Synthesis and Antiproliferative Effects of Grossheimin-Derived Aminoanalogues
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
Isolation of Grossheimin
2.2. Synthetic Part
2.3. Antiproliferative Assay
2.3.1. Cell Lines and Their Maintenance
2.3.2. MTT Assay
2.4. Molecular Docking Method
2.4.1. Preparation of the Crystal Structures
2.4.2. Docking Study (CDocker)
3. Results and Discussion
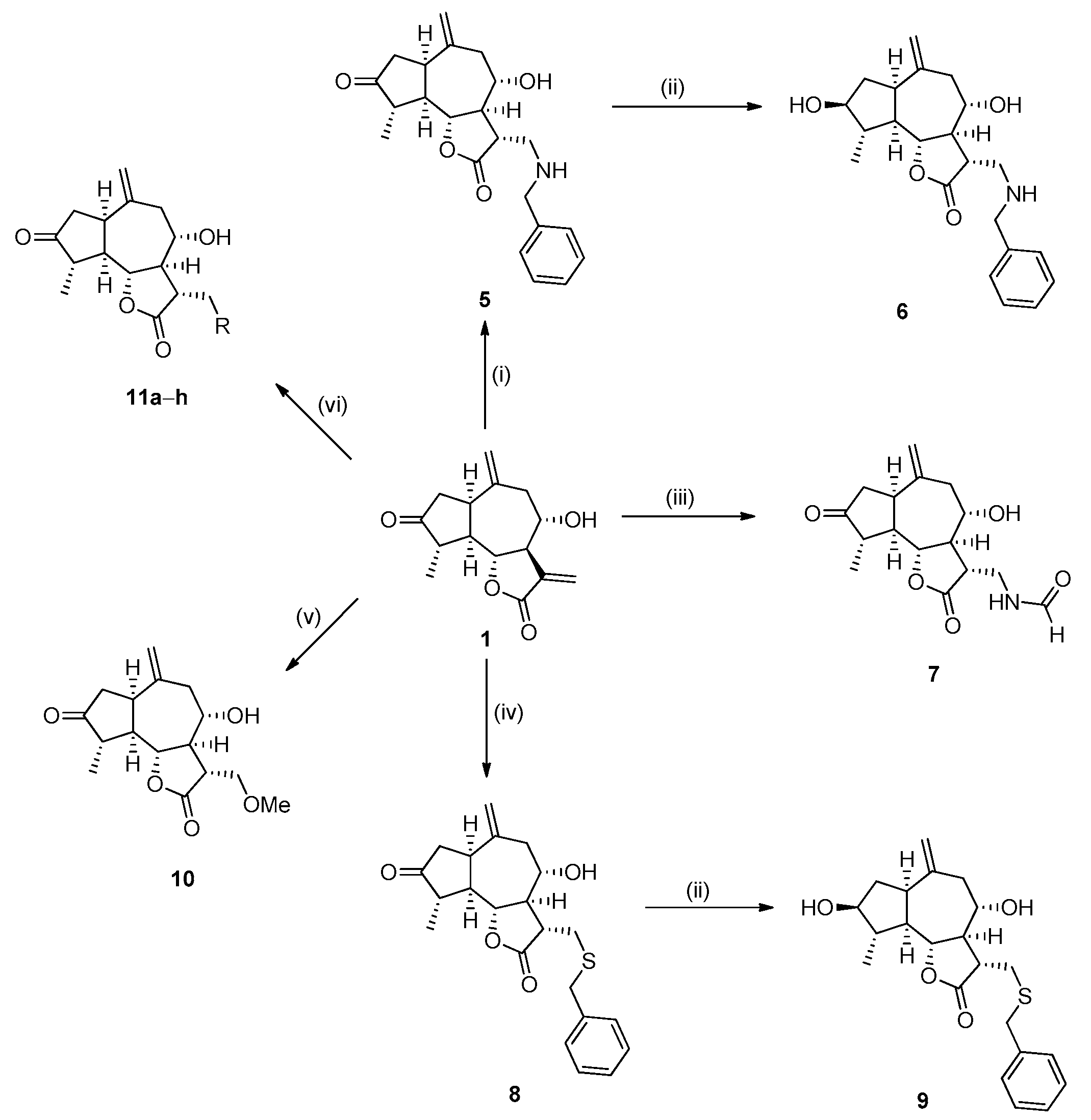
3.1. Chemical Protocols
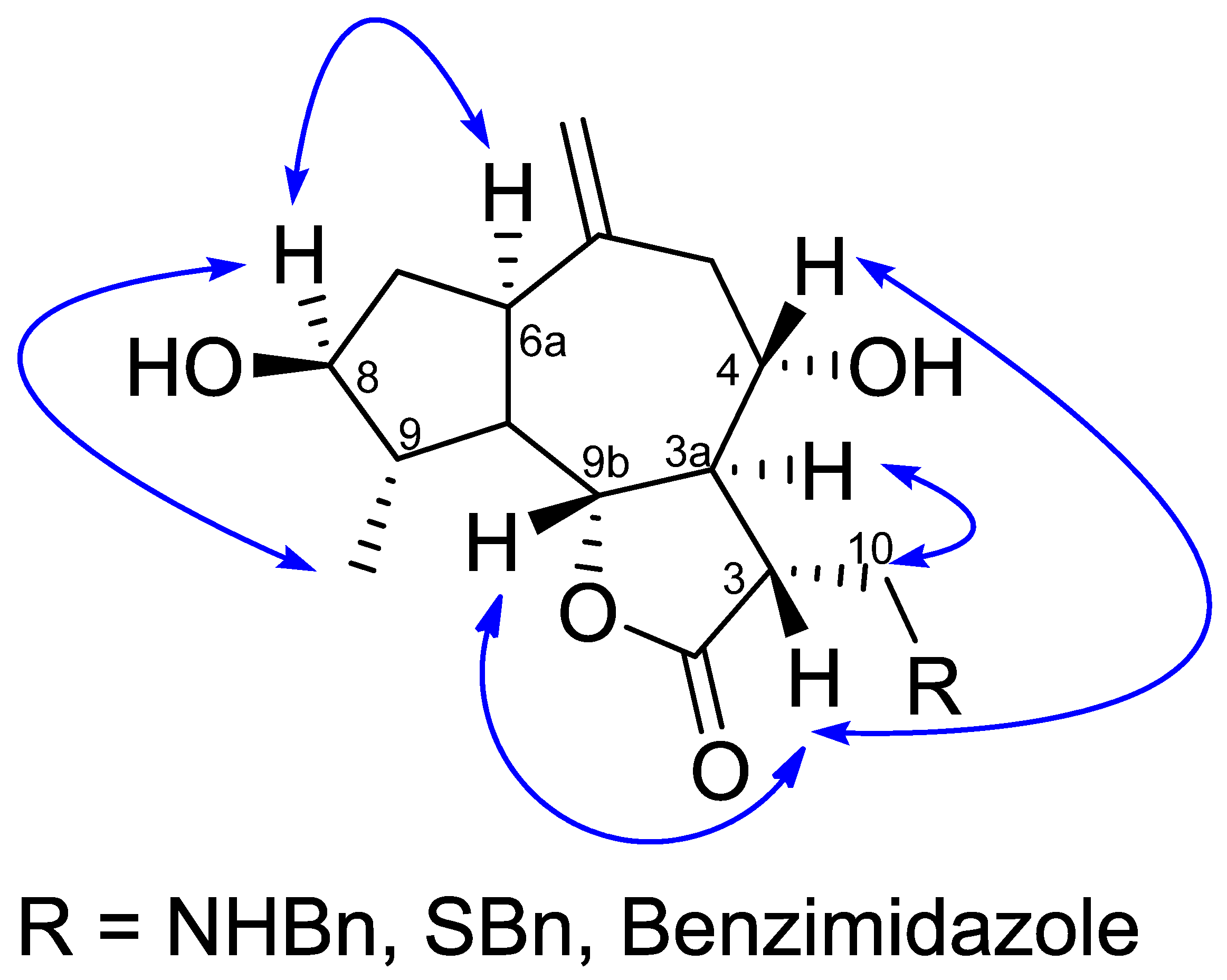
3.2. Determination of Relative Configuration
3.3. Determination of Cytotoxic Effect
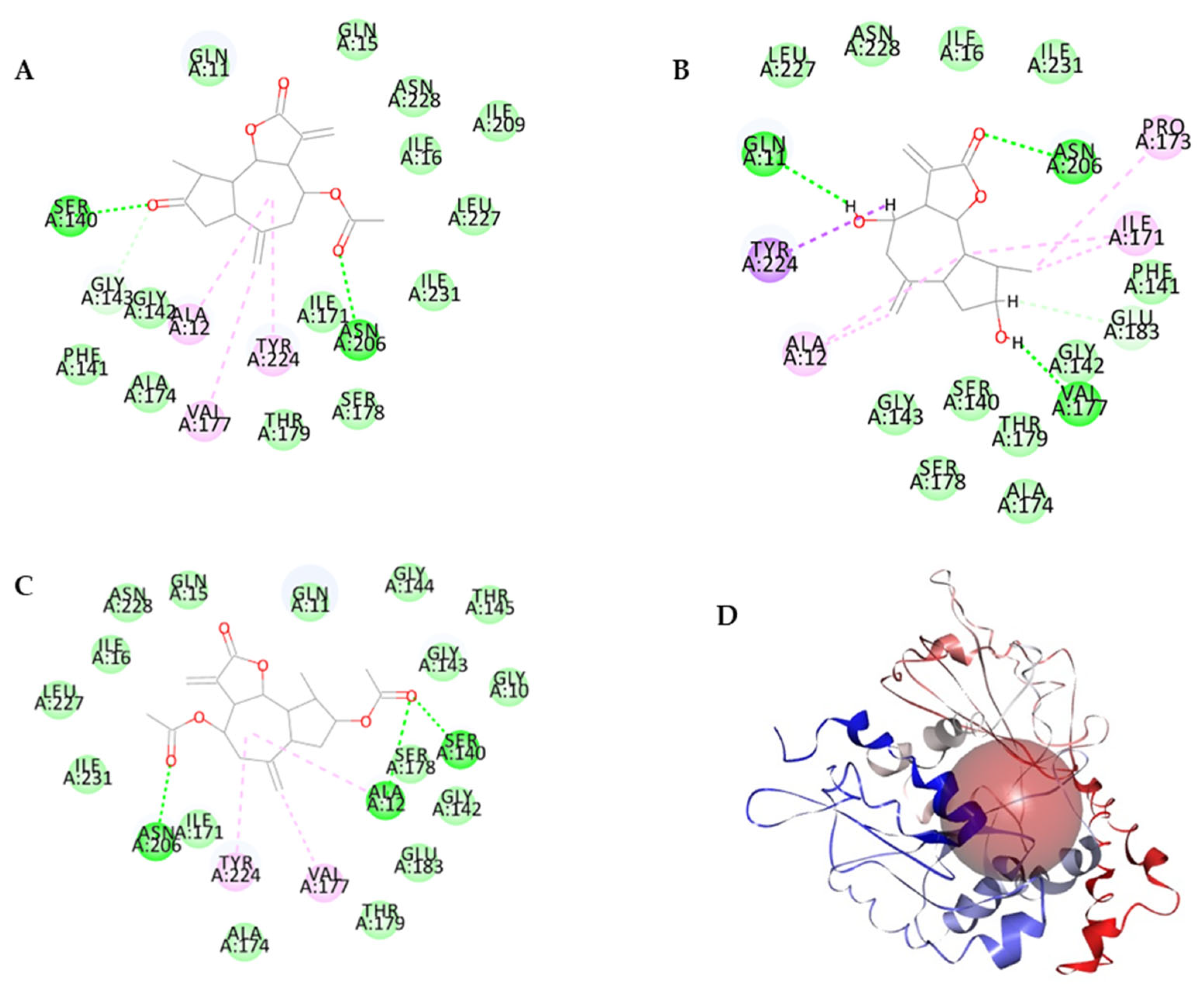
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Z.; Xie, Q.; Zhang, Z.; Zhang, H.; Ma, J. Natural Products for Treating Colorectal Cancer: A Mechanistic Review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Therapeut. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qiao, X.; Li, F.; Ma, H.; Xie, L.; Xu, X. Diastereoselective Total Synthesis of 8-Epigrosheimin. Tetrahedron Lett. 2009, 50, 1110–1112. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Shaimerdenova, Z.R.; Adekenova, K.S.; Kishkentayeva, A.S. Synthesis and Biological Evaluation of New Derivatives of Grossheimin. Fitoterapia 2022, 158, 105154. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised Checklist of Endemic Vascular Plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Fernández, I.P.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Trasobares, S.; Macías, F.A. Encapsulation of Cynara Cardunculus Guaiane-Type Lactones in Fully Organic Nanotubes Enhances Their Phytotoxic Properties. J. Agric. Food Chem. 2022, 70, 3644–3653. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Mauromicale, G.; Macías, F.A. Effect of Shading on the Sesquiterpene Lactone Content and Phytotoxicity of Cultivated Cardoon Leaf Extracts. J. Agric. Food Chem. 2020, 68, 11946–11953. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Mauromicale, G.; Macias, F.A. Influence of Genotype and Harvest Time on the Cynara cardunculus L. Sesquiterpene Lactone Profile. J. Agric. Food Chem. 2019, 67, 6487–6496. [Google Scholar] [CrossRef]
- Rial, C.; García, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macías, F.A. The Joint Action of Sesquiterpene Lactones from Leaves as an Explanation for the Activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Novaes, P.; Varela, R.M.; Molinillo, J.M.G.; Macias, F.A. Phytotoxicity of Cardoon (Cynara cardunculus) Allelochemicals on Standard Target Species and Weeds. J. Agric. Food Chem. 2014, 62, 6699–6706. [Google Scholar] [CrossRef] [PubMed]
- Kasenova, S.B.; Atazhanova, G.A.; Sagintaeva, Z.I.; Kasenov, B.K.; Kishkentaeva, A.S.; Adekenov, S.M. Thermodynamic Properties of Sesquiterpene Lactone Grossheimin. Russ. J. Phys. Chem. A 2016, 90, 1521–1524. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory Effects of Cynaropicrin and Related Sesquiterpene Lactones from Leaves of Artichoke (Cynara scolymus L.) on Induction of iNOS in RAW264.7 Cells and Its High-Affinity Proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The Natural Sesquiterpene Lactones Arglabin, Grosheimin, Agracin, Parthenolide, and Estafiatin Inhibit T Cell Receptor (TCR) Activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Adekenov, S.M. Study of Antiopisthorchiasis Activity of Sesquiterpene Lactones and Their Derivatives. Fitoterapia 2019, 133, 200–211. [Google Scholar] [CrossRef]
- Adekenova, A.S.; Sakenova, P.Y.; Ivasenko, S.A.; Khabarov, I.A.; Adekenov, S.M.; Berthod, A. Gram-Scale Purification of Two Sesquiterpene Lactones from Chartolepsis Intermedia Boiss. Chromatographia 2016, 79, 37–43. [Google Scholar] [CrossRef]
- Grothe, T.; Roemer, E.; Wabnitz, P. Use of Tricyclic Sesquiterpene Lactones in the Treatment of Obesity and Related Diseases and Non-Therapeutic Treatable Conditions 2020. U.S. Patent Application No. 13/522,110, 21 July 2011. [Google Scholar]
- Yae, E.; Yahara, S.; El-Aasr, M.; Ikeda, T.; Yoshimitsu, H.; Masuoka, C.; Ono, M.; Hide, I.; Nakata, Y.; Nohara, T. Studies on the Constituents of Whole Plants of Youngia japonica. Chem. Pharm. Bull. 2009, 57, 719–723. [Google Scholar] [CrossRef]
- Shimoda, H.; Ninomiya, K.; Nishida, N.; Yoshino, T.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Anti-Hyperlipidemic Sesquiterpenes and New Sesquiterpene Glycosides from the Leaves of Artichoke (Cynara scolymus L.): Structure Requirement and Mode of Action. Bioorg. Med. Chem. Lett. 2003, 13, 223–228. [Google Scholar] [CrossRef]
- Zhao, Q.-S.; Li, X.; Qian, P.; Liu, Z.; Zhao, Y.; Xu, G.; Tao, D.; Sun, H. The Sesquiterpenoids from Cynara scolymus. Heterocycles 2005, 65, 287–291. [Google Scholar] [CrossRef]
- Mukhametzhanova, G.; Asanova, G.; Adekenova, G.S.; Medeubayeva, B.; Kishkentayeva, A.; Adekenov, S. Chartolepis intermedia Boiss. and Centaurea ruthenica Lam.—New Medicina Plants Containing Pharmacologically Active Compounds. Open Access Maced. J. Med. Sci. 2022, 10, 56–64. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Moharana, S.; Khatun, G.N. Recent Advances in the Syntheses of Guaianolides. Org. Biomol. Chem. 2023, 21, 6652–6670. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What Made Sesquiterpene Lactones Reach Cancer Clinical Trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M. Natural Sesquiterpene Lactones as Renewable Chemical Materials for New Medicinal Products. Eurasian Chem.-Technol. J. 2013, 15, 163–174. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A. Bimolecular Compounds Based on Natural Metabolites. Chem. Nat. Compd. 2018, 54, 464–470. [Google Scholar] [CrossRef]
- Kulyyasov, A.T.; Adekenov, S.M.; Seitembetov, T.S.; Rakhimov, K.D. Chemical Modification of Grosshemin in the Lactone Ring. Chem. Nat. Compd. 1995, 31, 192–195. [Google Scholar] [CrossRef]
- Rybalko, K.S.; Evstratova, R.I.; Sheichenko, V.I. Production of a Pyridine Derivative of Grosshemin. Chem. Nat. Compd. 1976, 12, 272–274. [Google Scholar] [CrossRef]
- Jalmakhanbetova, R.I.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M.; Suleimen, Y.M. Synthesis and Molecular Docking of Some Grossgemin Amino Derivatives as Tubulin Inhibitors Targeting Colchicine Binding Site. J. Chem. 2021, 2021, 5586515. [Google Scholar] [CrossRef]
- Fardella, G.; Barbetti, P.; Grandolini, G.; Chiappini, I.; Ambrogi, V.; Scarcia, V.; Furlani Candiani, A. Phenylthio-Derivatives of α-Methylene-γ-Lactones as pro-Drugs of Cytotoxic Agents§. Eur. J. Med. Chem. 1999, 34, 515–523. [Google Scholar] [CrossRef]
- Barbetti, P.; Fardella, G.; Chiappini, I.; Scarcia, V.; Furlani Candiani, A. New Cytotoxic Selenoderivatives of Guaianolides. Eur. J. Med. Chem. 1989, 24, 299–305. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Kishkentayeva, A.S.; Khasenova, A.B.; Shul’ts, E.E.; Gatilov, Y.V.; Bagryanskaya, I.Y. New Arylhalo-Derivatives of Grosshemin. Chem. Nat. Compd. 2021, 57, 685–690. [Google Scholar] [CrossRef]
- Woods, J.R.; Mo, H.; Bieberich, A.A.; Alavanja, T.; Colby, D.A. Amino-Derivatives of the Sesquiterpene Lactone Class of Natural Products as Prodrugs. Med. Chem. Commun. 2013, 4, 27–33. [Google Scholar] [CrossRef]
- Ivasenko, S.A.; Kulyyasov, A.T.; Adekenov, S.M. Synthesis of a New Chloroderivative of Grosshemin Guaianolide. Chem. Nat. Compd. 2003, 39, 601–602. [Google Scholar] [CrossRef]
- Liya, M.; Weiling, L.; Jialu, Q.; Qian, P.; Xiansheng, Y.; Binlian, S.; Kanghong, H. Chemical Composition of Youngia japonica and Its Antitumor Activity. Biot. Resour. 2023, 45, 267–275. [Google Scholar] [CrossRef]
- Rampogu, S.; Baek, A.; Son, M.; Park, C.; Yoon, S.; Parate, S.; Lee, K.W. Discovery of Lonafarnib-Like Compounds: Pharmacophore Modeling and Molecular Dynamics Studies. ACS Omega 2020, 5, 1773–1781. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T Cell Receptor Activation by Semi-Synthetic Sesquiterpene Lactone Derivatives and Molecular Modeling of Their Interaction with Glutathione and Tyrosine Kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef]
- Yayli, N.; Baltaci, C.; Gök, Y.; Aydin, E.; Üçüncü, O. Sesquiterpene Lactones from Centaurea helenioides Boiss. Turk. J. Chem. 2006, 30, 229–233. [Google Scholar]
- Scotti, L.; Scotti, M.; Ishiki, H.; Ferreira, M.; Emerenciano, V.; Desmenezes, C.; Ferreira, E. Quantitative Elucidation of the Structure–Bitterness Relationship of Cynaropicrin and Grosheimin Derivatives. Food Chem. 2007, 105, 77–83. [Google Scholar] [CrossRef]
- Cala, A.; Zorrilla, J.G.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Easy Access to Alkoxy, Amino, Carbamoyl, Hydroxy, and Thiol Derivatives of Sesquiterpene Lactones and Evaluation of Their Bioactivity on Parasitic Weeds. J. Agric. Food Chem. 2019, 67, 10764–10773. [Google Scholar] [CrossRef]
- Lone, S.H.; Bhat, K.A.; Shakeel-u-Rehman; Majeed, R.; Hamid, A.; Khuroo, M.A. Synthesis and Biological Evaluation of Amino Analogs of Ludartin: Potent and Selective Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2013, 23, 4931–4934. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F. Sesquiterpene Lactones as Potent and Broad Spectrum Antiviral Compounds Against All Genotypes of Hepatitis c Virus (Hcv). Patent WO-2016169573-A1, 20 April 2015. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/WO-2016169573-A1 (accessed on 5 October 2024).
- Hegazy, M.-E.; Ibrahim, A.; Mohamed, T.; Shahat, A.; El Halawany, A.; Abdel-Azim, N.; Alsaid, M.; Paré, P. Sesquiterpene Lactones from Cynara cornigera: Acetyl Cholinesterase Inhibition and In Silico Ligand Docking. Planta Med. 2015, 82, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kitson, R.R.A.; Millemaggi, A.; Taylor, R.J.K. The Renaissance of α-Methylene-γ-butyrolactones: New Synthetic Approaches. Angew. Chem. Int. Ed. 2009, 48, 9426–9451. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Winssinger, N. Synthesis of α-Exo-Methylene-γ-Butyrolactones: Recent Developments and Applications in Natural Product Synthesis. Synthesis 2021, 53, 3977–3990. [Google Scholar] [CrossRef]
- Khdar, Z.A.; Le, T.M.; Schelz, Z.; Zupkó, I.; Szakonyi, Z. Stereoselective Synthesis and Application of Gibberellic Acid-Derived Aminodiols. Int. J. Mol. Sci. 2022, 23, 10366. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into Tubulin Regulation from a Complex with Colchicine and a Stathmin-like Domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]


| Entry | Compound | R | Yield (%) |
|---|---|---|---|
| 1 | 11a | Imidazole | 53 |
| 2 | 11b | Pyrazole | 42 |
| 3 | 11c | 1,2,4-Triazole | 67 |
| 4 | 11d | 1,2,3-Triazole | 66 |
| 5 | 11e | Indole | 35 |
| 6 | 11f | Benzimidazole | 50 |
| 7 | 11g | Indazole | 64 |
| 8 | 11h | Benzotriazole | 34 |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| Colo205 | Colo320 | CDD-19Lu | |
| 1 | 23.33 ± 2.05 | 27.89 ± 2.00 | >100 |
| 2 | 8.21 ± 0.51 | 9.44 ± 0.41 | >100 |
| 12 | 63.79 ± 1.32 | 53.65 ± 1.60 | >100 |
| 13 | 8.21 ± 0.51 | 6.98 ± 0.29 | 29.07 ± 0.46 |
| DOX | 3.21 ± 0.03 | 4.36 ± 0.08 | >8.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashimbayeva, M.; Szakonyi, Z.; Adekenov, S.M.; Szemerédi, N.; Spengler, G.; Le, T.M. Synthesis and Antiproliferative Effects of Grossheimin-Derived Aminoanalogues. Biomolecules 2025, 15, 578. https://doi.org/10.3390/biom15040578
Ashimbayeva M, Szakonyi Z, Adekenov SM, Szemerédi N, Spengler G, Le TM. Synthesis and Antiproliferative Effects of Grossheimin-Derived Aminoanalogues. Biomolecules. 2025; 15(4):578. https://doi.org/10.3390/biom15040578
Chicago/Turabian StyleAshimbayeva, Meruyert, Zsolt Szakonyi, Sergazy M. Adekenov, Nikoletta Szemerédi, Gabriella Spengler, and Tam Minh Le. 2025. "Synthesis and Antiproliferative Effects of Grossheimin-Derived Aminoanalogues" Biomolecules 15, no. 4: 578. https://doi.org/10.3390/biom15040578
APA StyleAshimbayeva, M., Szakonyi, Z., Adekenov, S. M., Szemerédi, N., Spengler, G., & Le, T. M. (2025). Synthesis and Antiproliferative Effects of Grossheimin-Derived Aminoanalogues. Biomolecules, 15(4), 578. https://doi.org/10.3390/biom15040578









