Persea americana Peel: A Promising Source of Nutraceutical for the Mitigation of Cardiovascular Risk in Arthritic Rats Through the Gut–Joint Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
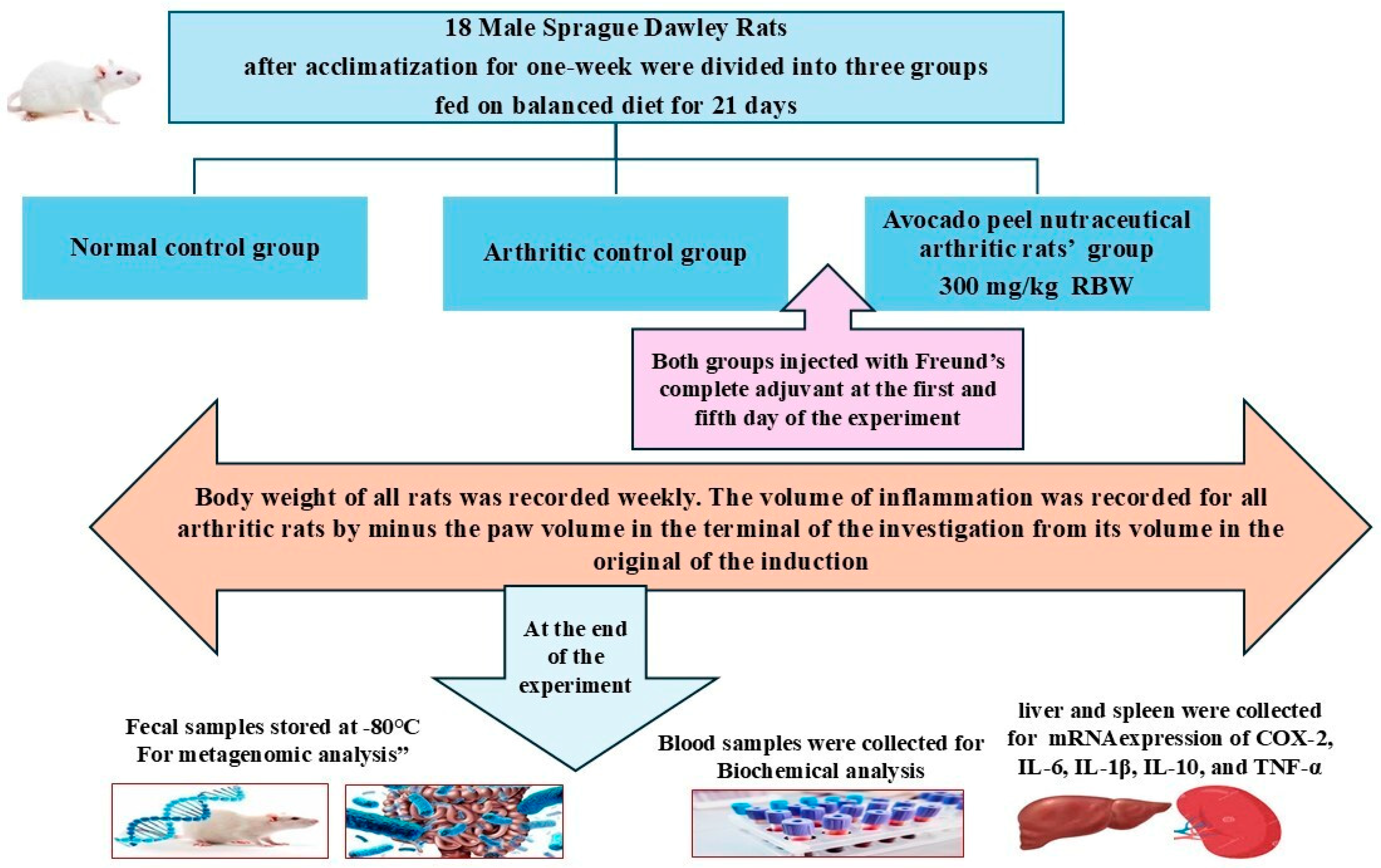
2.2. Animals
2.3. Extraction and Preparation of Avocado Peel Nutraceutical
2.4. Screening of the Active Compounds and Antioxidant Activity of Avocado Peel Nutraceutical
2.5. Evaluation of the Anti-Arthritic Effect of APN and Its Potential for the Mitigation of Cardiovascular Risk
2.6. Biochemical Analysis of Blood Samples of Different Experimental Groups
2.7. mRNA Expression of IL-6, IL-1β, IL-10, TNF-α, and COX-2
2.8. 16S rRNA Amplicon Sequencing Analysis of Colon Content
2.9. Molecular Docking Studies of Avocado Peel Nutraceutical
2.10. Statistical Analysis
3. Results
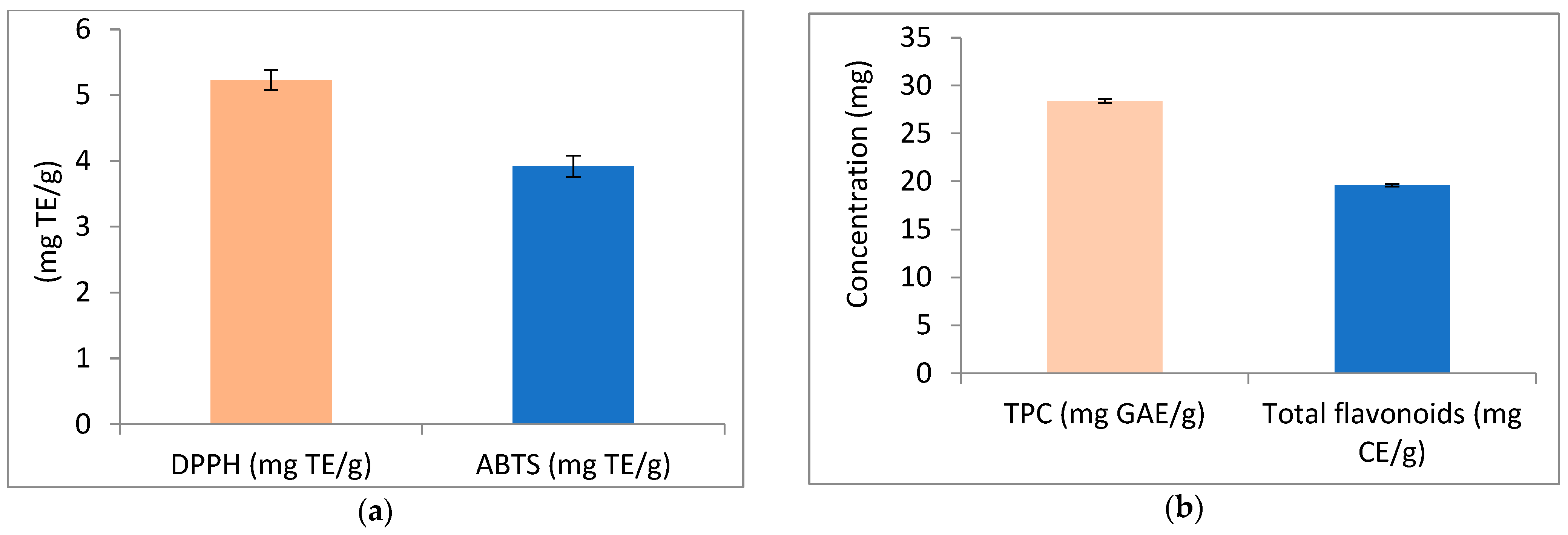
3.1. Screening of the Active Compounds of Avocado Peel Nutraceutical and Its Antioxidant Activity
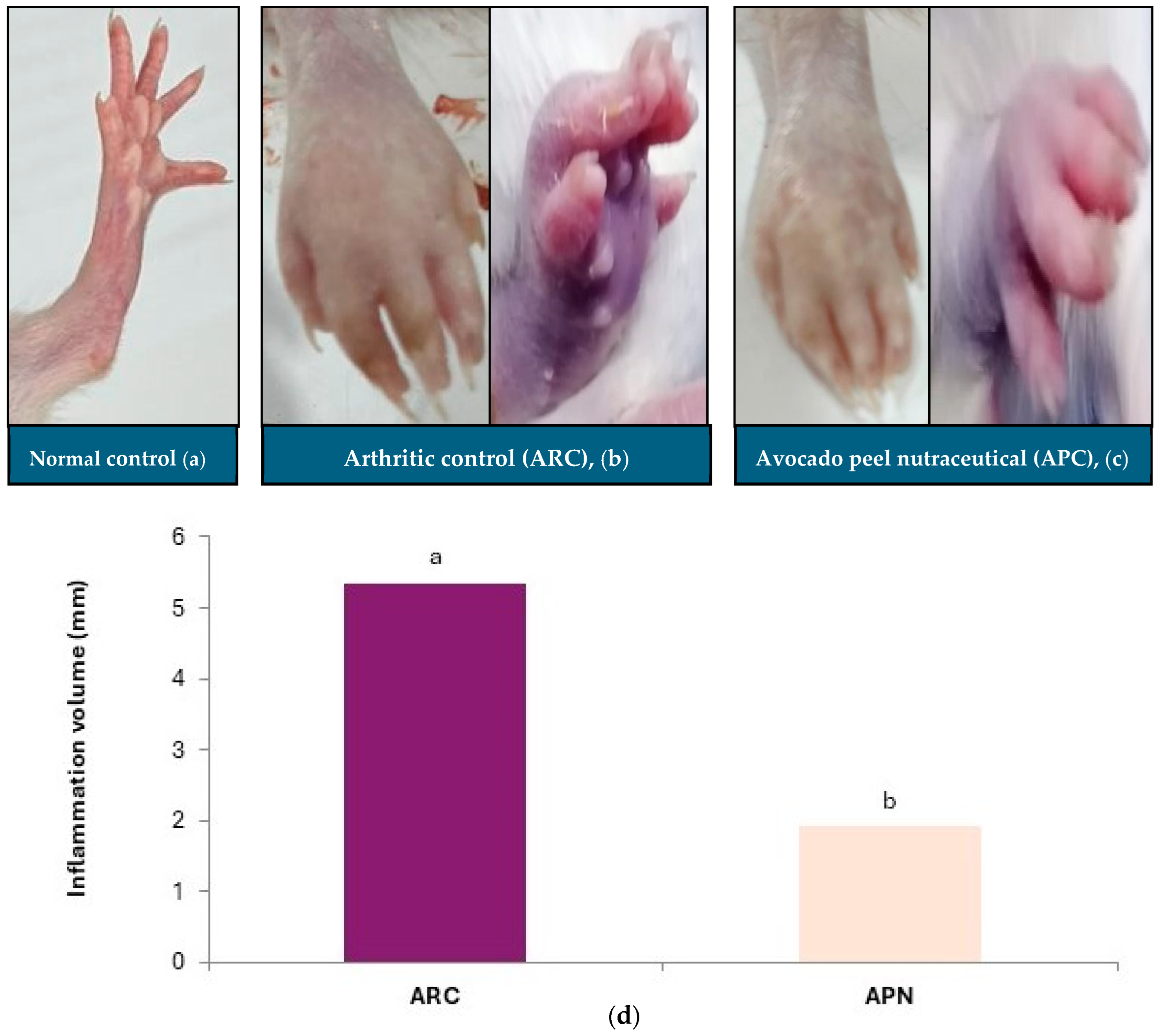
3.2. Impact of Avocado Peel Nutraceutical on Paw Inflammation Volume
3.3. Impact of Avocado Peel Nutraceutical on Different Biochemical Parameters in Normal and Arthritic Rats
3.4. Impact of Avocado Peel Nutraceutical on the Coronary Risk Index and Atherogenic Index
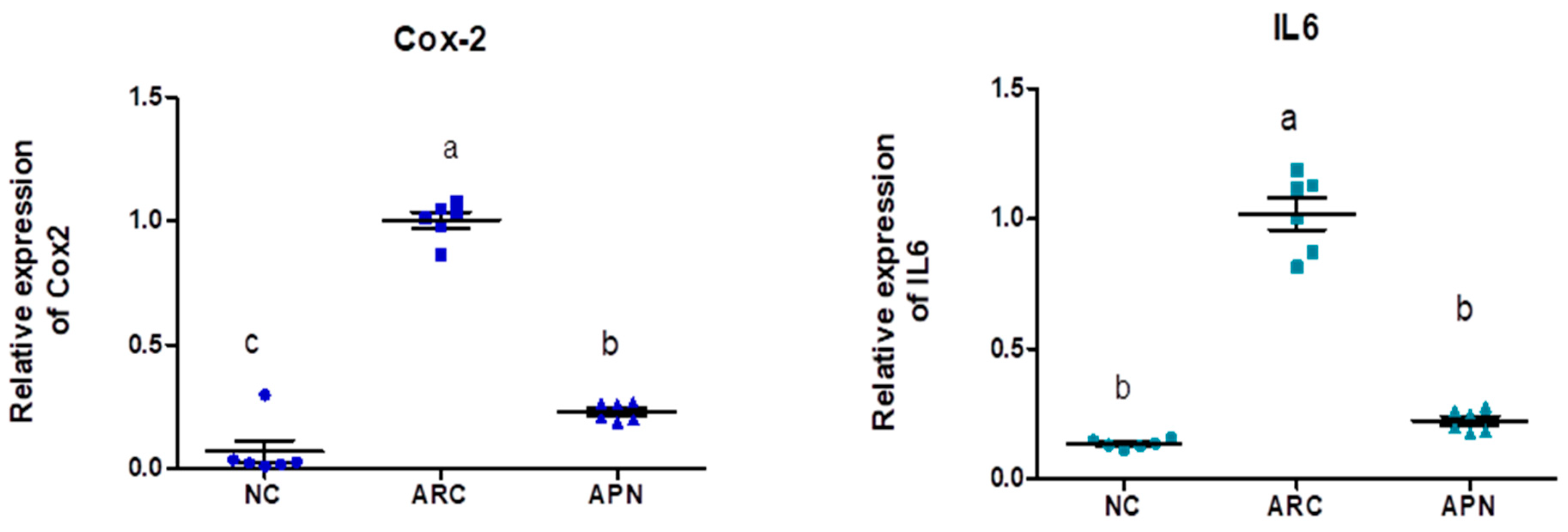
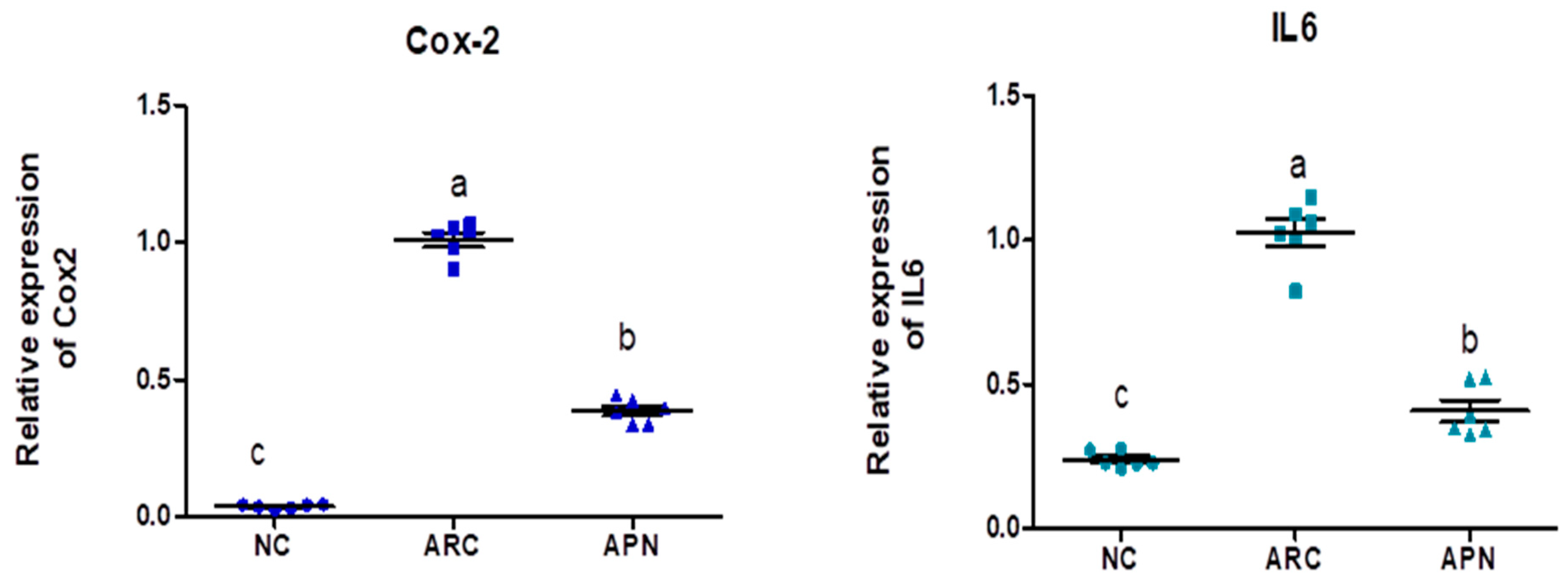
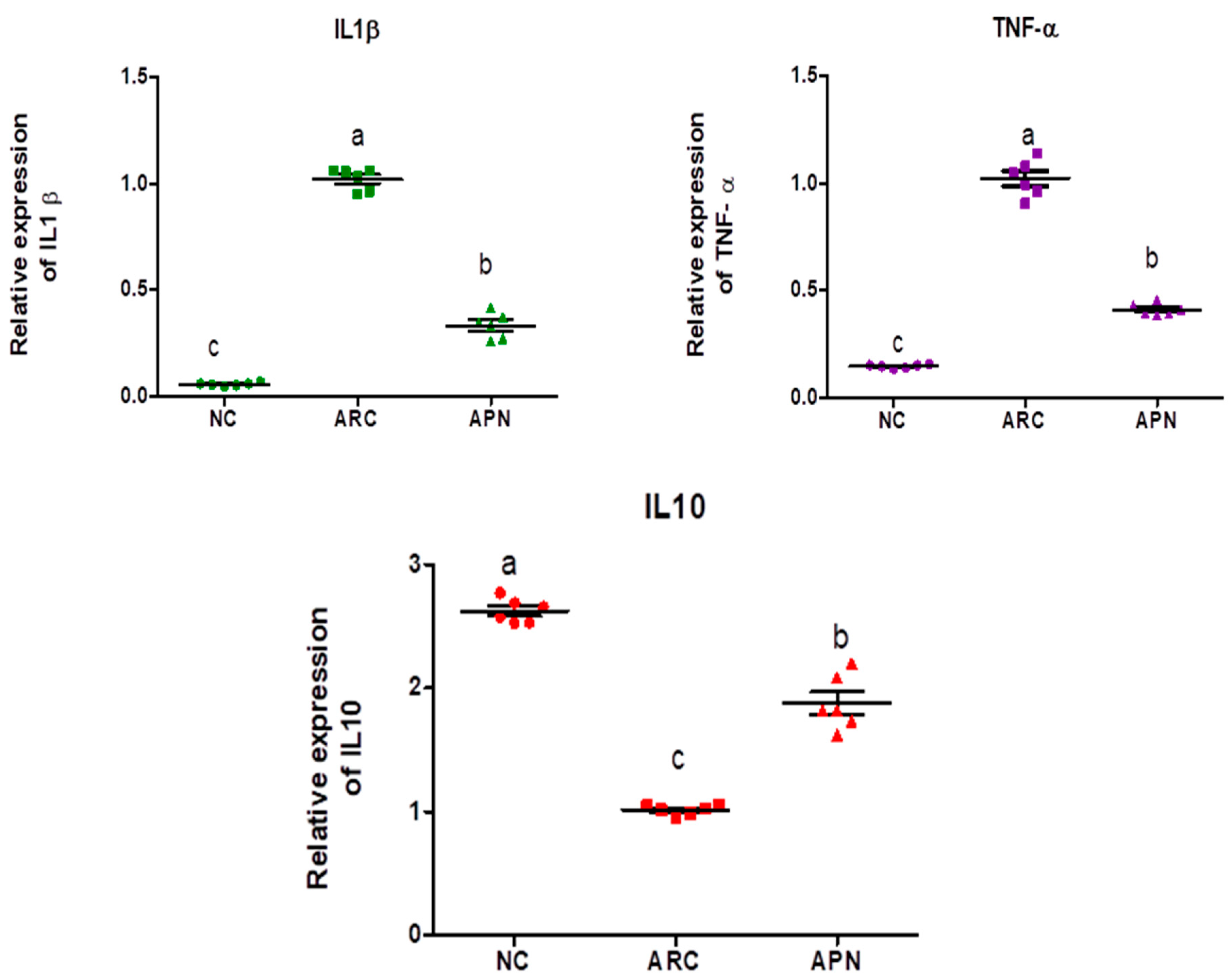
3.5. Impact of APN on the Expression of COX-2, IL-6, IL-1β, IL-10, and TNF-α in Different Rat Groups
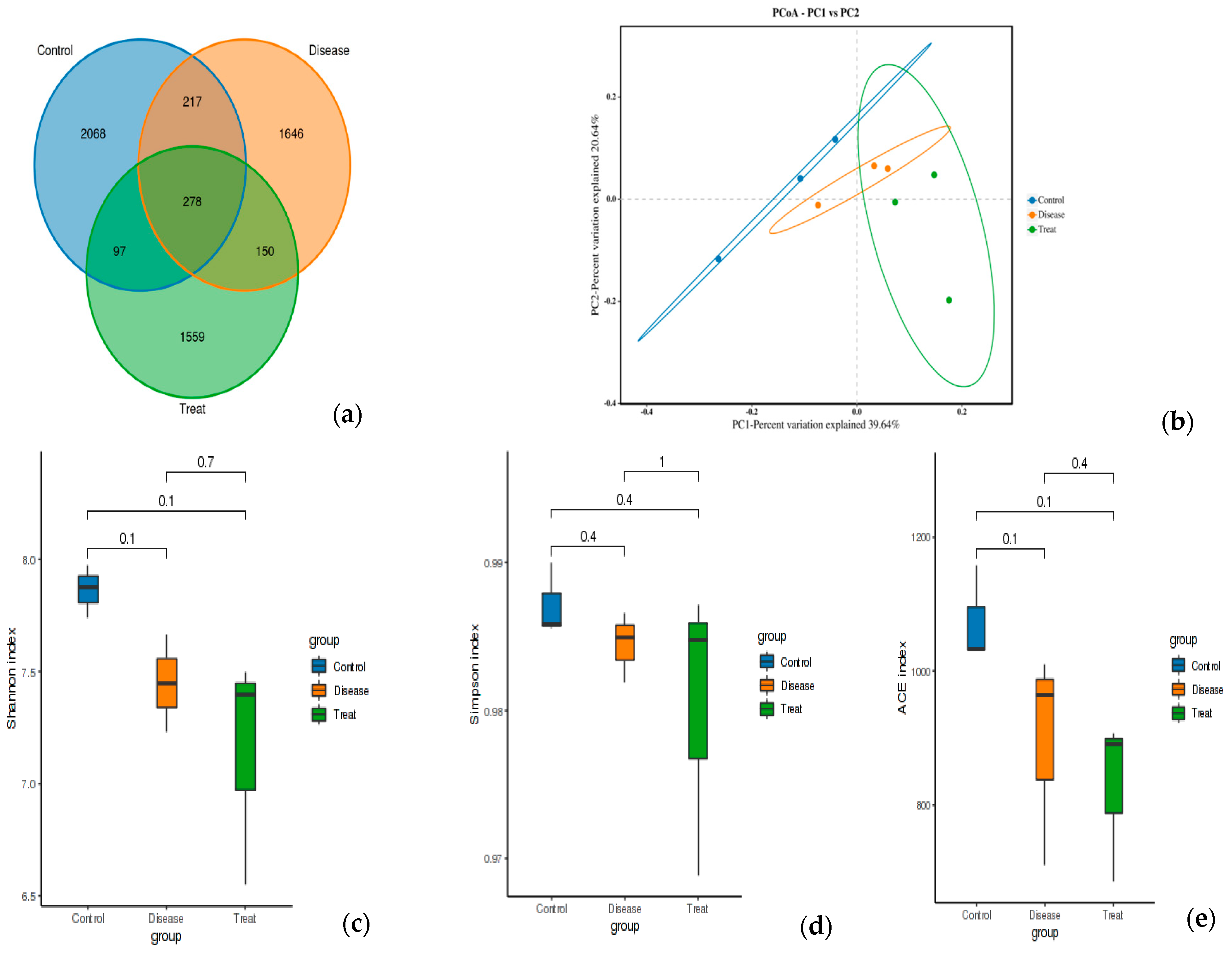
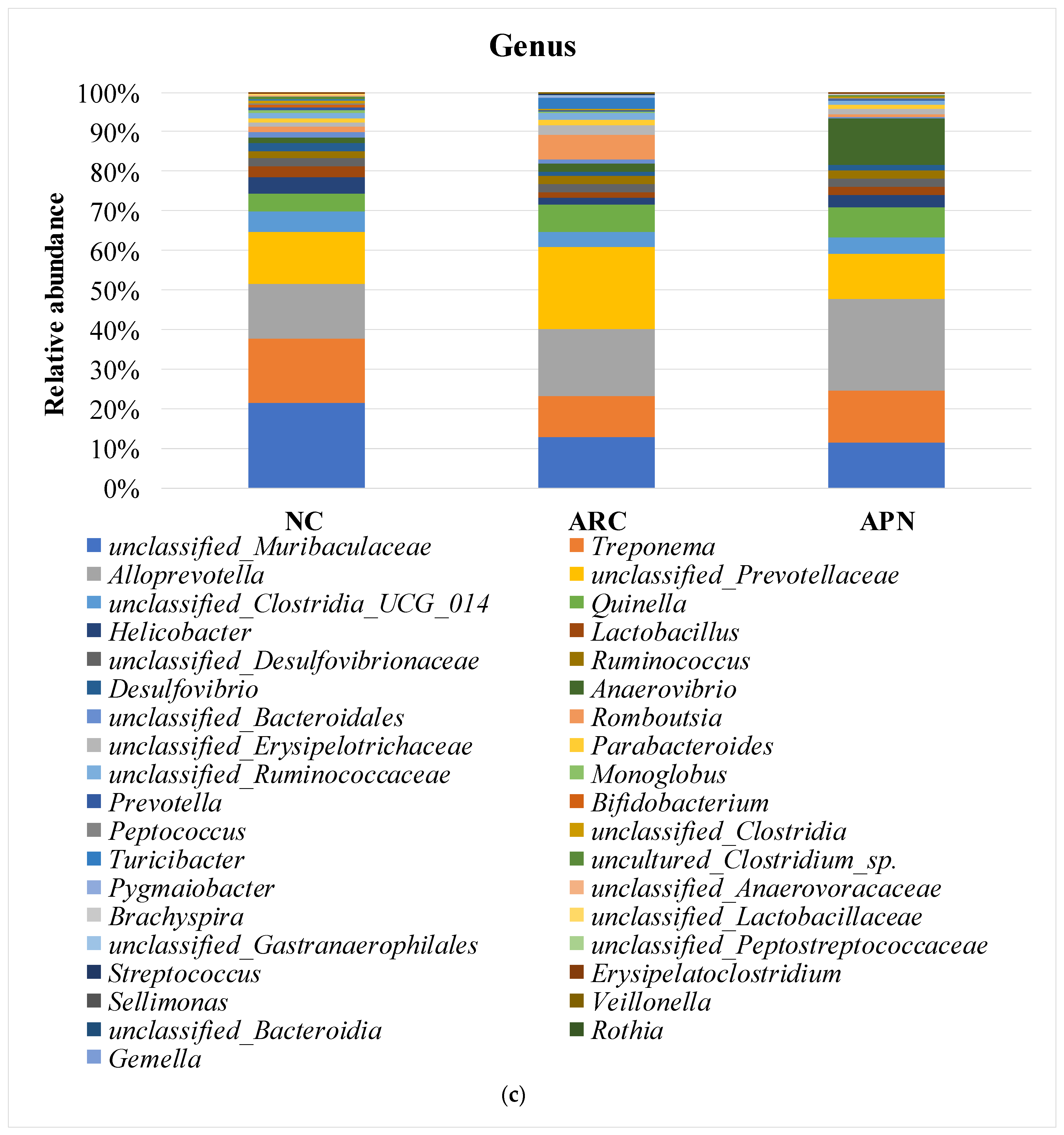
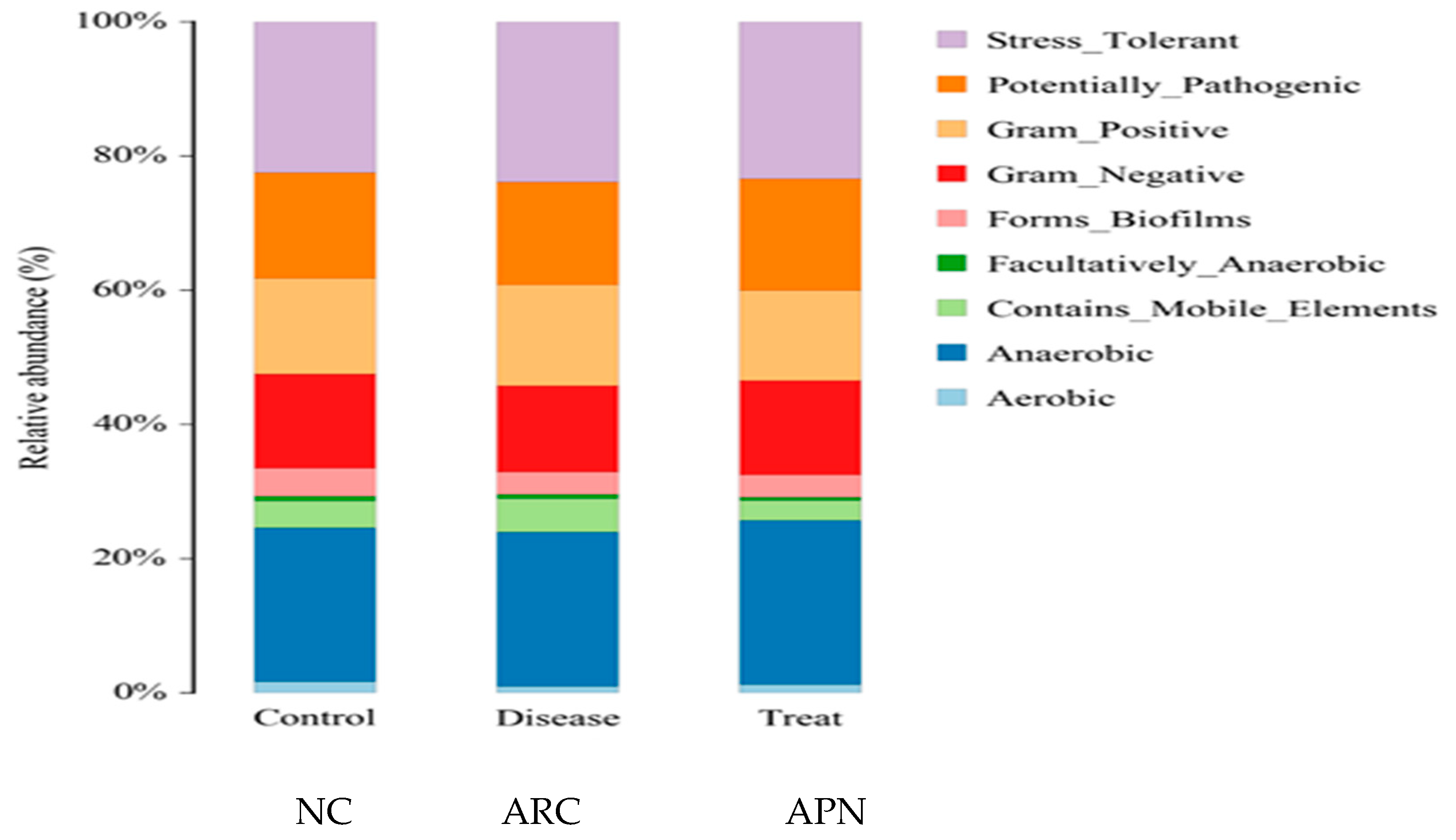
3.6. 16S rRNA Amplicon Sequencing Analysis of Colon Content
3.6.1. Diversity and Richness Analysis
3.6.2. Relative Abundance
3.7. Molecular Docking Studies of Avocado Peel Nutraceutical
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteleone, G.; Moscardelli, A.; Colella, A.; Marafini, I.; Salvatori, S. Immune-mediated inflammatory diseases: Common and different pathogenic and clinical features. Autoimmun. Rev. 2023, 22, 103410. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, M.; Cheng, Y.; Wang, X.; Wang, W. Prevention and treatment of rheumatoid arthritis through traditional Chinese medicine: Role of the gut microbiota. Front. Immunol. 2023, 14, 1233994. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Q.; Zhang, X.; Liu, Z. Rheumatoid arthritis and the intestinal microbiome: Probiotics as a potential therapy. Front. Immunol. 2024, 15, 1331486. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Scher, J.U.; Abramson, S.B.; Attur, M. Role of Intestinal Dysbiosis and Nutrition in Rheumatoid Arthritis. Cells 2022, 11, 2436. [Google Scholar] [CrossRef]
- Sun, X.; Qian, Y.; Cheng, W.; Ye, D.; Liu, B.; Zhou, D.; Wen, C.; Andreassen, O.A.; Mao, Y. Characterizing the polygenic overlap and shared loci between rheumatoid arthritis and cardiovascular diseases. BMC Med. 2024, 22, 152. [Google Scholar] [CrossRef]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, K.; Xiong, Q.; Zhang, J.; Cai, B.; Huang, Z.; Yang, B.; Wei, B.; Chen, J.; Niu, Q. Gut microbiota in pre-clinical rheumatoid arthritis: From pathogenesis to preventing progression. J. Autoimmun. 2023, 141, 103001. [Google Scholar] [CrossRef]
- Luo, Y.; Tong, Y.; Wu, L.; Niu, H.; Li, Y.; Su, L.C.; Wu, Y.; Bozec, A.; Zaiss, M.M.; Qing, P.; et al. Alteration of gut microbiota in individuals at high-Risk for rheumatoid arthritis associated with disturbed metabolome and the initiation of arthritis through the triggering of mucosal immunity imbalance. Arthritis Rheumatol. 2023, 75, 1736–1748. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.-X.; Chang, M.-J.; Qiao, J.; Wang, C.-H.; Li, X.-F.; Yu, Q.; He, P.-F. Characteristics of the gut microbiome and its relationship with peripheral cd4+ T cell subpopulations and cytokines in rheumatoid arthritis. Front. Microbiol. 2022, 13, 799602. [Google Scholar] [CrossRef]
- Gomez, A.; Luckey, D.; Yeoman, C.J.; Marietta, E.V.; Berg Miller, M.E.; Murray, J.A.; White, B.A.; Taneja, V. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE. 2012, 7, e36095. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Abramson, S.B. Periodontal disease, Porphyromonas gingivalis, and rheumatoid arthritis: What triggers autoimmunity and clinical disease? Arthritis Res. Ther. 2013, 15, 122. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Wang, Z.; Chen, Q.; Chen, X.; Xu, Y.; Dai, M.; Wu, B.; Li, Y. The bridge of the gut-joint axis: Gut microbial metabolites in rheumatoid arthritis. Front. Immunol. 2022, 13, 1007610. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Du, J.; Pu, X.; Zheng, L.; Chen, S.; Wang, N.; Li, J.; Chen, S.; Pan, S.; Shen, B. The gut microbiome and metabolites are altered and interrelated in patients with rheumatoid arthritis. Front. Cell. Infect. Microbiol. 2021, 11, 763507. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chu, Y.; Li, J.; Meng, Q.; Liu, Y.; Jin, J.; Wang, Y.; Wang, J.; Huang, B.; Shi, L.; et al. Intestinal butyrate-metabolizing species contribute to autoantibody production and bone erosion in rheumatoid arthritis. Sci. Adv. 2022, 8, eabm1511. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef]
- Izu, G.O.; Mfotie Njoya, E.; Tabakam, G.T.; Nambooze, J.; Otukile, K.P.; Tsoeu, S.E.; Fasiku, V.O.; Adegoke, A.M.; Erukainure, O.L.; Mashele, S.S.; et al. Unravelling the Influence of Chlorogenic Acid on the Antioxidant Phytochemistry of Avocado (Persea americana Mill.) Fruit Peel. Antioxidants 2024, 13, 456. [Google Scholar] [CrossRef]
- Ejiofor, N.C.; Ezeagu, I.E.; Ayoola, M.B.; Umera, E.A. Determination of the Chemical Composition of Avocado (Persea americana) Seed. Adv. Food Technol. Nutr. Sci. SE 2018, 2, S51–S55. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules 2023, 28, 2557. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of avocado processing industry by-product: A review on future prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, D.; Silva-Platas, C.; Rojo, R.P.; García, N.; Cisneros-Zevallos, L.; García-Rivas, G.; Hernández-Brenes, C. Activity-guided identification of acetogenins as novel lipophilic antioxidants present in avocado pulp (Persea americana). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 942, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Donoso, C.; Raluca, M.A.; Chávez-Jinez, S.; Vera, E. Hass Avocado (Persea americana Mill.) Peel Extract Reveals Antimicrobial and Antioxidant Properties against Verticillium theobromae, Colletotrichum musae, and Aspergillus niger Pathogens Affecting Musa acuminata Colla Species, in Ecuador. Microorganisms 2024, 12, 1929. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Bas, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidant 2019, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The Pulp, Peel, Seed, and Food Products of Persea americana as Sources of Bioactive Phytochemicals with Cardioprotective Properties: A Review. Int. J. Mol. Sci. 2024, 25, 13622. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fuentes, E.; Cádiz-Gurrea, M.L.; Rodriguez, L.; Villegas-Aguilar, M.D.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Biological Evaluation of Avocado Residues as a Potential Source of Bioactive Compounds. Antioxidants 2022, 11, 1049. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agri Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Zhishen, J.; Mencheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Mabrok, H.B.; Ramadan, A.A.; Elbakry, H.F. Alkaline Diets Potential Role in Prevention of Kidney Stone Formation: In-vivo and Molecular Docking Studies. Food Funct. 2024, 15, 12033–12046. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D.A. Spirulina-Enriched Pasta as Functional Food Rich in Protein and Antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 22nd ed.; AOAC: Gaithersburg, WA, USA, 2023. [Google Scholar]
- Mohamed, D.A.; Hamed, I.M.; Mohammed, S.E. Utilization of grape and apricot fruits by-products as cheap source for biologically active compounds for health promotion. Egypt. J. Chem. 2021, 64, 2037–2045. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Hammond, E.G. Analysis of Oleate, Linoleate and Linolenate Hydroperoxides in Oxidized Ester Mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free redical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hassan, M.; Abdel-Moniem, S.; Mahmoud, E.A.; Mohamed, D.A. Antioxidant, anti-cancer and anti-arthritic activities of acetogenin-rich extract of avocado pulp. Egypt. J. Chem. 2022, 65, 539–550. [Google Scholar] [CrossRef]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chun, J.M.; Kim, H.S.; Lee, A.Y.; Kim, S.H.; Kim, H.K. Anti-inflammatory and anti-osteoarthritis effects of Saposhnikovia divaricata ethanol extract: In vitro and in vivo studies. Evid. Based Complement. Altern. Med. 2016, 2016, 1984238. [Google Scholar] [CrossRef]
- Khan, H.A.; Abdelhalim, M.K.; Alhomida, A.S.; Al Ayed, M.S. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet. Mol. Res. 2013, 12, 5851–5857. [Google Scholar] [CrossRef]
- Edgar Robert, C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Kebede, B.; Ting, V.; Eyres, G.; Oey, I. Volatile changes during storage of shelf stable Apple juice: Integrating GC-MS fingerprinting and chemometrics. Foods 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komthong, P.; Noriyuki, I.; Mitsuya, S. Effect of ascorbic acid on the odours of cloudy apple juice. Food Chem. 2007, 100, 1342–1349. [Google Scholar] [CrossRef]
- Abdel-Moein, N.M.; Abdel-Moniem, E.A.; Mohamed, D.A.; Hanfy, E.A. Evaluation of the Anti-inflammatory and Anti-arthritic Effects of Some Plants Extracts. Grasas y Aceites 2011, 62, 365–374. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Kandil, E.; Abo-Zeid, M.A.; Mohammed, S.E.; Ahmed, E.K. Anti-inflammatory activity of two varieties of pumpkin seed oil in an adjuvant arthritis model in rats. Grasas y Aceites 2017, 68, e180. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Hanfy, E.A.; Fouda, K. Evaluation of Antioxidant, Anti-inflammatory and Anti-arthritic Activities of Yarrow (Achillea millefolium). J. Biol. Sci. 2018, 18, 317–328. [Google Scholar] [CrossRef]
- Liu, N.; Fan, X.; Shao, Y.; Chen, S.; Wang, T.; Yao, T.; Chen, X. Resveratrol attenuates inflammation and fibrosis in rheumatoid arthritis-associated interstitial lung disease via the AKT/TMEM175 pathway. J. Transl. Med. 2024, 22, 457. [Google Scholar] [CrossRef]
- Ranjbar, M.; Shab-Bidar, S.; Rostamian, A.; Mohammadi, H.; Djafarian, K. The effects of intermittent fasting diet on quality of life, clinical symptoms, inflammation, and oxidative stress in overweight and obese postmenopausal women with rheumatoid arthritis: Study protocol of a randomized controlled trial. Trials 2024, 25, 168. [Google Scholar] [CrossRef]
- Rutz, S.; Ouyang, W. Regulation of Interleukin-10 Expression. Adv. Exp. Med. Biol. 2016, 941, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Laragione, T.; Harris, C.; Azizgolshani, N.; Beeton, C.; Bongers, G.; Gulko, P.S. Magnesium increases numbers of Foxp3+ Treg cells and reduces arthritis severity and joint damage in an IL-10-dependent manner mediated by the intestinal microbiome. EBioMedicine 2023, 92, 104603. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Saksena, A.; Pal, R.; Jaiswal, R.; Kumar, R. Effect of Boswellia Serrata extract on acute inflammatory parameters and tumor necrosis factor-α in complete Freund’s adjuvant-induced animal model of rheumatoid arthritis. Int. J. Appl. Basic Med. Res. 2019, 9, 100–106. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, R.; Jin, X.; Tan, X. Antiarthritic activity of diallyl disulfide against Freund’s adjuvant-induced arthritic rat model. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 291–303. [Google Scholar] [CrossRef]
- Chen, G.; Song, Y.; Ma, F.; Ma, Y. Anti-arthritic activity of D-carvone against complete Freund’s adjuvant-induced arthritis in rats through modulation of inflammatory cytokines. Korean J. Physiol. Pharmacol. 2020, 24, 453–462. [Google Scholar] [CrossRef]
- Sanghavi, N.; Ingrassia, J.P.; Korem, S.; Ash, J.; Pan, S.; Wasserman, A. Cardiovascular Manifestations in Rheumatoid Arthritis. Cardiol. Rev. 2024, 32, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, S.; Han, L.; Ba, X.; Shen, P.; Lin, W.; Li, T.; Zhang, R.; Huang, Y.; Huang, Y.; et al. Dyslipidemia in rheumatoid arthritis: The possible mechanisms. Front Immunol. 2023, 14, 1254753. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; Rezus, E.; Badescu, M.C.; Dima, N.; Seritean Isac, P.N.; Dragoi, I.T.; Rezus, C. Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy. Life 2023, 13, 319. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI QTOF-MSandHPLC-FLD-MSasvaluable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; XiaoHui, Z. Chlorogenic acid (CGA): A pharmaco logical review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Ismael, A.I.; Ibrahim, A.R. Studying the anti-inflammatory and biochemical effects of wheat germ oil. Dtsch. Leb. Rundsch. 2005, 101, 66–72. [Google Scholar]
- Jayedi, A.; Shab-Bidar, S. Fish consumption and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Adv. Nutr. 2020, 11, 1123–1133. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Mohammed, S.E.; Hamed, I.M. Chia seeds oil enriched with phytosterols and mucilage as a cardioprotective dietary supplement towards inflammation, oxidative stress, and dyslipidemia. J. Herbmed. Pharmacol. 2022, 11, 83–90. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138 Pt A, 109774. [Google Scholar] [CrossRef]
- Alkhalaf, M.; Alansari, W.; Ibrahim, E.; ELhalwagy, M. Antioxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (Seeds and Peels) of Avocado (Persea americana Mill.) Cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Paz, B.; Yahia, E.M. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef]
- Marović, R.; Badanjak Sabolović, M.; Brnčić, M.; Ninčević Grassino, A.; Kljak, K.; Voća, S.; Karlović, S.; Rimac Brnčić, S. The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes. Foods 2024, 13, 2003. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Momot-Ruppert, M.; Stawicka, B.; Szydłowska-Czerniak, A. Health Benefits, Antioxidant Activity, and Sensory Attributes of Selected Cold-Pressed Oils. Molecules 2023, 28, 5484. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, M.; Zou, L.; Yin, L.; Zhong, C.; Zha, Y.; Zhu, X.; Zhang, L.; Ning, K.; Han, J. Hidden link in gut–joint axis: Gut microbes promote rheumatoid arthritis at early stage by enhancing ascorbate degradation. Gut 2022, 71, 1041–1043. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, S.; Xu, H.; Sun, S.; Wang, Z.; Li, M.; Zou, L.; Zhang, Y.; Zhao, Y.; Cui, Y.; et al. Vitamin C alleviates rheumatoid arthritis by modulating gut microbiota balance. Biol. Sci. Trends 2024, 18, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, G.; Ding, Y.; Gui, X.; Tong, H.; Xu, X.; Zhang, S.; Sun, Z.; Ju, W.; Li, Y.; et al. Protein tyrosine phosphatase PTPN22 negatively modulates platelet function and thrombus formation. Blood 2022, 140, 1038–1051. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mannaa, M.; Kim, Y.; Kim, J.; Kim, G.T.; Seo, Y.S. Comparative Analysis of Fecal Microbiota Composition Between Rheumatoid Arthritis and Osteoarthritis Patients. Genes 2019, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Q.; Zeng, B.; Fang, Y.; Wei, H. Analysis of Fecal Lactobacillus Community Structure in Patients with Early Rheumatoid Arthritis. Curr. Microbiol. 2013, 67, 170–176. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, J.; Ren, S.; Cao, Q.; Kong, H.; Xu, Q.; Liu, R. High-fat diet stimulated butyric acid metabolism dysbiosis, altered microbiota, and aggravated inflammatory response in collagen-induced arthritis rats. Nutr. Metab. 2024, 21, 95. [Google Scholar] [CrossRef]
- Gámez-Macías, P.E.; Félix-Soriano, E.; Samblas, M.; Sáinz, N.; Moreno-Aliaga, M.J.; González-Muniesa, P. Intestinal Permeability, Gut Inflammation, and Gut Immune System Response Are Linked to Aging-Related Changes in Gut Microbiota Composition: A Study in Female Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae045. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Isobe, J.; Hattori, K.; Hosonuma, M.; Baba, Y.; Murayama, M.; Narikawa, Y.; Toyoda, H.; Funayama, E.; Tajima, K.; et al. Turicibacter and Acidaminococcuspredict immune-related adverse events and efficacy of immune checkpoint inhibitor. Front. Immunol. 2023, 14, 1164724. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Cao, R.; Gao, T.; Yue, J.; Sun, G.; Yang, X. Disordered gut microbiome and alterations in metabolic patterns are associated with hypertensive left ventricular hypertrophy. J. Am. Heart Assoc. 2024, 13, e034230. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Zhao, L.; Bai, F.; Liu, X. Comparison of intestinal microbes in noninfectious anterior scleritis patients with and without rheumatoid arthritis. Front. Microbiol. 2022, 13, 925929. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kitabatake, M.; Kayano, S.I.; Ito, T. Dietary phenolic compounds: Their health benefits and association with the gut microbiota. Antioxidants 2023, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Taylor, K.D.; Wood, A.C.; Rotter, J.I.; Guo, X.; Herrington, D.M.; Johnson, W.C.; Post, W.; Tracy, R.P.; Rich, S.S.; Malik, S. Metagenomic Study of the MESA: Detection of Gemella Morbillorumand association with coronary heart disease. J. Am. Heart Assoc. 2024, 13, e035693. [Google Scholar] [CrossRef]
- Medina, G.; Vera-Lastra, O.; Peralta-Amaro, A.L.; Jiménez-Arellano, M.P.; Saavedra, M.A.; Cruz-Domínguez, M.P.; Jara, L.J. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol. Res. 2018, 133, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Bartels, L.; Pedersen, A.; Kristensen, N.; Jepsen, P.; Vilstrup, H.; Stengaard-Pedersen, K.; Dahlerup, J. Helicobacter pylori infection is not associated with rheumatoid arthritis. Scand. J. Rheumatol. 2019, 48, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Pan, F.; Shi, M.; He, C.; He, B.; Wang, R.; Ren, G.; Yang, S.; Zhang, S. 2-Monoacylglycerol Mimetic Liposomes to Promote Intestinal Lymphatic Transport for Improving Oral Bioavailability of Dihydroartemisinin. Int. J. Nanomed. 2024, 19, 5273–5295. [Google Scholar] [CrossRef] [PubMed]
- Dimple, P.; Fatemeh, A.; Hossein, Z. Intestinal permeability enhancement of levothyroxine sodium by straight chain fatty acids studied in MDCK epithelial cell line. Eur. J. Pharm. Sci. 2010, 40, 466–472. [Google Scholar] [CrossRef]
- Gumus, C.E.; Gharibzahedi, M.T. Yogurts supplemented with lipid emulsions rich in omega-3 fatty acids: New insights into the fortification, microencapsulation, quality properties, and health-promoting effects. Trends Food Sci. Technol. 2021, 110, 267–279. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Bigels as Delivery Systems of Bioactive Fatty Acids Present in Functional Edible Oils: Coconut, Avocado, and Pomegranate. Gels 2023, 9, 349. [Google Scholar] [CrossRef]
- Deng, H.; Jiang, J.; Zhang, S.; Wu, L.; Zhang, Q.; Sun, W. Network pharmacology and experimental validation to identify the potential mechanism of Hedyotis diffusa Willd against rheumatoid arthritis. Sci. Rep. 2023, 13, 1425. [Google Scholar] [CrossRef]
- Radu, A.-F.; Negru, P.A.; Radu, A.; Tarce, A.G.; Bungau, S.G.; Bogdan, M.A.; Tit, D.M.; Uivaraseanu, B. Simulation-Based Research on Phytoconstituents of Embelia ribes Targeting Proteins with Pathophysiological Implications in Rheumatoid Arthritis. Life 2023, 13, 1467. [Google Scholar] [CrossRef]
- Gong, N.; Wang, L.; An, L.; Xu, Y. Exploring the active ingredients and potential mechanisms of action of sinomenium acutum in the treatment of rheumatoid arthritis based on systems biology and network pharmacology. Front. Mol. Biosci. 2023, 10, 1065171. [Google Scholar] [CrossRef]
- Remuzgo-Martínez, S.; Genre, F.; Castañeda, S.; Corrales, A.; Moreno-Fresneda, P.; Ubilla, B.; Mijares, V.; Portilla, V.; González-Vela, J.; Pina, T.; et al. Protein tyrosine phosphatase non-receptor 22 and C-Src tyrosine kinase genes are down-regulated in patients with rheumatoid arthritis. Sci. Rep. 2017, 7, 10525. [Google Scholar] [CrossRef]
- Sharp, R.C.; Beg, S.A.; Naser, S.A. Polymorphisms in Protein Tyrosine Phosphatase Non-receptor Type 2 and 22 (PTPN2/22) Are Linked to Hyper-Proliferative T-Cells and Susceptibility to Mycobacteria in Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Noa, Y.; Hernández-Bello, J.; Llamas-Covarrubias, M.A.; Palafox-Sánchez, C.A.; Oregon-Romero, E.; Sánchez-Hernández, P.E.; Ramírez-Dueñas, M.G.; Parra-Rojas, I.; Muñoz-Valle, J.F. PTPN22 1858C>T polymorphism is associated with increased CD154 expression and higher CD4+ T cells percentage in rheumatoid arthritis patients. J. Clin. Lab. Anal. 2019, 33, e22710. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Lang, S.; Gottier, C.; Dai, X.; Rawlings, D.J.; Chan, A.C.; Rogler, G.; Scharl, M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017, 13, 1590–1601. [Google Scholar] [CrossRef]
- Alippe, Y.; Mbalaviele, G. Omnipresence of Inflammasome Activities in Inflammatory Bone Diseases. Semin. Immunopathol. 2019, 41, 607–618. [Google Scholar] [CrossRef]
- Pertovaara, M.; Raitala, A.; Juonala, M.; Kähönen, M.; Lehtimäki, T.; Viikari, J.S.; Raitakari, O.T.; Hurme, M. Autoimmunity and atherosclerosis: Functional polymorphism of PTPN22 is associated with phenotypes related to the risk of atherosclerosis. The Cardiovascular Risk in Young Finns Study. Clin. Exp. Immunol. 2007, 147, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Saccucci, P.; Banci, M.; Cozzoli, E.; Neri, A.; Magrini, A.; Bottini, E.; Gloria-Bottini, F. Atherosclerosis and PTPN22: A study in coronary artery disease. Cardiology 2011, 119, 54–56. [Google Scholar] [CrossRef]
- Li, S.; Luo, Z.; Su, S.; Wen, L.; Xian, G.; Zhao, J.; Xu, X.; Xu, D.; Zeng, Q. Targeted inhibition of PTPN22 is a novel approach to alleviate osteogenic responses in aortic valve interstitial cells and aortic valve lesions in mice. BMC Med. 2023, 21, 252. [Google Scholar] [CrossRef]





| Target Genes | Sequence | Ref |
|---|---|---|
| COX-2 | FW (5′-TGGTGCCGGGTCTGATGATG-3′) RW (5′-GCAATGCGGTTCTGATACTG-3′) | [42] |
| IL6 | FW (5′-ACTGAACTTCGGGGTGATTG-3′) RW (5′-GCTTGGTGGTTTGCTACGAC-3′) | [43] |
| IL-1β | FW (5′-TGA TGG ATG CTT CCA AAC TG-3′) RW (5′-GAG CAT TGG AAG TTG GGG TA-3′) | [43] |
| IL10 | FW (5′-CTTACTGGCTGGAGTGA-3′) RW (5′-AGCTCTCGGAGCATGTG-3′) | This study |
| GAPDH | FW (5′-GTATTGGGCGCCTGGTCACC-3′) RW (5′-CGCTCCTGGAAGATGGTGATGG-3′) | [43] |
| Phenolic Compounds | APN(µg/g) |
|---|---|
| Gallic acid | 274.41 ± 0.076 |
| Chlorogenic acid | 8029.69 ± 0.067 |
| Methyl gallate | 200.84 ± 0.086 |
| Coffeic acid | 76.43 ± 0.054 |
| Syringic acid | 31.44 ± 0.066 |
| Pyro catechol | 235.61 ± 0.045 |
| Ellagic acid | 112.89 ± 0.095 |
| Coumaric acid | 92.23 ± 0.076 |
| Vanillin | 4.23 ± 0.052 |
| Ferulic acid | 13.54 ± 0.015 |
| Naringenin | 165.82 ± 0.041 |
| Rosmarinic acid | 49.35 ± 0.051 |
| Daidzein | 5.30 ± 0.0105 |
| Querectin | 66.70 ± 0.056 |
| Cinnamic acid | 0.72 ± 0.040 |
| Kaempferol | 14.32 ± 0.065 |
| Hesperetin | 14.29 ± 0.046 |
| Volatile Compounds | LRI a | Peel b | Aroma Description c |
|---|---|---|---|
| Ethyl ethanoate | 601 | 1.59 ± 0.001 | |
| Ethanol | 617 | 0.57 ± 0.001 | Herbaceous, fruity |
| Ethyl acetate | 648 | 13.25 ± 0.001 | Fruity ester |
| Methyl propanoate | 657 | 1.46 ± 0.001 | |
| Butanal | 675 | 11.08 ± 0.002 | Malty, green odor |
| Propyl acetate | 701 | 2.16 ± 0.001 | |
| Methyl butyrate | 726 | 1.34 ± 0.001 | |
| 1-Penten-3-ol | 731 | 0.08 ± 0.001 | Fresh, mild fruity |
| (E)-2-Hexenal | 775 | 3.79 ± 0.001 | Green, fruity |
| Pentanol | 778 | 0.07 ± 0.001 | Fresh, mild fruity |
| Hexanal | 803 | 12.83 ± 0.002 | Oily avocado |
| 2-Furfural | 835 | 8.27 ± 0.001 | Sweet, coffee |
| Isobutyl propanoate | 865 | 4.37 ± 0.001 | |
| Isoamyl acetate | 874 | 2.38 ± 0.001 | |
| Methional | 896 | 6.07 ± 0.001 | |
| Benzaldehyde | 984 | 8.26 ± 0.001 | |
| Ethyl hexanoate | 1019 | 4.67 ± 0.001 | |
| γ-Terpinene | 1067 | 2.06 ± 0.001 | |
| (Z)-7-Decenol | 1196 | 0.06 ± 0.001 | |
| Decanal | 1217 | 9.52 ± 0.001 | Orange peel |
| (E,Z)-2,4-Decadienal | 1296 | 3.56 ± 0.001 | |
| α-Humulene | 1459 | 0.12 ± 0.001 | Mild woody, earthy |
| (E,E) α-Farnesene | 1512 | 0.29 ± 0.001 | |
| δ-Cadinene | 1526 | 1.03 ± 0.001 | |
| Z-Nerolidol | 1539 | 0.08 ± 0.001 |
| Parameters | APN |
|---|---|
| Fatty acids (as a percentage of total fatty acids) | |
| Palmitic acid (C16:0) | 13.69 ± 0.055 |
| Oleic acid (C18:1) | 73.62 ± 0.064 |
| Linoleic acid (C18:2) (ω − 6) | 0.857 ± 0.004 |
| Linolenic acid (C18:3) (ω − 3) | 5.42 ± 0.066 |
| Total saturated fatty acids | 13.69 ± 0.055 |
| Total unsaturated fatty acids | 79.897 ± 0.011 |
| Ratio of ω − 3:ω − 6 | 6.3 ± 0.002 |
| COX = (C18: 1 + 10.3 × C18: 2 + 21.6 × C18: 3)/100 | 5.07 ± 0.001 |
| AI = (C12: 0 + 4 × C14: 0 + C16: 0)/(ΣMUFA + Σ(ω − 3) + Σ(ω − 6)) | 0.171 ± 0.001 |
| TI = (C14: 0 + C16: 0 + C18: 0)/(0.5 × MUFA) + (0.5 × Σ(ω − 6) + (3 × Σ(ω − 3)) + (ω − 3/ω − 6)) | 0.229 ± 0.001 |
| H/H ratio = (C18:1 + C18:2 + C18:3)/(C16:0) | 5.836 ± 0.002 |
| Triterpenoids and Phytosterols (as a percentage of total unsaponifiable matter) | |
| β-Sitosterol | 7.96 ± 0.007 |
| Stigmasterol | 16.13 ± 0.01 |
| Campesterol | 10.18 ± 0.008 |
| β-amyrine | 12.79 ± 0.011 |
| Total phytosterols | 34.27 ± 0.011 |
| Parameters | NC | ARC | APN |
|---|---|---|---|
| Oxidative stress status | |||
| MDA (nmol/mL) | 7.85 c ± 0.34 | 19.00 a ± 0.97 | 11.83 b ± 0.60 |
| CAT (U/L) | 614.17 a ± 8.98 | 304.17 c ± 7.57 | 519.17 b ± 7.57 |
| Inflammatory markers | |||
| TNF-α (pg/mL) | 15.5 c ± 0.43 | 33.17 a ± 0.91 | 20.50 b ± 0.76 |
| IL-1β (pg/mL) | 39.14 b ± 0.35 | 42.75 a ± 0.99 | 36.13 c ± 0.39 |
| IL-6 (pg/mL) | 62.42 b ± 0.71 | 80.82 a ± 2.20 | 65.68 b ± 1.79 |
| Lipid profile | |||
| T-Ch (mg/dL) | 84.83 b ± 2.12 | 113.90 a ± 3.16 | 90.83 b ± 1.72 |
| TG (mg/dL) | 76.22 b ± 3.74 | 130.49 a ± 2.57 | 88.50 b ± 1.84 |
| HDL-Ch (mg/dL) | 44.33 a ± 0.49 | 26.50 b ± 0.76 | 43.00 a ± 0.52 |
| LDL-Ch (mg/dL) | 25.26 b ± 2.78 | 61.30 a ± 3.63 | 30.13 b ± 1.81 |
| Oxi-LDL (pg/mL) | 27.50 c ± 0.76 | 52.50 a ± 1.12 | 35.67 b ± 1.09 |
| Liver and kidney functions | |||
| AST (IU/L) | 35.83 b ± 3.60 | 62.00 a ± 3.16 | 37.33 b ± 4.68 |
| ALT (IU/L) | 5.33 b ± 0.84 | 10.00 a ± 0.89 | 5.67 b ± 0.80 |
| Urea (mg/dL) | 49.18 b ± 1.75 | 58.96 a ± 1.14 | 50.72 b ± 1.59 |
| Creatinine (mg/dL) | 0.65 b ± 0.02 | 0.98 a ± 0.08 | 0.64 b ± 0.02 |
| Nutritional parameters | |||
| Initial Weight (g) | 105.67 a ± 1.48 | 105.67 a ± 1.84 | 106.17 a ± 2.21 |
| Final Weight (g) | 167.2 a ± 2.87 | 145.7 b ± 1.56 | 162.2 a ± 4.20 |
| Body Weight Gain (g) | 61.5 a ± 3.66 | 40.00 b ± 2.73 | 56.00 a ± 2.78 |
| Spleen % | 0. 70 b ± 0.01 | 0.98 a ± 0.03 | 0.71 b ± 0.03 |
| Liver % | 3.22 a ± 0.12 | 3.93 a ± 0.17 | 3.33 a ± 0.13 |
| Name of the Ligand | Binding Free Energy (kcal/mol) |
|---|---|
| Ellagic acid | −9.2 |
| β-amyrine | −8.6 |
| Quercetin | −8.4 |
| Naringenin | −8.2 |
| Campesterol | −8.1 |
| Hesperetin | −8 |
| Kaempferol | −8 |
| Stigmasterol | −8 |
| β-Sitosterol | −8 |
| Daidzein | −7.5 |
| Rosmarinic | −7.5 |
| Chlorogenic | −6.7 |
| Ferulic acid | −6.6 |
| Caffeic acid | −6.4 |
| Cinnamic acid | −5.9 |
| Coumaric acid | −5.8 |
| Gallic acid | −5.8 |
| Methyl gallate | −5.8 |
| Vanillin | −5.5 |
| Syringic acid | −5.5 |
| Linoleic acid | −5.2 |
| Oleic acid | −5.1 |
| Pyro-chatechol | −4.8 |
| Linolenic acid | −4.8 |
| Benzaldehyde | −4.7 |
| Ethyl hexanoate | −4.7 |
| Palmitic | −4.5 |
| 2-Furfural | −4.4 |
| Decanal | −4.3 |
| Isobutyl propanoate | −4.1 |
| Pentanol | −4.1 |
| Hexanal | −3.6 |
| Ethyl acetate | −3.2 |
| Methyl propanoate | −3.2 |
| Methional | −3.2 |
| Ligands | PTPN22 | |
|---|---|---|
| 2D | 3D | |
| Ellagic acid | 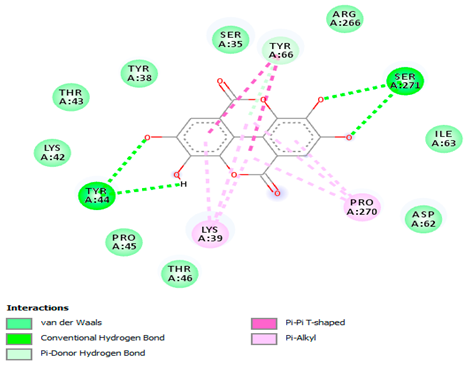 | 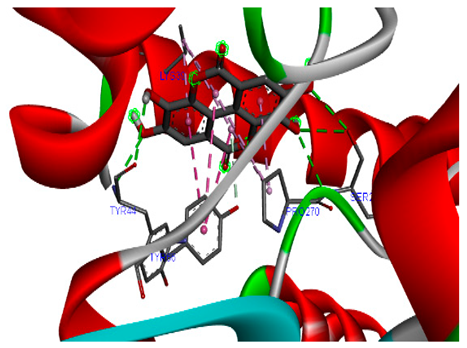 |
| Quercetin | 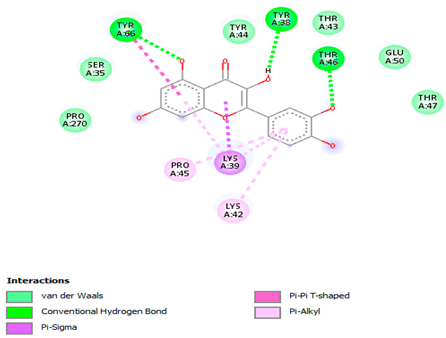 | 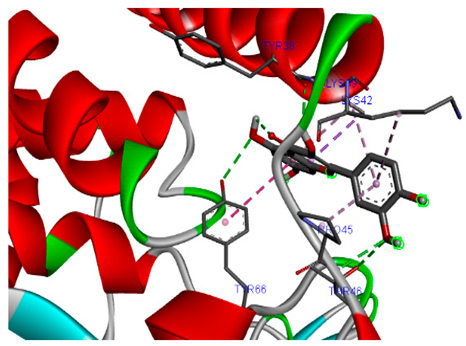 |
| Naringenin |  |  |
| β-amyrine |  |  |
| Campesterol | 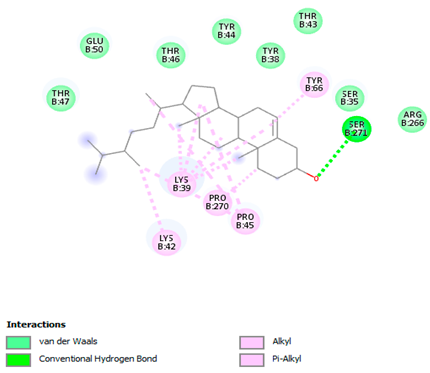 | 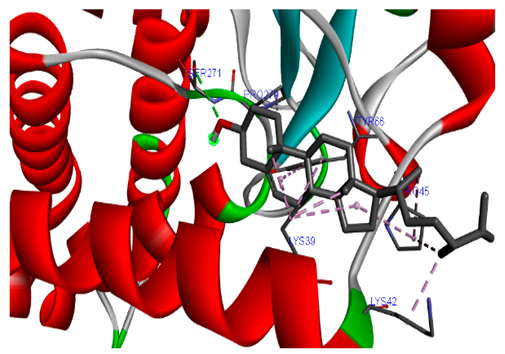 |
| β-Sitosterol | 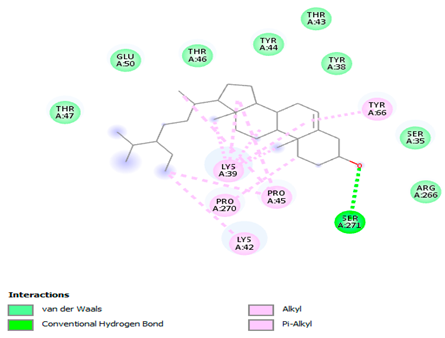 | 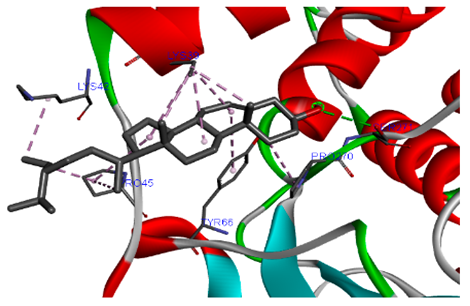 |
| Stigmasterol | 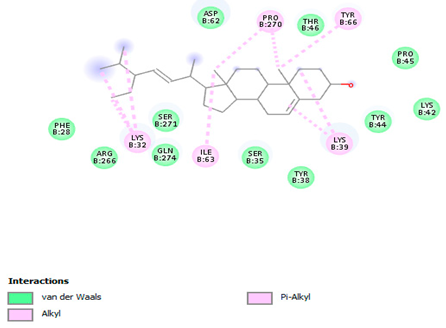 | 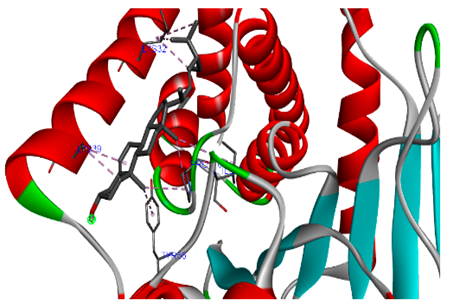 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, D.A.; Ramadan, A.A.; Mabrok, H.B.; Ibrahim, G.E.; Mohammed, S.E. Persea americana Peel: A Promising Source of Nutraceutical for the Mitigation of Cardiovascular Risk in Arthritic Rats Through the Gut–Joint Axis. Biomolecules 2025, 15, 590. https://doi.org/10.3390/biom15040590
Mohamed DA, Ramadan AA, Mabrok HB, Ibrahim GE, Mohammed SE. Persea americana Peel: A Promising Source of Nutraceutical for the Mitigation of Cardiovascular Risk in Arthritic Rats Through the Gut–Joint Axis. Biomolecules. 2025; 15(4):590. https://doi.org/10.3390/biom15040590
Chicago/Turabian StyleMohamed, Doha A., Asmaa A. Ramadan, Hoda B. Mabrok, Gamil E. Ibrahim, and Shaimaa E. Mohammed. 2025. "Persea americana Peel: A Promising Source of Nutraceutical for the Mitigation of Cardiovascular Risk in Arthritic Rats Through the Gut–Joint Axis" Biomolecules 15, no. 4: 590. https://doi.org/10.3390/biom15040590
APA StyleMohamed, D. A., Ramadan, A. A., Mabrok, H. B., Ibrahim, G. E., & Mohammed, S. E. (2025). Persea americana Peel: A Promising Source of Nutraceutical for the Mitigation of Cardiovascular Risk in Arthritic Rats Through the Gut–Joint Axis. Biomolecules, 15(4), 590. https://doi.org/10.3390/biom15040590






