The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment
Abstract
1. Introduction
2. Microbiome-Derived Metabolites and Their Role in Inflammation-Related Cancer
2.1. Major Microbial Metabolites and Their Functions
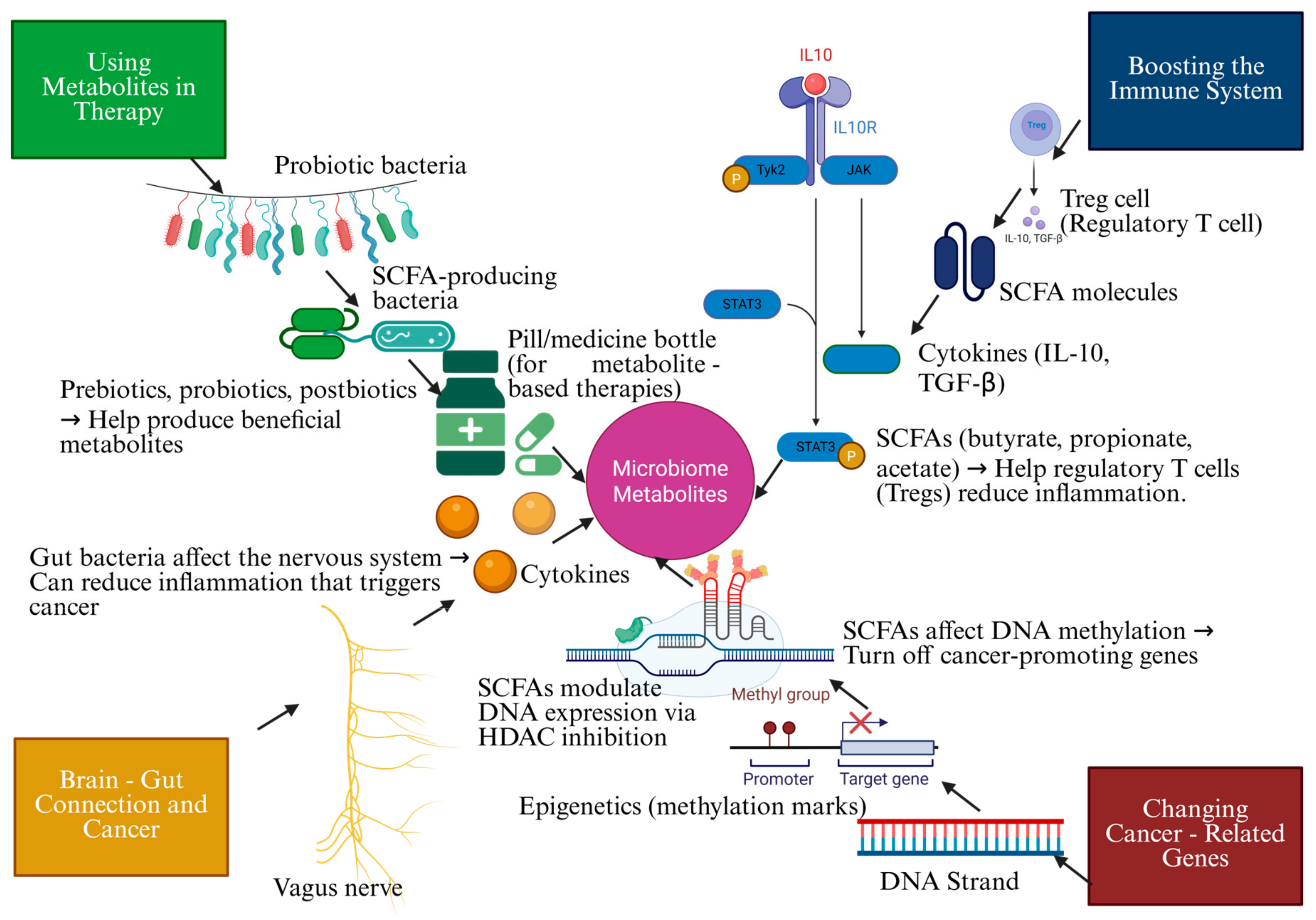
2.1.1. Short-Chain Fatty Acids (SCFAs)
2.1.2. Polyamines
2.1.3. Indoles and Tryptophan Metabolites
2.1.4. Bile Acid Derivatives
2.1.5. Lipopolysaccharide (LPS)—The Pro-Inflammatory Metabolite
3. Molecular Mechanisms Linking Microbiome Metabolites to Cancer Prevention
3.1. Immunomodulation and Anti-Inflammatory Pathways
3.1.1. SCFAs and Regulatory T Cell Activation
3.1.2. Gut Microbiota and Tumor Microenvironment Modulation
3.2. Epigenetic and Metabolic Reprogramming
3.2.1. Microbiome Influence on Oncogene Expression and DNA Methylation
3.2.2. Metabolite-Driven Epigenetic Changes in Inflammation
3.3. Gut–Brain Axis and Systemic Effects on Cancer
Neuroimmune Interactions and Inflammatory Signaling Pathways
4. Microbiome Metabolites in Cancer Therapy: Innovations and Emerging Strategies
4.1. Prebiotic- and Probiotic-Based Therapies
4.2. Postbiotics and Metabolite Supplementation
4.3. Synthetic Microbiome Engineering for Precision Medicine
4.4. Metabolite-Targeted Drug Development
5. Case Studies and Controversies in Microbiome Metabolite Research
5.1. Clinical Trials on SCFAs in Colorectal Cancer Prevention
5.2. Challenges and Controversies in Metabolite-Based Cancer Therapy
6. Complications, Limitations, and Future Perspectives
6.1. Complications and Limitations
6.1.1. Variability in Individual Microbiomes
6.1.2. Bioavailability and Stability of Metabolites
6.1.3. Regulatory and Ethical Considerations
6.2. Future Research Directions
6.2.1. Personalized Microbiome-Based Therapies
6.2.2. Advanced Multi-Omics Approaches for Precision Medicine
6.2.3. Integration of Microbiome Metabolites with Immunotherapy and Chemotherapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFAs | Short-chain fatty acids |
| AHR | Aryl hydrocarbon receptor |
| LCA | Lithocholic acid |
| Tregs | Regulatory T cells |
| HDAC | Histone deacetylase |
| NK | Natural killer |
| AI | Artificial intelligence |
References
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Pan, M.; Yang, F.; Yu, Y.; Qian, Z. Role of the Gut Microbiota in Tumorigenesis and Treatment. Theranostics 2024, 14, 2304–2328. [Google Scholar] [CrossRef]
- Anwer, E.K.E.; Ajagbe, M.; Sherif, M.; Musaibah, A.S.; Mahmoud, S.; ElBanbi, A.; Abdelnaser, A. Gut Microbiota Secondary Metabolites: Key Roles in GI Tract Cancers and Infectious Diseases. Biomedicines 2025, 13, 100. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA. Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Mullen, N.J.; Singh, P.K. Nucleotide Metabolism: A Pan-Cancer Metabolic Dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A Systematic Framework for Understanding the Microbiome in Human Health and Disease: From Basic Principles to Clinical Translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Rebeck, O.N.; Dantas, G. Complex Interactions between the Microbiome and Cancer Immune Therapy. Crit. Rev. Clin. Lab. Sci. 2019, 56, 567–585. [Google Scholar] [CrossRef]
- Valdés-González, J.A.; Sánchez, M.; Moratilla-Rivera, I.; Iglesias, I.; Gómez-Serranillos, M.P. Immunomodulatory, Anti-Inflammatory, and Anti-Cancer Properties of Ginseng: A Pharmacological Update. Molecules 2023, 28, 3863. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Getsina, M.; Chernevskaya, E.; Beloborodova, N.; Golovnya, E.; Polyakov, P.; Kushlinskii, N. Features of Metabolites and Biomarkers in Inflammatory and Infectious Complications of Childhood Cancers. Biomedicines 2024, 12, 2101. [Google Scholar] [CrossRef]
- Nemoto, H.; Otake, M.; Matsumoto, T.; Izutsu, R.; Jehung, J.P.; Goto, K.; Osaki, M.; Mayama, M.; Shikanai, M.; Kobayashi, H.; et al. Prevention of Tumor Progression in Inflammation-Related Carcinogenesis by Anti-Inflammatory and Anti-Mutagenic Effects Brought about by Ingesting Fermented Brown Rice and Rice Bran with Aspergillus Oryzae (FBRA). J. Funct. Foods 2022, 88, 104907. [Google Scholar] [CrossRef]
- Mederle, A.L.; Semenescu, A.; Drăghici, G.A.; Dehelean, C.A.; Vlăduț, N.-V.; Nica, D.V. Sodium Butyrate: A Multifaceted Modulator in Colorectal Cancer Therapy. Medicina 2025, 61, 136. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Liao, R.; Chen, X.; Cao, Q.; Bai, L.; Ma, C.; Dai, Z.; Dong, C. AMD1 Promotes Breast Cancer Aggressiveness via a Spermidine-EIF5A Hypusination-TCF4 Axis. Breast Cancer Res. 2024, 26, 70. [Google Scholar] [CrossRef]
- Conteduca, V.; Caffo, O.; Scarpi, E.; Sepe, P.; Galli, L.; Fratino, L.; Maines, F.; Chiuri, V.E.; Santoni, M.; Zanardi, E.; et al. Immune Modulation in Prostate Cancer Patients Treated with Androgen Receptor (AR)-Targeted Therapy. J. Clin. Med. 2020, 9, 1950. [Google Scholar] [CrossRef]
- Malhotra, P.; Palanisamy, R.; Caparros-Martin, J.A.; Falasca, M. Bile Acids and Microbiota Interplay in Pancreatic Cancer. Cancers 2023, 15, 3573. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-Chain Fatty Acids in Cancer Pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, W.; Zhang, X.; Li, Y.; Wang, Y.; Peng, A.; Cao, D. Gut Microbiota and Its Metabolites in Non-Small Cell Lung Cancer and Brain Metastasis: From Alteration to Potential Microbial Markers and Drug Targets. Front. Cell. Infect. Microbiol. 2024, 13, 1211855. [Google Scholar] [CrossRef]
- Tian, C.; Deng, S.; Yang, M.; Bai, B.; Pan, Y.; Xie, G.; Zhao, D.; Wei, L. Indole-3-Carbinol and Its Main Derivative 3,3′-Diindolylmethane: Regulatory Roles and Therapeutic Potential in Liver Diseases. Biomed. Pharmacother. 2024, 180, 117525. [Google Scholar] [CrossRef]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A. Polyamines and Cancer: Implications for Chemotherapy and Chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef]
- Mowat, C.; Dhatt, J.; Bhatti, I.; Hamie, A.; Baker, K. Short Chain Fatty Acids Prime Colorectal Cancer Cells to Activate Antitumor Immunity. Front. Immunol. 2023, 14, 1190810. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, C.; Wang, L.; Xie, F.; Wu, H.-Y.; Li, T.; Bian, C.-W.; Wu, R.-L. Lipopolysaccharide Promotes Cancer Cell Migration and Invasion through METTL3/PI3K/AKT Signaling in Human Cholangiocarcinoma. Heliyon 2024, 10, e29683. [Google Scholar] [CrossRef]
- Stø, K.; Valeur, J.; Ueland, T.; Malmstrøm, G.H.; Bjerkeli, V.; Trøseid, M.; Hov, J.R.; Holm, K.; Vestad, B.; Halvorsen, B.; et al. Fecal Level of Butyric Acid, a Microbiome-Derived Metabolite, Is Increased in Patients with Severe Carotid Atherosclerosis. Sci. Rep. 2022, 12, 22378. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chen, Y.-T.; Ko, J.-L.; Chen, J.-Y.; Zheng, J.-Y.; Liao, J.-W.; Ou, C.-C. Butyrate Modulates Gut Microbiota and Anti-Inflammatory Response in Attenuating Cisplatin-Induced Kidney Injury. Biomed. Pharmacother. 2024, 181, 117689. [Google Scholar] [CrossRef]
- Klys, Y.G.; Kerimov, T.R.; Savosko, S.I.; Osadchuk, Y.S.; Smirnov, S.M.; Natrus, L.V. Unfolded Protein Response in Gastric Glandulocytes of Rats with the Pharmacological Correction of Type 2 Diabetes. Gastroenterology 2023, 57, 127–134. [Google Scholar] [CrossRef]
- Ramesh, V.; Gollavilli, P.N.; Pinna, L.; Siddiqui, M.A.; Turtos, A.M.; Napoli, F.; Antonelli, Y.; Leal-Egaña, A.; Havelund, J.F.; Jakobsen, S.T.; et al. Propionate Reinforces Epithelial Identity and Reduces Aggressiveness of Lung Carcinoma. EMBO Mol. Med. 2023, 15, e17836. [Google Scholar] [CrossRef]
- Carriche, G.M.; Almeida, L.; Stüve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. Regulating T-Cell Differentiation through the Polyamine Spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348.e11. [Google Scholar] [CrossRef]
- Ruggieri, V.; Scaricamazza, S.; Bracaglia, A.; D’Ercole, C.; Parisi, C.; D’Angelo, P.; Proietti, D.; Cappelletti, C.; Macone, A.; Lozanoska-Ochser, B.; et al. Polyamine Metabolism Dysregulation Contributes to Muscle Fiber Vulnerability in ALS. Cell Rep. 2025, 44, 115123. [Google Scholar] [CrossRef]
- Jiang, D.; Ji, C.; Zhou, X.; Wang, Z.; Sun, Q.; Wang, X.; An, X.; Ling, W.; Kang, B. Pathway Analysis of Spermidine Anti-Oxidative Stress and Inducing Autophagy in Granulosa Cells of Sichuan White Geese. Theriogenology 2024, 215, 290–301. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Liu, S. Subtype-Specific Transcription Factors Affect Polyamine Metabolism and the Tumor Microenvironment in Breast Cancer. Cancer Innov. 2025, 4, e138. [Google Scholar] [CrossRef]
- Vazquez-Medina, A.; Rodriguez-Trujillo, N.; Ayuso-Rodriguez, K.; Marini-Martinez, F.; Angeli-Morales, R.; Caussade-Silvestrini, G.; Godoy-Vitorino, F.; Chorna, N. Exploring the Interplay between Running Exercises, Microbial Diversity, and Tryptophan Metabolism along the Microbiota-Gut-Brain Axis. Front. Microbiol. 2024, 15, 1326584. [Google Scholar] [CrossRef]
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Ciudad, M.T.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; et al. Tryptophan-Derived Microbial Metabolites Activate the Aryl Hydrocarbon Receptor in Tumor-Associated Macrophages to Suppress Anti-Tumor Immunity. Immunity 2022, 55, 324–340.e8. [Google Scholar] [CrossRef]
- Raizandha, M.A.; Hidayatullah, F.; Kloping, Y.P.; Rizaldi, F. Neutrophil-Lymphocyte Ratio and Fournier Gangrene Severity Index Are Not Prognostic Factors of Mortality in Fournier Gangrene Patients. Universa Med. 2022, 41, 71–78. [Google Scholar] [CrossRef]
- Suraya, R.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Microbiome as a Target for Cancer Therapy. Integr. Cancer Ther. 2020, 19, 1534735420920721. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, X.; Yu, Y.; Cui, Y.; Wang, W.; Luo, H.; Stergiadis, S.; Wang, B. Rumen Microbiome-Driven Insight into Bile Acid Metabolism and Host Metabolic Regulation. ISME J. 2024, 18, wrae098. [Google Scholar] [CrossRef]
- Choi, S.I.; Son, J.H.; Kim, N.; Kim, Y.S.; Nam, R.H.; Park, J.H.; Song, C.-H.; Yu, J.E.; Lee, D.H.; Yoon, K.; et al. Changes in Cecal Microbiota and Short-Chain Fatty Acid During Lifespan of the Rat. J. Neurogastroenterol. Motil. 2021, 27, 134–146. [Google Scholar] [CrossRef]
- Kumar, R.; Grinberg, A.V.; Li, H.; Kuo, T.-H.; Sako, D.; Krishnan, L.; Liharska, K.; Li, J.; Grenha, R.; Maguire, M.C.; et al. Functionally Diverse Heteromeric Traps for Ligands of the Transforming Growth Factor-β Superfamily. Sci. Rep. 2021, 11, 18341. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, B.; Liu, X.; Li, A. The Mediating Role of Metabolites between Gut Microbiome and Hirschsprung Disease: A Bidirectional Two-Step Mendelian Randomization Study. Front. Pediatr. 2024, 12, 1371933. [Google Scholar] [CrossRef]
- Liu, C.; Fu, L.; Wang, Y.; Yang, W. Influence of the Gut Microbiota on Immune Cell Interactions and Cancer Treatment. J. Transl. Med. 2024, 22, 939. [Google Scholar] [CrossRef]
- Jaouhari, Y.; Ferreira-Santos, P.; Disca, V.; Oliveira, H.; Martoccia, M.; Travaglia, F.; Gullón, B.; Mateus, N.; Coïsson, J.D.; Bordiga, M. Carbohydrases Treatment on Blueberry Pomace: Influence on Chemical Composition and Bioactive Potential. LWT 2024, 206, 116573. [Google Scholar] [CrossRef]
- Ranf, S.; Scheel, D.; Lee, J. Challenges in the Identification of Microbe-associated Molecular Patterns in Plant and Animal Innate Immunity: A Case Study with Bacterial Lipopolysaccharide. Mol. Plant Pathol. 2016, 17, 1165–1169. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and Function: Lipid A Modifications in Commensals and Pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Mo, C.; Lou, X.; Xue, J.; Shi, Z.; Zhao, Y.; Wang, F.; Chen, G. The Influence of Akkermansia Muciniphila on Intestinal Barrier Function. Gut Pathog. 2024, 16, 41. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and Function of Faecalibacterium Prausnitzii in Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Tiwari, A.; Ika Krisnawati, D.; Susilowati, E.; Mutalik, C.; Kuo, T.-R. Next-Generation Probiotics and Chronic Diseases: A Review of Current Research and Future Directions. J. Agric. Food Chem. 2024, 72, 27679–27700. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome Is Associated With Favorable Responses to Anti–Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Modulation of the Neuro–Cancer Connection by Metabolites of Gut Microbiota. Biomolecules 2025, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Su, W.; Tan, Z.; Zhang, J.; Dong, W. The Interaction between Microbiota and Immune in Intestinal Inflammatory Diseases: Global Research Status and Trends. Front. Cell. Infect. Microbiol. 2023, 13, 1128249. [Google Scholar] [CrossRef]
- Yu, Y.; Zi, Y.; Fu, R.; Fu, B.; Li, C.; Lv, Y.; Li, Z.; Wang, H.; Leng, J. Effects of Dietary Energy Levels on Microorganisms and Short-Chain Fatty Acids of Rumen and Tight Junction Proteins in Honghe Yellow Cattle. Front. Microbiol. 2024, 15, 1335818. [Google Scholar] [CrossRef]
- Maseda, D.; Manfredo-Vieira, S.; Payne, A.S. T Cell and Bacterial Microbiota Interaction at Intestinal and Skin Epithelial Interfaces. Discov. Immunol. 2023, 2, kyad024. [Google Scholar] [CrossRef]
- Ju, H.J.; Song, W.H.; Shin, J.H.; Lee, J.H.; Bae, J.M.; Lee, Y.B.; Lee, M. Characterization of Gut Microbiota in Patients with Active Spreading Vitiligo Based on Whole-Genome Shotgun Sequencing. Int. J. Mol. Sci. 2025, 26, 2939. [Google Scholar] [CrossRef]
- Mishima, R.; Ejima, R.; Arai, S.; Horigome, A.; Mitsuyama, E.; Kaneko, H.; Yamaguchi, K.; Kamezaki, K.; Togashi, Y.; Nakamura, K.; et al. Synbiotic Combination of Bifidobacterium Longum BB536 and Lactulose Improves the Presenteeism of Healthy Adults Associated with Aromatic Lactic Acids—A Single-Arm, Open-Label Study. Gut Microbes Rep. 2025, 2. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liao, B.; Wang, M.; Lin, H.; Hu, B.; Lan, X.; Shu, Z.; Zhang, C.; Yu, M.; et al. Orally Administrated Hydrogel Harnessing Intratumoral Microbiome and Microbiota-Related Immune Responses for Potentiated Colorectal Cancer Treatment. Research 2024, 7, 0364. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, W.; Feng, Y.; Wang, Y.; Sun, T.; Xu, J. Microbial Metabolites Affect Tumor Progression, Immunity and Therapy Prediction by Reshaping the Tumor Microenvironment (Review). Int. J. Oncol. 2024, 65, 73. [Google Scholar] [CrossRef]
- Vojdani, A.; Koksoy, S.; Vojdani, E.; Engelman, M.; Benzvi, C.; Lerner, A. Natural Killer Cells and Cytotoxic T Cells: Complementary Partners against Microorganisms and Cancer. Microorganisms 2024, 12, 230. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Kumari, S.; Srilatha, M.; Nagaraju, G.P. Effect of Gut Dysbiosis on Onset of GI Cancers. Cancers 2024, 17, 90. [Google Scholar] [CrossRef]
- Niam, M.S.; Nastiti, N.A.; Dradjat, R.S.; Rudijanto, A.; Sujuti, H. Do Tumor Locations and Stages at Diagnosis Predict the 5-Year Survival Outcome in Patients with Colorectal Cancer? Open Access Maced. J. Med. Sci. 2022, 10, 1663–1666. [Google Scholar] [CrossRef]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-Chain Fatty Acid Metabolites Propionate and Butyrate Are Unique Epigenetic Regulatory Elements Linking Diet, Metabolism and Gene Expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.-L.; Xiong, Y.; Ye, D. Metabolite Regulation of Epigenetics in Cancer. Cell Rep. 2024, 43, 114815. [Google Scholar] [CrossRef]
- Reva, K.; Laranjinha, J.; Rocha, B.S. Epigenetic Modifications Induced by the Gut Microbiota May Result from What We Eat: Should We Talk about Precision Diet in Health and Disease? Metabolites 2023, 13, 375. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel Epigenetic Therapeutic Strategies and Targets in Cancer. Biochim. Biophys. Acta—Mol. Basis Dis. 2022, 1868, 166552. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the Link between Chronic Inflammation and Cancer. Metab. Open 2025, 25, 100347. [Google Scholar] [CrossRef]
- Huang, Z.; Boekhorst, J.; Fogliano, V.; Capuano, E.; Wells, J.M. Impact of High-Fiber or High-Protein Diet on the Capacity of Human Gut Microbiota To Produce Tryptophan Catabolites. J. Agric. Food Chem. 2023, 71, 6956–6966. [Google Scholar] [CrossRef]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Li, Y.; Zou, Q.; Yang, M.; Li, H.; Niu, R.; Lai, H.; Wang, J.; Yang, X.; Zhou, L. Gut Microbiota and Serum Metabolomic Alterations in Modulating the Impact of Fecal Microbiota Transplantation on Ciprofloxacin-Induced Seizure Susceptibility. Front. Microbiol. 2024, 15, 1403892. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Effendi, R.M.R.A.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia Muciniphila and Faecalibacterium Prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium Prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Jan, T.; Negi, R.; Sharma, B.; Kumar, S.; Singh, S.; Rai, A.K.; Shreaz, S.; Rustagi, S.; Chaudhary, N.; Kaur, T.; et al. Next Generation Probiotics for Human Health: An Emerging Perspective. Heliyon 2024, 10, e35980. [Google Scholar] [CrossRef]
- Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. [Google Scholar] [CrossRef]
- Williams, L.M.; Cao, S. Harnessing and Delivering Microbial Metabolites as Therapeutics via Advanced Pharmaceutical Approaches. Pharmacol. Ther. 2024, 256, 108605. [Google Scholar] [CrossRef]
- Alkhaldy, A.A. Awareness, Knowledge, and Beliefs about Probiotics and Prebiotics among Saudi Adults: A Cross-Sectional Study. Front. Immunol. 2024, 15, 1464622. [Google Scholar] [CrossRef]
- Barber, C.; Sabater, C.; Dolores Frutos, M.; Vallejo, F.; Guyonnet, D.; Daniel, N.; Guarner, F.; Carlos Espín, J.; Margolles, A.; Azpiroz, F. Effects of a (Poly)Phenol-Rich Berry Mix on Gas Production in Healthy Individuals: An Integrated Clinical, Metagenomic, and Metabolomic Proof-of-Concept Study. J. Funct. Foods 2024, 113, 106032. [Google Scholar] [CrossRef]
- Tian, Q.; Ye, H.; Zhou, X.; Wang, J.; Zhang, L.; Sun, W.; Duan, C.; Fan, M.; Zhou, W.; Bi, C.; et al. Evaluating the Health Risk of Probiotic Supplements from the Perspective of Antimicrobial Resistance. Microbiol. Spectr. 2025, 13, e0001924. [Google Scholar] [CrossRef]
- Nama, A.S.A.; Sandeepa, G.M.; Buddolla, V.; Mastan, A. Advances in Understanding Therapeutic Mechanisms of Probiotics in Cancer Management, with Special Emphasis on Breast Cancer: A Comprehensive Review. Eur. J. Pharmacol. 2025, 995, 177410. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhang, X.; Wang, R.; Han, S.; Qin, P.; Xing, X.-H.; Zhang, C. Gut Lumen-Targeted Oral Delivery System for Bioactive Agents to Regulate Gut Microbiome. J. Futur. Foods 2022, 2, 307–325. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- LaPointe, G.; Rogers, M.A. Microorganisms Special Issue “How Do Food and Probiotics Influence the Composition and Activity of the Gut Microbiota? ” Microorganisms 2022, 10, 2097. [Google Scholar] [CrossRef]
- Ghosh, S.; Yang, X.; Wang, L.; Zhang, C.; Zhao, L. Active Phase Prebiotic Feeding Alters Gut Microbiota, Induces Weight-Independent Alleviation of Hepatic Steatosis and Serum Cholesterol in High-Fat Diet-Fed Mice. Comput. Struct. Biotechnol. J. 2021, 19, 448–458. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Poveshchenko, A.F.; Kabakov, A.V.; Bodrova, N.R.; Kapustina, V.I.; Kazakov, O.V.; Koldysheva, E.V.; Cherkas, V.N.; Afonyushkin, V.N. Microbiota of the Mammary Gland in Wistar Rats with Chemically Induced Breast Cancer after Treatment. Bull. Exp. Biol. Med. 2024, 178, 223–226. [Google Scholar] [CrossRef]
- Islam, F.; Azmat, F.; Imran, A.; Zippi, M.; Hong, W.; Tariq, F.; Shehzadi, U.; Fatima, A.; Safdar, M.; Ahmed, H.; et al. Role of Postbiotics in Food and Health: A Comprehensive Review. CyTA—J. Food 2024, 22, 2386412. [Google Scholar] [CrossRef]
- Attebury, H.; Daley, D. The Gut Microbiome and Pancreatic Cancer Development and Treatment. Cancer J. 2023, 29, 49–56. [Google Scholar] [CrossRef]
- Xiu, W.; Dong, H.; Chen, X.; Wan, L.; Lu, L.; Yang, K.; Yuwen, L.; Li, Q.; Ding, M.; Zhang, Y.; et al. Metabolic Modulation-Mediated Antibiotic and Immune Activation for Treatment of Chronic Lung Infections. ACS Nano 2024, 18, 15204–15217. [Google Scholar] [CrossRef]
- Tanaka, T.; Sugiyama, R.; Sato, Y.; Kawaguchi, M.; Honda, K.; Iwaki, H.; Okano, K. Precise Microbiome Engineering Using Natural and Synthetic Bacteriophages Targeting an Artificial Bacterial Consortium. Front. Microbiol. 2024, 15, 1403903. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Cornista, A.M.; Giolito, M.V.; Baker, K.; Hazime, H.; Dufait, I.; Datta, J.; Khumukcham, S.S.; De Ridder, M.; Roper, J.; Abreu, M.T.; et al. Colorectal Cancer Immunotherapy: State of the Art and Future Directions. Gastro Hep Adv. 2023, 2, 1103–1119. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef]
- Su, A.C.Y.; Ding, X.; Lau, H.C.H.; Kang, X.; Li, Q.; Wang, X.; Liu, Y.; Jiang, L.; Lu, Y.; Liu, W.; et al. Lactococcus Lactis HkyuLL 10 Suppresses Colorectal Tumourigenesis and Restores Gut Microbiota through Its Generated Alpha-Mannosidase. Gut 2024, 73, 1478–1488. [Google Scholar] [CrossRef]
- DiMattia, Z.; Damani, J.; Rogers, C.J. 75 Effect of Probiotic Supplementation on Intestinal Permeability in Subjects with Overweight and Obesity: A Systematic Review of Randomized Controlled Trials. J. Clin. Transl. Sci. 2023, 7, 20–21. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a Promising Therapeutic Target in Cancer: From Pathogenesis to Clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Soliman, S.M.; Haukka, M.; Al-Shaalan, N.H.; Alkharboush, A.A.; Barakat, A. Synthesis, Characterization, and Cytotoxicity of New Spirooxindoles Engrafted Furan Structural Motif as a Potential Anticancer Agent. ACS Omega 2022, 7, 35743–35754. [Google Scholar] [CrossRef]
- Bober, J.R.; Beisel, C.L.; Nair, N.U. Synthetic Biology Approaches to Engineer Probiotics and Members of the Human Microbiota for Biomedical Applications. Annu. Rev. Biomed. Eng. 2018, 20, 277–300. [Google Scholar] [CrossRef]
- Yadegar, A.; Salahi-Niri, A.; Wang, Y.-D.; Ochoa-Repáraz, J. Editorial: Gut Microbiota and Gastrointestinal Disorders, Volume II. Front. Med. 2025, 12, 1576152. [Google Scholar] [CrossRef]
- Liu, J.; Tian, R.; Sun, C.; Guo, Y.; Dong, L.; Li, Y.; Song, X. Microbial Metabolites Are Involved in Tumorigenesis and Development by Regulating Immune Responses. Front. Immunol. 2023, 14, 1290414. [Google Scholar] [CrossRef]
- Kang, X.; Lau, H.C.-H.; Yu, J. Modulating Gut Microbiome in Cancer Immunotherapy: Harnessing Microbes to Enhance Treatment Efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef]
- Javdan, B.; Lopez, J.G.; Chankhamjon, P.; Lee, Y.-C.J.; Hull, R.; Wu, Q.; Wang, X.; Chatterjee, S.; Donia, M.S. Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell 2020, 181, 1661–1679.e22. [Google Scholar] [CrossRef]
- Schwarcz, S.; Nyerges, P.; Bíró, T.I.; Janka, E.; Bai, P.; Mikó, E. Cytostatic Bacterial Metabolites Interfere with 5-Fluorouracil, Doxorubicin and Paclitaxel Efficiency in 4T1 Breast Cancer Cells. Molecules 2024, 29, 3073. [Google Scholar] [CrossRef]
- Shanmugam, G.; Rakshit, S.; Sarkar, K. HDAC Inhibitors: Targets for Tumor Therapy, Immune Modulation and Lung Diseases. Transl. Oncol. 2022, 16, 101312. [Google Scholar] [CrossRef]
- Agarwal, D.S.; Mazumdar, S.; Italiya, K.S.; Chitkara, D.; Sakhuja, R. Bile-Acid-Appended Triazolyl Aryl Ketones: Design, Synthesis, In Vitro Anticancer Activity and Pharmacokinetics in Rats. Molecules 2021, 26, 5741. [Google Scholar] [CrossRef]
- Goering, A.W.; McClure, R.A.; Doroghazi, J.R.; Albright, J.C.; Haverland, N.A.; Zhang, Y.; Ju, K.-S.; Thomson, R.J.; Metcalf, W.W.; Kelleher, N.L. Metabologenomics: Correlation of Microbial Gene Clusters with Metabolites Drives Discovery of a Nonribosomal Peptide with an Unusual Amino Acid Monomer. ACS Cent. Sci. 2016, 2, 99–108. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Ahasan, M.T.; Sarkar, N.; Khan, H.; Hasan, A.M.; Cavalu, S.; Rauf, A. Microbiome in Cancer: Role in Carcinogenesis and Impact in Therapeutic Strategies. Biomed. Pharmacother. 2022, 149, 112898. [Google Scholar] [CrossRef]
- Jotshi, A.; Sukla, K.K.; Haque, M.M.; Bose, C.; Varma, B.; Koppiker, C.B.; Joshi, S.; Mishra, R. Exploring the Human Microbiome—A Step Forward for Precision Medicine in Breast Cancer. Cancer Rep. 2023, 6, e1877. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Y.; Wang, Y. Microbiome Analysis Reveals Dynamic Changes of Gut Microbiota in Guizhou Horse and Dutch Warmblood Horses. Front. Microbiol. 2025, 16, 1562482. [Google Scholar] [CrossRef]
- Dai, J.-H.; Tan, X.-R.; Qiao, H.; Liu, N. Emerging Clinical Relevance of Microbiome in Cancer: Promising Biomarkers and Therapeutic Targets. Protein Cell 2024, 15, 239–260. [Google Scholar] [CrossRef]
- Saravanan, C.; Gopinath, N.K.; Ganesan, R.; Thirumurugan, D. Challenges and Limitations in Using Bacterial Metabolites as Immunomodulators. Front. Cell. Infect. Microbiol. 2025, 15, 1535394. [Google Scholar] [CrossRef]
- Moniri, N.H.; Farah, Q. Short-Chain Free-Fatty Acid G Protein-Coupled Receptors in Colon Cancer. Biochem. Pharmacol. 2021, 186, 114483. [Google Scholar] [CrossRef]
- Tsukanov, V.V.; Vasyutin, A.V.; Tonkikh, J.L. Risk Factors, Prevention and Screening of Colorectal Cancer: A Rising Problem. World J. Gastroenterol. 2025, 31, 98629. [Google Scholar] [CrossRef]
- Rana, A.K.; Kumar Saraswati, S.S.; Anang, V.; Singh, A.; Singh, A.; Verma, C.; Natarajan, K. Butyrate Induces Oxidative Burst Mediated Apoptosis via Glucose-6-Phosphate Dehydrogenase (G6PDH) in Macrophages during Mycobacterial Infection. Microbes Infect. 2024, 26, 105271. [Google Scholar] [CrossRef]
- Bin Lee, D.; Hwang, I.S. Macronutrient Balance Determines the Human Gut Microbiome Eubiosis: Insights from in Vitro Gastrointestinal Digestion and Fermentation of Eight Pulse Species. Front. Microbiol. 2025, 15, 1512217. [Google Scholar] [CrossRef]
- Sayol-Altarriba, A.; Aira, A.; Villasante, A.; Albarracín, R.; Faneca, J.; Casals, G.; Villanueva-Cañas, J.L.; Casals-Pascual, C. Normalization of Short-Chain Fatty Acid Concentration by Bacterial Count of Stool Samples Improves Discrimination between Eubiotic and Dysbiotic Gut Microbiota Caused by Clostridioides Difficile Infection. Gut Microbes 2024, 16, 2415488. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Albaladejo-Riad, N.; El Qendouci, M.; Cuesta, A.; Esteban, M.Á. Ability of Short-Chain Fatty Acids to Reduce Inflammation and Attract Leucocytes to the Inflamed Skin of Gilthead Seabream (Sparus aurata L.). Sci. Rep. 2024, 14, 31404. [Google Scholar] [CrossRef]
- Sun, M.; Ji, W.; Ye, H.; Cai, Y.; Yun, Y.; Wei, X.; Wang, C.; Mao, H. Sodium Butyrate Administration Improves Intestinal Development of Suckling Lambs. J. Anim. Sci. 2024, 102, skae028. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Kaneko, M.; Narukawa, M. Factors Associated with Successful Phase III Trials for Solid Tumors: A Systematic Review. Contemp. Clin. Trials Commun. 2021, 24, 100855. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lynch, E.; Tillman, A.; Lewis, K.; Jin, Z.; Uhlemann, A.-C.; Abrams, J.A.; Freedberg, D.E. A Phase 2 Randomized, Placebo-Controlled Trial of Inulin for the Prevention of Gut Pathogen Colonization and Infection among Patients Admitted to the Intensive Care Unit for Sepsis. Crit. Care 2025, 29, 21. [Google Scholar] [CrossRef]
- Molan, K.; Ambrožič Avguštin, J.; Likar, M.; Pongrac Barlovic, D.; Žgur Bertok, D.; Starčič Erjavec, M. Fecal Short-Chain Fatty Acids Are Associated with Obesity in Gestational Diabetes. Biomedicines 2025, 13, 387. [Google Scholar] [CrossRef]
- Peters, V.B.M.; Arulkumaran, N.; Melis, M.J.; Gaupp, C.; Roger, T.; Shankar-Hari, M.; Singer, M. Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis. Life 2022, 12, 2034. [Google Scholar] [CrossRef]
- Reim, D.; Novotny, A.; Friess, H.; Slotta-Huspenina, J.; Weichert, W.; Ott, K.; Dislich, B.; Lorenzen, S.; Becker, K.; Langer, R. Significance of Tumour Regression in Lymph Node Metastases of Gastric and Gastro-oesophageal Junction Adenocarcinomas. J. Pathol. Clin. Res. 2020, 6, 263–272. [Google Scholar] [CrossRef]
- Tian, T.; Zhao, Y.; Yang, Y.; Wang, T.; Jin, S.; Guo, J.; Liu, Z. The Protective Role of Short-Chain Fatty Acids Acting as Signal Molecules in Chemotherapy- or Radiation-Induced Intestinal Inflammation. Am. J. Cancer Res. 2020, 10, 3508–3531. [Google Scholar]
- Machado, D.T.; Dias, B.d.C.; Cayô, R.; Gales, A.C.; Marques de Carvalho, F.; Vasconcelos, A.T.R. Uncovering New Firmicutes Species in Vertebrate Hosts through Metagenome-Assembled Genomes with Potential for Sporulation. Microbiol. Spectr. 2024, 12, e0211324. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Laborda-Illanes, A.; Otero, A.; Ordóñez, R.; González-González, A.; Plaza-Andrades, I.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Relationships of Gut Microbiota Composition, Short-Chain Fatty Acids and Polyamines with the Pathological Response to Neoadjuvant Radiochemotherapy in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 9549. [Google Scholar] [CrossRef]
- Yue, F.; Li, W.; Zou, J.; Jiang, X.; Xu, G.; Huang, H.; Liu, L. Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res. 2017, 77, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer Pharmacoprevention: Targeting Polyamine Metabolism to Manage Risk Factors for Colon Cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Liang, J.; Bian, Y.; Shan, G.; Huang, Y.; Lu, T.; Zhang, H.; Jin, X.; Chen, Z.; Zhao, M.; et al. Polyamine-Mediated Ferroptosis Amplification Acts as a Targetable Vulnerability in Cancer. Nat. Commun. 2024, 15, 2461. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine Metabolism and Cancer: Treatments, Challenges and Opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Geck, R.C.; Foley, J.R.; Murray Stewart, T.; Asara, J.M.; Casero, R.A.; Toker, A. Inhibition of the Polyamine Synthesis Enzyme Ornithine Decarboxylase Sensitizes Triple-Negative Breast Cancer Cells to Cytotoxic Chemotherapy. J. Biol. Chem. 2020, 295, 6263–6277. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-Inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Roy, S.; Kumaravel, S.; Sharma, A.; Duran, C.L.; Bayless, K.J.; Chakraborty, S. Hypoxic Tumor Microenvironment: Implications for Cancer Therapy. Exp. Biol. Med. 2020, 245, 1073–1086. [Google Scholar] [CrossRef]
- Firoozi, D.; Masoumi, S.J.; Mohammad-Kazem Hosseini Asl, S.; Labbe, A.; Razeghian-Jahromi, I.; Fararouei, M.; Lankarani, K.B.; Dara, M. Effects of Short-Chain Fatty Acid-Butyrate Supplementation on Expression of Circadian-Clock Genes, Sleep Quality, and Inflammation in Patients with Active Ulcerative Colitis: A Double-Blind Randomized Controlled Trial. Lipids Health Dis. 2024, 23, 216. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, J.; Zhao, R.; Li, H.; Cui, H.; Yuan, Z.; Ren, H.; Cao, B.; Wei, B. Akkermansia Muciniphila-Derived Pentadecanoic Acid Enhances Oxaliplatin Sensitivity in Gastric Cancer by Modulating Glycolysis. Pharmacol. Res. 2024, 206, 107278. [Google Scholar] [CrossRef]
- Bradlow, H.L.; Michnovicz, J.J.; Telang, N.T.; Osborne, M.P. Effects of Dietary Indole-3-Carbinol on Estradiol Metabolism and Spontaneous Mammary Tumors in Mice. Carcinogenesis 1991, 12, 1571–1574. [Google Scholar] [CrossRef]
- Mousa, W.K.; Mousa, S.; Ghemrawi, R.; Obaid, D.; Sarfraz, M.; Chehadeh, F.; Husband, S. Probiotics Modulate Host Immune Response and Interact with the Gut Microbiota: Shaping Their Composition and Mediating Antibiotic Resistance. Int. J. Mol. Sci. 2023, 24, 13783. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.; Shiryan, I.; Borenstein, E. Multi-Omic Integration of Microbiome Data for Identifying Disease-Associated Modules. Nat. Commun. 2024, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Mladosievicova, B.; Mego, M. Microbiome in Cancer Development and Treatment. Microorganisms 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Auster, A.; Cho, S.; Lai, Z. Dissecting the Human Gut Microbiome to Better Decipher Drug Liability: A Once-Forgotten Organ Takes Center Stage. J. Adv. Res. 2023, 52, 171–201. [Google Scholar] [CrossRef]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the Concept of Gut Microbiota Dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. Human Microbiome Variance Is Underestimated. Curr. Opin. Microbiol. 2023, 73, 102288. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of Short-Chain Fatty Acids (SCFAs) as Chemicals or Substrates for Microbes to Obtain Biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 96. [Google Scholar] [CrossRef]
- Hardwick, A.; Cummings, C.; Graves, J.; Kuzma, J. Can Societal and Ethical Implications of Precision Microbiome Engineering Be Applied to the Built Environment? A Systematic Review of the Literature. Environ. Syst. Decis. 2024, 44, 215–238. [Google Scholar] [CrossRef]
- Manrique, P.; Montero, I.; Fernandez-Gosende, M.; Martinez, N.; Cantabrana, C.H.; Rios-Covian, D. Past, Present, and Future of Microbiome-Based Therapies. Microbiome Res. Rep. 2024, 3, 23. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-Derived Short-Chain Fatty Acids and Modulation of Host-Derived Peptides Formation: Focused on Host Defense Peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Das, B. Microbiome-Based Therapeutics: Opportunity and Challenges. Prog. Mol. Biol. Transl. Sci. 2022, 191, 229–262. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The Influence of Antibiotics and Dietary Components on Gut Microbiota. Gastroenterol. Rev. 2018, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-Based Interventions to Modulate Gut Ecology and the Immune System. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Jain, N. The Need for Personalized Approaches to Microbiome Modulation. Front. Public Health 2020, 8, 144. [Google Scholar] [CrossRef]
- Yan, Q.; Jia, S.; Li, D.; Yang, J. The Role and Mechanism of Action of Microbiota-Derived Short-Chain Fatty Acids in Neutrophils: From the Activation to Becoming Potential Biomarkers. Biomed. Pharmacother. 2023, 169, 115821. [Google Scholar] [CrossRef]
- Rahman, S. Gut Microbial Metabolites and Its Impact on Human Health. Ann. Gastroenterol. 2023, 36, 360–368. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Fekete, E.E.; Figeys, D.; Zhang, X. Microbiota-Directed Biotherapeutics: Considerations for Quality and Functional Assessment. Gut Microbes 2023, 15, 2186671. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Berg, G.; Cernava, T.; Champomier-Vergès, M.-C.; Charles, T.; Cocolin, L.; Cotter, P.; D’Hondt, K.; Kostic, T.; Maguin, E.; et al. Microbiome Ethics, Guiding Principles for Microbiome Research, Use and Knowledge Management. Environ. Microbiome 2022, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Elinav, E. Harnessing the Microbiota for Therapeutic Purposes. Am. J. Transplant. 2020, 20, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.A.; Monemi, M.; Asli, S.; Mohammadi, S.; Foroozanmehr, B.; Haghmorad, D.; Oksenych, V.; Eslami, M. Using New Technologies to Analyze Gut Microbiota and Predict Cancer Risk. Cells 2024, 13, 1987. [Google Scholar] [CrossRef]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef]
- Patil, A.; Singh, N.; Patwekar, M.; Patwekar, F.; Patil, A.; Gupta, J.K.; Elumalai, S.; Priya, N.S.; Sahithi, A. AI-Driven Insights into the Microbiota: Figuring out the Mysterious World of the Gut. Intell. Pharm. 2024, 3, 46–52. [Google Scholar] [CrossRef]
- Li, P.; Luo, H.; Ji, B.; Nielsen, J. Machine Learning for Data Integration in Human Gut Microbiome. Microb. Cell Fact. 2022, 21, 241. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; He, L.; Sun, X. The Critical Role of Tumor Microbiome in Cancer Immunotherapy. Cancer Biol. Ther. 2024, 25, 2301801. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, D.; Zhang, Z.; Xian, J.; Bai, X. Effect of Gut Microbiota-Derived Metabolites on Immune Checkpoint Inhibitor Therapy: Enemy or Friend? Molecules 2022, 27, 4799. [Google Scholar] [CrossRef]
- Soleimani, N.; Javadi, M.M. Future Prospects of Bacteria-Mediated Cancer Therapies: Affliction or Opportunity? Microb. Pathog. 2022, 172, 105795. [Google Scholar] [CrossRef]
- Lu, S.; Wang, C.; Ma, J.; Wang, Y. Metabolic Mediators: Microbial-Derived Metabolites as Key Regulators of Anti-Tumor Immunity, Immunotherapy, and Chemotherapy. Front. Immunol. 2024, 15, 1456030. [Google Scholar] [CrossRef]

| Major Findings | Metabolites Studied | Cancer Type |
|---|---|---|
| Butyrate induces apoptosis | SCFAs | Colorectal [12] |
| Propionate suppresses inflammation | SCFAs | Liver [13] |
| Spermidine regulates tumor growth | Polyamines | Breast [14] |
| Indoles modulate immune responses | Indole derivatives | Prostate [15] |
| Bile acids impact gut microbiota | Secondary bile acids | Pancreatic [16] |
| SCFAs enhance gut barrier function | SCFAs | Gastric [17] |
| Microbiome-derived metabolites impact metastasis | Various | Lung [18] |
| Indole derivatives affect inflammatory markers | Indoles | Ovarian [19] |
| Polyamine levels correlate with tumor progression | Polyamines | Multiple [20] |
| SCFAs modulate T cell differentiation | SCFAs | Colorectal [21] |
| LPS promotes chronic inflammation and tumor progression via TLR4 and METTL3/PI3K/AKT signaling | LPS | Cholangiocarcinoma [22] |
| Approach | Target | Cancer Type |
|---|---|---|
| Probiotic supplementation | Gut microbiota balance | Colorectal [85] |
| Prebiotic fiber therapy | SCFA production | Liver [86] |
| SCFA-based treatment | Tumor suppression | Gastric [87] |
| Engineered microbiome therapies | Precision medicine | Breast [88] |
| Postbiotic metabolites as drugs | Anti-inflammatory effects | Ovarian [89] |
| Microbiome modification | Tumor microenvironment | Pancreatic [90] |
| Metabolite-targeted drugs | Immune modulation | Lung [91] |
| Synthetic microbiota engineering | Personalized therapy | Multiple [92] |
| Bile acid modulation | Tumor immunotherapy | Liver [93] |
| SCFAs combined with immunotherapy | Combination therapy | Colorectal [94] |
| Trial Phase | Findings |
|---|---|
| Phase I | SCFAs reduced inflammation [120] |
| Phase II | Butyrate improved immune response [121] |
| Phase III | Mixed results on tumor regression [122] |
| Phase I | SCFA bioavailability drawbacks [123] |
| Phase II | Combination therapy with SCFAs [124] |
| Phase III | SCFA-based diets showed limited efficacy [125] |
| Phase II | Butyrate supplementation improved outcomes [126] |
| Phase III | No significant tumor regression observed [127] |
| Phase II | SCFAs enhanced chemotherapy response [128] |
| Phase III | SCFAs reduced side effects of treatment [129] |
| Complication | Findings |
|---|---|
| Microbiome variability | Microbiome composition differs across populations [147] |
| Bioavailability issues | SCFAs degrade quickly in circulation [148] |
| Ethical concerns | Engineering gut microbiota raises safety issues [149] |
| Regulatory barriers | Lack of FDA-approved microbiome therapies [150] |
| Individual response variability | Different patients respond differently to SCFAs [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. https://doi.org/10.3390/biom15050688
Mafe AN, Büsselberg D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules. 2025; 15(5):688. https://doi.org/10.3390/biom15050688
Chicago/Turabian StyleMafe, Alice N., and Dietrich Büsselberg. 2025. "The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment" Biomolecules 15, no. 5: 688. https://doi.org/10.3390/biom15050688
APA StyleMafe, A. N., & Büsselberg, D. (2025). The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules, 15(5), 688. https://doi.org/10.3390/biom15050688








