Vitamin D and Acute Kidney Injury: A Reciprocal Relationship
Abstract
1. Introduction
2. Methodology
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search
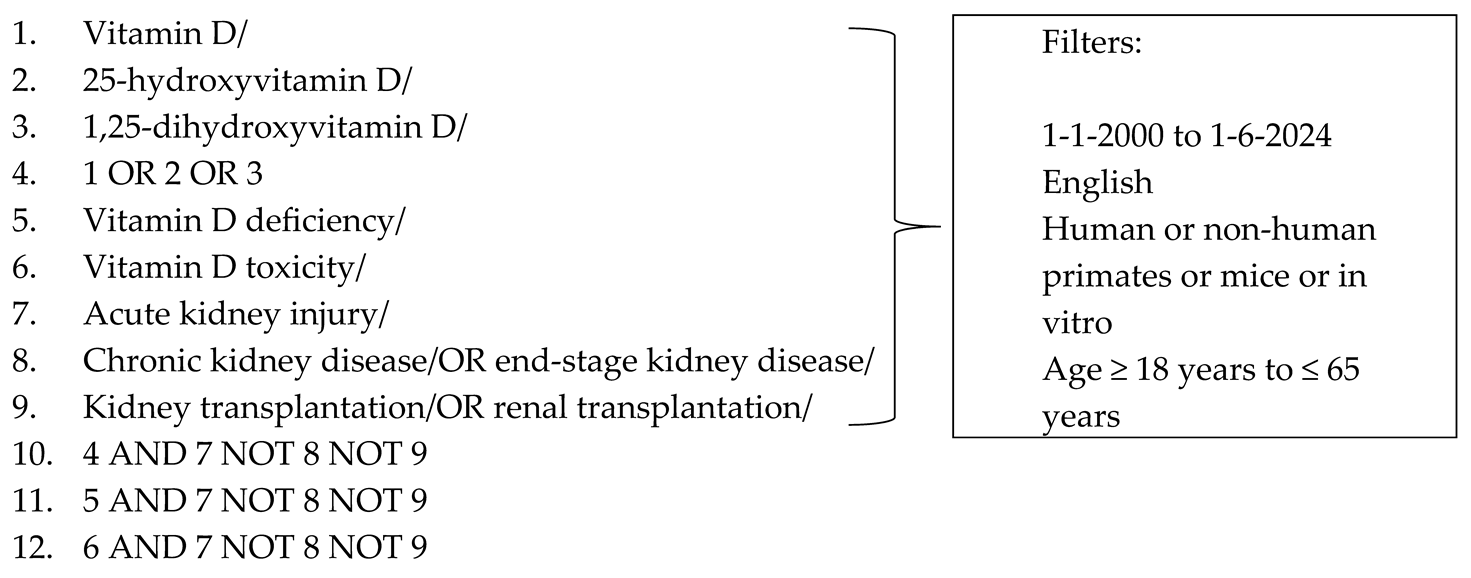
2.4. Selection of Sources of Evidence
2.5. Data Charting/Extraction
2.6. Synthesis of Data
3. Role of Vitamin D in Health
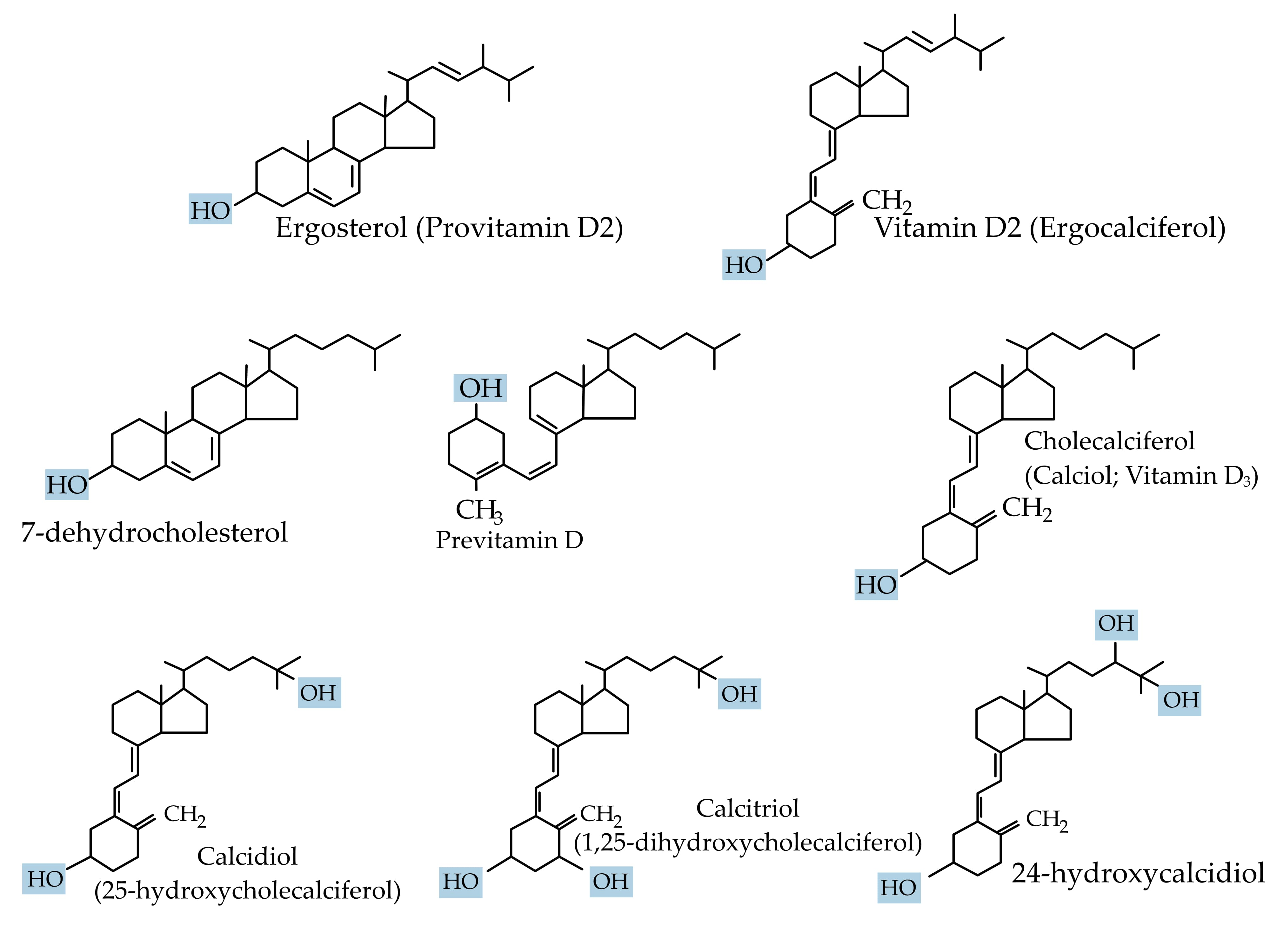
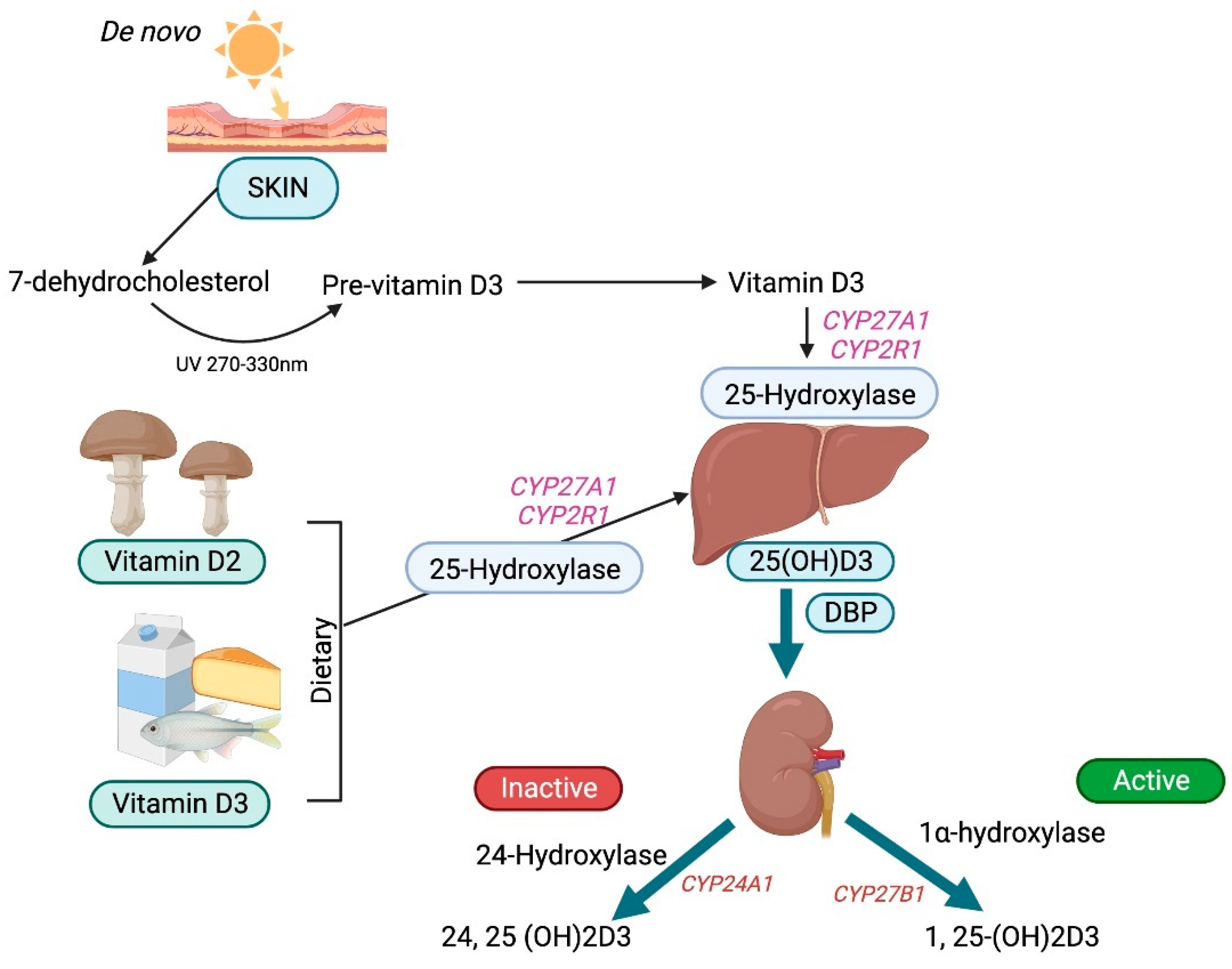
3.1. Vitamin D Synthesis, Transport, and Bioavailability
3.2. One Alpha-Hydroxylase
3.3. Mechanism of Vitamin D Action
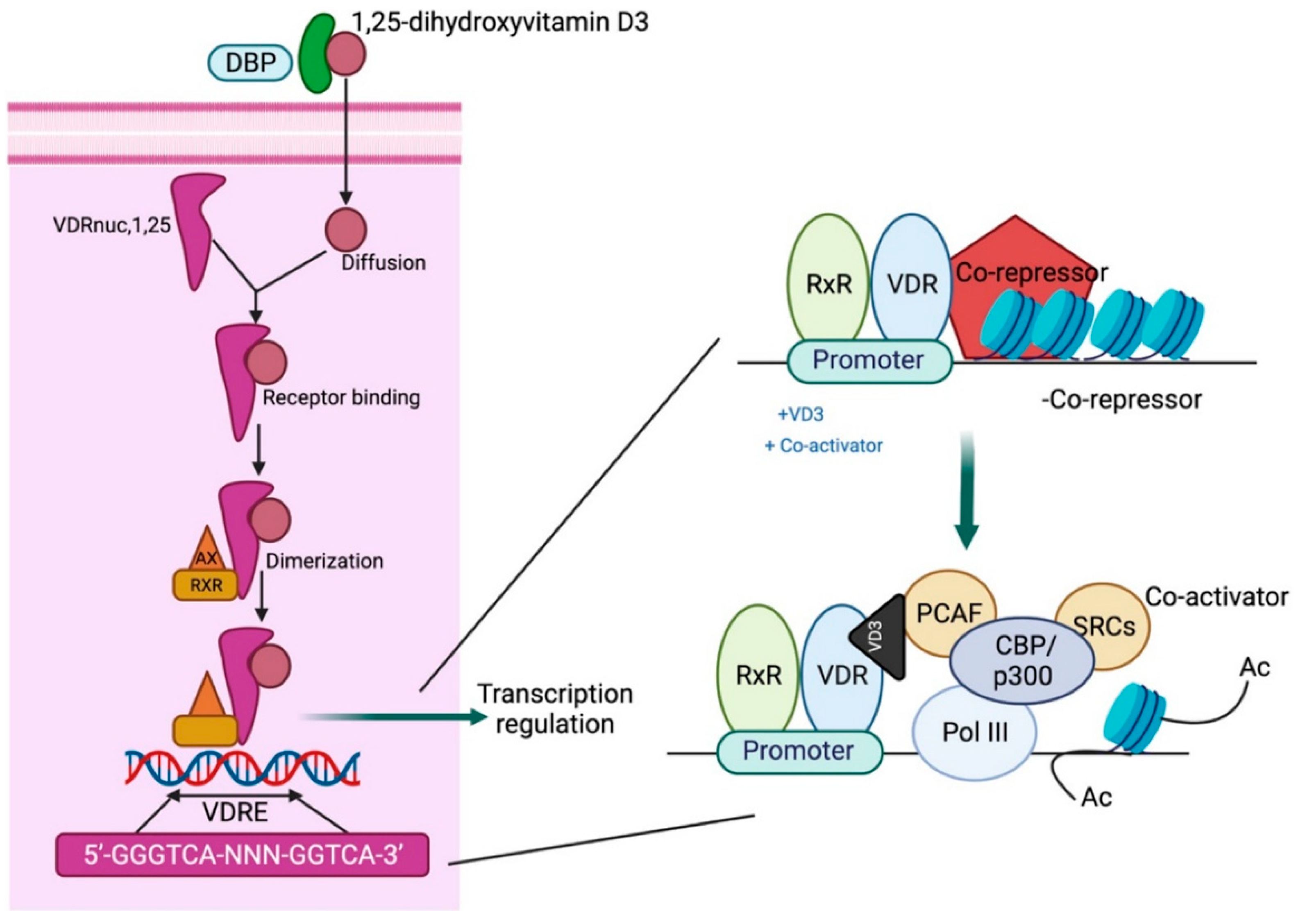
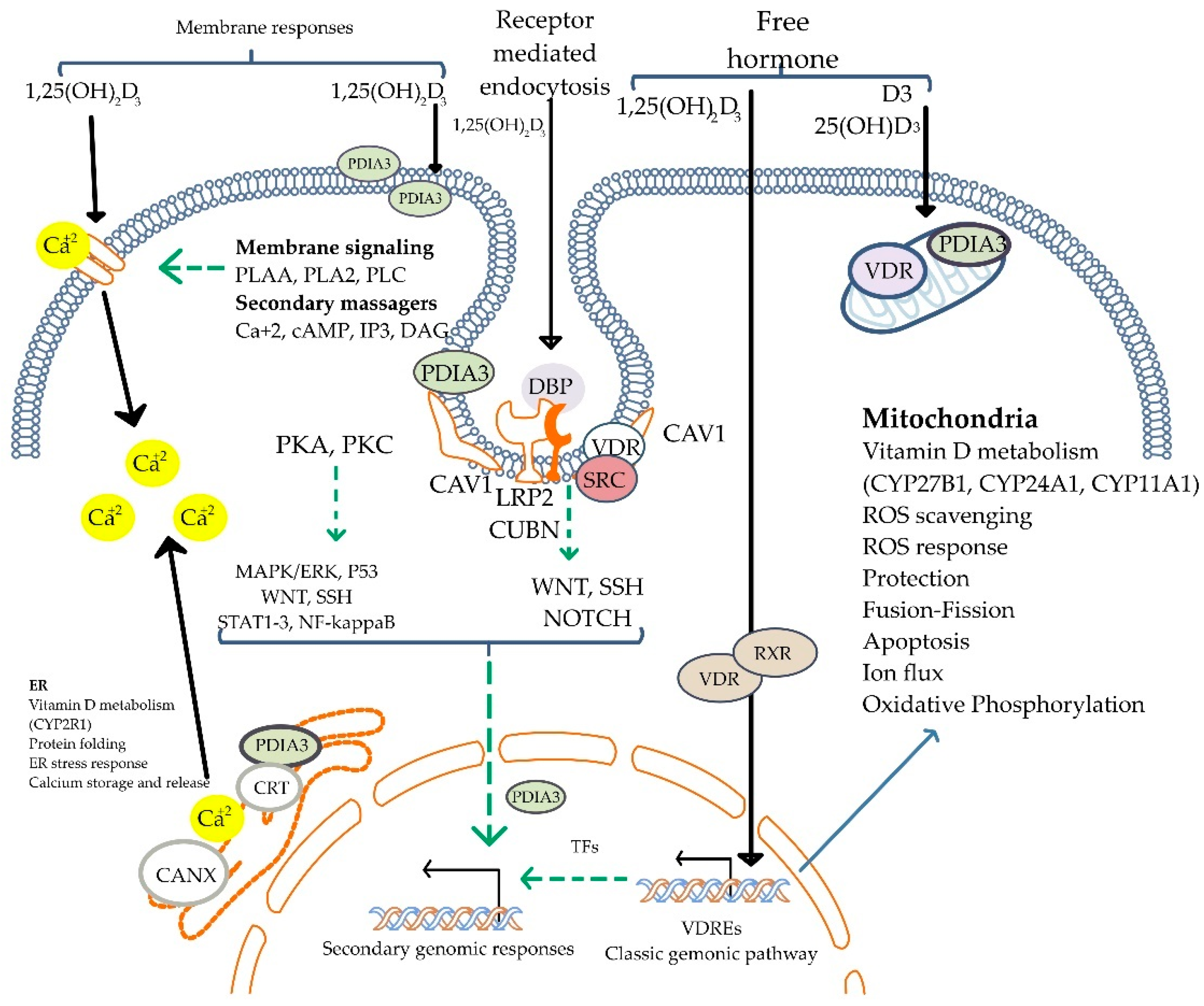
3.4. Biological Effects
3.5. Vitamin D Regulation
3.6. Inactivation of Vitamin D
3.7. Vitamin D Analogs
3.8. Analysis and Quantification of Vitamin D Metabolites
4. Role of Vitamin D in AKI
4.1. Dysregulation of Vitamin D in AKI
4.1.1. Development of Hypovitaminosis D in AKI
4.1.2. Development of Hypervitaminosis D in AKI
4.2. Vitamin D Deficiency and AKI
4.2.1. Vitamin D and Critical Illness-Related AKI
4.2.2. Vitamin D and Sepsis-Induced AKI
4.2.3. Vitamin D and Contrast-Induced AKI
4.2.4. Vitamin D and Aminoglycoside-Induced AKI
4.2.5. Vitamin D and Cisplatin-Induced AKI
4.2.6. Vitamin D and Rhabdomyolysis
4.2.7. Vitamin D and Ischemia-Reperfusion Injury
4.3. Hypervitaminosis and AKI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine; Food and Nutrition Board; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Valle, H.B.D.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cheng, J.B.; Levine, M.A.; Bell, N.H.; Mangelsdorf, D.J.; Russell, D.W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA 2004, 101, 7711–7715. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Kato, S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci. Biotechnol. Biochem. 2011, 75, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gu, B.; Gu, Y.; Groome, L.J.; Sun, J.; Wang, Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Liu, W.C.; Wu, C.C.; Hung, Y.M.; Liao, M.T.; Shyu, J.F.; Lin, Y.F.; Lu, K.C.; Yeh, K.C. Pleiotropic effects of vitamin D in chronic kidney disease. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 453, 1–12. [Google Scholar] [CrossRef]
- Li, T.; Higgins, J.; Deeks, J.J. Chapter 5: Collecting data. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Steenbock, H. The induction of growth promoting and calcifying properites in a ration by exposure to light. Science 1924, 60, 224–225. [Google Scholar] [CrossRef]
- Armas, L.A.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T., Jr.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 132–137. [Google Scholar] [CrossRef]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Barbour, G.L.; Coburn, J.W.; Slatopolsky, E.; Norman, A.W.; Horst, R.L. Hypercalcemia in an anephric patient with sarcoidosis: Evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N. Engl. J. Med. 1981, 305, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Garmyn, M.; Verstuyf, A.; Segaert, S.; Casteels, K.; Mathieu, C. Paracrine role for calcitriol in the immune system and skin creates new therapeutic possibilities for vitamin D analogs. Eur. J. Endocrinol. 1995, 133, 7–16. [Google Scholar] [CrossRef]
- Brenza, H.L.; DeLuca, H.F. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 2000, 381, 143–152. [Google Scholar] [CrossRef]
- Sánchez-Martínez, R.; Zambrano, A.; Castillo, A.I.; Aranda, A. Vitamin D-dependent recruitment of corepressors to vitamin D/retinoid X receptor heterodimers. Mol. Cell. Biol. 2008, 28, 3817–3829. [Google Scholar] [CrossRef]
- Yang, S.; Li, A.; Wang, J.; Liu, J.; Han, Y.; Zhang, W.; Li, Y.C.; Zhang, H. Vitamin D Receptor: A Novel Therapeutic Target for Kidney Diseases. Curr. Med. Chem. 2018, 25, 3256–3271. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Carlberg, C. Genome-Wide (Over)View on the Actions of Vitamin D. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/24808867/?tool=EBI (accessed on 7 December 2022).
- Singh, P.K.; van den Berg, P.R.; Long, M.D.; Vreugdenhil, A.; Grieshober, L.; Ochs-Balcom, H.M.; Wang, J.; Delcambre, S.; Heikkinen, S.; Carlberg, C.; et al. Integration of VDR genome wide binding and GWAS genetic variation data reveals co-occurrence of VDR and NF-κB binding that is linked to immune phenotypes. BMC Genom. 2017, 18, 132. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Neme, A.; Seuter, S.; Carlberg, C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Liu, Y.; Li, Y.; Xu, C.; Liu, Y.; Gao, M.; Dong, J. Calcitriol inhibits COX-1 and COX-2 expressions of renal vasculature in hypertension: Reactive oxygen species involved? Clin. Exp. Hypertens. 2021, 43, 91–100. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Thadhani, R. Vitamin D receptor activation: Cardiovascular and renal implications. Kidney Int. Suppl. 2013, 3, 427–430. [Google Scholar] [CrossRef]
- Dou, D.; Yang, B.; Gan, H.; Xie, D.; Lei, H.; Ye, N. Vitamin D supplementation for the improvement of vascular function in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. Int. Urol. Nephrol. 2019, 51, 851–858. [Google Scholar] [CrossRef]
- Arfian, N.; Budiharjo, S.; Wibisono, D.P.; Setyaningsih, W.A.W.; Romi, M.M.; Saputri, R.; Rofiah, E.K.; Rahmanti, T.; Agustin, M.; Sari, D.C.R. Vitamin D Ameliorates Kidney Ischemia Reperfusion Injury via Reduction of Inflammation and Myofibroblast Expansion. Kobe J. Med. Sci. 2020, 65, E138–E143. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Sardar, S.; Chakraborty, A.; Chatterjee, M. Comparative effectiveness of vitamin D3 and dietary vitamin E on peroxidation of lipids and enzymes of the hepatic antioxidant system in Sprague–Dawley rats. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam. Und Ernahrungsforsch. J. Int. De Vitaminol. De Nutr. 1996, 66, 39–45. [Google Scholar]
- Henry, H. Regulation of vitamin D metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zierold, C.; Mings, J.A.; DeLuca, H.F. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA 2001, 98, 13572–13576. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Nesbitt, T.; Drezner, M.K. Insulin-like growth factor-I regulation of renal 25-hydroxyvitamin D-1-hydroxylase activity. Endocrinology 1993, 132, 133–138. [Google Scholar] [CrossRef]
- Kawashima, H.; Torikai, S.; Kurokawa, K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature 1981, 291, 327–329. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef]
- Esvelt, R.P.; De Luca, H.F. Calcitroic acid: Biological activity and tissue distribution studies. Arch. Biochem. Biophys. 1981, 206, 403–413. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Bröking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef]
- Gui-Dong, Z.; Yongjun, C.; Xiaoming, Z.; Vandewalle, M.; De Clercq, P.J.; Bouillon, R.; Verstuyf, A. Synthesis of CD-ring modified 1α,25-dihydroxy vitamin D analogues: C-ring analogues. Bioorg. Med. Chem. Lett. 1996, 6, 1703–1708. [Google Scholar] [CrossRef]
- Vogeser, M. Quantification of circulating 25-hydroxyvitamin D by liquid chromatography–tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2010, 121, 565–573. [Google Scholar] [CrossRef]
- Duncan, M.W.; Nedelkov, D.; Walsh, R.; Hattan, S.J. Applications of MALDI Mass Spectrometry in Clinical Chemistry. Clin. Chem. 2016, 62, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Graidis, S.; Papavramidis, T.S.; Papaioannou, M. Vitamin D and Acute Kidney Injury: A Two-Way Causality Relation and a Predictive, Prognostic, and Therapeutic Role of Vitamin D. Front. Nutr. 2021, 7, 630951. [Google Scholar] [CrossRef] [PubMed]
- Omdahl, J.L.; Morris, H.A.; May, B.K. Hydroxylase enzymes of the vitamin D pathway: Expression, function, and regulation. Annu. Rev. Nutr. 2002, 22, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Nagano, N.; Urakawa, I.; Yamazaki, Y.; Iijima, K.; Fujita, T.; Yamashita, T.; Fukumoto, S.; Shimada, T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010, 78, 975–980. [Google Scholar] [CrossRef]
- Tan, S.J.; Smith, E.R.; Hewitson, T.D.; Holt, S.G.; Toussaint, N.D. The importance of klotho in phosphate metabolism and kidney disease. Nephrology 2014, 19, 439–449. [Google Scholar] [CrossRef]
- Akmal, M.; Bishop, J.E.; Telfer, N.; Norman, A.W.; Massry, S.G. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J. Clin. Endocrinol. Metab. 1986, 63, 137–142. [Google Scholar] [CrossRef]
- Zapatero, A.; Dot, I.; Diaz, Y.; Gracia, M.P.; Pérez-Terán, P.; Climent, C.; Masclans, J.R.; Nolla, J. Severe vitamin D deficiency upon admission in critically ill patients is related to acute kidney injury and a poor prognosis. Med. Intensiv. (Engl. Ed.) 2018, 42, 216–224. [Google Scholar] [CrossRef]
- Wani, M.; Wani, I.; Banday, K.; Ashraf, M. The other side of vitamin D therapy—A case series of acute kidney injury due to malpractice-related vitamin D intoxication. Clin. Nephrol. 2016, 86, 236–241. [Google Scholar] [CrossRef]
- Sharma, L.K.; Dutta, D.; Sharma, N.; Gadpayle, A.K. The increasing problem of subclinical and overt hypervitaminosis D in India: An institutional experience and review. Nutrition 2017, 34, 76–81. [Google Scholar] [CrossRef]
- Putzu, A.; Belletti, A.; Cassina, T.; Clivio, S.; Monti, G.; Zangrillo, A.; Landoni, G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J. Crit. Care 2017, 38, 109–114. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Li, J.G.; Mao, Z.; Zeng, X.T. Randomised trials of vitamin D(3) for critically ill patients in adults: Systematic review and meta-analysis with trial sequential analysis. Intensive Care Med. 2017, 43, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Y.; Shi, N.; Lu, Y.-Q. Serum vitamin D levels and acute kidney injury: A systemic review and meta-analysis. Sci. Rep. 2022, 12, 20365. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Qian, J.; Yang, Y.; Xie, Q.; You, H.; Zhou, Y.; Ma, S.; Hao, C.; Gu, Y.; Ding, F. Is the serum vitamin D level at the time of hospital-acquired acute kidney injury diagnosis associated with prognosis? PLoS ONE 2013, 8, e64964. [Google Scholar] [CrossRef]
- Amrein, K.; Sourij, H.; Wagner, G.; Holl, A.; Pieber, T.R.; Smolle, K.H.; Stojakovic, T.; Schnedl, C.; Dobnig, H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit. Care 2011, 15, R104. [Google Scholar] [CrossRef]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef]
- Vijayan, A.; Li, T.; Dusso, A.; Jain, S.; Coyne, D.W. Relationship of 1,25 dihydroxy Vitamin D Levels to Clinical Outcomes in Critically Ill Patients with Acute Kidney Injury. J. Nephrol. Ther. 2015, 5, 190. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.H.; Tan, Z.X.; Xie, D.D.; Zhang, C.; Zhang, Z.H.; Wang, H.; Zhao, H.; Yu, D.X.; Xu, D.X. Vitamin D3 pretreatment regulates renal inflammatory responses during lipopolysaccharide-induced acute kidney injury. Sci. Rep. 2015, 5, 18687. [Google Scholar] [CrossRef]
- Du, J.; Jiang, S.; Hu, Z.; Tang, S.; Sun, Y.; He, J.; Li, Z.; Yi, B.; Wang, J.; Zhang, H.; et al. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am. J. Physiol. Ren. Physiol. 2019, 316, F1068–F1077. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.; Quiroz, Y.; Zhang, Z.; Zhang, Y.; Bravo, Y.; Weisinger, J.R.; Li, Y.C.; Rodriguez-Iturbe, B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008, 74, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Ari, E.; Kedrah, A.E.; Alahdab, Y.; Bulut, G.; Eren, Z.; Baytekin, O.; Odabasi, D. Antioxidant and renoprotective effects of paricalcitol on experimental contrast-induced nephropathy model. Br. J. Radiol. 2012, 85, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Luchi, W.M.; Shimizu, M.H.; Canale, D.; Gois, P.H.; de Bragança, A.C.; Volpini, R.A.; Girardi, A.C.; Seguro, A.C. Vitamin D deficiency is a potential risk factor for contrast-induced nephropathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R215–R222. [Google Scholar] [CrossRef]
- Katzberg, R.W.; Pabico, R.C.; Morris, T.W.; Hayakawa, K.; McKenna, B.A.; Panner, B.J.; Ventura, J.A.; Fischer, H.W. Effects of contrast media on renal function and subcellular morphology in the dog. Investig. Radiol. 1986, 21, 64–70. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand. J. Clin. Lab. Investig. Suppl. 2008, 241, 89–94. [Google Scholar] [CrossRef]
- Taber, S.S.; Mueller, B.A. Drug-associated renal dysfunction. Crit. Care Clin. 2006, 22, 357–374. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Suh, S.; Lee, K.; Park, J.W.; Kim, I.; Kim, O.; Kim, C.; Choi, J.; Bae, E.; Ma, S.; Lee, J.; et al. Antiapoptotic Effect of Paricalcitol in Gentamicin-induced Kidney Injury. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2013, 17, 435–440. [Google Scholar] [CrossRef]
- Hur, E.; Garip, A.; Camyar, A.; Ilgun, S.; Ozisik, M.; Tuna, S.; Olukman, M.; Narli Ozdemir, Z.; Yildirim Sozmen, E.; Sen, S.; et al. The effects of vitamin d on gentamicin-induced acute kidney injury in experimental rat model. Int. J. Endocrinol. 2013, 2013, 313528. [Google Scholar] [CrossRef]
- Baliga, R.; Zhang, Z.; Baliga, M.; Ueda, N.; Shah, S.V. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998, 53, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Adigun, R. Rhabdomyolysis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Soares, T.J.; Costa, R.S.; Volpini, R.A.; Da Silva, C.G.; Coimbra, T.M. Long-term evolution of the acute tubular necrosis (ATN) induced by glycerol: Role of myofibroblasts and macrophages. Int. J. Exp. Pathol. 2002, 83, 165–172. [Google Scholar] [CrossRef]
- Reis, N.G.; Francescato, H.D.C.; de Almeida, L.F.; Silva, C.; Costa, R.S.; Coimbra, T.M. Protective effect of calcitriol on rhabdomyolysis-induced acute kidney injury in rats. Sci. Rep. 2019, 9, 7090. [Google Scholar] [CrossRef]
- Gonçalves, J.G.; de Bragança, A.C.; Canale, D.; Shimizu, M.H.M.; Sanches, T.R.; Moysés, R.M.A.; Andrade, L.; Seguro, A.C.; Volpini, R.A. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. PLoS ONE 2014, 9, e107228. [Google Scholar] [CrossRef]
- Yu, W.; Sheng, M.; Xu, R.; Yu, J.; Cui, K.; Tong, J.; Shi, L.; Ren, H.; Du, H. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med. 2013, 11, 24. [Google Scholar] [CrossRef]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1α, 25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell. Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef]
- Bragança, A.C.; Volpini, R.A.; Mehrotra, P.; Andrade, L.; Basile, D.P. Vitamin D deficiency contributes to vascular damage in sustained ischemic acute kidney injury. Physiol. Rep. 2016, 4, e12829. [Google Scholar] [CrossRef]
- Li, Y.; Spataro, B.C.; Yang, J.; Dai, C.; Liu, Y. 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005, 68, 1500–1510. [Google Scholar] [CrossRef]
- Branisteanu, D.; Leenaerts, P.; Van Damme, B.; Bouillon, R. Partial prevention of active Heymann nephritis by lα, 25 dihydroxyvitamin D3. Clin. Exp. Immunol. 1993, 94, 412–417. [Google Scholar] [CrossRef]
- Lemire, J.M.; Ince, A.; Takashima, M. 1,25-dihydroxyvitamin D3 attenuates of expression of experimental murine lupus of MRL/1 mice. Autoimmunity 1992, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.; Haas, C.S.; Gross, M.L.; Reulbach, U.; Holzinger, M.; Schwarz, U.; Ritz, E.; Amann, K. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am. J. Physiol. Ren. Physiol. 2004, 286, F526–F533. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.; Amann, K.; Orth, S.R.; Simonaviciene, A.; Wessels, S.; Ritz, E. Effect of 1,25(OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998, 53, 1696–1705. [Google Scholar] [CrossRef]
- Eren, Z.; Günal, M.Y.; Bakir, E.A.; Coban, J.; Çağlayan, B.; Ekimci, N.; Ethemoglu, S.; Albayrak, O.; Akdeniz, T.; Demirel, G.Y.; et al. Effects of paricalcitol and aliskiren combination therapy on experimental diabetic nephropathy model in rats. Kidney Blood Press. Res. 2014, 39, 581–590. [Google Scholar] [CrossRef]
- Tan, X.; Li, Y.; Liu, Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 2006, 17, 3382–3393. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Morrissey, J.; Finch, J.L.; Martin, D.R.; Liapis, H.; Akizawa, T.; Slatopolsky, E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J. Am. Soc. Nephrol. 2007, 18, 1796–1806. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef]
- Taylor, P.N.; Davies, J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22 (Suppl. S2), V28–V33. [Google Scholar] [CrossRef]
- De Francesco Daher, E.; Mesquita Martiniano, L.V.; Lopes Lima, L.L.; Viana Leite Filho, N.C.; de Oliveira Souza, L.E.; Duarte Fernandes, P.H.; da Silva, S.L.; da Silva Junior, G.B. Acute kidney injury due to excessive and prolonged intramuscular injection of veterinary supplements containing vitamins A, D and E: A series of 16 cases. Nefrologia 2017, 37, 61–67. [Google Scholar] [CrossRef]
- Anık, A.; Çatlı, G.; Abacı, A.; Dizdarer, C.; Böber, E. Acute vitamin D intoxication possibly due to faulty production of a multivitamin preparation. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A. Primer on Kidney Diseases E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Markowitz, G.S.; Perazella, M.A. Acute phosphate nephropathy. Kidney Int. 2009, 76, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.; Sedivy, M.; Lindner, G.; Schwarz, C. Successful treatment with denosumab for two cases with hypercalcemia due to vitamin D intoxication and associated acute kidney injury. CEN Case Rep. 2022, 11, 141–145. [Google Scholar] [CrossRef]
| Aspect | Current Evidence | Clinical Implications |
|---|---|---|
| Vitamin D Deficiency | Common in AKI, correlates with worse outcomes but lacks strong causal evidence [50,53,55,57,58,63,66,73,75,78,83] | Routine vitamin D monitoring in AKI patients may be beneficial but requires further study |
| Vitamin D Supplementation | Mixed results from RCTs, with some showing benefit and others no impact [53,54,55,58,60,61] | Individualized approach needed; avoid universal supplementation without monitoring |
| Hypervitaminosis D | Can cause hypercalcemia, nephrocalcinosis, and AKI, especially with high-dose IM injections [51,52,91,92,98] | Controlled administration essential; avoid excessive dosing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annamalai, C.; Viswanathan, P. Vitamin D and Acute Kidney Injury: A Reciprocal Relationship. Biomolecules 2025, 15, 586. https://doi.org/10.3390/biom15040586
Annamalai C, Viswanathan P. Vitamin D and Acute Kidney Injury: A Reciprocal Relationship. Biomolecules. 2025; 15(4):586. https://doi.org/10.3390/biom15040586
Chicago/Turabian StyleAnnamalai, Chandrashekar, and Pragasam Viswanathan. 2025. "Vitamin D and Acute Kidney Injury: A Reciprocal Relationship" Biomolecules 15, no. 4: 586. https://doi.org/10.3390/biom15040586
APA StyleAnnamalai, C., & Viswanathan, P. (2025). Vitamin D and Acute Kidney Injury: A Reciprocal Relationship. Biomolecules, 15(4), 586. https://doi.org/10.3390/biom15040586






