Vitamin D and Retinoic Acid Require Protein Kinase C Activity and Reactive Oxygen Species as Opposing Signals Regulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture Cells
2.3. Quantitative Real-Time RT-PCR (RT-qPCR)
2.4. Measurement of Cellular and Mitochondrial Reactive Oxygen Species
2.5. Measurement of Mitochondrial Membrane Potential (Ψm)
2.6. Statistics
3. Results
3.1. Effects of VD and RA on GPRC5A Expression
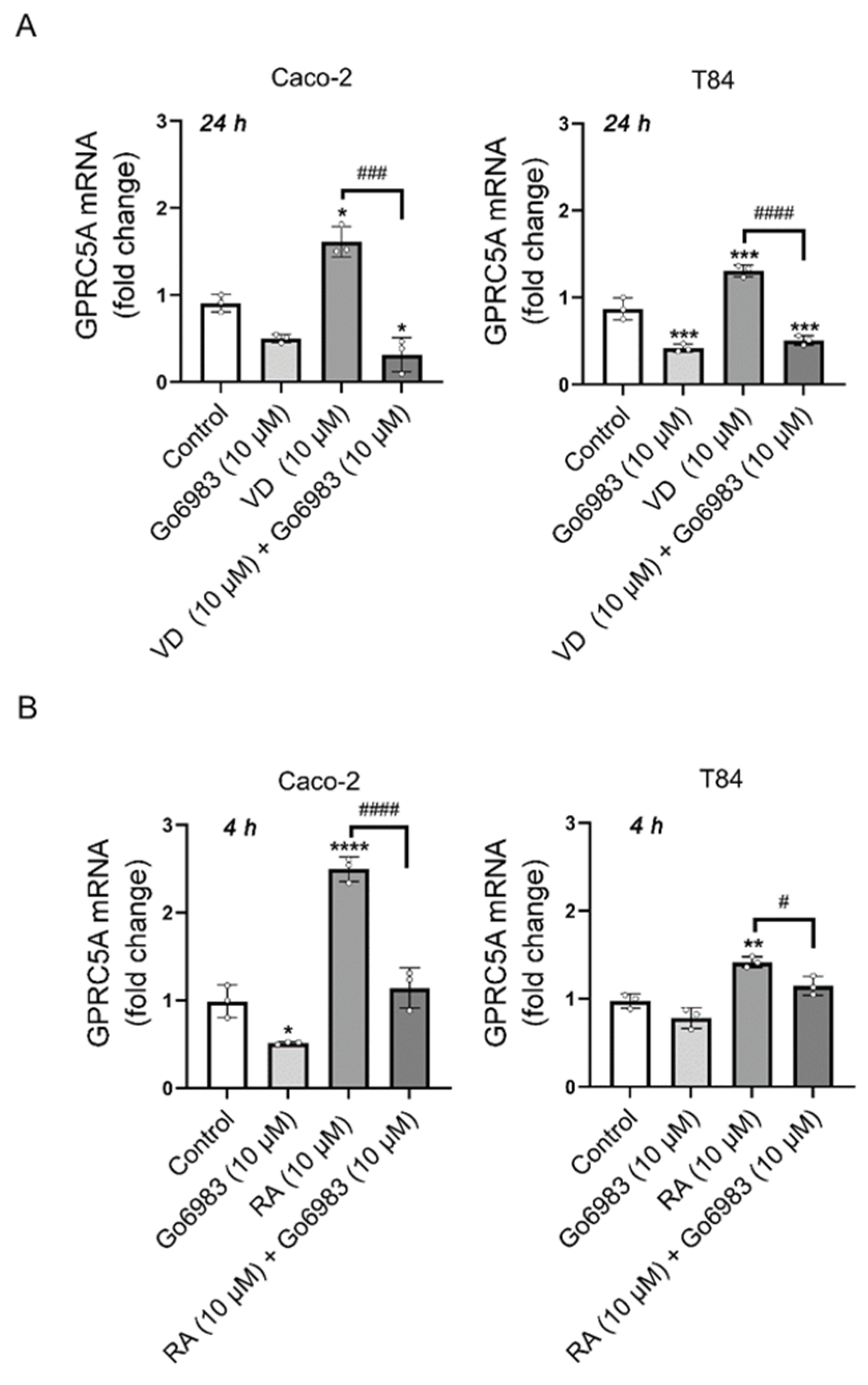
3.2. PKC Inhibition with Gö6983 Completely Blocks VD Effects on GPRC5A mRNA Expression
3.3. Effects of VD and RA on ROS Levels and Mitochondrial Membrane Potential (Ψm) in Caco-2 and T84 Cells
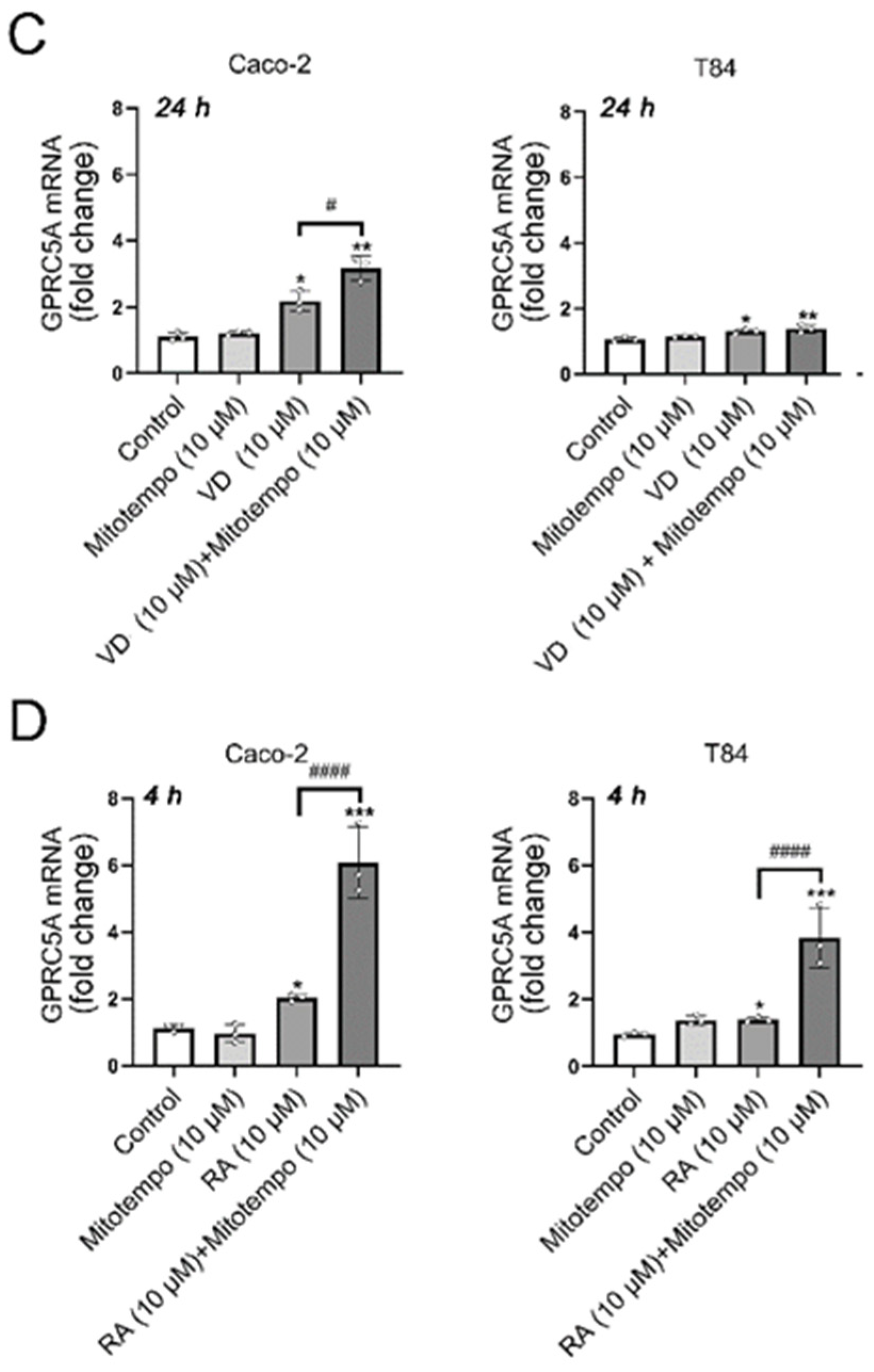
3.4. Effects of the ROS Scavengers NAC or MitoTEMPO on GPRC5A mRNA Expression After VD or RA Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Xu, X.; Wu, M.; Guan, Z.; Su, X.; Chen, S.; Wang, H.; Teng, L. GPRC5A: An Emerging Biomarker in Human Cancer. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Iglesias-González, P.A.; Valdivieso, Á.G.; Santa-Coloma, T.A. The G protein-coupled receptor GPRC5A—a phorbol ester and retinoic acid-induced orphan receptor with roles in cancer, inflammation, and immunity. Biochem. Cell Biol. 2023, 101, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Cafferata, E.G.; Gonzalez-Guerrico, A.M.; Pivetta, O.H.; Santa-Coloma, T.A. Identification by differential display of a mRNA specifically induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in T84 human colon carcinoma cells. Cell Mol. Biol 1996, 42, 797–804. [Google Scholar] [PubMed]
- Cafferata, E.G.A. Regulación del Gen CFTR (Afectado en Fibrosis Quística) en Células de Carcinoma de Colon Humano T84. Ph.D. Thesis, School of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina, 2002. [Google Scholar]
- Mori, C. Regulación del gen PEIG-1/GPRC5A por 12-O-tetradecanoilforbol-13-acetato. Ph.D. Thesis, School of Exact Sciences, National University of La Plata, Buenos Aires, Argentina, 2020. [Google Scholar] [CrossRef]
- Mori, C.; Valdivieso, Á.G.; Clauzure, M.; Massip-Copiz, M.M.; Aguilar, M.Á.; Cafferata, E.G.; Santa Coloma, T.A. Identification and characterization of human PEIG-1/GPRC5A as a 12-O-tetradecanoyl phorbol-13-acetate (TPA) and PKC-induced gene. Arch. Biochem. Biophys. 2020, 687, 108375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lotan, R. Molecular Cloning and Characterization of a Novel Retinoic Acid-inducible Gene That Encodes a Putative G Protein-coupled Receptor. J. Biol. Chem. 1998, 273, 35008–35015. [Google Scholar] [CrossRef]
- Robbins, M.; Michalovich, D.; Hill, J.; Calver, A.; Medhurst, A.; Gloger, I.; Sims, M.; Middlemiss, D.; Pangalos, M. Molecular Cloning and Characterization of Two Novel Retinoic Acid-Inducible Orphan G-Protein-Coupled Receptors (GPRC5B and GPRC5C). Genomics 2000, 67, 8–18. [Google Scholar] [CrossRef]
- Bräuner-Osborne, H.; A Jensen, A.; O Sheppard, P.; Brodin, B.; Krogsgaard-Larsen, P.; O’hara, P. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim. et Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1518, 237–248. [Google Scholar] [CrossRef]
- Xu, J.; Tian, J.; Shapiro, S.D. Normal Lung Development in RAIG1-Deficient Mice Despite Unique Lung Epithelium–Specific Expression. Am. J. Respir. Cell Mol. Biol. 2005, 32, 381–387. [Google Scholar] [CrossRef]
- Lin, X.; Zhong, S.; Ye, X.; Liao, Y.; Yao, F.; Yang, X.; Sun, B.; Zhang, J.; Li, Q.; Gao, Y.; et al. EGFR phosphorylates and inhibits lung tumor suppressor GPRC5A in lung cancer. Mol. Cancer 2014, 13, 233. [Google Scholar] [CrossRef]
- Yang, L.; Ma, T.; Zhang, J. GPRC5A exerts its tumor-suppressive effects in breast cancer cells by inhibiting EGFR and its downstream pathway. Oncol. Rep. 2016, 36, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Gao, G.; Wei, G.; Zheng, Y.; Wang, C.; Gao, N.; Zhao, Y.; Deng, J.; Chen, H.; et al. Elevation of GPRC5A expression in colorectal cancer promotes tumor progression through VNN-1 induced oxidative stress. Int. J. Cancer 2017, 140, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Bagley, C.; Heesom, K.J.; Gurevich, D.B.; Gay, D.; Bond, M.; Collard, T.J.; Paraskeva, C.; Martin, P.; Sansom, O.J.; et al. Cancer cell adaptation to hypoxia involves a HIF-GPRC5A-YAP axis. EMBO Mol. Med. 2018, 10, e8699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Chen, S.; Li, G.; Pu, P.; Yang, Y.; Wu, W.; Geng, Y.; Liu, Y. GPRC5A promotes gallbladder cancer metastasis by upregulating TNS4 via the JAK2–STAT3 pathway. Cancer Lett. 2024, 598, 217067. [Google Scholar] [CrossRef]
- Slater, S.J.; Kelly, M.B.; Taddeo, F.J.; Larkin, J.D.; Yeager, M.D.; McLane, J.A.; Ho, C.; Stubbs, C.D. Direct Activation of Protein Kinase C by 1α,25-Dihydroxyvitamin D3. J. Biol. Chem. 1995, 270, 6639–6643. [Google Scholar] [CrossRef]
- Cheskis, B.; Freedman, L.P. Ligand Modulates the Conversion of DNA-Bound Vitamin D3 Receptor (VDR) Homodimers into VDR-Retinoid X Receptor Heterodimers. Mol. Cell. Biol. 1994, 14. [Google Scholar] [CrossRef]
- Ye, X.; Tao, Q.; Wang, Y.; Cheng, Y.; Lotan, R. Mechanisms underlying the induction of the putative human tumor suppressor GPRC5A by Retinoic acid. Cancer Biol. Ther. 2009, 8, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Bouzinbasegard, H.; Fan, X.; Perderiset, M.; Castagna, M. Synergy Between Phorbol Esters and Retinoic Acid in Inducing Protein Kinase C Activation. Biochem. Biophys. Res. Commun. 1994, 204, 112–119. [Google Scholar] [CrossRef]
- Kambhampati, S.; Li, Y.; Verma, A.; Sassano, A.; Majchrzak, B.; Deb, D.K.; Parmar, S.; Giafis, N.; Kalvakolanu, D.V.; Rahman, A.; et al. Activation of Protein Kinase Cδ by All-trans-retinoic Acid. J. Biol. Chem. 2003, 278, 32544–32551. [Google Scholar] [CrossRef]
- Radominska-Pandya, A.; Chen, G.; Czernik, P.J.; Little, J.M.; Samokyszyn, V.M.; Carter, C.A.; Nowak, G. Direct Interaction of All-trans-retinoic Acid with Protein Kinase C (PKC). J. Biol. Chem. 2000, 275, 22324–22330. [Google Scholar] [CrossRef]
- Iglesias-González, P.A.; Mori, C.; Valdivieso, Á.G.; Santa-Coloma, T.A. Vitamin D and Retinoic Acid Induce Reactive Oxygen Species (ROS) and PKC Signaling as Opposed Signals Upregulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells. SSRN. 2024. August 15. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4916839 (accessed on 15 August 2024).
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. Methods Inmolecularbiol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Kauffman, M.; Traore, K.; Zhu, H.; Trush, M.; Jia, Z.; Li, Y. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087361. [Google Scholar] [CrossRef] [PubMed]
- Show the dots in plots. Nat. Biomed. Eng. 2017, 1. [CrossRef]
- Vantieghem, K.; Overbergh, L.; Carmeliet, G.; De Haes, P.; Bouillon, R.; Segaert, S. UVB-induced 1,25(OH)2D3 production and vitamin D activity in intestinal CaCo-2 cells and in THP-1 macrophages pretreated with a sterol Δ7-reductase inhibitor. J. Cell. Biochem. 2006, 99, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, S.-W.; Kim, J.-M.; Jho, E.-H.; Park, S.-Y.; Oh, D.-Y.; Yun-Choi, H.-S. PKC inhibitors RO 31-8220 and Gö 6983 enhance epinephrine-induced platelet aggregation in catecholamine hypo-responsive platelets by enhancing Akt phosphorylation. BMB Rep. 2011, 44, 140–145. [Google Scholar] [CrossRef]
- Bissonnette, M.; Tien, X.Y.; Niedziela, S.M.; Hartmann, S.C.; Frawley, B.P.; Roy, H.K.; Sitrin, M.D.; Perlman, R.L.; Brasitus, T.A. 1,25(OH)2 vitamin D3 activates PKC-alpha in Caco-2 cells: A mechanism to limit secosteroid-induced rise in [Ca2+]i. Am. J. Physiol. Liver Physiol. 1994, 267, G465–G475. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, W.; Zhang, T.; Li, S.; Guo, J.; Liu, L. Identification of a ferroptosis-related gene signature for prognosis prediction in colorectal cancer patients and relationship with vitamin D. J. Steroid Biochem. Mol. Biol. 2022, 227, 106234. [Google Scholar] [CrossRef]
- El-Baba, C.; Eid, A.H.; Shaito, A.; Kobeissy, F.; Darwiche, N. Retinoids and Reactive Oxygen Species in Cancer Cell Death and Therapeutics. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–22. [Google Scholar]
- Mandal, J.P.; Shiue, C.-N.; Chen, Y.-C.; Lee, M.-C.; Yang, H.-H.; Chang, H.-H.; Hu, C.-T.; Liao, P.-C.; Hui, L.-C.; You, R.-I.; et al. PKCδ mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free. Radic. Biol. Med. 2021, 163, 69–87. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Zhou, J.; Terluk, M.R.; Orchard, P.J.; Cloyd, J.C.; Kartha, R.V. N-Acetylcysteine Reverses the Mitochondrial Dysfunction Induced by Very Long-Chain Fatty Acids in Murine Oligodendrocyte Model of Adrenoleukodystrophy. Biomedicines 2021, 9, 1826. [Google Scholar] [CrossRef]
- Mukem, S.; Thongbuakaew, T.; Khornchatri, K. Mito-Tempo suppresses autophagic flux via the PI3K/Akt/mTOR signaling pathway in neuroblastoma SH-SY5Y cells. Heliyon 2021, 7, e07310. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-González, P.A.; Dib, C.; Núñez, N.; Limpias del Valle, T.M.; Mori, C.; Valdivieso, Á.G.; Santa-Coloma, T.A. Abstract- GPRC5A Regulation by Vitamin D3 and Cigarette Smoke Extract in Colon Cancer Cell Lines. In Proceedings of the Reunión Conjunta Saic Sai&Faic Safis 2022, Mar del Plata, Argentina, 16–19 November 2022; p. 311. Available online: https://www.medicinabuenosaires.com/PMID/36368022.pdf (accessed on 15 August 2024).
- Tao, Q.; Fujimoto, J.; Men, T.; Ye, X.; Deng, J.; Lacroix, L.; Clifford, J.L.; Mao, L.; Van Pelt, C.S.; Lee, J.J.; et al. Identification of the Retinoic Acid-Inducible Gprc5a As a New Lung Tumor Suppressor Gene. JNCI J. Natl. Cancer Inst. 2007, 99, 1668–1682. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Franceschi, R.T.; Wood, R.J. Characterization of the vitamin D receptor from the Caco-2 human colon carcinoma cell line: Effect of cellular differentiation. Arch. Biochem. Biophys. 1991, 285, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bartik, L.; Whitfield, G.K.; Kaczmarska, M.; Lowmiller, C.L.; Moffet, E.W.; Furmick, J.K.; Hernandez, Z.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Curcumin: A novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J. Nutr. Biochem. 2010, 21, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Clauzure, M.; Valdivieso, Á.G.; Dugour, A.V.; Mori, C.; Massip-Copiz, M.M.; Aguilar, M.Á.; Sotomayor, V.; Asensio, C.J.A.; Figueroa, J.M.; Santa-Coloma, T.A. NLR family pyrin domain containing 3 (NLRP3) and caspase 1 (CASP1) modulation by intracellular Cl– concentration. Immunology 2021, 163, 493–511. [Google Scholar] [CrossRef]
- Clauzure, M.; Valdivieso, A.G.; Copiz, M.M.M.; Schulman, G.; Teiber, M.L.; Santa-Coloma, T.A. Disruption of Interleukin-1β Autocrine Signaling Rescues Complex I Activity and Improves ROS Levels in Immortalized Epithelial Cells with Impaired Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Function. PLoS ONE 2014, 9, e99257. [Google Scholar] [CrossRef]
- Clauzure, M.; Valdivieso, Á.G.; Massip-Copiz, M.M.; Mori, C.; Dugour, A.V.; Figueroa, J.M.; Santa-Coloma, T.A. Intracellular Chloride Concentration Changes Modulate IL-1β Expression and Secretion in Human Bronchial Epithelial Cultured Cells. J. Cell. Biochem. 2017, 118, 2131–2140. [Google Scholar] [CrossRef]
- Massip-Copiz, M.M.; Clauzure, M.; Valdivieso, Á.G.; Santa-Coloma, T.A. CFTR impairment upregulates c-Src activity through IL-1β autocrine signaling. Arch. Biochem. Biophys. 2017, 616, 1–12. [Google Scholar] [CrossRef]
- Song, H.; Song, H.; Ye, X.; Ye, X.; Liao, Y.; Liao, Y.; Zhang, S.; Zhang, S.; Xu, D.; Xu, D.; et al. NF-κB represses retinoic acid receptor–mediated GPRC5A transactivation in lung epithelial cells to promote neoplasia. J. Clin. Investig. 2023, 8, e153976. [Google Scholar] [CrossRef]
- Sampei, C.; Kato, K.; Arasaki, Y.; Kimura, Y.; Konno, T.; Otsuka, K.; Kohara, Y.; Noda, M.; Ezura, Y.; Hayata, T. Gprc5a is a novel parathyroid hormone-inducible gene and negatively regulates osteoblast proliferation and differentiation. J. Cell. Physiol. 2024, 239. [Google Scholar] [CrossRef]
- Zhong, S.; Yin, H.; Liao, Y.; Yao, F.; Li, Q.; Zhang, J.; Jiao, H.; Zhao, Y.; Xu, D.; Liu, S.; et al. Lung Tumor Suppressor GPRC5A Binds EGFR and Restrains Its Effector Signaling. Cancer Res. 2015, 75, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Roche, N.S.; Sporn, M.B. Selective inhibition of the anchorage-independent growth of myc-transfected fibroblasts by retinoic acid. Nature 1985, 315, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.M.; Hofer, H.; Ellinger, A.; Peterlik, M.; Cross, H.S. Mechanism of antimitogenic action of vitamin D in human colon carcinoma cells: Relevance for suppression of epidermal growth factor-stimulated cell growth. Oncol. Res. 1999, 11, 77–84. [Google Scholar] [PubMed]
- Palanca, A.; Ampudia-Blasco, F.J.; Real, J.T. The Controversial Role of Vitamin D in Thyroid Cancer Prevention. Nutrients 2022, 14, 2593. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias González, P.A.; Mori, C.; Valdivieso, Á.G.; Santa Coloma, T.A. Vitamin D and Retinoic Acid Require Protein Kinase C Activity and Reactive Oxygen Species as Opposing Signals Regulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells. Biomolecules 2025, 15, 711. https://doi.org/10.3390/biom15050711
Iglesias González PA, Mori C, Valdivieso ÁG, Santa Coloma TA. Vitamin D and Retinoic Acid Require Protein Kinase C Activity and Reactive Oxygen Species as Opposing Signals Regulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells. Biomolecules. 2025; 15(5):711. https://doi.org/10.3390/biom15050711
Chicago/Turabian StyleIglesias González, Pablo A., Consuelo Mori, Ángel G. Valdivieso, and Tomás A. Santa Coloma. 2025. "Vitamin D and Retinoic Acid Require Protein Kinase C Activity and Reactive Oxygen Species as Opposing Signals Regulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells" Biomolecules 15, no. 5: 711. https://doi.org/10.3390/biom15050711
APA StyleIglesias González, P. A., Mori, C., Valdivieso, Á. G., & Santa Coloma, T. A. (2025). Vitamin D and Retinoic Acid Require Protein Kinase C Activity and Reactive Oxygen Species as Opposing Signals Regulating PEIG-1/GPRC5A Expression in Caco-2 and T84 Colon Carcinoma Cells. Biomolecules, 15(5), 711. https://doi.org/10.3390/biom15050711






