A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
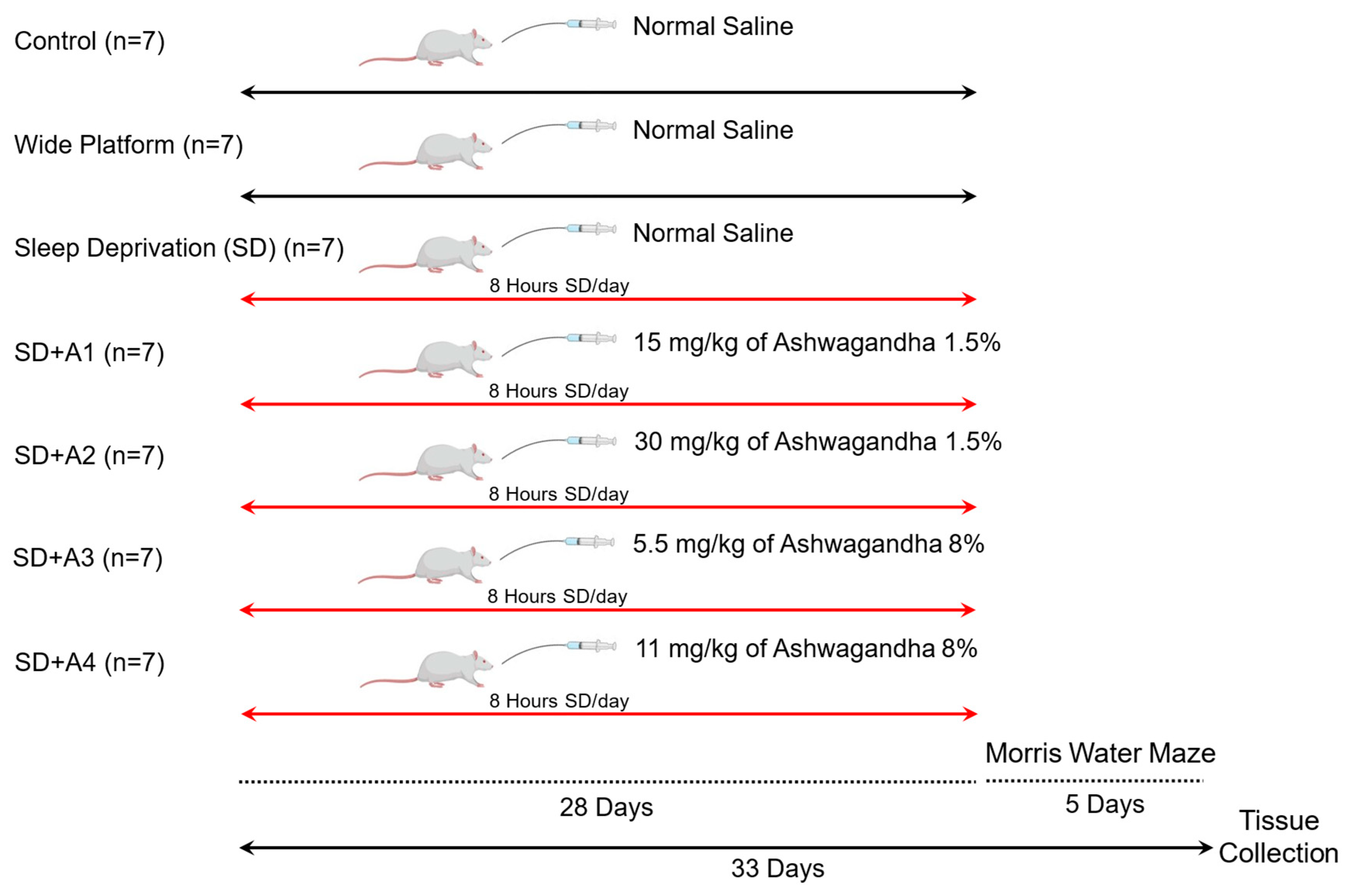
2.1. Animals and Experimental Design
2.2. Induction of Sleep Deprivation
2.3. Morris Water Maze
2.4. Sample Collection
2.5. Biochemical Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Body Weight and Serum Biochemical Parameters
3.2. Serum Hormone Levels
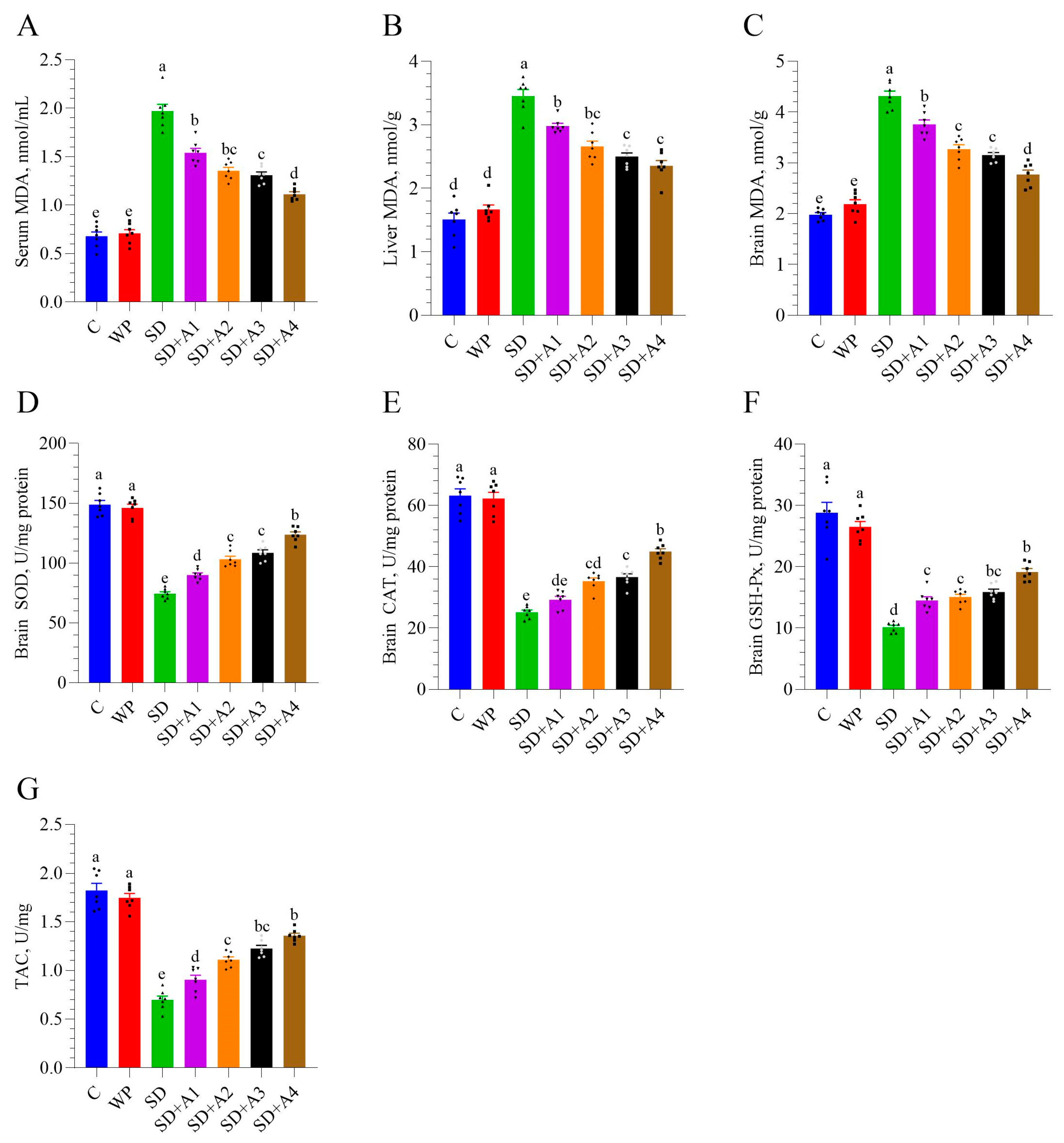
3.3. Malondialdehyde and Antioxidant Enzymes
3.4. Morris Water Maze
3.5. Brain Neurotrophic Factors
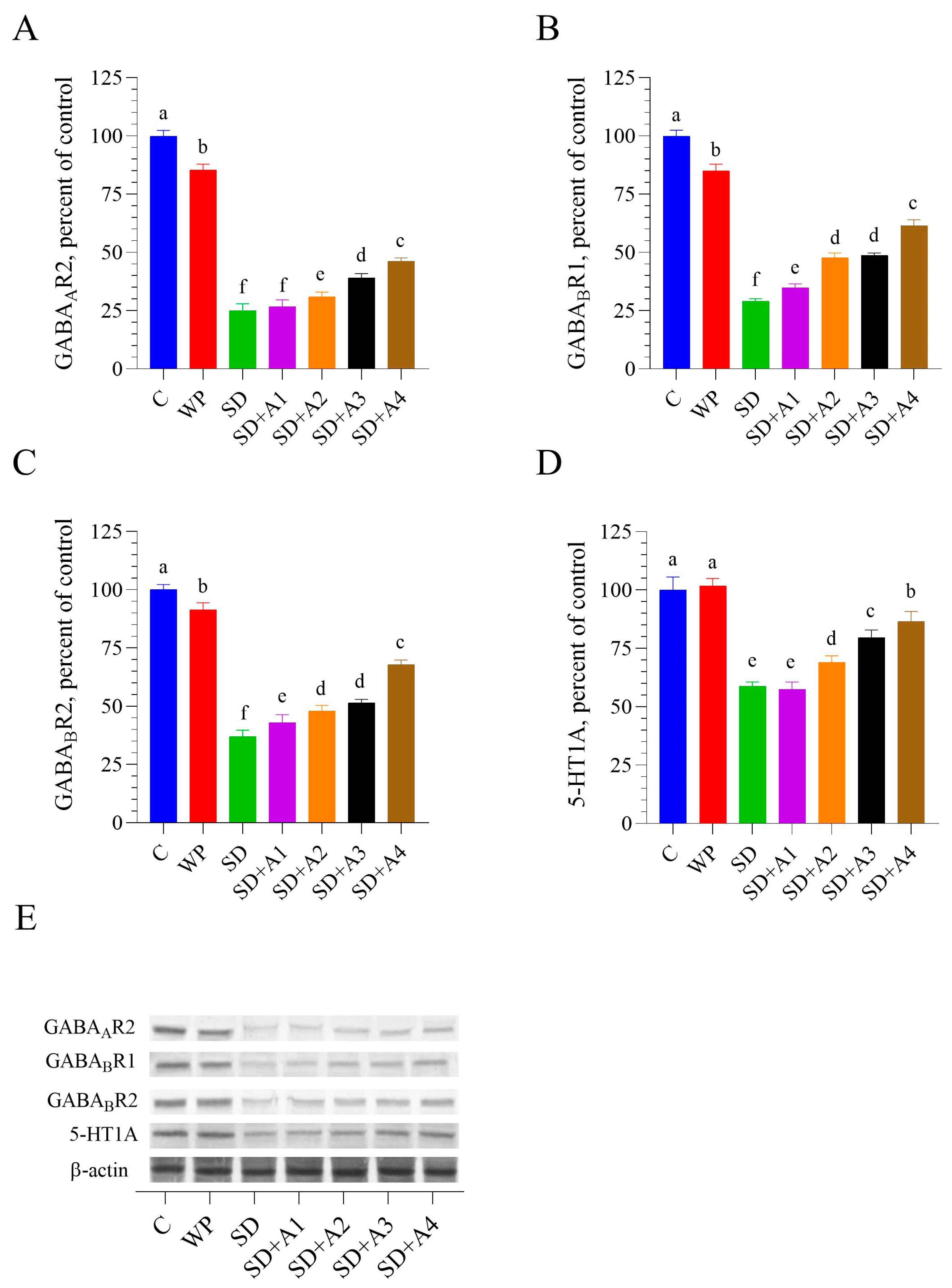
3.6. Brain GABAergic and Serotonergic Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohari, A.; Baumann, B.; Jen, R.; Ayas, N. Sleep Deficiency: Epidemiology and Effects. Clin. Chest Med. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The Sleep-Deprived Human Brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Alrousan, G.; Hassan, A.; Pillai, A.A.; Atrooz, F.; Salim, S. Early Life Sleep Deprivation and Brain Development: Insights From Human and Animal Studies. Front. Neurosci. 2022, 16, 833786. [Google Scholar] [CrossRef]
- Prpar Mihevc, S.; Majdič, G. Canine Cognitive Dysfunction and Alzheimer’s Disease—Two Facets of the Same Disease? Front. Neurosci. 2019, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.L.; Fernandez, E.J.; Handley, K.N.; Hazel, S.J. Non-Pharmacological Interventions for the Treatment of Canine Cognitive Dysfunction: A Scoping Review. Appl. Anim. Behav. Sci. 2023, 269, 106097. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Why Stress Is Bad for Your Brain. Science 1996, 273, 749–750. [Google Scholar] [CrossRef]
- Grillon, C.; Duncko, R.; Covington, M.F.; Kopperman, L.; Kling, M.A. Acute Stress Potentiates Anxiety in Humans. Biol. Psychiatry 2007, 62, 1183–1186. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef]
- Vaseghi, S.; Arjmandi-Rad, S.; Eskandari, M.; Ebrahimnejad, M.; Kholghi, G.; Zarrindast, M.-R. Modulating Role of Serotonergic Signaling in Sleep and Memory. Pharmacol. Rep. 2022, 74, 1–26. [Google Scholar] [CrossRef]
- Mishra, R.; Manchanda, S.; Gupta, M.; Kaur, T.; Saini, V.; Sharma, A.; Kaur, G. Tinospora Cordifolia Ameliorates Anxiety-like Behavior and Improves Cognitive Functions in Acute Sleep Deprived Rats. Sci. Rep. 2016, 6, 25564. [Google Scholar] [CrossRef]
- Frey, D.J.; Fleshner, M.; Wright, K.P. The Effects of 40 Hours of Total Sleep Deprivation on Inflammatory Markers in Healthy Young Adults. Brain. Behav. Immun. 2007, 21, 1050–1057. [Google Scholar] [CrossRef]
- Sahin, K.; Korkusuz, A.K.; Sahin, E.; Orhan, C.; Er, B.; Morde, A.; Padigaru, M.; Kilic, E. The Effect of Water-Soluble Alpinia Galanga Extract on Sleep and the Activation of the GABAAergic/Serotonergic Pathway in Mice. Pharmaceuticals 2024, 17, 1649. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A Prospective, Randomized Double-Blind, Placebo-Controlled Study of Safety and Efficacy of a High-Concentration Full-Spectrum Extract of Ashwagandha Root in Reducing Stress and Anxiety in Adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Hong, K.-B.; Suh, H.J.; Ahn, Y. Sleep-Promoting Activity of Amylase-Treated Ashwagandha (Withania somnifera L. Dunal) Root Extract via GABA Receptors. J. Food Drug Anal. 2023, 31, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, M.T.; Hegazy, M.A.; Mohamed, E.H. Major Biochemical Constituents of Withania Somnifera (Ashwagandha) Extract: A Review of Chemical Analysis. Rev. Anal. Chem. 2023, 42, 20220055. [Google Scholar] [CrossRef]
- United States Pharmacopeia Ashwagandha Root Dry Extract. 2020. Available online: https://doi.usp.org/USPNF/USPNF_M2789_08_01.html (accessed on 2 May 2025).
- Konar, A.; Shah, N.; Singh, R.; Saxena, N.; Kaul, S.C.; Wadhwa, R.; Thakur, M.K. Protective Role of Ashwagandha Leaf Extract and Its Component Withanone on Scopolamine-Induced Changes in the Brain and Brain-Derived Cells. PLoS ONE 2011, 6, e27265. [Google Scholar] [CrossRef]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-Blind, Randomized, Placebo-Controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed]
- Dudhat, K.; Bhalodiya, M.; Dudhrejiya, A.; Shah, S.; Parmar, R.; Baldaniya, L.; Dhaval, M. Application of Amorphous Solid Dispersion Technology for Improving the Physicochemical Properties, Saturation Solubility, and In Vitro Dissolution of Withania somnifera Methanolic Root Powder Extract. J. Pharm. Innov. 2023, 18, 1338–1349. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Salah, H.A.; Abu Rashid, B.E. The Combined Effect of Sleep Deprivation and Western Diet on Spatial Learning and Memory: Role of BDNF and Oxidative Stress. J. Mol. Neurosci. 2013, 50, 124–133. [Google Scholar] [CrossRef]
- Grahnstedt, S.; Ursin, R. Platform Sleep Deprivation Affects Deep Slow Wave Sleep in Addition to REM Sleep. Behav. Brain Res. 1985, 18, 233–239. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Karatoprak, S.; Tuzcu, M.; Deeh, P.B.D.; Ozercan, I.H.; Sahin, N.; Bozoglan, M.Y.; Sylla, S.; Ojalvo, S.P.; et al. Therapeutic Effects of a Novel Form of Biotin on Propionic Acid-Induced Autistic Features in Rats. Nutrients 2022, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Nikolic, A.; Fahlbusch, P.; Riffelmann, N.-K.; Wahlers, N.; Jacob, S.; Hartwig, S.; Kettel, U.; Schiller, M.; Dille, M.; Al-Hasani, H.; et al. Chronic Stress Alters Hepatic Metabolism and Thermodynamic Respiratory Efficiency Affecting Epigenetics in C57BL/6 Mice. iScience 2024, 27, 109276. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.A.; Griffith, D.M.; Thorpe, R.J. Stress and the Kidney. Adv. Chronic Kidney Dis. 2015, 22, 46–53. [Google Scholar] [CrossRef]
- Jain, V.; Chaturvedi, S.; Jamil, S.; Tyagi, R.; Arya, S.; Madan, S. Ashwagandha: Botanic Occurrence, Conventional Uses, and Significance in Heart, Metabolic, Renal and Hepatic Disorder. Nutr. Food Sci. 2024, 54, 1337–1355. [Google Scholar] [CrossRef]
- Devkar, S.T.; Kandhare, A.D.; Zanwar, A.A.; Jagtap, S.D.; Katyare, S.S.; Bodhankar, S.L.; Hegde, M.V. Hepatoprotective Effect of Withanolide-Rich Fraction in Acetaminophen-Intoxicated Rat: Decisive Role of TNF-α, IL-1β, COX-II and iNOS. Pharm. Biol. 2016, 54, 2394–2403. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Mastorakos, G.; Bixler, E.O.; Kales, A.; Gold, P.W.; Chrousos, G.P. Sleep Deprivation Effects on the Activity of the Hypothalamic-Pituitary-Adrenal and Growth Axes: Potential Clinical Implications. Clin. Endocrinol. 1999, 51, 205–215. [Google Scholar] [CrossRef]
- Della Porta, M.; Maier, J.A.; Cazzola, R. Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review. Nutrients 2023, 15, 5015. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Goel, R.K.; Kaur, R.; Ghosal, S. Anti-Stress Activity of Sitoindosides VII and VIII, New Acylsterylglucosides from Withania somnifera. Phytother. Res. 1987, 1, 32–37. [Google Scholar] [CrossRef]
- Ghosal, S.; Lal, J.; Srivastava, R.; Bhattacharya, S.K.; Upadhyay, S.N.; Jaiswal, A.K.; Chattopadhyay, U. Immunomodulatory and CNS Effects of Sitoindosides IX and X, Two New Glycowithanolides from Withania somnifera. Phytother. Res. 1989, 3, 201–206. [Google Scholar] [CrossRef]
- Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Kurant, D.; Gryczka, K.; Falkowski, M.; Wiśniewska, M.; Słupski, M.; Ohla, J.; Zabrzyński, J. Can Ashwagandha Benefit the Endocrine System?-A Review. Int. J. Mol. Sci. 2023, 24, 16513. [Google Scholar] [CrossRef] [PubMed]
- Longordo, F.; Kopp, C.; Lüthi, A. Consequences of Sleep Deprivation on Neurotransmitter Receptor Expression and Function. Eur. J. Neurosci. 2009, 29, 1810–1819. [Google Scholar] [CrossRef]
- Dawane, J.; Seok, S.; Dhande, P.; Langade, D.; Han, H.; Kim, S.-B.; Ju, J.-Y. Evaluation of the Anxiolytic and Antidepressant Effects of Standardized Ashwagandha (Withania somnifera) Root Extract in Wistar Rats. Prev. Nutr. Food Sci. 2024, 29, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.-S.; Han, K.; Sim, W.; Suh, H.J.; Ahn, Y. Ashwagandha (Withania somnifera (L.) Dunal) Root Extract Containing Withanolide a Alleviates Depression-like Behavior in Mice by Enhancing the Brain-Derived Neurotrophic Factor Pathway under Unexpected Chronic Mild Stress. J. Ethnopharmacol. 2025, 340, 119224. [Google Scholar] [CrossRef]
- Tyagi, A.; Choi, Y.-Y.; Shan, L.; Vinothkanna, A.; Lee, E.-S.; Chelliah, R.; Barathikannan, K.; Raman, S.T.; Park, S.J.; Jia, A.-Q.; et al. Limosilactobacillus Reuteri Fermented Brown Rice Alleviates Anxiety Improves Cognition and Modulates Gut Microbiota in Stressed Mice. NPJ Sci. Food 2025, 9, 5. [Google Scholar] [CrossRef]
- Sahin, K.; Gencoglu, H.; Korkusuz, A.K.; Orhan, C.; Aldatmaz, İ.E.; Erten, F.; Er, B.; Morde, A.; Padigaru, M.; Kilic, E. Impact of a Novel Valerian Extract on Sleep Quality, Relaxation, and GABA/Serotonin Receptor Activity in a Murine Model. Antioxidants 2024, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Abdelfatah, S.; Efferth, T. Induction of Stress Resistance and Extension of Lifespan in Chaenorhabditis Elegans Serotonin-Receptor Knockout Strains by Withanolide A. Phytomedicine 2021, 84, 153482. [Google Scholar] [CrossRef]
- Bassareo, V.; Talani, G.; Frau, R.; Porru, S.; Rosas, M.; Kasture, S.B.; Peana, A.T.; Loi, E.; Sanna, E.; Acquas, E. Inhibition of Morphine- and Ethanol-Mediated Stimulation of Mesolimbic Dopamine Neurons by Withania somnifera. Front. Neurosci. 2019, 13, 545. [Google Scholar] [CrossRef]
- Guo, S.; Rezaei, M.J. The Benefits of Ashwagandha (Withania somnifera) Supplements on Brain Function and Sports Performance. Front. Nutr. 2024, 11, 1439294. [Google Scholar] [CrossRef]
- Suganya, K.; Kayalvizhi, E.; Yuvaraj, R.; Chandrasekar, M.; Kavitha, U.; Konakanchi Suresh, K. Effect of Withania somnifera on the Antioxidant and Neurotransmitter Status in Sleep Deprivation Induced Wistar Rats. Bioinformation 2020, 16, 631–637. [Google Scholar] [CrossRef]
- Konakanchi, S.; Raavi, V.; Ml, H.K.; Shankar MS, V. Impact of Chronic Sleep Deprivation and Sleep Recovery on Hippocampal Oligodendrocytes, Anxiety-like Behavior, Spatial Learning and Memory of Rats. Brain Res. Bull. 2023, 193, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Kallubai, M.; Subramanyam, R. Improving the Inhibition of β-Amyloid Aggregation by Withanolide and Withanoside Derivatives. Int. J. Biol. Macromol. 2021, 173, 56–65. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Withanoside IV and Its Active Metabolite, Sominone, Attenuate Abeta(25-35)-Induced Neurodegeneration. Eur. J. Neurosci. 2006, 23, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific Basis for the Therapeutic Use of Withania somnifera (Ashwagandha): A Review. Altern. Med. Rev. J. Clin. Ther. 2000, 5, 334–346. [Google Scholar]
- Gladen-Kolarsky, N.; Monestime, O.; Bollen, M.; Choi, J.; Yang, L.; Magaña, A.A.; Maier, C.S.; Soumyanath, A.; Gray, N.E. Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer’s Disease. Antioxidants 2024, 13, 1164. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, R.; Nazmi, A.; Lakhanpal, D.; Kataria, H.; Kaur, G. Glioprotective Effects of Ashwagandha Leaf Extract against Lead Induced Toxicity. BioMed Res. Int. 2014, 2014, 182029. [Google Scholar] [CrossRef]
- Kataria, H.; Wadhwa, R.; Kaul, S.C.; Kaur, G. Withania somnifera Water Extract as a Potential Candidate for Differentiation Based Therapy of Human Neuroblastomas. PLoS ONE 2013, 8, e55316. [Google Scholar] [CrossRef]
- Yan, X.; Huang, G.; Liu, Q.; Zheng, J.; Chen, H.; Huang, Q.; Chen, J.; Huang, H. Withaferin A Protects against Spinal Cord Injury by Inhibiting Apoptosis and Inflammation in Mice. Pharm. Biol. 2017, 55, 1171–1176. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dongare, S.; Mathur, R.; Mohanty, I.R.; Srivastava, S.; Mathur, S.; Nag, T.C. Genistein Ameliorates Cardiac Inflammation and Oxidative Stress in Streptozotocin-Induced Diabetic Cardiomyopathy in Rats. Mol. Cell. Biochem. 2015, 408, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Munni, Y.A.; Dash, R.; Sultana, A.; Moon, I.S. Unveiling the Effect of Withania somnifera on Neuronal Cytoarchitecture and Synaptogenesis: A Combined in Vitro and Network Pharmacology Approach. Phytother. Res. 2022, 36, 2524–2541. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kwon, T.K. Withaferin A Inhibits Tumor Necrosis Factor-Alpha-Induced Expression of Cell Adhesion Molecules by Inactivation of Akt and NF-kappaB in Human Pulmonary Epithelial Cells. Int. Immunopharmacol. 2009, 9, 614–619. [Google Scholar] [CrossRef]
- Candelario, M.; Cuellar, E.; Reyes-Ruiz, J.M.; Darabedian, N.; Feimeng, Z.; Miledi, R.; Russo-Neustadt, A.; Limon, A. Direct Evidence for GABAergic Activity of Withania somnifera on Mammalian Ionotropic GABAA and GABAρ Receptors. J. Ethnopharmacol. 2015, 171, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Bhattacharya, A.; Sairam, K.; Ghosal, S. Anxiolytic-Antidepressant Activity of Withania somnifera Glycowithanolides: An Experimental Study. Phytomedicine 2000, 7, 463–469. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Sharma, D.; Salvi, M. Neuroprotective Effects of Withania somnifera Dunal.: A Possible Mechanism. Neurochem. Res. 2009, 34, 1975–1983. [Google Scholar] [CrossRef]



| Items | C | WP | SD | SD + A1 | SD + A2 | SD + A3 | SD + A4 |
|---|---|---|---|---|---|---|---|
| Initial BW, g | 235.30 ± 5.51 | 232.90 ± 4.16 | 233.90 ± 6.116 | 231.90 ± 5.587 | 230.70 ± 5.768 | 233.00 ± 3.823 | 234.70 ± 5.172 |
| Final BW, g | 297.00 ± 4.99 a | 293.90 ± 3.06 a | 234.6 ± 3.54 c | 244.90 ± 6.32 bc | 250.1 ± 5.36 bc | 253.3 ± 7.22 bc | 258.6 ± 5.08 b |
| Glucose | 97.23 ± 2.57 d | 101.1 ± 3.66 d | 146.40 ± 2.78 a | 136.1 ± 3.04 ab | 128.2 ± 1.83 bc | 130.7 ± 1.56 bc | 121.9 ± 2.42 c |
| Cholesterol | 67.22 ± 1.97 | 66.96 ± 2.29 | 65.13 ± 1.21 | 64.59 ± 1.52 | 66.74 ± 1.36 | 67.35 ± 2.07 | 65.26 ± 2.72 |
| Triglyceride | 73.93 ± 2.65 | 74.39 ± 2.40 | 75.98 ± 1.97 | 73.96 ± 1.80 | 73.76 ± 2.06 | 74.45 ± 2.86 | 70.01 ± 1.85 |
| AST, U/L | 115.10 ± 1.97 ab | 116.00 ± 1.90 ab | 122.90 ± 2.25 a | 115.40 ± 2.64 ab | 112.70 ± 1.57 b | 114.00 ± 1.78 ab | 110.40 ± 2.15 b |
| ALT, U/L | 83.71 ± 2.18 | 85 ± 2.16 | 89.71 ± 1.95 | 86.14 ± 2.40 | 87 ± 2.29 | 84.14 ± 1.68 | 81.86 ± 2.20 |
| BUN | 31.07 ± 0.92 a | 30.72 ± 0.92 a | 23.1 ± 0.79 c | 24.29 ± 0.41 bc | 24.55 ± 0.53 bc | 25.31 ± 0.41 bc | 26.98 ± 0.27 b |
| Creatinine | 0.61 ± 0.016 | 0.60 ± 0.015 | 0.62 ± 0.021 | 0.58 ± 0.019 | 0.58 ± 0.017 | 0.56 ± 0.022 | 0.56 ± 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er, B.; Ozmen, B.; Sahin, E.; Orhan, C.; Sahin, N.; Morde, A.A.; Padigaru, M.; Sahin, K. A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model. Biomolecules 2025, 15, 710. https://doi.org/10.3390/biom15050710
Er B, Ozmen B, Sahin E, Orhan C, Sahin N, Morde AA, Padigaru M, Sahin K. A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model. Biomolecules. 2025; 15(5):710. https://doi.org/10.3390/biom15050710
Chicago/Turabian StyleEr, Besir, Busra Ozmen, Emre Sahin, Cemal Orhan, Nurhan Sahin, Abhijeet A. Morde, Muralidhara Padigaru, and Kazim Sahin. 2025. "A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model" Biomolecules 15, no. 5: 710. https://doi.org/10.3390/biom15050710
APA StyleEr, B., Ozmen, B., Sahin, E., Orhan, C., Sahin, N., Morde, A. A., Padigaru, M., & Sahin, K. (2025). A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model. Biomolecules, 15(5), 710. https://doi.org/10.3390/biom15050710







