Interplay Between Vascular Dysfunction and Neurodegenerative Pathology: New Insights into Molecular Mechanisms and Management
Abstract
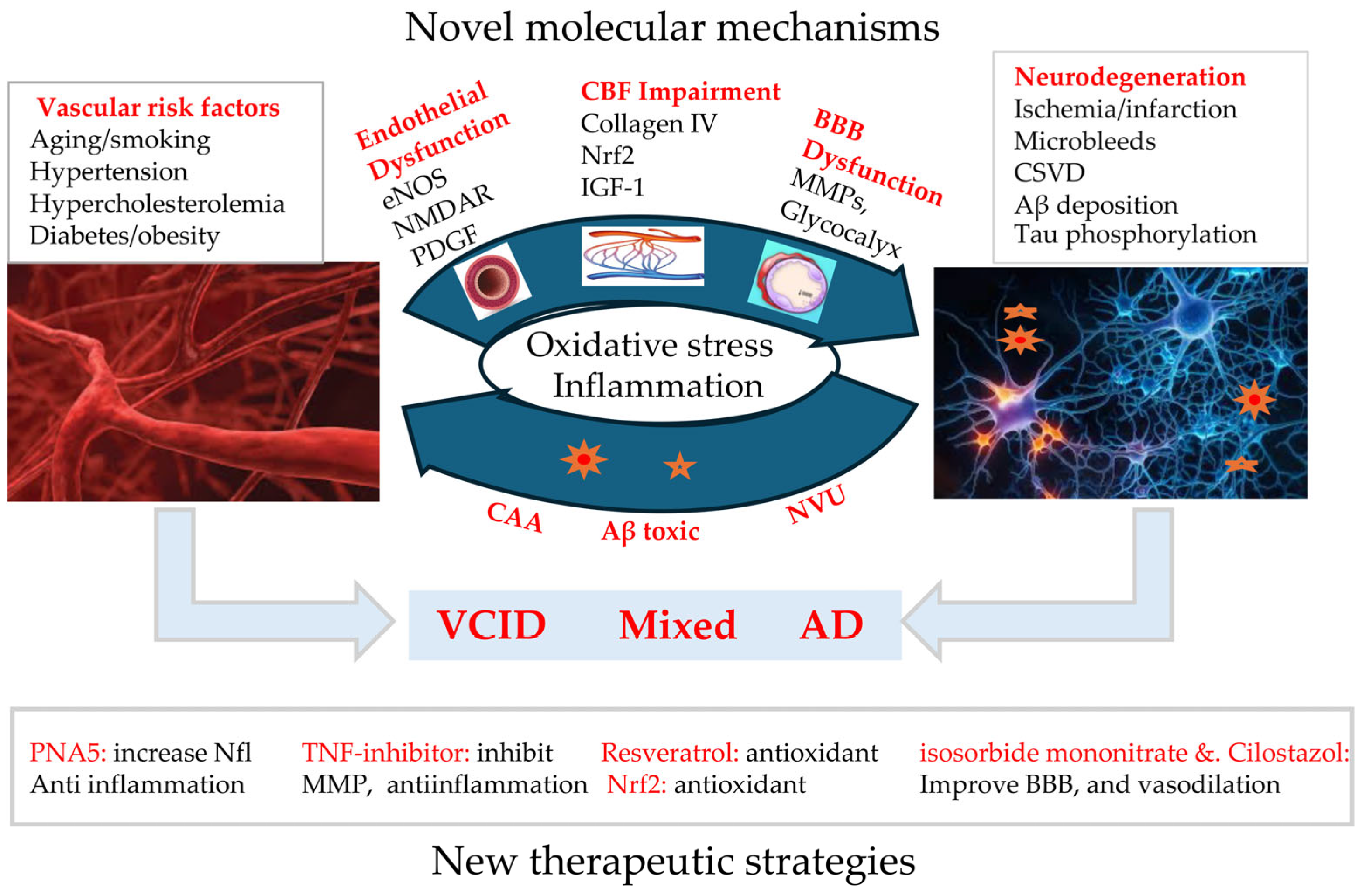
1. Introduction
2. Vascular Aging and Its Impact on Cognitive Decline and Dementia
2.1. Aging-Induced Systemic and Cerebral Vascular Dysfunction
2.2. The Role of Aging-Related Vascular Dysfunction in AD
2.3. Vascular Contributions to Cognitive Impairment and Dementia (VCID)
2.4. Interplay Between Vascular Pathology and Neurodegeneration
3. Recent Progression in Molecular Mechanisms and Potential Targets
3.1. Mechanisms Related to BBB Disruption
3.2. Mechanisms Related to Endothelial Dysfunction
3.3. Mechanism Related to CBF
4. Implications in Management
4.1. Diagnostic Techniques
4.2. Biomarkers
4.3. Therapy
4.4. Prevention
5. Limitations and Future Directions
5.1. Therapeutic Targets
5.2. Biomarkers for Vascular Dysfunction
5.3. Interplay Mechanisms
5.4. Translational Research
6. Conclusions
| Mechanism | Study Model | Main Findings | References |
|---|---|---|---|
| Chronic cerebral hypoperfusion | Rodents and humans | - Rotterdam Study, published in 2005, was one of the first studies to establish a connection between cerebral hypoperfusion and cognitive impairment with risk of dementia by measuring cerebral blood flow velocity and cognitive decline - White matter hyperintensities are a direct manifestation of chronic cerebral hypoperfusion and are negatively correlated with local perfusion and cerebral blood flow - CCH also plays a role in Aβ deposition in the cerebrovascular area and brain parenchyma - Reduced levels of MAG:PLP1 and increased VEGF (vascular endothelial growth factor) in postmortem brain tissue in VaD and AD | [6,39,63,65,115] |
| White Matter Changes | Human | - White matter lesions are a classic manifestation of VCID and are associated with lower motor function in people with average and low activity - Increased total and periventricular white matter hyperintensity burden associated with decreased gait performance over time - White matter hyperintensity volume is also a SVD biomarker | [27,63,76,116] |
| Blood–brain barrier disruptions | - Blood–brain barrier disturbances tend to be found early in chronic cerebral hypoperfusion, contributing to the deterioration of white matter and the development of cognitive impairment - BBB permeability can increase due to the possible excess production of matrix metalloproteinases due to chronic hypoxia and inflammation - Matrix metalloproteinases have a major role in the breakdown of the extracellular matrix and tight junctions of the blood–brain barrier | [6,63,65,77] | |
| Inflammation | Rodent | - Hyperhomocysteinemia diet induced vascular inflammation, microhemorrhages, and cognitive decline - Elevated serum cholesterol in atherosclerosis can advance the onset of inflammation - Conditions such as atrial fibrillation and sleep apnea can increase systemic inflammation | [65,106,117,118] |
| Increased Oxidative stress | - Reactive oxygen species inhibit anti-inflammatory nitric oxide, which is essential for vasculature protection - Improper functioning of NRF2 causes endothelial cells to lose protection against physiologic ROS production - Reactive oxygen species inhibit anti-inflammatory nitric oxide, which is essential for vasculature protection as it plays a role in the inhibition of platelet aggregation, inhibition of endothelial apoptosis, and the preservation of endothelial progenitor cells | [6,61] | |
| Endothelial dysfunction | - Endothelial dysfunction during chronic cerebral hypoperfusion may cause increased serum antibodies against the N-methyl-d-aspartate (NMDA) receptor - Inhibition of eNOS in cultured human brain microvascular endothelial cells caused significant upregulation of amyloid precursor proteins and beta-site amyloid precursor protein cleaving enzyme 1 - Decreased pericyte concentration is correlated with disordered neurovascular uncoupling, along with hypoxia, through reduced oxygen supply to the brain, further resulting in the loss of structural stability - Impaired hemodynamic response in neurodegenerative diseases like AD can also be caused by defective endothelial NMDAR signaling | [27,63,71] | |
| Cerebral small vessel disease (CSVD) | Human | - Affects arterioles, capillaries, and venules with major pathologies, such as arteriosclerosis and cerebral amyloid angiopathy - Features of small vessel disease include white matter hyperintensities, recent small subcortical infarcts, lacunes of presumed vascular origin, enlarged perivascular space, microbleeds, and brain atrophy | [35] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| Aβ | Beta-amyloid |
| ACEis | Angiotensin-converting enzyme inhibitors |
| ACAS | Asymmetrical carotid artery stenosis |
| AD | Alzheimer’s disease |
| Anti-BACE1 | Beta-Site APP Cleaving Enzyme 1 |
| APP | Amyloid precursor protein |
| ARBs | Angiotensin receptor blockers |
| ATVs | Antibody transport vehicles |
| BCAL | Bilateral carotid artery ligation |
| BCAS | Bilateral common carotid artery stenosis |
| BBB | Blood–brain barrier |
| CAA | Cerebral amyloid angiopathy |
| CBF | Cerebral blood flow |
| CNS | Central nervous system |
| CSVD | Cerebral small vessel disease |
| DIAN-TU | Dominantly inherited Alzheimer Network Trials Unit |
| DTI | Diffusion tensor imaging |
| ECM | Extracellular matrix |
| ENIGMA | Enhancing Neuro-Imaging Genetics through Meta-Analysis |
| eNOS | Endothelial nitric oxide synthase |
| Gal-3 | Galectin-3 |
| HIF-1α | Hypoxia-inducible factor 1-α |
| IGF-1 | Insulin-like growth factor 1 |
| MMP | Matrix metalloproteinases |
| NfL | Neurofilament light chain |
| NMDAR | N-methyl-D-aspartate receptor |
| NO | Nitric oxide |
| NOC-18 | NO donor 2,2′-(hydroxynitrosohydrazino)bis-ethanamine |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NVU | Neurovascular unit |
| PDGF | Platelet-derived growth factor |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| SAH | Subarachnoid hemorrhage |
| SHRSP | Spontaneously hypertensive stroke-prone |
| SMARRT | Systemic Multi-Domain Alzheimer’s Risk Reduction Trial |
| SPRINT-MIND | Systolic Blood Pressure Intervention Memory Trial and Cognition in Decreased Hypertension |
| SVD | Small vessel disease |
| TIA | Transient ischemic attacks |
| VaD | Vascular dementia |
| VCID | Vascular contributions to cognitive impairment and dementia |
| VICCCS | Vascular Impairment of Cognition Classification Consensus Study |
| VSMC | Vascular smooth muscle cells |
References
- Sabbatinelli, J.; Ramini, D.; Giuliani, A.; Recchioni, R.; Spazzafumo, L.; Olivieri, F. Connecting vascular aging and frailty in Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111444. [Google Scholar] [CrossRef] [PubMed]
- Rius-Perez, S.; Tormos, A.M.; Perez, S.; Talens-Visconti, R. Vascular pathology: Cause or effect in Alzheimer disease? Neurologia 2018, 33, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Capuano, A.W.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol. 2016, 15, 934–943. [Google Scholar] [CrossRef]
- Eisenmenger, L.B.; Peret, A.; Famakin, B.M.; Spahic, A.; Roberts, G.S.; Bockholt, J.H.; Johnson, K.M.; Paulsen, J.S. Vascular contributions to Alzheimer’s disease. Transl. Res. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Lu, Z.; Leak, R.K.; Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2017, 34, 15–29. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Nagai, A. The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 2169. [Google Scholar] [CrossRef]
- Khan, F.; Qiu, H. Amyloid-beta: A potential mediator of aging-related vascular pathologies. Vasc. Pharmacol. 2023, 152, 107213. [Google Scholar] [CrossRef]
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The Implications of Aging on Vascular Health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Depre, C.; Ghosh, K.; Resuello, R.G.; Natividad, F.F.; Rossi, F.; Peppas, A.; Shen, Y.T.; Vatner, D.E.; Vatner, S.F. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation 2007, 116, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Tian, B.; Resuello, R.G.; Natividad, F.F.; Peppas, A.; Shen, Y.T.; Vatner, D.E.; Vatner, S.F.; Depre, C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol. Genom. 2007, 29, 169–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, B.; Melton, E.; Wiener, R.; Zhou, N.; Wu, W.; Lai, L.; Wang, C.; Costa, K.D.; Qiu, H. Age and Blood Pressure Contribute to Aortic Cell and Tissue Stiffness Through Distinct Mechanisms. Hypertension 2022, 79, 1777–1788. [Google Scholar] [CrossRef]
- Kim, H.L. Arterial stiffness and hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- Onuh, J.O.; Qiu, H. New progress on the study of aortic stiffness in age-related hypertension. J. Hypertens. 2020, 38, 1871–1877. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Bickel, M.A.; Sherry, D.M.; Bullen, E.C.; Vance, M.L.; Jones, K.L.; Howard, E.W.; Conley, S.M. Microvascular smooth muscle cells exhibit divergent phenotypic switching responses to platelet-derived growth factor and insulin-like growth factor 1. Microvasc. Res. 2024, 151, 104609. [Google Scholar] [CrossRef]
- Manning, D.; Rivera, E.J.; Santana, L.F. The life cycle of a capillary: Mechanisms of angiogenesis and rarefaction in microvascular physiology and pathologies. Vasc. Pharmacol. 2024, 156, 107393. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Pinto, J.; Sparks, S.N.; Wang, C.; Suri, S.; Bulte, D.P. Vascular smooth muscle cell dysfunction in neurodegeneration. Front. Neurosci. 2022, 16, 1010164. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.R.; Bickel, M.A.; Tarantini, S.; Runion, M.E.; Matacchiera, Z.; Vance, M.L.; Hibbs, C.; Vaden, H.; Nagykaldi, D.; Martin, T.; et al. IGF1R deficiency in vascular smooth muscle cells impairs myogenic autoregulation and cognition in mice. Front. Aging Neurosci. 2024, 16, 1320808. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, L.; Lin, G.; Wang, Z.; Kodali, M.C.; Li, M.; Chen, H.; Lebovitz, S.G.; Ortyl, T.C.; Li, L.; et al. White matter damage as a consequence of vascular dysfunction in a spontaneous mouse model of chronic mild chronic hypoperfusion with eNOS deficiency. Mol. Psychiatry 2022, 27, 4754–4769. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Gottesman, R.F.; Bernstein, K.E.; Seshadri, S.; McKee, A.; Snyder, H.; Greenberg, S.M.; Yaffe, K.; Schaffer, C.B.; Yuan, C.; et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimer’s Dement. 2020, 16, 1714–1733. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef]
- Hayden, M.R. Brain endothelial cell activation and dysfunction associate with and contribute to the development of enlarged perivascular spaces and cerebral small vessel disease. Histol. Histopathol. 2024, 39, 1565–1586. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef]
- Ullah, R.; Lee, E.J. Advances in Amyloid-β Clearance in the Brain and Periphery: Implications for Neurodegenerative Diseases. Exp. Neurobiol. 2023, 32, 216–246. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Bell, R.D.; Zlokovic, B.V. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a011452. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Hernandez, H.; Cuevas, E.; Raymick, J.B.; Robinson, B.L.; Sarkar, S. Impaired Amyloid Beta Clearance and Brain Microvascular Dysfunction are Present in the Tg-SwDI Mouse Model of Alzheimer’s Disease. Neuroscience 2020, 440, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Inoue, Y.; Shue, F.; Bu, G.; Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 46. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Gilson, A.; Rost, N.S.; Rosand, J.; Viswanathan, A.; Smith, E.E.; Greenberg, S.M. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 2009, 72, 1230–1235. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, X.; Liu, J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int. J. Mol. Sci. 2022, 23, 6224. [Google Scholar] [CrossRef]
- Rajeev, V.; Chai, Y.L.; Poh, L.; Selvaraji, S.; Fann, D.Y.; Jo, D.G.; De Silva, T.M.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V.; et al. Chronic cerebral hypoperfusion: A critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol. Commun. 2023, 11, 93. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Hu, J.; Wu, J.; Huang, Y. A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis. J. Clin. Med. 2022, 11, 4742. [Google Scholar] [CrossRef]
- Gannon, O.J.; Naik, J.S.; Riccio, D.; Mansour, F.M.; Abi-Ghanem, C.; Salinero, A.E.; Kelly, R.D.; Brooks, H.L.; Zuloaga, K.L. Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia. Biol. Sex. Differ. 2023, 14, 34. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 2016, 1862, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Fan, F.; Border, J.J.; Roman, R.J. Cerebrovascular Dysfunction in Alzheimer’s Disease and Transgenic Rodent Models. J. Exp. Neurol. 2024, 5, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Valis, M.; Kuca, K.; Angelucci, F. Vascular Cognitive Impairment: Information from Animal Models on the Pathogenic Mechanisms of Cognitive Deficits. Int. J. Mol. Sci. 2019, 20, 2405. [Google Scholar] [CrossRef] [PubMed]
- Gooch, J.; Wilcock, D.M. Animal Models of Vascular Cognitive Impairment and Dementia (VCID). Cell Mol. Neurobiol. 2016, 36, 233–239. [Google Scholar] [CrossRef]
- Vasilkovska, T.; Salajeghe, S.; Vanreusel, V.; Van Audekerke, J.; Verschuuren, M.; Hirschler, L.; Warnking, J.; Pintelon, I.; Pustina, D.; Cachope, R.; et al. Longitudinal alterations in brain perfusion and vascular reactivity in the zQ175DN mouse model of Huntington’s disease. J. Biomed. Sci. 2024, 31, 37. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef]
- Shirzadi, Z.; Boyle, R.; Yau, W.W.; Coughlan, G.; Fu, J.F.; Properzi, M.J.; Buckley, R.F.; Yang, H.S.; Scanlon, C.E.; Hsieh, S.; et al. Vascular contributions to cognitive decline: Beyond amyloid and tau in the Harvard Aging Brain Study. J. Cereb. Blood Flow Metab. 2024, 44, 1319–1328. [Google Scholar] [CrossRef]
- Vestergaard, S.B.; Damsbo, A.G.; Pedersen, N.L.; Zachariassen, K.; Drasbek, K.R.; Ostergaard, L.; Andersen, G.; Dalby, R.B.; Mortensen, J.K. Exploring vascular contributions to cognitive impairment and dementia (ENIGMA): Protocol for a prospective observational study. BMC Neurol. 2024, 24, 110. [Google Scholar] [CrossRef]
- Madigan, J.B.; Wilcock, D.M.; Hainsworth, A.H. Vascular Contributions to Cognitive Impairment and Dementia: Topical Review of Animal Models. Stroke 2016, 47, 1953–1959. [Google Scholar] [CrossRef]
- Havrylov, S.; Chrystal, P.; van Baarle, S.; French, C.R.; MacDonald, I.M.; Avasarala, J.; Rogers, R.C.; Berry, F.B.; Kume, T.; Waskiewicz, A.J.; et al. Pleiotropy in FOXC1-attributable phenotypes involves altered ciliation and cilia-dependent signaling. Sci. Rep. 2024, 14, 20278. [Google Scholar] [CrossRef]
- Solis, E., Jr.; Hascup, K.N.; Hascup, E.R. Alzheimer’s Disease: The Link Between Amyloid-beta and Neurovascular Dysfunction. J. Alzheimer’s Dis. 2020, 76, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Woods, A.S.; Cotter, R.J.; Gowing, E.; Ball, M.J. Beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 10836–10840. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Olichney, J.M.; Thal, L.J.; Mirra, S.S.; Morris, J.C.; Beekly, D.; Heyman, A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: The CERAD experience, Part XV. Neurology 1996, 46, 1592–1596. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Choi, C.; Pomakian, J.; Vinters, H.V. High-definition characterization of cerebral beta-amyloid angiopathy in Alzheimer’s disease. Hum. Pathol. 2010, 41, 1601–1608. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Lyros, E.; Bakogiannis, C.; Liu, Y.; Fassbender, K. Molecular links between endothelial dysfunction and neurodegeneration in Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 18–26. [Google Scholar] [CrossRef]
- Linh, T.T.D.; Hsieh, Y.C.; Huang, L.K.; Hu, C.J. Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. Int. J. Mol. Sci. 2022, 23, 11067. [Google Scholar] [CrossRef]
- Pellegrini, C.; D’Antongiovanni, V.; Fornai, M.; Duranti, E.; Baldacci, F.; Bernardini, N.; Taddei, S.; Virdis, A.; Blandizzi, C.; Masi, S.; et al. Donepezil improves vascular function in a mouse model of Alzheimer’s disease. Pharmacol. Res. Perspect. 2021, 9, e00871. [Google Scholar] [CrossRef]
- Bar, K.J.; Boettger, M.K.; Seidler, N.; Mentzel, H.J.; Terborg, C.; Sauer, H. Influence of galantamine on vasomotor reactivity in Alzheimer’s disease and vascular dementia due to cerebral microangiopathy. Stroke 2007, 38, 3186–3192. [Google Scholar] [CrossRef]
- MacLean, M.A.; Muradov, J.H.; Greene, R.; Van Hameren, G.; Clarke, D.B.; Dreier, J.P.; Okonkwo, D.O.; Friedman, A. Memantine inhibits cortical spreading depolarization and improves neurovascular function following repetitive traumatic brain injury. Sci. Adv. 2023, 9, eadj2417. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, F.; Ikonomovic, M.; Yang, T. The Role of NRF2 in Cerebrovascular Protection: Implications for Vascular Cognitive Impairment and Dementia (VCID). Int. J. Mol. Sci. 2024, 25, 3833. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, L.; Huang, T.; Zhang, Y.; Zhou, J.; Qi, B.; Wang, X.; Chen, Z.; Li, P. Aging Neurovascular Unit and Potential Role of DNA Damage and Repair in Combating Vascular and Neurodegenerative Disorders. Front. Neurosci. 2019, 13, 778. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bai, Q.; Dong, Q.; Guo, M.; Cui, M. Blood-Brain Barrier Dysfunction and the Potential Mechanisms in Chronic Cerebral Hypoperfusion Induced Cognitive Impairment. Front. Cell Neurosci. 2022, 16, 870674. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Linton, A.E.; Weekman, E.M.; Wilcock, D.M. Pathologic sequelae of vascular cognitive impairment and dementia sheds light on potential targets for intervention. Cereb. Circ. Cogn. Behav. 2021, 2, 100030. [Google Scholar] [CrossRef]
- Feng, D.; Zhou, J.; Liu, H.; Wu, X.; Li, F.; Zhao, J.; Zhang, Y.; Wang, L.; Chao, M.; Wang, Q.; et al. Astrocytic NDRG2-PPM1A interaction exacerbates blood-brain barrier disruption after subarachnoid hemorrhage. Sci. Adv. 2022, 8, eabq2423. [Google Scholar] [CrossRef]
- Shi, S.M.; Suh, R.J.; Shon, D.J.; Garcia, F.J.; Buff, J.K.; Atkins, M.; Li, L.; Lu, N.; Sun, B.; Luo, J.; et al. Glycocalyx dysregulation impairs blood-brain barrier in ageing and disease. Nature 2025, 639, 985–994. [Google Scholar] [CrossRef]
- Brix, B.; Mesters, J.R.; Pellerin, L.; Johren, O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1alpha-mediated target gene activation. J. Neurosci. 2012, 32, 9727–9735. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y. Collagen IV contributes to nitric oxide-induced angiogenesis of lung endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C979–C988. [Google Scholar] [CrossRef]
- Rabkin, S.W. Collagen type IV as the link between arterial stiffness and dementia. Am. J. Transl. Res. 2023, 15, 5961–5971. [Google Scholar]
- Soda, T.; Brunetti, V.; Berra-Romani, R.; Moccia, F. The Emerging Role of N-Methyl-D-Aspartate (NMDA) Receptors in the Cardiovascular System: Physiological Implications, Pathological Consequences, and Therapeutic Perspectives. Int. J. Mol. Sci. 2023, 24, 3914. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, Y.; Wu, Y.; Yi, F. Targeting NMDA receptor signaling for therapeutic intervention in brain disorders. Rev. Neurosci. 2023, 34, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Intson, K.; Geissah, S.; McCullumsmith, R.E.; Ramsey, A.J. A role for endothelial NMDA receptors in the pathophysiology of schizophrenia. Schizophr. Res. 2022, 249, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Ding, R.; Hase, Y.; Ameen-Ali, K.E.; Ndung’u, M.; Stevenson, W.; Barsby, J.; Gourlay, R.; Akinyemi, T.; Akinyemi, R.; Uemura, M.T.; et al. Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer’s disease. Brain Pathol. 2020, 30, 1087–1101. [Google Scholar] [CrossRef]
- Fleischman, D.A.; Yang, J.; Arfanakis, K.; Arvanitakis, Z.; Leurgans, S.E.; Turner, A.D.; Barnes, L.L.; Bennett, D.A.; Buchman, A.S. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology 2015, 84, 1294–1300. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, F. Targeting Transcription Factor Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) for the Intervention of Vascular Cognitive Impairment and Dementia. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 97–116. [Google Scholar] [CrossRef]
- Raghavan, S.; Przybelski, S.A.; Lesnick, T.G.; Fought, A.J.; Reid, R.I.; Gebre, R.K.; Windham, B.G.; Algeciras-Schimnich, A.; Machulda, M.M.; Vassilaki, M.; et al. Vascular risk, gait, behavioral, and plasma indicators of VCID. Alzheimer’s Dement. 2024, 20, 1201–1213. [Google Scholar] [CrossRef]
- Shen, J.; Xu, G.; Zhu, R.; Yuan, J.; Ishii, Y.; Hamashima, T.; Matsushima, T.; Yamamoto, S.; Takatsuru, Y.; Nabekura, J.; et al. PDGFR-β restores blood-brain barrier functions in a mouse model of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2019, 39, 1501–1515. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Perez, J.M.; Evans, A.C.; Alzheimer’s Disease Neuroimaging, I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- Jiang, C.; Li, S.; Wang, Y.; Lai, Y.; Bai, Y.; Zhao, M.; He, L.; Kong, Y.; Guo, X.; Li, S.; et al. Diastolic Blood Pressure and Intensive Blood Pressure Control on Cognitive Outcomes: Insights From the SPRINT MIND Trial. Hypertension 2023, 80, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Palmer, K.; Hoang, T.D.; Yaffe, K. Trials and Treatments for Vascular Brain Health: Risk Factor Modification and Cognitive Outcomes. Stroke 2022, 53, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Alladi, S.; Black, S.E.; Claassen, J.; DeCarli, C.; Hughes, T.M.; Moonen, J.; Pajewski, N.M.; Price, B.R.; Satizabal, C.; et al. Clinical trials in vascular cognitive impairment following SPRINT-MIND: An international perspective. Cell Rep. Med. 2023, 4, 101089. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimer’s Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef]
- Bateman, R.J.; Benzinger, T.L.; Berry, S.; Clifford, D.B.; Duggan, C.; Fagan, A.M.; Fanning, K.; Farlow, M.R.; Hassenstab, J.; McDade, E.M.; et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimer’s Dement. 2017, 13, 8–19. [Google Scholar] [CrossRef]
- Yang, W.; Luo, H.; Ma, Y.; Si, S.; Zhao, H. Effects of Antihypertensive Drugs on Cognitive Function in Elderly Patients with Hypertension: A Review. Aging Dis. 2021, 12, 841–851. [Google Scholar] [CrossRef]
- Yasmin, S.; Ashique, S.; Taj, T.; Garg, A.; Das, J.; Shorog, E.; Bhui, U.; Pal, R.; Selim, S.; Panigrahy, U.P.; et al. The role of ACE inhibitors and ARBs in preserving cognitive function via hypertension Management: A critical Update. Brain Res. 2025, 1850, 149400. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Turner, N.; Howden, B.; Jarutyte, L.; Clegg, S.L.; Malone, I.B.; Barnes, J.; Nielsen, C.; Sudre, C.H.; Wilson, A.; et al. Safety and efficacy of losartan for the reduction of brain atrophy in clinically diagnosed Alzheimer’s disease (the RADAR trial): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2021, 20, 895–906. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Farghaly, H.S.M.; Ahmed, A.M.; El-Mokhtar, M.A.; Hemida, F.K. Advancing combination treatment with cilostazol and caffeine for Alzheimer’s disease in high fat-high fructose-STZ induced model of amnesia. Eur. J. Pharmacol. 2022, 921, 174873. [Google Scholar] [CrossRef]
- Salloway, S.P.; Sevingy, J.; Budur, K.; Pederson, J.T.; DeMattos, R.B.; Von Rosenstiel, P.; Paez, A.; Evans, R.; Weber, C.J.; Hendrix, J.A.; et al. Advancing combination therapy for Alzheimer’s disease. Alzheimer’s Dement. 2020, 6, e12073. [Google Scholar] [CrossRef]
- McConlogue, L.; Buttini, M.; Anderson, J.P.; Brigham, E.F.; Chen, K.S.; Freedman, S.B.; Games, D.; Johnson-Wood, K.; Lee, M.; Zeller, M.; et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J. Biol. Chem. 2007, 282, 26326–26334. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Jiang, H.; Mahan, T.E.; Maloney, S.E.; Wozniak, D.F.; Diamond, M.I.; Holtzman, D.M. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann. Clin. Transl. Neurol. 2015, 2, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Pooler, A.M.; Polydoro, M.; Maury, E.A.; Nicholls, S.B.; Reddy, S.M.; Wegmann, S.; William, C.; Saqran, L.; Cagsal-Getkin, O.; Pitstick, R.; et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 2015, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Williams, B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: More Similar Than Different. JAMA 2018, 320, 1749–1750. [Google Scholar] [CrossRef]
- Cooper, C.G.; Kafetzis, K.N.; Patabendige, A.; Tagalakis, A.D. Blood-brain barrier disruption in dementia: Nano-solutions as new treatment options. Eur. J. Neurosci. 2024, 59, 1359–1385. [Google Scholar] [CrossRef]
- Daraban, B.S.; Popa, A.S.; Stan, M.S. Latest Perspectives on Alzheimer’s Disease Treatment: The Role of Blood-Brain Barrier and Antioxidant-Based Drug Delivery Systems. Molecules 2024, 29, 4056. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Toyama, K.; Spin, J.M.; Mogi, M.; Tsao, P.S. Therapeutic perspective on vascular cognitive impairment. Pharmacol. Res. 2019, 146, 104266. [Google Scholar] [CrossRef]
- Hoyer-Kimura, C.; Konhilas, J.P.; Mansour, H.M.; Polt, R.; Doyle, K.P.; Billheimer, D.; Hay, M. Neurofilament light: A possible prognostic biomarker for treatment of vascular contributions to cognitive impairment and dementia. J. Neuroinflamm. 2021, 18, 236. [Google Scholar] [CrossRef]
- Fagerli, E.; Jackson, C.W.; Escobar, I.; Ferrier, F.J.; Perez Lao, E.J.; Saul, I.; Gomez, J.; Dave, K.R.; Bracko, O.; Perez-Pinzon, M.A. Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion. Antioxidants 2024, 13, 984. [Google Scholar] [CrossRef]
- Lewis, C.R.; Talboom, J.S.; De Both, M.D.; Schmidt, A.M.; Naymik, M.A.; Håberg, A.K.; Rundek, T.; Levin, B.E.; Hoscheidt, S.; Bolla, Y.; et al. Smoking is associated with impaired verbal learning and memory performance in women more than men. Sci. Rep. 2021, 11, 10248. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Egle, M.; Groechel, R.C.; Mughal, A. Blood pressure and the brain: The conundrum of hypertension and dementia. Cardiovasc. Res. 2025, 120, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Buie, J.J.; Watson, L.S.; Smith, C.J.; Sims-Robinson, C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 2019, 132, 104580. [Google Scholar] [CrossRef]
- Lyu, F.; Wu, D.; Wei, C.; Wu, A. Vascular cognitive impairment and dementia in type 2 diabetes mellitus: An overview. Life Sci. 2020, 254, 117771. [Google Scholar] [CrossRef]
- Fan, Y.C.; Hsu, J.L.; Tung, H.Y.; Chou, C.C.; Bai, C.H. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimer’s Res. Ther. 2017, 9, 7. [Google Scholar] [CrossRef]
- Duong, M.T.; Nasrallah, I.M.; Wolk, D.A.; Chang, C.C.Y.; Chang, T.Y. Cholesterol, Atherosclerosis, and APOE in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Potential Mechanisms and Therapy. Front. Aging Neurosci. 2021, 13, 647990. [Google Scholar] [CrossRef]
- Weekman, E.M.; Johnson, S.N.; Rogers, C.B.; Sudduth, T.L.; Xie, K.; Qiao, Q.; Fardo, D.W.; Bottiglieri, T.; Wilcock, D.M. Atorvastatin rescues hyperhomocysteinemia-induced cognitive deficits and neuroinflammatory gene changes. J. Neuroinflamm. 2023, 20, 199. [Google Scholar] [CrossRef]
- Gannon, O.J.; Robison, L.S.; Custozzo, A.J.; Zuloaga, K.L. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019, 127, 38–55. [Google Scholar] [CrossRef]
- Salinero, A.E.; Robison, L.S.; Gannon, O.J.; Riccio, D.; Mansour, F.; Abi-Ghanem, C.; Zuloaga, K.L. Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID. FASEB J. 2020, 34, 15108–15122. [Google Scholar] [CrossRef]
- Tong, X.K.; Trigiani, L.J.; Hamel, E. High cholesterol triggers white matter alterations and cognitive deficits in a mouse model of cerebrovascular disease: Benefits of simvastatin. Cell Death Dis. 2019, 10, 89. [Google Scholar] [CrossRef]
- Soares, L.C.; Al-Dalahmah, O.; Hillis, J.; Young, C.C.; Asbed, I.; Sakaguchi, M.; O’Neill, E.; Szele, F.G. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells 2021, 10, 3047. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Alam, H.; Siddiqui, S.; Shaikh, M.F.; Sharma, A.; Rehman, A.; Baban, B.; Arbab, A.S.; Hess, D.C. Exercise Improves Cerebral Blood Flow and Functional Outcomes in an Experimental Mouse Model of Vascular Cognitive Impairment and Dementia (VCID). Transl. Stroke Res. 2024, 15, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Saks, D.G.; Sachdev, P.S. Monogenic causes of cerebral small vessel disease- models for vascular cognitive impairment and dementia? Curr. Opin. Psychiatry 2025, 38, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Stepicheva, N.A.; Weng, Z.; Cao, S.; Foley, L.M.; Cao, G. A Mouse Model for Vascular Cognitive Impairment and Dementia Based on Needle-guided Asymmetric Bilateral Common Carotid Artery Stenosis. J. Vis. Exp. 2024, 213, e67092. [Google Scholar] [CrossRef]
- Tayler, H.; Miners, J.S.; Güzel, Ö.; MacLachlan, R.; Love, S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer’s disease, vascular dementia and mixed dementia. Brain Pathol. 2021, 31, e12935. [Google Scholar] [CrossRef]
- Silbert, L.C.; Nelson, C.; Howieson, D.B.; Moore, M.M.; Kaye, J.A. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008, 71, 108–113. [Google Scholar] [CrossRef]
- Fekete, M.; Liotta, E.M.; Molnar, T.; Fülöp, G.A.; Lehoczki, A. The role of atrial fibrillation in vascular cognitive impairment and dementia: Epidemiology, pathophysiology, and preventive strategies. Geroscience 2025, 47, 287–300. [Google Scholar] [CrossRef]
- Culebras, A.; Anwar, S. Sleep Apnea Is a Risk Factor for Stroke and Vascular Dementia. Curr. Neurol. Neurosci. Rep. 2018, 18, 53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekala, A.; Qiu, H. Interplay Between Vascular Dysfunction and Neurodegenerative Pathology: New Insights into Molecular Mechanisms and Management. Biomolecules 2025, 15, 712. https://doi.org/10.3390/biom15050712
Mekala A, Qiu H. Interplay Between Vascular Dysfunction and Neurodegenerative Pathology: New Insights into Molecular Mechanisms and Management. Biomolecules. 2025; 15(5):712. https://doi.org/10.3390/biom15050712
Chicago/Turabian StyleMekala, Avanthika, and Hongyu Qiu. 2025. "Interplay Between Vascular Dysfunction and Neurodegenerative Pathology: New Insights into Molecular Mechanisms and Management" Biomolecules 15, no. 5: 712. https://doi.org/10.3390/biom15050712
APA StyleMekala, A., & Qiu, H. (2025). Interplay Between Vascular Dysfunction and Neurodegenerative Pathology: New Insights into Molecular Mechanisms and Management. Biomolecules, 15(5), 712. https://doi.org/10.3390/biom15050712







