Between PT-Modified Position and 3′ End of Probe Sequence
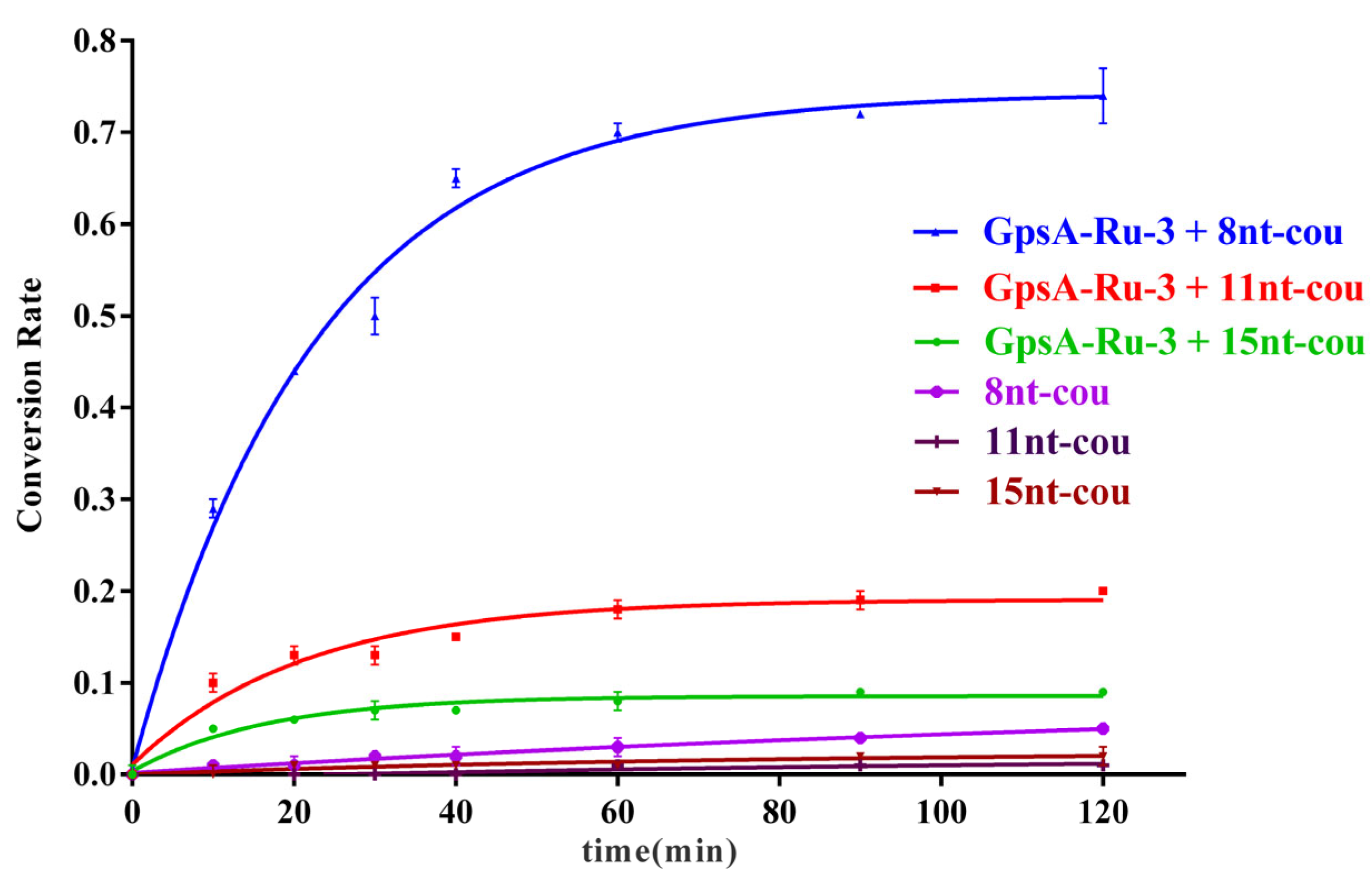
3.3. Effect of Probe Length on PT Detection
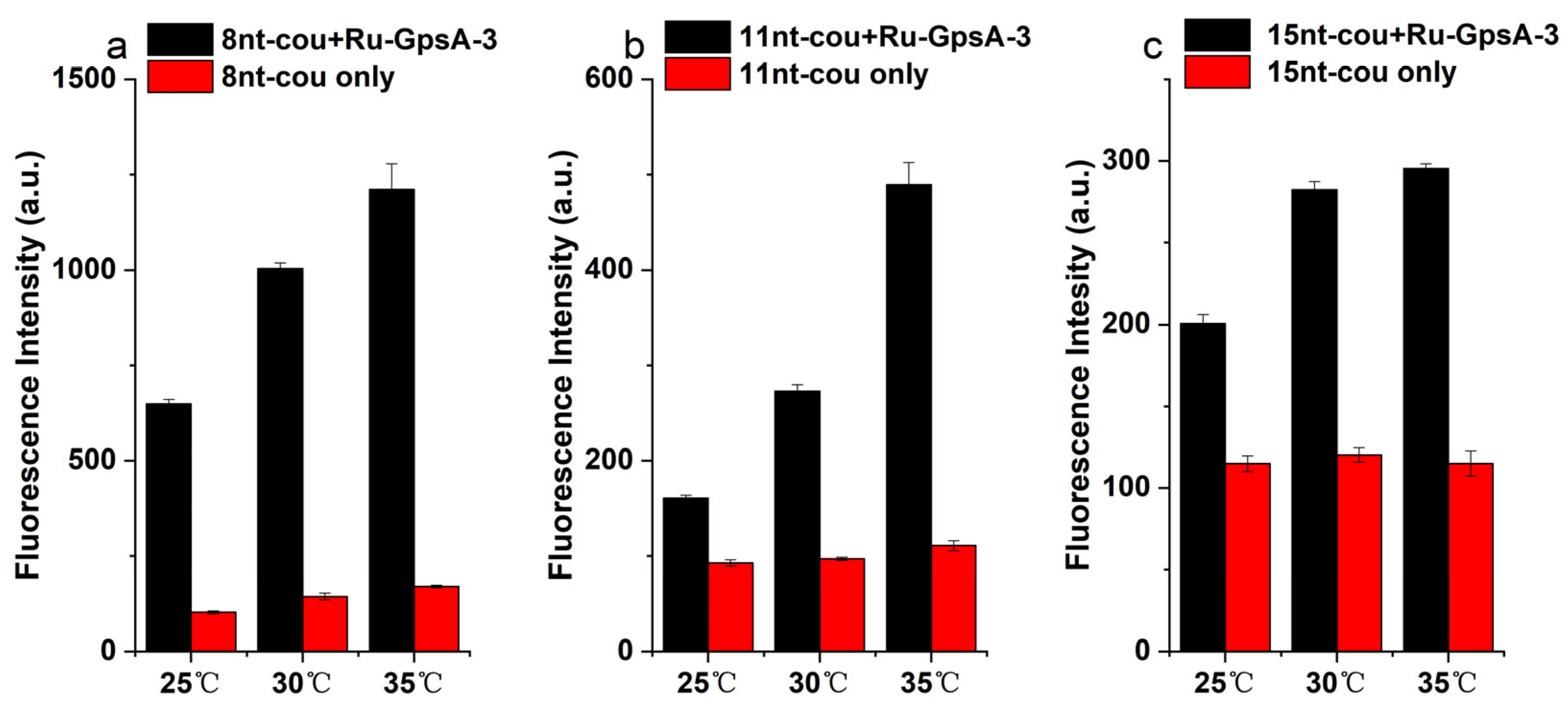
3.4. Effect of Temperature on Detection Performance
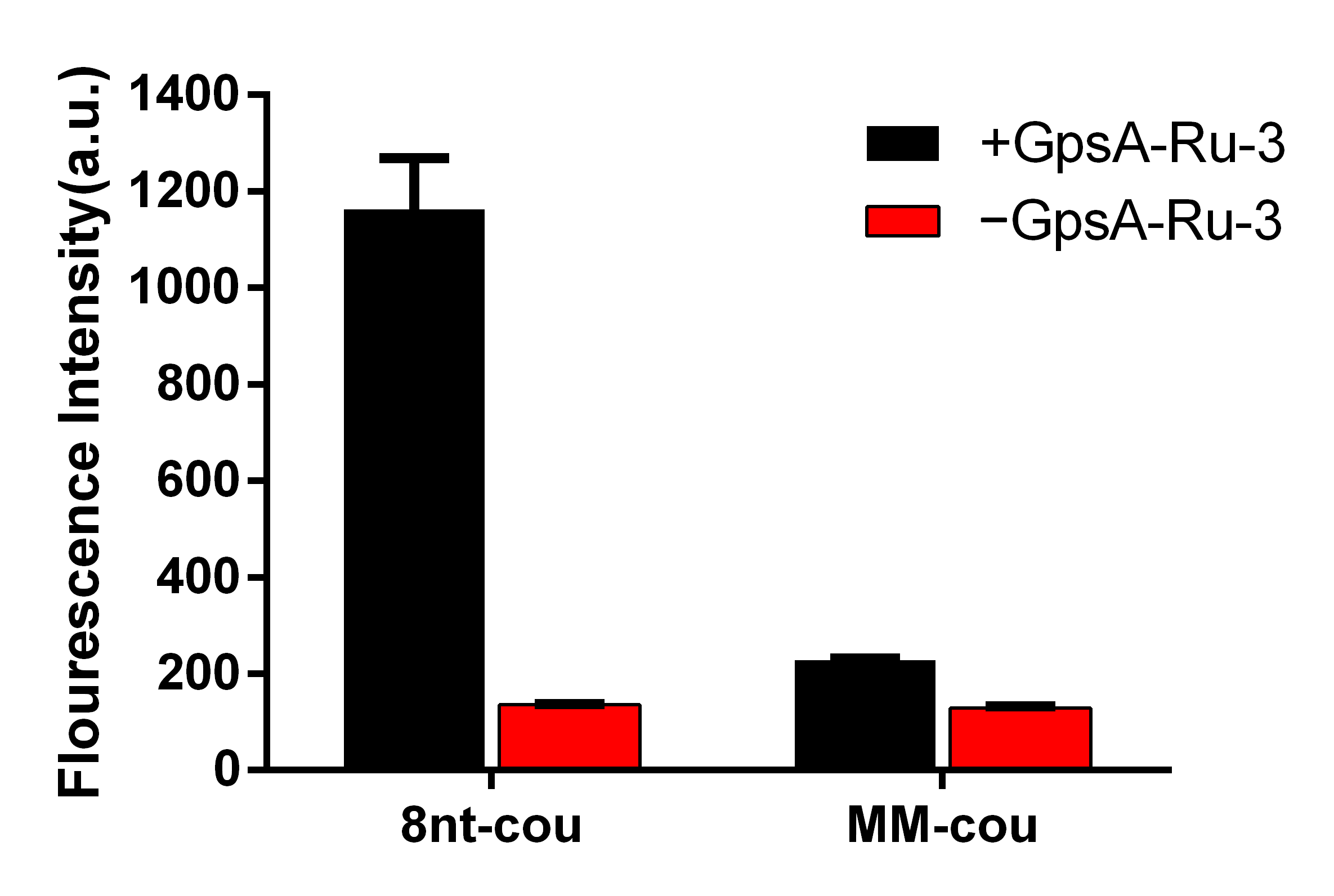
3.5. Sequence Selectivity of PT Detection Using 8nt-cou
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Travers, A.; Muskhelishvili, G. DNA structure and function. FEBS J. 2015, 282, 2279–2295. [Google Scholar] [CrossRef]
- Eckstein, F.; Gish, G. Phosphorothioates in molecular biology. Trends Biochem. Sci. 1989, 14, 97–100. [Google Scholar] [CrossRef]
- Eckstein, F. Nucleoside phosphorothioates. Annu. Rev. Biochem. 1985, 54, 367–402. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Steitz, T.A. Structural principles for the inhibition of the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I by phosphorothioates. J. Mol. Biol. 1998, 277, 363–377. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.-H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioates, Essential Components of Therapeutic Oligonucleotides. Nucleic Acid Ther. 2014, 24, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Deng, Z.; Firmin, J.L.; Hopwood, D.A.; Kieser, T. Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffers contaminated with ferrous iron. Nucleic Acids Res. 1988, 16, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, X.; Liang, J.; Li, A.; Xu, T.; Kieser, T.; Helmann, J.D.; Deng, Z. A novel DNA modification by sulphur. Mol. Microbiol. 2005, 57, 1428–1438. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Vergin, K.L.; Giovannoni, S.J.; Chan, S.W.; DeMott, M.S.; Taghizadeh, K.; Cordero, O.X.; Cutler, M.; Timberlake, S.; et al. DNA phosphorothioation is widespread and quantized in bacterial genomes. Proc. Natl. Acad. Sci. USA 2011, 108, 2963–2968. [Google Scholar] [CrossRef]
- LaPlanche, L.A.; James, T.L.; Powell, C.; Wilson, W.D.; Uznanski, B.; Stec, W.J.; Summers, M.F.; Zon, G. Phosphorothioate-modified oligodeoxyribonucleotides. III. NMR and UV spectroscopic studies of the Rp-Rp, Sp-Sp, and Rp-Sp duplexes, [d(GGSAATTCC)]2, derived from diastereomeric O-ethyl phosphorothioates. Nucleic Acids Res. 1986, 14, 9081–9093. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, S.; Deng, Z.; Dedon, P.C.; Chen, S. DNA phosphorothioate modification—A new multi-functional epigenetic system in bacteria. FEMS Microbiol. Rev. 2018, 43, 109–122. [Google Scholar] [CrossRef]
- Tong, T.; Chen, S.; Wang, L.; Tang, Y.; Ryu, J.Y.; Jiang, S.; Wu, X.; Chen, C.; Luo, J.; Deng, Z.; et al. Occurrence, evolution, and functions of DNA phosphorothioate epigenetics in bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E2988–E2996. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Deng, Z. Twenty years hunting for sulfur in DNA. Protein Cell 2010, 1, 14–21. [Google Scholar] [CrossRef][Green Version]
- Ou, H.-Y.; He, X.; Shao, Y.; Tai, C.; Rajakumar, K.; Deng, Z. dndDB: A Database Focused on Phosphorothioation of the DNA Backbone. PLoS ONE 2009, 4, e5132. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zheng, X.; Cheng, Q.; Yao, F.; Zheng, T.; Ramesh Babu, I.; Zhou, H.; Dedon, P.; You, D. In vitro analysis of phosphorothioate modification of DNA reveals substrate recognition by a multiprotein complex. Sci. Rep. 2015, 5, 12513. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, G.; Liang, J.; He, Y.; Xiong, L.; Li, H.; Bartlett, D.; Deng, Z.; Wang, Z.; Xiao, X. DNA Backbone Sulfur-Modification Expands Microbial Growth Range under Multiple Stresses by its anti-oxidation function. Sci. Rep. 2017, 7, 3516. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liang, J.; Pu, T.; Xu, F.; Yao, F.; Yang, Y.; Zhao, Y.L.; You, D.; Zhou, X.; Deng, Z.; et al. Phosphorothioate DNA as an antioxidant in bacteria. Nucleic Acids Res. 2012, 40, 9115–9124. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Deng, Z.; Chen, S. DNA Phosphorothioate Modification Systems and Associated Phage Defense Systems. Annu. Rev. Microbiol. 2024, 78, 447–462. [Google Scholar] [CrossRef]
- Gan, R.; Wu, X.; He, W.; Liu, Z.; Wu, S.; Chen, C.; Chen, S.; Xiang, Q.; Deng, Z.; Liang, D.; et al. DNA phosphorothioate modifications influence the global transcriptional response and protect DNA from double-stranded breaks. Sci. Rep. 2014, 4, 6642. [Google Scholar] [CrossRef]
- Liu, G.; Fu, W.; Zhang, Z.; He, Y.; Yu, H.; Wang, Y.; Wang, X.; Zhao, Y.-L.; Deng, Z.; Wu, G.; et al. Structural basis for the recognition of sulfur in phosphorothioated DNA. Nat. Commun. 2018, 9, 4689. [Google Scholar] [CrossRef]
- Xiao, L.; Xiang, Y. Quantification of total phosphorothioate in bacterial DNA by a bromoimane-based fluorescent method. Biotechnol. J. 2016, 11, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Gish, G.; Eckstein, F. DNA and RNA Sequence Determination Based on Phosphorothioate Chemistry. Science 1988, 240, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Nakamaye, K.L.; Gish, G.; Eckstein, F.; Vosberg, H.-P. Direct sequencing of polymerase chain reaction amplified DNA fragments through the incorporation of deoxynucleoside α-thiotriphosphates. Nucleic Acids Res. 1988, 16, 9947–9959. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zheng, T.; Kong, L.; Zhu, S.; Sun, Y.; Deng, Z.; Yang, L.; You, D.; Song, C. Quantitative mapping of DNA phosphorothioatome reveals phosphorothioate heterogeneity of low modification frequency. PLoS Genet. 2019, 15, e1008026. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, T.; Li, J.; Cui, J.; Deng, Z.; You, D.; Yang, L. Novel Iodine-induced Cleavage Real-time PCR Assay for Accurate Quantification of Phosphorothioate Modified Sites in Bacterial DNA. Sci. Rep. 2019, 9, 7485. [Google Scholar] [CrossRef]
- Marcello, A.; Nicolas, W. Turn On of a Ruthenium(II) Photocatalyst by DNA-Templated Ligation. Chemistry 2018, 25, 334–342. [Google Scholar]

| Name | Sequence (5′–3′) |
|---|---|
| GpsA-0 | 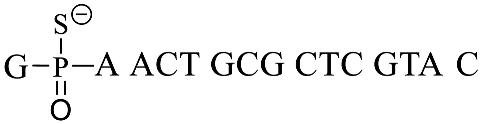 |
| GpsA-template | GAG AGA ACT GCG CTC GTA C |
| GpsA-Ru-1 |  |
| GpsA-Ru-2 | 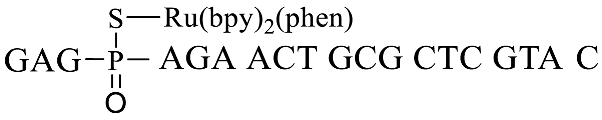 |
| GpsA-Ru-3 | 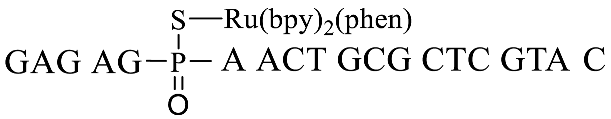 |
| 8nt-cou | GCG CAG TT-(7-AzC) |
| 11nt-cou | CGA GCG CAG TT-(7-AzC) |
| 15nt-cou | CGA GCG CAG TT-(7-AzC) |
| MM-cou | GCG CTG TT-(7-AzC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, N.; Qin, Y.; Fan, X.; Wang, Q.; Wang, J.; You, F.; Tang, X. A Convenient Fluorogenic Detection Strategy for Phosphorothioate Modification of DNA Through Photocatalytic Oligonucleotide-Templated Reaction. Biomolecules 2025, 15, 752. https://doi.org/10.3390/biom15060752
Jing N, Qin Y, Fan X, Wang Q, Wang J, You F, Tang X. A Convenient Fluorogenic Detection Strategy for Phosphorothioate Modification of DNA Through Photocatalytic Oligonucleotide-Templated Reaction. Biomolecules. 2025; 15(6):752. https://doi.org/10.3390/biom15060752
Chicago/Turabian StyleJing, Nannan, Yantian Qin, Xinli Fan, Qian Wang, Jing Wang, Fuping You, and Xinjing Tang. 2025. "A Convenient Fluorogenic Detection Strategy for Phosphorothioate Modification of DNA Through Photocatalytic Oligonucleotide-Templated Reaction" Biomolecules 15, no. 6: 752. https://doi.org/10.3390/biom15060752
APA StyleJing, N., Qin, Y., Fan, X., Wang, Q., Wang, J., You, F., & Tang, X. (2025). A Convenient Fluorogenic Detection Strategy for Phosphorothioate Modification of DNA Through Photocatalytic Oligonucleotide-Templated Reaction. Biomolecules, 15(6), 752. https://doi.org/10.3390/biom15060752







