HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery
Abstract
1. Introduction
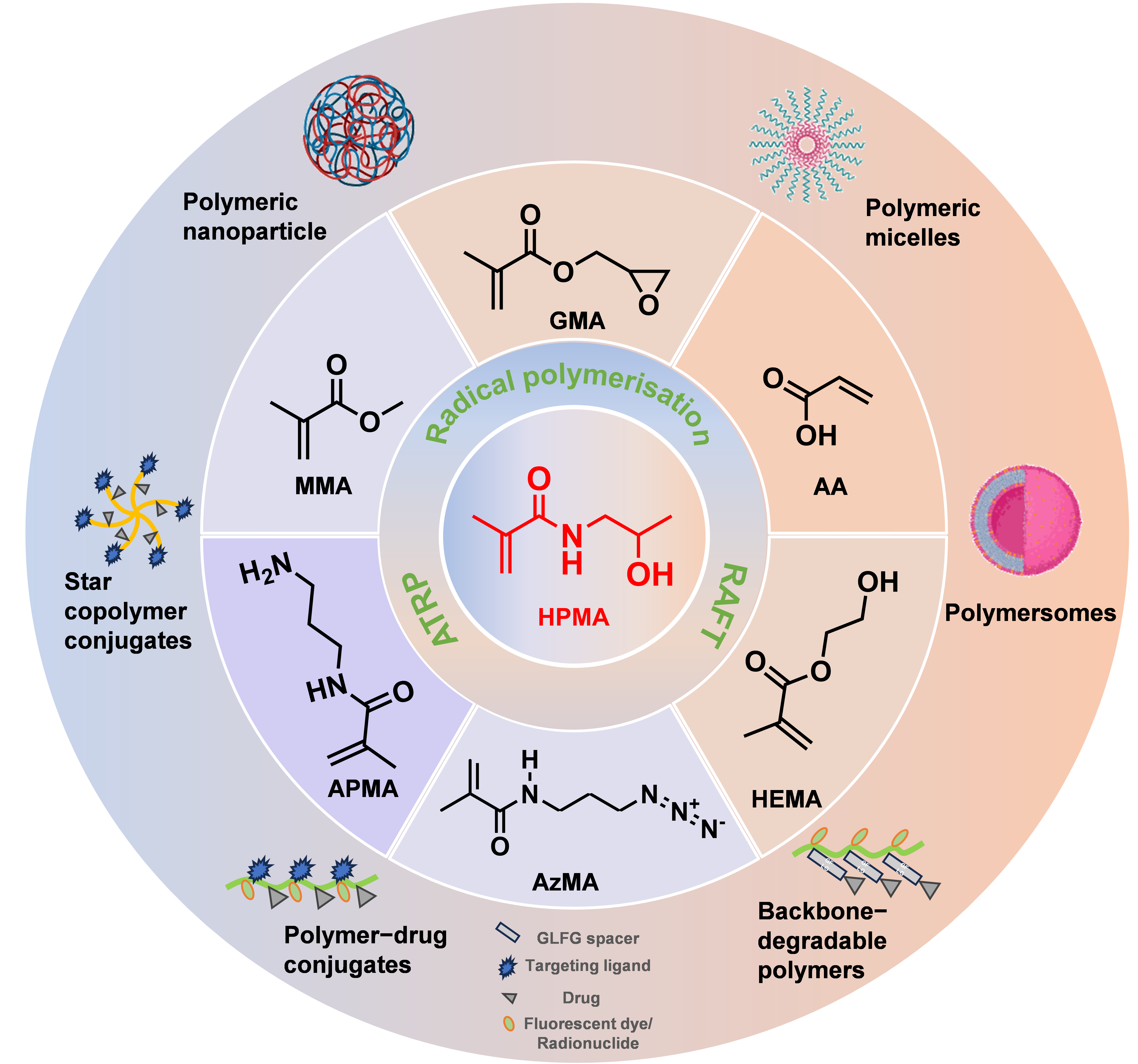
2. Advantages of HPMA as the Delivery System
2.1. Tunable Chemical Structure
2.2. Molecular Weight
2.3. Solubility
2.4. Biocompatibility
3. HPMA Copolymers as Drug Delivery System for Peptides
3.1. Active Targeted Drug Delivery System
3.1.1. Bombesin
3.1.2. Cell-Penetrating Peptides
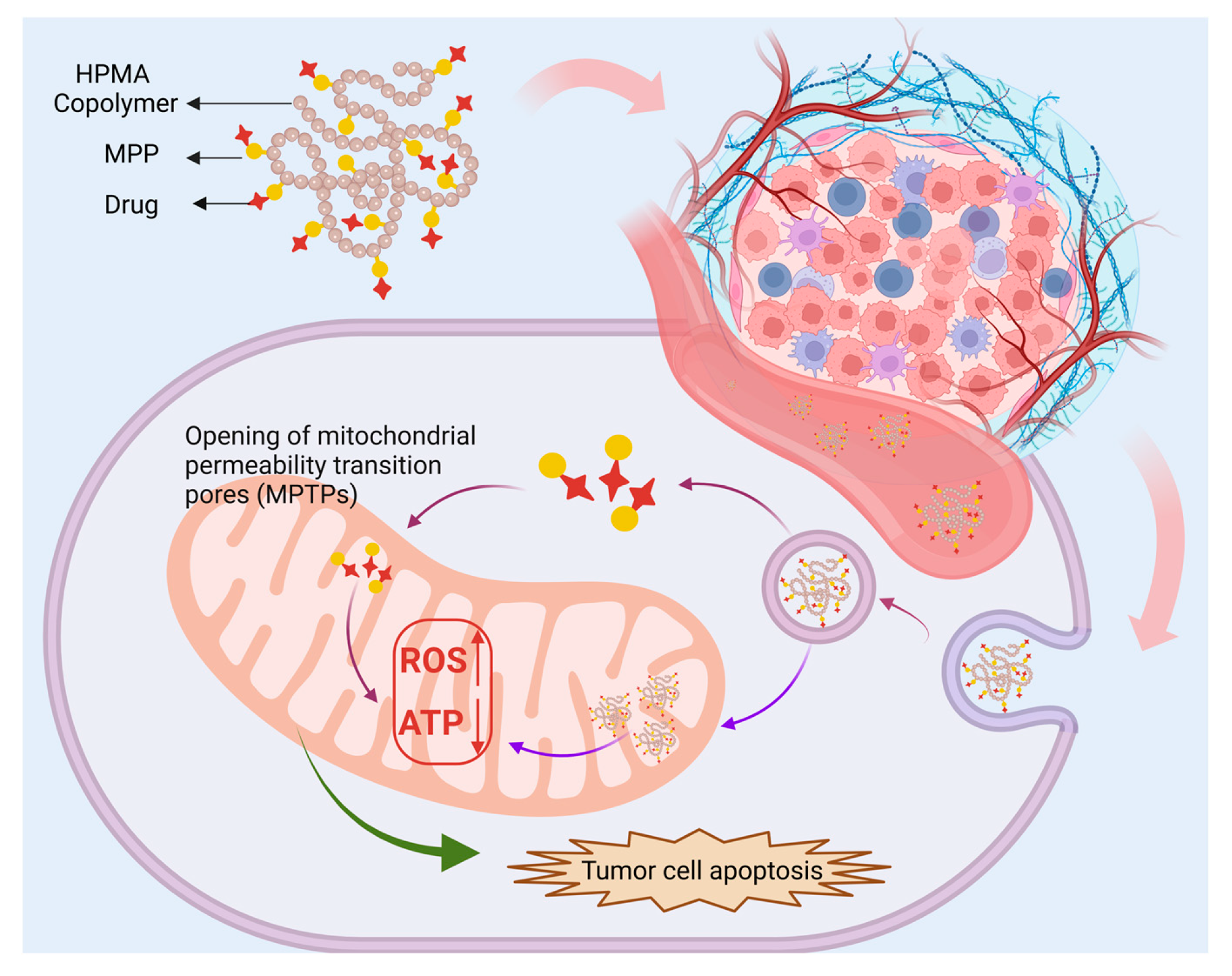
3.1.3. Mitochondria-Targeted Peptides
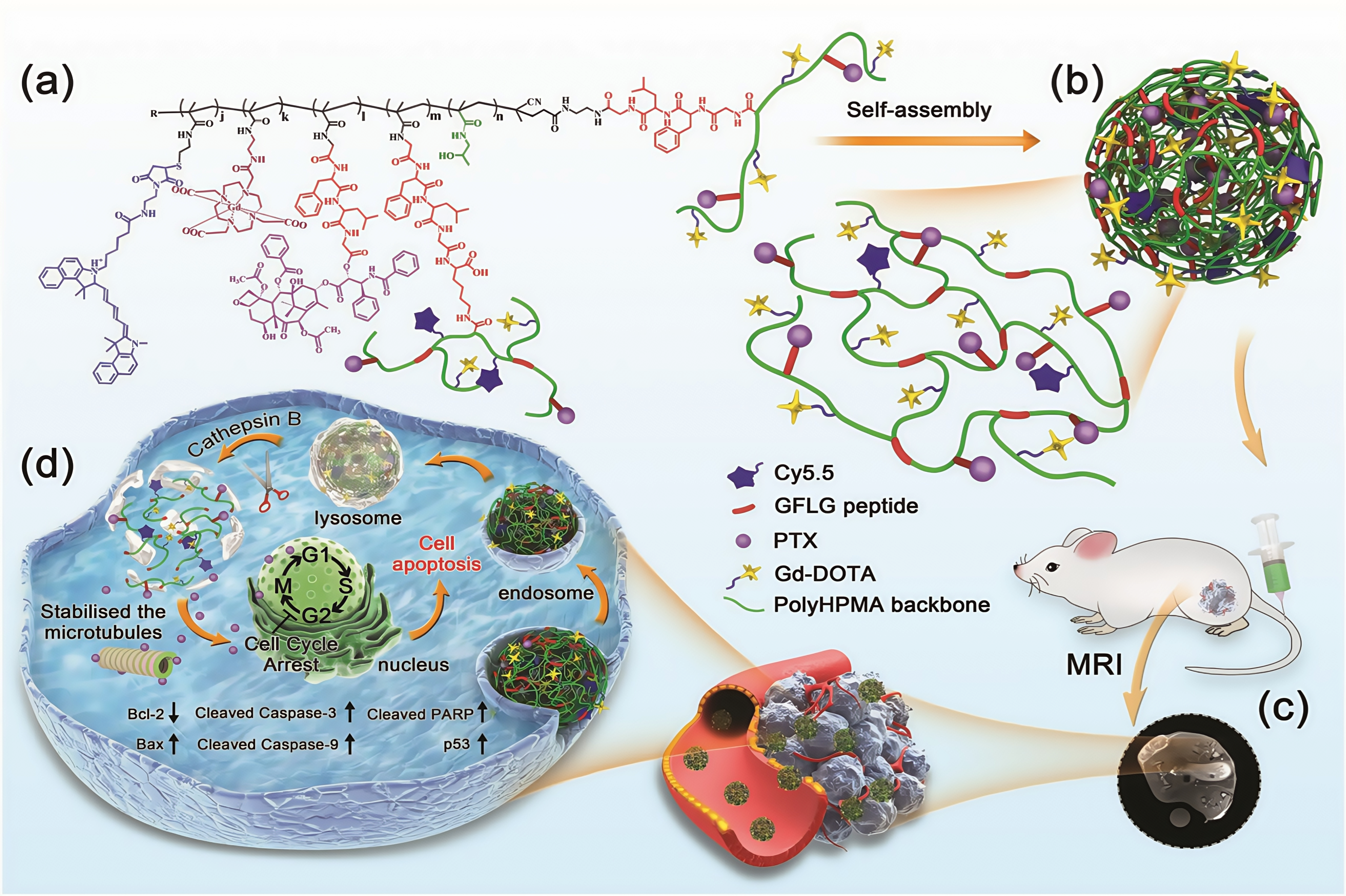
3.1.4. αVβ3 Targeting Peptides
3.1.5. Nucleus-Targeting Peptide (NrTP6)
| Peptides | Therapeutics | Imaging Probes | Cell Line | Types of Cancer | Outcomes | Refs |
|---|---|---|---|---|---|---|
| BBN | 177Lu | PC-3 | Prostate | The positively charged conjugates were more efficiently taken up by PC-3 cells than the negatively charged ones but were cleared from the blood within 4 h in normal CF-1 mice. | [36] | |
| Bradykinin | / | / | C26 | Colon | pH-responsive HPMA-BK copolymers increased blood flow to tumor tissue by 1.7-fold and selective accumulation in tumor tissue by 3-fold. | [118] |
| Collagen hybridizing peptides (CHPs) | / | Cy5 | MDA-MB-231 | Breast | HPMA copolymer-CHP exhibited a selective affinity for denatured collagen, and the conjugates increased tumor localization and showed higher retention compared to free CHP. | [41] |
| ACPP | Ad5 | / | A549, MDA-MB-231, HepG2 and HBE | / | ACPP promotes pc-Ad-eGFP to directly cross the cell membrane and enter the cytoplasm, facilitating the efficient expression of therapeutic adenovirus. | [91] |
| dNP2 | DOX | / | HeLa | Cervical | The DNA damage ability of P-(dNP2)-DOX is 10.5 times higher than that of P-DOX, and the inhibition rate of 3D tumor spheroids is 78%. | [92] |
| SVS-1 | DOX | Cy5 | HeLa | Cervical | SVS-1-P-DOX showed 2.4-fold increased cytotoxicity compared with P-DOX and exhibited prolonged blood circulation and preferential tumor accumulation compared with free DOX. | [37] |
| SS20 | / | FITC, Cy5 | HeLa | Cervical | Tumor accumulation of P-SS20-Cy5 was higher than that of P-Cy5 (approximately 1.69-fold) within 96 h after administration. | [100] |
| SS20 + dNP2 | α-TOS | FITC | HeLa | Cervical | The combination of these two functional peptides resulted in a 7.6-fold increase in cellular uptake and accumulation in mitochondria, increasing apoptosis and necrosis. | [101] |
| R8 + MTS | DOX | / | 4T1, MDA-MB-231 | Breast | P-D-R8MTS exhibited the highest anti-tumor efficacy of 86.8% and tumor accumulation was 2.98-fold higher than that of free DOX. | [102] |
| MPP | DOX | / | 4T1 | Breast | pH-responsive destruction of cell nuclei and mitochondria exerts anti-tumor and anti-metastasis effects. | [107] |
| KLA | / | FITC, Cy5 | B16F10 | Melanoma | The cellular uptake and mitochondrial targeting abilities of the targeted copolymer increased by 4.3-fold and 23.8-fold, respectively; the in vivo tumor inhibition rate reached a peak of 82.9%. | [119] |
| FQS | DOX | / | B16F10 | Melanoma | The antitumor efficiency of FQS-HPMA-DOX (83.9%) was significantly higher than that of HPMA-DOX (44.9%). | [112] |
| RGD | / | 111In | LLC1 | Lung | HPMA copolymers of mono-RGDfK and di-cyclized RGD4C both enhance tumor uptake and reduce background accumulation. | [120] |
| Aminohexyl geldanamycin (AH-GDM) | / | PC-3 | Prostate | The conjugate exhibited similar binding activity to the free peptide and was better tolerated in vivo than the free drug. | [121] | |
| / | DU-145 | Prostate | The higher permeability of the RGDfK-targeted conjugate at a lower dose showed higher anti-tumor activity in nude mice. | [122] | ||
| / | Indocyanine green azido derivative (ICG) | U87-MG LN-18 | Glioblastoma | The targeted fluorescent nanoprobe showed a 35% increase in accumulation in glioblastoma compared to nontargeted controls. | [123] | |
| NrTP6 | H1-S6A, F8A (H1) | FITC | HeLa | Cervical | NrTP6-modified HPMA copolymer-H1 peptide conjugates could improve internalization and nuclear accumulation of H1 peptide by 2.2 and 37.1-fold; tumor growth inhibition rate of 77.0%. | [117] |
| Microtubule-dependent nuclear-targeting peptide (MT) | H1-S6A, F8A (H1) | FITC | HeLa | Cervical | The apoptotic cells induced by H1, P-H1, and P-H1-MT were 20.8%, 26.5%, and 45.4%; nuclear drug accumulation was 15.8-fold higher than that of polymers without peptides. | [124] |
| Nuclear-homing cell-penetrating peptide (R8NLS) | DOX | FITC, Cy5 | HeLa | Cervical | It exhibited 4.5-fold higher nuclear accumulation than HPMA-Dox and inhibited tumor growth by 75%. | [125] |
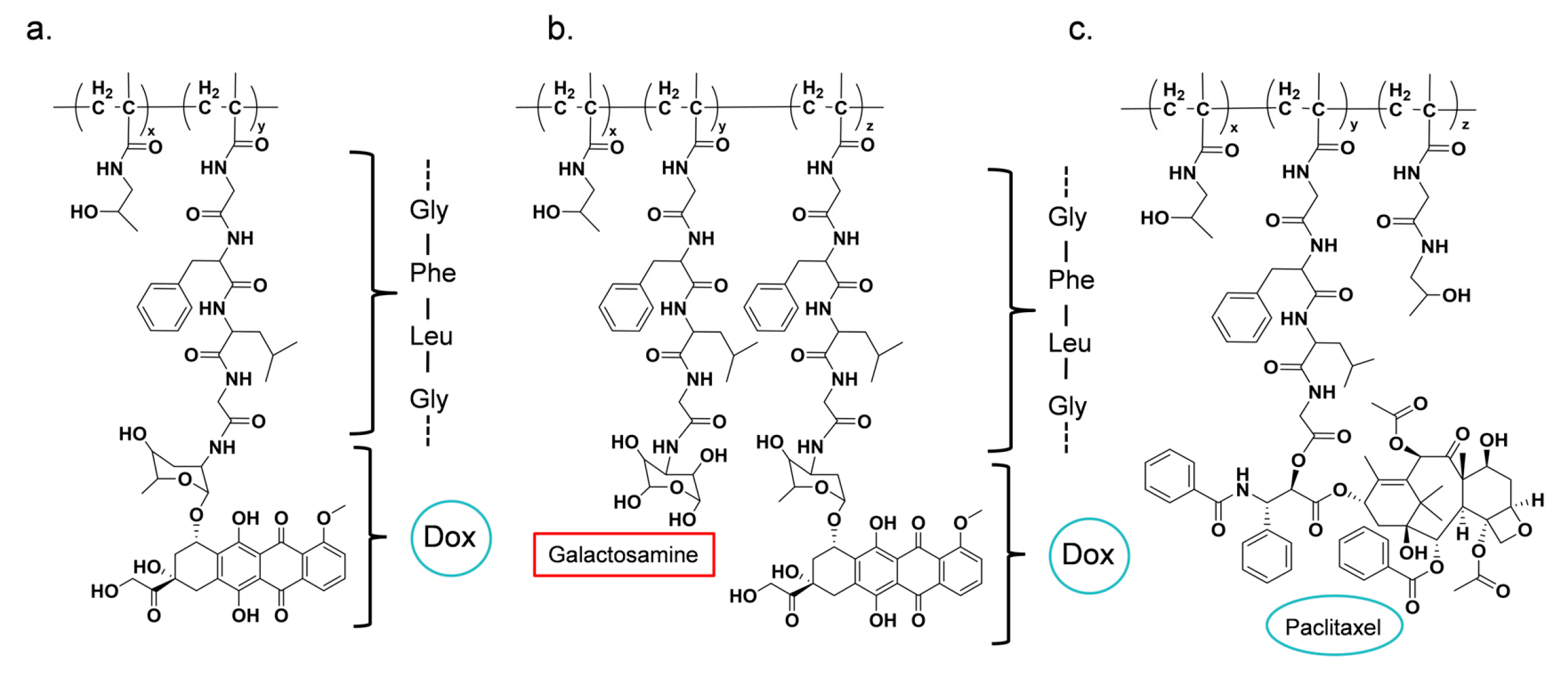
3.2. Peptide-Triggered Drug Release System
| Conjugate | Drug | Spacer | Refs |
|---|---|---|---|
| PK1 (FCE28068) | DOX | GFLG | [131,134,135] |
| PK2 (FCE28069) | DOX (Galactosamine) | GFLG | [136,137,138] |
| PNU166945 | PTX | GFLG | [65] |
| MAG–CPT (PNU 166148) | CPT | / | [139] |
| AP5280 | Carbo platinate | GFLG | [140,141] |
| ProLindacTM (AP-5346) | Oxaliplatin | / | [142] |
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPMA | N-(2-Hydroxypropyl) Methacrylamide |
| PEG | Polyethylene Glycol |
| EPR | Higher permeability and retention |
| MMA | Methyl Methacrylate |
| GMA | Glycidyl Methacrylate |
| MA-AP-TT | 3-(3-methacrylamidopropanoyl) thiazolidine-2-thione |
| APMA | N-(3-Aminopropyl) Methacrylamide |
| HEMA | 2-Hydroxyethyl Methacrylate |
| MAGG | N-Methacryloylglycylglycine |
| AA | Acrylic acid |
| AzMA | N-(3-azidopropyl)methacrylamide |
| RAFT | Reversible addition-fragmentation chain transfer |
| ATRP | Atom transfer radical polymerization |
| APIs | Active pharmaceutical ingredients |
| PTX | Paclitaxel |
| BA | Betulinic acid |
| RES | Reticuloendothelial system |
| RBC | Red blood cell |
| BBN | Bombesin |
| GRPR | Gastrin-releasing peptide receptor |
| CPPs | Cell-penetrating peptides |
| ACPP | Activatable cell-penetrating peptide |
| MMPs | Matrix metalloproteinases |
| DOX | Doxorubicin |
| AMPs | Antimicrobial peptides |
| ATP | Adenosine triphosphate |
| α-TOS | A-Tocopherol Succinate |
| ROS | Reactive oxygen species |
| MDR | Multidrug resistance |
| MPTP | Mitochondrial permeability transition pore |
| ECM | Extracellular matrix |
| MTD | Maximum tolerated dose |
References
- Mohtavinejad, N.; Shafiee Ardestani, M.; Khalaj, A.; Pormohammad, A.; Najafi, R.; Bitarafan-Rajabi, A.; Hajiramezanali, M.; Amanlou, M. Application of Radiolabeled Peptides in Tumor Imaging and Therapy. Life Sci. 2020, 258, 118206. [Google Scholar] [CrossRef]
- Hodolic, M.; Wu, W.-Y.; Zhao, Z.; Yu, F.; Virgolini, I.; Wang, F. Safety and Tolerability of 68Ga-NT-20.3, a Radiopharmaceutical for Targeting Neurotensin Receptors, in Patients with Pancreatic Ductal Adenocarcinoma: The First in-Human Use. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, N.; Egbu, R.; Onyekuru, L.; Javaheri, H.; Tee Khaw, P.R.; Williams, G.; Brocchini, S.; Awwad, S. Injectables and Depots to Prolong Drug Action of Proteins and Peptides. Pharmaceutics 2020, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, P.; Kaloudi, A.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands. Pharmaceutics 2020, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Valkema, R.; Krenning, E.P.; De Jong, M.; Maina, T. Impact of Clinically Tested NEP/ACE Inhibitors on Tumor Uptake of [111In-DOTA]MG11—First Estimates for Clinical Translation. EJNMMI Res. 2016, 6, 15. [Google Scholar] [CrossRef]
- Rock, B.M.; Foti, R.S. Pharmacokinetic and Drug Metabolism Properties of Novel Therapeutic Modalities. Drug Metab. Dispos. 2019, 47, 1097–1099. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Yu, Y.; He, C.; Tan, L.; Shen, Y.-M. RGD Peptide-Decorated Micelles Assembled from Polymer–Paclitaxel Conjugates towards Gastric Cancer Therapy. Colloids Surf. B Biointerfaces 2019, 180, 58–67. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Williams, C.C.; Leong, N.J.; Chan, L.J.; Butcher, N.J.; Feeney, O.M.; Porter, C.J.H.; Tyssen, D.; Tachedjian, G.; Ascher, D.B. A 30 kDa Polyethylene Glycol-Enfuvirtide Complex Enhances the Exposure of Enfuvirtide in Lymphatic Viral Reservoirs in Rats. Eur. J. Pharm. Biopharm. 2019, 137, 218–226. [Google Scholar] [CrossRef]
- Wu, H.; Nan, J.; Yang, L.; Park, H.J.; Li, J. Insulin-Loaded Liposomes Packaged in Alginate Hydrogels Promote the Oral Bioavailability of Insulin. J. Control. Release 2023, 353, 51–62. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, K.; Lin, T.; Zhao, P.; Wang, H.; Su, Z. Versatile Hydrogel Drug Carrier Loading with Peptide-Carbon Dots Conjugates for Efficient Targeting and Synergistic Suppression of Breast Cancer Cell. Mater. Today Commun. 2024, 41, 110404. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, S.-T.; Chen, X.-X.; Wang, H. Fluorescent Carbon Dots and Nanodiamonds for Biological Imaging: Preparation, Application, Pharmacokinetics and Toxicity. Curr. Drug Metab. 2012, 13, 1046–1056. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Li, S.; Qin, T.; Chen, T.; Wang, J.; Zeng, Q. Poly(Vinyl Alcohol)/Poly(Hydroxypropyl Methacrylate-Co-Methacrylic Acid) as pH-Sensitive Semi-IPN Hydrogels for Oral Insulin Delivery: Preparation and Characterization. Iran. Polym. J. 2021, 30, 343–353. [Google Scholar] [CrossRef]
- Cui, Y.; Shan, W.; Liu, M.; Wu, L.; Huang, Y. A Strategy for Developing Effective Orally-Delivered Nanoparticles through Modulation of the Surface “Hydrophilicity/Hydrophobicity Balance”. J. Mater. Chem. B 2017, 5, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, Z.; Li, L.; Yang, Q.; Yang, Y.; Guan, S.; Zhang, J.; Zhu, X.; Jin, Y.; Huang, Y. Comparison of Active and Passive Targeting of Doxorubicin for Somatostatin Receptor 2 Positive Tumor Models by Octreotide-Modified HPMA Copolymer-Doxorubicin Conjugates. Drug Deliv. 2016, 23, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Noack, A.K.; Lucas, H.; Chytil, P.; Etrych, T.; Mäder, K.; Mueller, T. Intratumoral Distribution and pH-Dependent Drug Release of High Molecular Weight HPMA Copolymer Drug Conjugates Strongly Depend on Specific Tumor Substructure and Microenvironment. Int. J. Mol. Sci. 2020, 21, 6029. [Google Scholar] [CrossRef]
- Yuan, J.; Miao, C.; Peng, F.; Zeng, X.; Guo, H.; Wang, X.; Liao, S.; Xie, X. Synthesis and Characterization of Poly(HPMA)-APMA-DTPA- 99m Tc for Imaging-guided Drug Delivery in Hepatocellular Carcinoma. J. Appl. Polym. Sci. 2013, 127, 4549–4556. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer-Drug Conjugates: Recent Advances and Future Perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The Accelerated Blood Clearance (ABC) Phenomenon: Clinical Challenge and Approaches to Manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef]
- Fu, J.; Wu, E.; Li, G.; Wang, B.; Zhan, C. Anti-PEG Antibodies: Current Situation and Countermeasures. Nano Today 2024, 55, 102163. [Google Scholar] [CrossRef]
- Subasic, C.N.; Ardana, A.; Chan, L.J.; Huang, F.; Scoble, J.A.; Butcher, N.J.; Meagher, L.; Chiefari, J.; Kaminskas, L.M.; Williams, C.C. Poly(HPMA-Co-NIPAM) Copolymer as an Alternative to Polyethylene Glycol-Based Pharmacokinetic Modulation of Therapeutic Proteins. Int. J. Pharm. 2021, 608, 121075. [Google Scholar] [CrossRef]
- Chytil, P.; Kostka, L.; Etrych, T. HPMA Copolymer-Based Nanomedicines in Controlled Drug Delivery. J. Pers. Med. 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhao, G.; Wei, X.; Kong, D.; Sun, Y.; Zhou, Y.; Lele, S.M.; Fehringer, E.V.; Garvin, K.L.; Goldring, S.R.; et al. Structural Optimization of HPMA Copolymer-Based Dexamethasone Prodrug for Improved Treatment of Inflammatory Arthritis. J. Control. Release 2020, 324, 560–573. [Google Scholar] [CrossRef]
- Lane, D.D.; Su, F.Y.; Chiu, D.Y.; Srinivasan, S.; Wilson, J.T.; Ratner, D.M.; Stayton, P.S.; Convertine, A.J. Dynamic Intracellular Delivery of Antibiotics via pH-Responsive Polymersomes. Polym. Chem. 2015, 6, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, Y.; Zhao, J.; Shi, Q.; Zhang, D.; Sun, J.; Zhang, C. Design and Fabrication of Poly(HPMA)–6-Mercaptopurine Conjugates and Their in Vitro Anticancer Effect. New J. Chem. 2018, 42, 19729–19733. [Google Scholar] [CrossRef]
- Verma, V.S.; Pandey, A.; Jha, A.K.; Badwaik, H.K.R.; Alexander, A.; Ajazuddin. Polyethylene Glycol-Based Polymer-Drug Conjugates: Novel Design and Synthesis Strategies for Enhanced Therapeutic Efficacy and Targeted Drug Delivery. Appl. Biochem. Biotechnol. 2024, 196, 7325–7361. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. P(MMA-
co-HPMA)-b-POEGMA Copolymers: Synthesis, Micelle Formation in Aqueous Media and Drug Encapsulation. Polym. Int. 2021, 70, 1508–1522. [Google Scholar] [CrossRef] - Shao, T.; Gong, Y.; Chen, X.; Chen, L. Preparation and Properties of Novel Self-Crosslinking Long Fluorocarbon Acrylate (MMA–BA–DFMA–HPMA) Polymer Latex with Mixed Surfactants. Chem. Pap. 2021, 75, 5561–5569. [Google Scholar] [CrossRef]
- Gao, J.; Wen, J.; Hu, D.; Liu, K.; Zhang, Y.; Zhao, X.; Wang, K. Bottlebrush Inspired Injectable Hydrogel for Rapid Prevention of Postoperative and Recurrent Adhesion. Bioact. Mater. 2022, 16, 27–46. [Google Scholar] [CrossRef]
- Orakdogen, N.; Sanay, B. Poly(Hydroxypropyl Methacrylate-Co-Glycidyl Methacrylate): Facile Synthesis of Well-Defined Hydrophobic Gels Containing Hydroxy-Functional Methacrylates. Polym. Degrad. Stab. 2017, 144, 251–263. [Google Scholar] [CrossRef]
- Bojarová, P.; Chytil, P.; Mikulová, B.; Bumba, L.; Konefał, R.; Pelantová, H.; Krejzová, J.; Slámová, K.; Petrásková, L.; Kotrchová, L.; et al. Glycan-Decorated HPMA Copolymers as High-Affinity Lectin Ligands. Polym. Chem. 2017, 8, 2647–2658. [Google Scholar] [CrossRef]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefał, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible Glyconanomaterials Based on HPMA-Copolymer for Specific Targeting of Galectin-3. J. Nanobiotechnol. 2018, 16, 73. [Google Scholar] [CrossRef]
- Alsuraifi, A.; Mathew, E.; Lamprou, D.A.; Curtis, A.; Hoskins, C. Thermally Reactive N-(2-Hydroxypropyl)Methacrylamide (HPMA) Amphiphiles for Drug Solubilisation. Int. J. Pharm. 2021, 601, 120570. [Google Scholar] [CrossRef]
- Sincari, V.; Petrova, S.L.; Konefał, R.; Hruby, M.; Jäger, E. Microwave-Assisted RAFT Polymerization of N-(2-Hydroxypropyl) Methacrylamide and Its Relevant Copolymers. React. Funct. Polym. 2021, 162, 104875. [Google Scholar] [CrossRef]
- Alshehri, S.; Fan, W.; Zhang, W.; Garrison, J.C. In Vitro Evaluation and Biodistribution Studies of HPMA Copolymers Targeting the Gastrin Releasing Peptide Receptor in Prostate Cancer. Pharm. Res. 2020, 37, 229. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Chen, L.; Zhou, R.; Huang, Y. Enhanced Intracellular and Intranuclear Drug Delivery Mediated by Biomimetic Peptide SVS-1 for Anticancer Therapy. Int. J. Pharm. 2019, 570, 118668. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, R.; Christopher Radford, D.; Kopeček, J. Design and Synthesis of FRET-Trackable HPMA-Based Biodegradable Conjugates for Drug/Gene Delivery. J. Control. Release 2015, 213, e58. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Zhou, Z.; Zhong, J.; Huang, Y. Doxorubicin-Loaded, Charge Reversible, Folate Modified HPMA Copolymer Conjugates for Active Cancer Cell Targeting. Biomaterials 2014, 35, 5171–5187. [Google Scholar] [CrossRef]
- Abasi, S.; Davis, R.; Podstawczyk, D.A.; Guiseppi-Elie, A. Distribution of Water States within Poly(HEMA-Co-HPMA)-Based Hydrogels. Polymer 2019, 185, 121978. [Google Scholar] [CrossRef]
- Subrahmanyam, N.; Yathavan, B.; Kessler, J.; Yu, S.M.; Ghandehari, H. HPMA Copolymer-Collagen Hybridizing Peptide Conjugates Targeted to Breast Tumor Extracellular Matrix. J. Control. Release 2023, 353, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Kopecková, P.; Kopecek, J. Self-Association Properties of HPMA Copolymers Containing an Amphipathic Heptapeptide. J. Drug Target. 2007, 15, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, M.F.; Schaffert, D.H.; Crowley, M.L.; Oupický, D.; Howard, K.A. Synthesis of Click-reactive HPMA Copolymers Using RAFT Polymerization for Drug Delivery Applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5091–5099. [Google Scholar] [CrossRef]
- Ebbesen, M.F.; Bienk, K.; Deleuran, B.W.; Howard, K.A. Extended Blood Circulation and Joint Accumulation of a p(HPMA-Co-AzMA)-Based Nanoconjugate in a Murine Model of Rheumatoid Arthritis. Mol. Cell Ther. 2014, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Koziolová, E.; Machová, D.; Pola, R.; Janoušková, O.; Chytil, P.; Laga, R.; Filippov, S.K.; Šubr, V.; Etrych, T.; Pechar, M. Micelle-Forming HPMA Copolymer Conjugates of Ritonavir Bound via a pH-Sensitive Spacer with Improved Cellular Uptake Designed for Enhanced Tumor Accumulation. J. Mater. Chem. B 2016, 4, 7620–7629. [Google Scholar] [CrossRef]
- Najafi, M.; Asadi, H.; van den Dikkenberg, J.; van Steenbergen, M.J.; Fens, M.H.A.M.; Hennink, W.E.; Vermonden, T. Conversion of an Injectable MMP-Degradable Hydrogel into Core-Cross-Linked Micelles. Biomacromolecules 2020, 21, 1739–1751. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, D.; Luo, K.; Li, L.; Gu, Z. Biodegradable and Amphiphilic Block Copolymer–Doxorubicin Conjugate as Polymeric Nanoscale Drug Delivery Vehicle for Breast Cancer Therapy. Biomaterials 2013, 34, 8430–8443. [Google Scholar] [CrossRef]
- Kramer, S.; Svatunek, D.; Alberg, I.; Gräfen, B.; Schmitt, S.; Braun, L.; Van Onzen, A.H.A.M.; Rossin, R.; Koynov, K.; Mikula, H.; et al. HPMA-Based Nanoparticles for Fast, Bioorthogonal iEDDA Ligation. Biomacromolecules 2019, 20, 3786–3797. [Google Scholar] [CrossRef]
- Chytil, P.; Koziolová, E.; Janoušková, O.; Kostka, L.; Ulbrich, K.; Etrych, T. Synthesis and Properties of Star HPMA Copolymer Nanocarriers Synthesised by RAFT Polymerisation Designed for Selective Anticancer Drug Delivery and Imaging. Macromol. Biosci. 2015, 15, 839–850. [Google Scholar] [CrossRef]
- Etrych, T.; Kovář, L.; Strohalm, J.; Chytil, P.; Říhová, B.; Ulbrich, K. Biodegradable Star HPMA Polymer–Drug Conjugates: Biodegradability, Distribution and Anti-Tumor Efficacy. J. Control. Release 2011, 154, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Edmans, J.G.; Harrison, S.; Hatton, P.V.; Murdoch, C.; Spain, S.G.; Colley, H.E. Electrospinning Polymersomes into Bead-on-String Polyethylene Oxide Fibres for the Delivery of Biopharmaceuticals to Mucosal Epithelia. Biomater. Adv. 2024, 157, 213734. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Říhová, B.; Kovář, M. Immunogenicity and Immunomodulatory Properties of HPMA-Based Polymers. Adv. Drug Deliv. Rev. 2010, 62, 184–191. [Google Scholar] [CrossRef]
- Noguchi, Y.; Wu, J.; Duncan, R.; Strohalm, J.; Ulbrich, K.; Akaike, T.; Maeda, H. Early Phase Tumor Accumulation of Macromolecules: A Great Difference in Clearance Rate between Tumor and Normal Tissues. Jpn. J. Cancer Res. 1998, 89, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, W.; Jia, Y.; Brusnahan, S.K.; Garrison, J.C. Investigation into the Biological Impact of Block Size on Cathepsin S-Degradable HPMA Copolymers. Mol. Pharm. 2017, 14, 1405–1417. [Google Scholar] [CrossRef]
- Fan, W.; Shi, W.; Zhang, W.; Jia, Y.; Zhou, Z.; Brusnahan, S.K.; Garrison, J.C. Cathepsin S-Cleavable, Multi-Block HPMA Copolymers for Improved SPECT/CT Imaging of Pancreatic Cancer. Biomaterials 2016, 103, 101–115. [Google Scholar] [CrossRef]
- Allmeroth, M.; Moderegger, D.; Biesalski, B.; Koynov, K.; Thews, O.; Zentel, R. Modifying the Body Distribution of HPMA-Based Copolymers by Molecular Weight and Aggregate Formation. Biomacromolecules 2011, 12, 2841–2849. [Google Scholar] [CrossRef]
- Pytlíková, S.; Pechar, M.; Chytil, P.; Studenovský, M.; Pola, R.; Kotrchová, L.; Konefał, R.; Čtveráčková, L.; Laga, R.; Pankrác, J.; et al. Highly Hydrophilic Methacrylamide-Based Copolymers as Precursors for Polymeric Nanomedicines Containing Anthracyclines. Eur. Polym. J. 2024, 205, 112756. [Google Scholar] [CrossRef]
- Barz, M.; Armiñán, A.; Canal, F.; Wolf, F.; Koynov, K.; Frey, H.; Zentel, R.; Vicent, M.J. P(HPMA)-Block-P(LA) Copolymers in Paclitaxel Formulations: Polylactide Stereochemistry Controls Micellization, Cellular Uptake Kinetics, Intracellular Localization and Drug Efficiency. J. Control. Release 2012, 163, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Morosi, L.; Lupi, M.; Capasso Palmiero, U. Poly(HPMA)-Based Copolymers with Biodegradable Side Chains Able to Self Assemble into Nanoparticles. RSC Adv. 2017, 7, 50981–50992. [Google Scholar] [CrossRef]
- Shalmani, A.A.; Ahmed, Z.; Sheybanifard, M.; Wang, A.; Weiler, M.; Buhl, E.M.; Klinkenberg, G.; Schmid, R.; Hennink, W.; Kiessling, F.; et al. Effect of Radical Polymerization Method on Pharmaceutical Properties of Π Electron-Stabilized HPMA-Based Polymeric Micelles. Biomacromolecules 2023, 24, 4444–4453. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Pun, S.H. Multivalent Polymers Displaying M2 Macrophage-Targeting Peptides Improve Target Binding Avidity and Serum Stability. ACS Biomater. Sci. Eng. 2017, 3, 2050–2053. [Google Scholar] [CrossRef]
- El Hamd, M.A.; Obaydo, R.H.; Nashed, D.; El-Maghrabey, M.; Lotfy, H.M. Hydrotropy and Co-Solvency: Sustainable Strategies for Enhancing Solubility of Poorly Soluble Pharmaceutical Active Ingredients. Talanta Open 2025, 11, 100391. [Google Scholar] [CrossRef]
- Wang, Y.; Van Steenbergen, M.J.; Beztsinna, N.; Shi, Y.; Lammers, T.; Van Nostrum, C.F.; Hennink, W.E. Biotin-Decorated All-HPMA Polymeric Micelles for Paclitaxel Delivery. J. Control. Release 2020, 328, 970–984. [Google Scholar] [CrossRef]
- Meerum Terwogt, J.M.; ten Bokkel Huinink, W.W.; Schellens, J.H.; Schot, M.; Mandjes, I.A.; Zurlo, M.G.; Rocchetti, M.; Rosing, H.; Koopman, F.J.; Beijnen, J.H. Phase I Clinical and Pharmacokinetic Study of PNU166945, a Novel Water-Soluble Polymer-Conjugated Prodrug of Paclitaxel. Anticancer. Drugs 2001, 12, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Banerjee, S.; Bishayee, A.; Da Silva, M.N.; Sukocheva, O.A.; Tse, E.; Casarcia, N.; Bishayee, A. Cellular and Molecular Mechanisms Underlying the Potential of Betulinic Acid in Cancer Prevention and Treatment. Phytomedicine 2024, 132, 155858. [Google Scholar] [CrossRef]
- Lomkova, E.A.; Chytil, P.; Janoušková, O.; Mueller, T.; Lucas, H.; Filippov, S.K.; Trhlíková, O.; Aleshunin, P.A.; Skorik, Y.A.; Ulbrich, K.; et al. Biodegradable Micellar HPMA-Based Polymer-Drug Conjugates with Betulinic Acid for Passive Tumor Targeting. Biomacromolecules 2016, 17, 3493–3507. [Google Scholar] [CrossRef]
- Naksuriya, O.; Shi, Y.; van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-Based Polymeric Micelles for Curcumin Solubilization and Inhibition of Cancer Cell Growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key Terminology in Biomaterials and Biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What Affects the Biocompatibility of Polymers? Adv. Colloid. Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Sprincl, L.; Exner, J.; Stĕrba, O.; Kopecek, J. New Types of Synthetic Infusion Solutions. III. Elimination and Retention of Poly-[N-(2-Hydroxypropyl)Methacrylamide] in a Test Organism. J. Biomed. Mater. Res. 1976, 10, 953–963. [Google Scholar] [CrossRef]
- Yu, Q.; Wei, Z.; Shi, J.; Guan, S.; Du, N.; Shen, T.; Tang, H.; Jia, B.; Wang, F.; Gan, Z. Polymer-Doxorubicin Conjugate Micelles Based on Poly(Ethylene Glycol) and Poly(N-(2-Hydroxypropyl) Methacrylamide): Effect of Negative Charge and Molecular Weight on Biodistribution and Blood Clearance. Biomacromolecules 2015, 16, 2645–2655. [Google Scholar] [CrossRef]
- Cai, H.; Dai, X.; Wang, X.; Tan, P.; Gu, L.; Luo, Q.; Zheng, X.; Li, Z.; Zhu, H.; Zhang, H.; et al. A Nanostrategy for Efficient Imaging-Guided Antitumor Therapy through a Stimuli-Responsive Branched Polymeric Prodrug. Adv. Sci. 2020, 7, 1903243. [Google Scholar] [CrossRef]
- Sun, H.; Gu, X.; Zhang, Q.; Xu, H.; Zhong, Z.; Deng, C. Cancer Nanomedicines Based on Synthetic Polypeptides. Biomacromolecules 2019, 20, 4299–4311. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Randárová, E.; Kudláčová, J.; Etrych, T. HPMA Copolymer-Antibody Constructs in Neoplastic Treatment: An Overview of Therapeutics, Targeted Diagnostics, and Drug-Free Systems. J. Control. Release 2020, 325, 304–322. [Google Scholar] [CrossRef]
- Anastasi, A.; Erspamer, V.; Bucci, M. Isolation and Structure of Bombesin and Alytesin, 2 Analogous Active Peptides from the Skin of the European Amphibians Bombina and Alytes. Experientia 1971, 27, 166–167. [Google Scholar] [CrossRef]
- Baratto, L.; Duan, H.; Mäcke, H.; Iagaru, A. Imaging the Distribution of Gastrin-Releasing Peptide Receptors in Cancer. J. Nucl. Med. 2020, 61, 792–798. [Google Scholar] [CrossRef]
- Carlucci, G.; Kuipers, A.; Ananias, H.J.K.; De Paula Faria, D.; Dierckx, R.A.J.O.; Helfrich, W.; Rink, R.; Moll, G.N.; De Jong, I.J.; Elsinga, P.H. GRPR-Selective PET Imaging of Prostate Cancer Using [18F]-Lanthionine-Bombesin Analogs. Peptides 2015, 67, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gao, R.; Zhao, Y.; Zhang, F.; Wang, X.; Li, B.; Wang, L.; Ma, L.; Du, J. pH-Responsive Graphene Oxide Loaded with Targeted Peptide and Anticancer Drug for OSCC Therapy. Front. Oncol. 2022, 12, 930920. [Google Scholar] [CrossRef]
- Marques, A.; Belchior, A.; Silva, F.; Marques, F.; Campello, M.P.C.; Pinheiro, T.; Santos, P.; Santos, L.; Matos, A.P.A.; Paulo, A. Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles. IJMS 2022, 23, 5279. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Mansi, R.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Ziaco, B.; Tesauro, D.; et al. Bombesin Peptide Antagonist for Target-Selective Delivery of Liposomal Doxorubicin on Cancer Cells. J. Drug Target. 2013, 21, 240–249. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Wang, K.; Wang, Y.; Yang, F.; Wang, H. Breast Cancer Targeted Chemotherapy Based on Doxorubicin-Loaded Bombesin Peptide Modified Nanocarriers. Drug Deliv. 2016, 23, 2697–2702. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shevchenko, K.G.; Deyev, S.M. Rediscovery of Mononuclear Phagocyte System Blockade for Nanoparticle Drug Delivery. Nat. Commun. 2024, 15, 4366. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Kong, X.; Xu, J.; Yang, X.; Zhai, Y.; Ji, J.; Zhai, G. Progress in Tumour-Targeted Drug Delivery Based on Cell-Penetrating Peptides. J. Drug Target. 2022, 30, 46–60. [Google Scholar] [CrossRef]
- Gori, A.; Lodigiani, G.; Colombarolli, S.G.; Bergamaschi, G.; Vitali, A. Cell Penetrating Peptides: Classification, Mechanisms, Methods of Study, and Applications. ChemMedChem 2023, 18, e202300236. [Google Scholar] [CrossRef]
- Timotievich, E.D.; Shilovskiy, I.P.; Khaitov, M.R. Cell-Penetrating Peptides as Vehicles for Delivery of Therapeutic Nucleic Acids. Mechanisms and Application in Medicine. Biochem. Mosc. 2023, 88, 1800–1817. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The Role of Cell-penetrating Peptides in Potential Anti-cancer Therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Xu, H.; Long, J.; Xie, X.; Zhang, Y. The Targeted Transduction of MMP-Overexpressing Tumor Cells by ACPP-HPMA Copolymer-Coated Adenovirus Conjugates. PLoS ONE 2014, 9, e100670. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Shan, W.; Huang, Y. Improved Anticancer Efficacy of Doxorubicin Mediated by Human-Derived Cell-Penetrating Peptide dNP2. Int. J. Pharm. 2018, 551, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Joanne, P.; Nicolas, P.; El Amri, C. Antimicrobial Peptides and Viral Fusion Peptides: How Different They Are? PPL 2009, 16, 743–750. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Cho, Y.-Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-Targeted Drug Delivery in Cancers. Biochim. Et. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165808. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, X.; Jin, Q.; Ji, J. ATP Suppression by pH-Activated Mitochondria-Targeted Delivery of Nitric Oxide Nanoplatform for Drug Resistance Reversal and Metastasis Inhibition. Small 2020, 16, 2001747. [Google Scholar] [CrossRef]
- O’Neill, K.L.; Huang, K.; Zhang, J.; Chen, Y.; Luo, X. Inactivation of Prosurvival Bcl-2 Proteins Activates Bax/Bak through the Outer Mitochondrial Membrane. Genes. Dev. 2016, 30, 973–988. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and Metabolic Dysfunction in Ageing and Age-Related Diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Dai, D.-F.; Hsieh, E.J.; Chen, T.; Menendez, L.G.; Basisty, N.B.; Tsai, L.; Beyer, R.P.; Crispin, D.A.; Shulman, N.J.; Szeto, H.H.; et al. Global Proteomics and Pathway Analysis of Pressure-Overload–Induced Heart Failure and Its Attenuation by Mitochondrial-Targeted Peptides. Circ. Heart Fail. 2013, 6, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Xiong, X.; Zhou, Z. Improved Mitochondrial Targeting Effect of HPMA Copolymer by SS20 Peptide Mediation and Nonendocytosis Pathway. J. Pept. Sci. 2019, 25, e3144. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Xiong, X.; Huang, Y.; Zhou, Z. Mitochondria-Targeting and Cell-Penetrating Peptides-Co-Modified HPMA Copolymers for Enhancing Therapeutic Efficacy of α-Tocopheryl Succinate. J. Mater. Chem. B 2018, 6, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Chen, C.; Lin, X.; Zhou, M.; Zhou, Z.; Huang, Y. A Novel Mitochondrial Targeted Hybrid Peptide Modified HPMA Copolymers for Breast Cancer Metastasis Suppression. J. Control. Release 2020, 325, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Yousif, L.F.; Stewart, K.M.; Horton, K.L.; Kelley, S.O. Mitochondria-Penetrating Peptides: Sequence Effects and Model Cargo Transport. Chembiochem 2009, 10, 2081–2088. [Google Scholar] [CrossRef]
- Horton, K.L.; Stewart, K.M.; Fonseca, S.B.; Guo, Q.; Kelley, S.O. Mitochondria-Penetrating Peptides. Chem. Biol. 2008, 15, 375–382. [Google Scholar] [CrossRef]
- Chen, W.-H.; Luo, G.-F.; Qiu, W.-X.; Lei, Q.; Liu, L.-H.; Zheng, D.-W.; Hong, S.; Cheng, S.-X.; Zhang, X.-Z. Tumor-Triggered Drug Release with Tumor-Targeted Accumulation and Elevated Drug Retention To Overcome Multidrug Resistance. Chem. Mater. 2016, 28, 6742–6752. [Google Scholar] [CrossRef]
- Zhou, M.; Li, L.; Li, L.; Lin, X.; Wang, F.; Li, Q.; Huang, Y. Overcoming Chemotherapy Resistance via Simultaneous Drug-Efflux Circumvention and Mitochondrial Targeting. Acta Pharm. Sin. B 2019, 9, 615–625. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Zhou, M.; Li, X.; Huang, Y.; Yang, N.; Zhou, Z. Concurrent Impairment of Nucleus and Mitochondria for Synergistic Inhibition of Cancer Metastasis. Int. J. Pharm. 2021, 608, 121077. [Google Scholar] [CrossRef]
- Pachane, B.C.; Selistre-de-Araujo, H.S. The Role of Avβ3 Integrin in Cancer Therapy Resistance. Biomedicines 2024, 12, 1163. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Sig Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, D.; Wilsch-Bräuninger, M.; Wong, F.K.; Heuer, H.; Huttner, W.B. Integrin Avβ3 and Thyroid Hormones Promote Expansion of Progenitors in Embryonic Neocortex. Development 2014, 141, 795–806. [Google Scholar] [CrossRef]
- Svensen, N.; Díaz-Mochón, J.J.; Bradley, M. Decoding a PNA Encoded Peptide Library by PCR: The Discovery of New Cell Surface Receptor Ligands. Chem. Biol. 2011, 18, 1284–1289. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Sun, W.; Li, L.; Liu, Y.; Huang, Y.; Zhou, Z. A Novel α V β 3 Ligand-Modified HPMA Copolymers for Anticancer Drug Delivery. J. Drug Target. 2018, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Pike, D.B.; Ghandehari, H. HPMA Copolymer-Cyclic RGD Conjugates for Tumor Targeting. Adv. Drug Deliv. Rev. 2010, 62, 167–183. [Google Scholar] [CrossRef]
- Peng, Z.-H.; Kopeček, J. Enhancing Accumulation and Penetration of HPMA Copolymer-Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. J. Am. Chem. Soc. 2015, 137, 6726–6729. [Google Scholar] [CrossRef] [PubMed]
- Tögel, L.; Nightingale, R.; Chueh, A.C.; Jayachandran, A.; Tran, H.; Phesse, T.; Wu, R.; Sieber, O.M.; Arango, D.; Dhillon, A.S.; et al. Dual Targeting of Bromodomain and Extraterminal Domain Proteins, and WNT or MAPK Signaling, Inhibits c-MYC Expression and Proliferation of Colorectal Cancer Cells. Mol. Cancer Ther. 2016, 15, 1217–1226. [Google Scholar] [CrossRef]
- Draeger, L.J.; Mullen, G.P. Interaction of the bHLH-Zip Domain of c-Myc with H1-Type Peptides. Characterization of Helicity in the H1 Peptides by NMR. J. Biol. Chem. 1994, 269, 1785–1793. [Google Scholar] [CrossRef]
- Xie, D.; Wang, F.; Xiang, Y.; Huang, Y. Enhanced Nuclear Delivery of H1-S6A, F8A Peptide by NrTP6-Modified Polymeric Platform. Int. J. Pharm. 2020, 580, 119224. [Google Scholar] [CrossRef]
- Appiah, E.; Nakamura, H.; Pola, R.; Grossmanová, E.; Lidický, O.; Kuniyasu, A.; Etrych, T.; Haratake, M. Acid-Responsive HPMA Copolymer-Bradykinin Conjugate Enhances Tumor-Targeted Delivery of Nanomedicine. J. Control. Release 2021, 337, 546–556. [Google Scholar] [CrossRef]
- Wang, F.; Sun, W.; Li, L.; Li, L.; Liu, Y.; Zhang, Z.; Huang, Y. Charge-Reversible Multifunctional HPMA Copolymers for Mitochondrial Targeting. ACS Appl. Mater. Interfaces 2017, 9, 27563–27574. [Google Scholar] [CrossRef]
- Mitra, A.; Coleman, T.; Borgman, M.; Nan, A.; Ghandehari, H.; Line, B.R. Polymeric Conjugates of Mono- and Bi-Cyclic αVβ3 Binding Peptides for Tumor Targeting. J. Control. Release 2006, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Borgman, M.P.; Ray, A.; Kolhatkar, R.B.; Sausville, E.A.; Burger, A.M.; Ghandehari, H. Targetable HPMA Copolymer–Aminohexylgeldanamycin Conjugates for Prostate Cancer Therapy. Pharm. Res. 2009, 26, 1407–1418. [Google Scholar] [CrossRef]
- Greish, K.; Ray, A.; Bauer, H.; Larson, N.; Malugin, A.; Pike, D.; Haider, M.; Ghandehari, H. Anticancer and Antiangiogenic Activity of HPMA Copolymer-Aminohexylgeldanamycin-RGDfK Conjugates for Prostate Cancer Therapy. J. Control. Release 2011, 151, 263–270. [Google Scholar] [CrossRef]
- Grosmanová, E.; Pola, R.; Filipová, M.; Henry, M.; Coll, J.; Etrych, T. Novel Strategies for Enhanced Fluorescence Visualization of Glioblastoma Tumors Based on HPMA Copolymers Conjugated with Tumor Targeting and/or Cell-penetrating Peptides. VIEW 2024, 5, 20230116. [Google Scholar] [CrossRef]
- Zhong, J.; Zhu, X.; Luo, K.; Li, L.; Tang, M.; Liu, Y.; Zhou, Z.; Huang, Y. Direct Cytoplasmic Delivery and Nuclear Targeting Delivery of HPMA-MT Conjugates in a Microtubules Dependent Fashion. Mol. Pharm. 2016, 13, 3069–3079. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Zhong, J.; Yang, Q.; Zhu, X.; Zhou, Z.; Zhang, Z.; Huang, Y. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv. Funct. Mater. 2015, 25, 4101–4113. [Google Scholar] [CrossRef]
- Han, T.; Hu, Y.; Fu, H. Research progress and consideration of polymeric prodrugs. J. China Pharm. Univ. 2019, 50, 397–404. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Moustoifa, E.-F.; Alouini, M.-A.; Salaün, A.; Berthelot, T.; Bartegi, A.; Albenque-Rubio, S.; Déléris, G. Novel Cyclopeptides for the Design of MMP Directed Delivery Devices: A Novel Smart Delivery Paradigm. Pharm. Res. 2010, 27, 1713–1721. [Google Scholar]
- Kopeček, J. Controlled Biodegradability of Polymers—A Key to Drug Delivery Systems. Biomaterials 1984, 5, 19–25. [Google Scholar] [CrossRef]
- Wei, X.; Luo, Q.; Sun, L.; Li, X.; Zhu, H.; Guan, P.; Wu, M.; Luo, K.; Gong, Q. Enzyme- and pH-Sensitive Branched Polymer–Doxorubicin Conjugate-Based Nanoscale Drug Delivery System for Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 11765–11778. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Ferry, D.R.; Kerr, D.J.; Rea, D.; Whitlock, M.; Poyner, R.; Boivin, C.; Hesslewood, S.; Twelves, C.; Blackie, R.; et al. Phase II Studies of Polymer-Doxorubicin (PK1, FCE28068) in the Treatment of Breast, Lung and Colorectal Cancer. Int. J. Oncol. 2009, 34, 1629–1636. [Google Scholar] [CrossRef]
- Duncan, R. Development of HPMA Copolymer-Anticancer Conjugates: Clinical Experience and Lessons Learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What Has Been Done and the Challenges Remain Ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Bilim, V. Technology Evaluation: PK1, Pfizer/Cancer Research UK. Curr. Opin. Mol. Ther. 2003, 5, 326–330. [Google Scholar]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2-Hydroxypropyl)Methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents-Drug-Polymer Conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J.; et al. Hepatic Drug Targeting: Phase I Evaluation of Polymer-Bound Doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [CrossRef]
- Julyan, P.J.; Seymour, L.W.; Ferry, D.R.; Daryani, S.; Boivin, C.M.; Doran, J.; David, M.; Anderson, D.; Christodoulou, C.; Young, A.M.; et al. Preliminary Clinical Study of the Distribution of HPMA Copolymers Bearing Doxorubicin and Galactosamine. J. Control Release 1999, 57, 281–290. [Google Scholar] [CrossRef]
- Hopewel, J.W.; Duncan, R.; Wilding, D.; Chakrabarti, K. Preclinical Evaluation of the Cardiotoxicity of PK2: A Novel HPMA Copolymer-Doxorubicin-Galactosamine Conjugate Antitumour Agent. Hum. Exp. Toxicol. 2001, 20, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.; Cassidy, J.; de Bono, J.S.; Muirhead, F.; Main, M.; Robson, L.; Fraier, D.; Magnè, M.L.; Pellizzoni, C.; Porro, M.G.; et al. Phase I and Pharmacokinetic (PK) Study of MAG-CPT (PNU 166148): A Polymeric Derivative of Camptothecin (CPT). Br. J. Cancer 2004, 91, 50–55. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Terret, C.; Howell, S.B.; Baud, C.M.; De Boer, R.F.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M.; Droz, J.-P. A Phase I and Pharmacological Study of the Platinum Polymer AP5280 given as an Intravenous Infusion Once Every 3 Weeks in Patients with Solid Tumors. Clin. Cancer Res. 2004, 10, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Bouma, M.; Nuijen, B.; Stewart, D.R.; Shannon, K.F.; St John, J.V.; Rice, J.R.; Harms, R.; Jansen, B.A.J.; van Zutphen, S.; Reedijk, J.; et al. Pharmaceutical Quality Control of the Investigational Polymer-Conjugated Platinum Anticancer Agent AP 5280. PDA J. Pharm. Sci. Technol. 2003, 57, 198–207. [Google Scholar]
- Nowotnik, D.P.; Cvitkovic, E. ProLindacTM (AP5346): A Review of the Development of an HPMA DACH Platinum Polymer Therapeutic. Adv. Drug Deliv. Rev. 2009, 61, 1214–1219. [Google Scholar] [CrossRef]
- Zhang, X.; Niebuur, B.-J.; Chytil, P.; Etrych, T.; Filippov, S.K.; Kikhney, A.; Wieland, D.C.F.; Svergun, D.I.; Papadakis, C.M. Macromolecular p HPMA-Based Nanoparticles with Cholesterol for Solid Tumor Targeting: Behavior in HSA Protein Environment. Biomacromolecules 2018, 19, 470–480. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xing, L.; Zhu, M.; Li, X.; Wei, F.; Sun, W.; Jia, Y. HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery. Biomolecules 2025, 15, 596. https://doi.org/10.3390/biom15040596
Li Y, Xing L, Zhu M, Li X, Wei F, Sun W, Jia Y. HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery. Biomolecules. 2025; 15(4):596. https://doi.org/10.3390/biom15040596
Chicago/Turabian StyleLi, Ya, Liangda Xing, Mingliang Zhu, Xian Li, Fangfang Wei, Wenyan Sun, and Yinnong Jia. 2025. "HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery" Biomolecules 15, no. 4: 596. https://doi.org/10.3390/biom15040596
APA StyleLi, Y., Xing, L., Zhu, M., Li, X., Wei, F., Sun, W., & Jia, Y. (2025). HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery. Biomolecules, 15(4), 596. https://doi.org/10.3390/biom15040596







