Etiologies of Multidrug-Resistant Epilepsy in Latin America: A Comprehensive Review of Structural, Genetic, Metabolic, Inflammatory, and Infectious Origins: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Language
2.3. Information Sources
2.4. Search Strategy
2.5. Data Management
2.6. Selection Process
2.7. Data Items
2.8. Bias Assessment
2.9. Effect Measures and Synthesis Methods
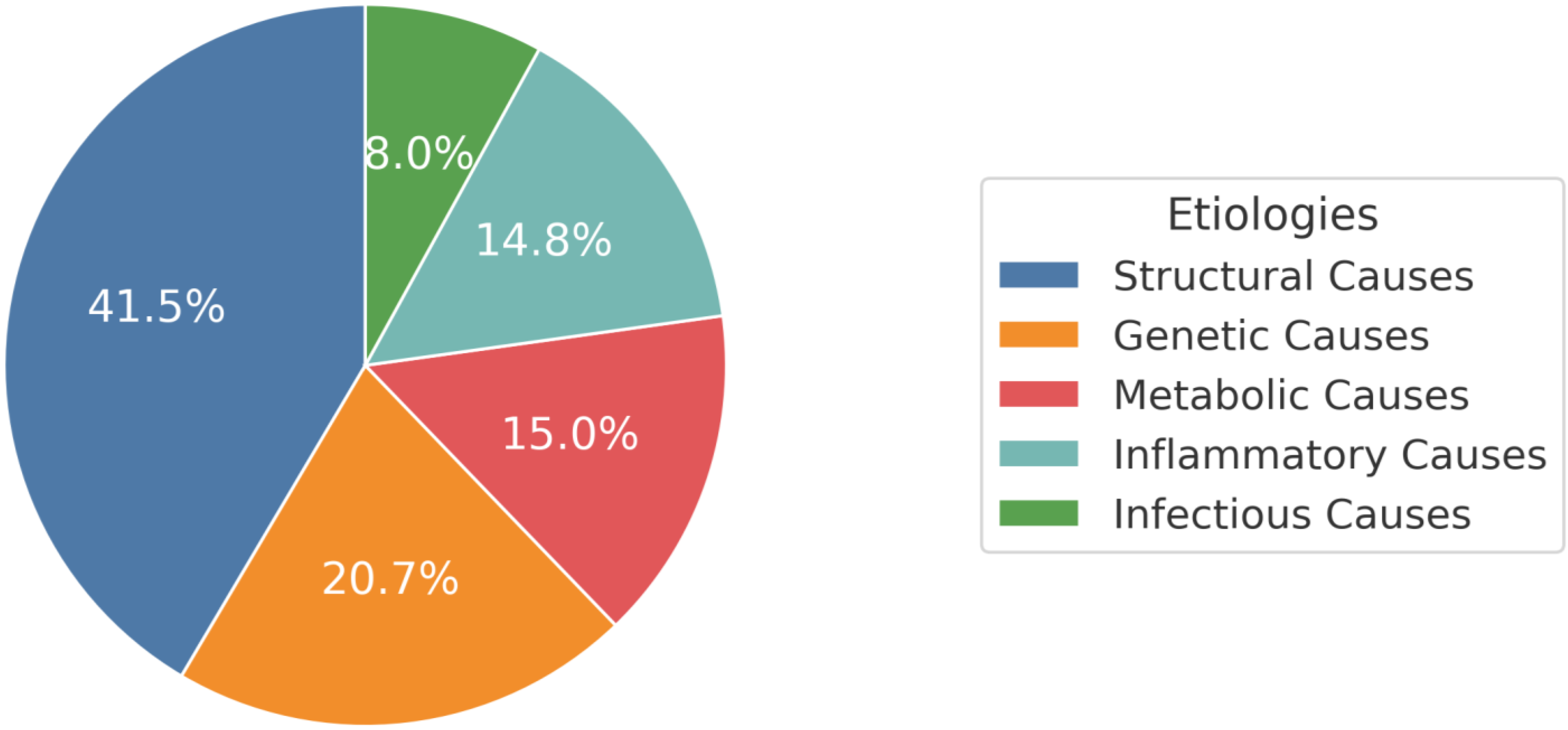
3. Results
3.1. Study Selection
3.2. Risk of Bias
3.3. Structural Causes
3.4. Genetic Causes
3.5. Metabolic Causes
3.6. Inflammatory Causes
3.7. Infectious Causes
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | ATP-Binding Cassette |
| ASM | Anti-seizure medication |
| BDNF | Brain-derived neurotrophic factor |
| CB | Cannabinoid receptor |
| CYP | Cytochrome |
| hK1 | Kallikrein 1 |
| ILAE | International League Against Epilepsy |
| MDRE | Multidrug-resistant epilepsy |
| MRI | Magnetic resonance imaging |
| MTLE | Mesial Temporal Lobe Epilepsy |
| NHLBI | National Heart, Lung, and Blood Institute |
| NTRK2 | Neurotrophic Tyrosine Kinase Receptor 2 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| P-gp | P-glycoprotein |
| SLC | Serotonin transporter gene |
| SNP | Single-nucleotide polymorphism |
| TEAC | TROLOX equivalent antioxidant capacity |
| TLE | Temporal lobe epilepsy |
| WOS | Web of Science |
| 5-HT | 5-hydroxytryptamine |
References
- Guery, D.; Rheims, S. Clinical Management of Drug Resistant Epilepsy: A Review on Current Strategies. Neuropsychiatr. Dis. Treat. 2021, 17, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The Epidemiology of Drug-Resistant Epilepsy: A Systematic Review and Meta-Analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef]
- Pacheco, N.; Alva-Diaz, C.; Rivera, A.L.; Neira, L.P.; Fernandez-Guzman, D.; Espino-Alvarado, P.; Rolston, J.; Burneo, J. Epidemiology of Drug-Resistant Epilepsy in Latin America and the Caribbean: Systematic Review Update and Meta-Analysis (P10-1.008). Neurology 2024, 102, 4055. [Google Scholar] [CrossRef]
- Rodríguez-Rivas, R.; Flisser, A.; Norcia, L.F.; Filho, P.T.H.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; Carpio, A.; Romo, M.L.; Fleury, A. Neurocysticercosis in Latin America: Current Epidemiological Situation Based on Official Statistics from Four Countries. PLoS Negl. Trop. Dis. 2022, 16, e0010652. [Google Scholar] [CrossRef] [PubMed]
- Aberastury, M.; Comas, B.; García, M.; Besocke, A.; Ciraolo, C.; Agosta, G.; Silva, W. Epilepsy Surgery in Children and Adolescents: Report on 43 Cases. Arch. Argent. Pediatr. 2016, 114, 458–463. [Google Scholar] [CrossRef]
- Alves-Leon, S.V.; Pinto, M.P.; Andraus, M.E.C.; Pereira, V.C.S.R.; Meira, I.D.; Oliveira, R.d.C.; Villas Boas, S.; Rêgo, C.C.d.S.; de Souza, J.P.B.M.; Pedrosa, R.C. Syncope in Patients with Drug-Resistant Epilepsy without Apparent Cardiovascular Disease. Arq. Neuropsiquiatr. 2013, 71, 925–930. [Google Scholar] [CrossRef]
- Bilevicius, E.; Yasuda, C.L.; Silva, M.S.; Guerreiro, C.A.M.; Lopes-Cendes, I.; Cendes, F. Antiepileptic Drug Response in Temporal Lobe Epilepsy: A Clinical and MRI Morphometry Study. Neurology 2010, 75, 1695–1701. [Google Scholar] [CrossRef]
- Duarte, J.T.C.; Jardim, A.P.; Comper, S.M.; De Marchi, L.R.; Gaça, L.B.; Garcia, M.T.F.C.; Sandim, G.B.; Assunção-Leme, I.B.; Carrete, H.J.; Centeno, R.S.; et al. The Impact of Epilepsy Duration in a Series of Patients with Mesial Temporal Lobe Epilepsy Due to Unilateral Hippocampal Sclerosis. Epilepsy Res. 2018, 147, 51–57. [Google Scholar] [CrossRef]
- Garcia, M.T.F.C.; Gaça, L.B.; Sandim, G.B.; Assunção Leme, I.B.; Carrete, H.J.; Centeno, R.S.; Sato, J.R.; Yacubian, E.M.T. Morphometric MRI Features Are Associated with Surgical Outcome in Mesial Temporal Lobe Epilepsy with Hippocampal Sclerosis. Epilepsy Res. 2017, 132, 78–83. [Google Scholar] [CrossRef]
- Kobayashi, E.; Lopes-Cendes, I.; Guerreiro, C.A.; Sousa, S.C.; Guerreiro, M.M.; Cendes, F. Seizure Outcome and Hippocampal Atrophy in Familial Mesial Temporal Lobe Epilepsy. Neurology 2001, 56, 166–172. [Google Scholar] [CrossRef]
- Ladino, L.D.; Gleadow, A.; Téllez-Zenteno, J.F. A Unique Ictal EEG Pattern in a Patient with the Coexistence of Generalized and Focal Epilepsy. Clin. EEG Neurosci. 2015, 46, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lara-Girón, J.C.; Callejas-Cordón, L.; Sanabria-Sanchinel, A.; Durón-Martínez, R.; Duarte, W.; Salatino, E.; Rodríguez, F.; Conti, V.; Jeréz-Magaña, Á. Histological Findings in Familial Temporal Epilepsy in a Mayan Descent Family|Hallazgos Histológicos en Epilepsia Temporal Familiar en una Familia de Ascendencia Maya. Rev. Ecuat. De Neurol. 2021, 29, 102–106. [Google Scholar] [CrossRef]
- Latini, M.F.; Oddo, S.; Anzulovich, A.C.; Kochen, S. Daily Rhythms in Right-Sided and Left-Sided Temporal Lobe Epilepsy. BMJ Neurol. Open 2022, 4, e000264. [Google Scholar] [CrossRef]
- Pinheiro-Martins, A.P.; Bianchin, M.M.; Velasco, T.R.; Terra, V.C.; Araújo, D.J.; Wichert-Ana, L.; Assirati, J.A.J.; Machado, H.R.; Carlotti, C.G.J.; Sakamoto, A.C. Independent Predictors and a Prognostic Model for Surgical Outcome in Refractory Frontal Lobe Epilepsy. Epilepsy Res. 2012, 99, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Quiroga-Padilla, P.J.; Briceño, C.; Mayor, L.C. Factors Associated with Initiation of the Modified Atkins Diet in Adults with Drug-Resistant Epilepsy. Epilepsy Behav. 2022, 129, 108620. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Henríquez-Varela, F.; Lara-Maier, S.; Monge-Bonilla, C.; Sittenfeld-Appel, M. Characteristics of Patients with Refractory Epilepsy Attended in a Tertiary Referral Center in Costa Rica|Caracterización de Los Pacientes con Epilepsia Refractaria de Un Hospital de Tercer Nivel En Costa Rica. Rev. Neurol. 2016, 63, 58–64. [Google Scholar] [CrossRef]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Pavón Fuentes, N.; Robinson Agramonte, M.d.L.A.; Serrano Sánchez, T.; Cruz-Xenes, R.M.; Díaz Hung, M.-L.; Estupiñán Díaz, B.; Báez Martín, M.M.; Orozco-Suárez, S. Follow-Up of Peripheral IL-1β and IL-6 and Relation with Apoptotic Death in Drug-Resistant Temporal Lobe Epilepsy Patients Submitted to Surgery. Behav. Sci. 2018, 8, 21. [Google Scholar] [CrossRef]
- Santos, R.O.; Secolin, R.; Barbalho, P.G.; Silva-Alves, M.S.; Alvim, M.K.M.; Yasuda, C.L.; Rogerio, F.; Velasco, T.R.; Sakamoto, A.C.; Teixeira, A.L.; et al. Multidimensional Approach Assessing the Role of Interleukin 1 Beta in Mesial Temporal Lobe Epilepsy. Front. Neurol. 2021, 12, 690847. [Google Scholar] [CrossRef]
- Rosa, D.V.; Rezende, V.B.; Costa, B.S.; Mudado, F.; Schütze, M.; Torres, K.C.; Martins, L.C.; Moreira-Filho, C.A.; Miranda, D.M.; Romano-Silva, M.A. Circulating CD4 and CD8 T Cells Expressing Pro-Inflammatory Cytokines in a Cohort of Mesial Temporal Lobe Epilepsy Patients with Hippocampal Sclerosis. Epilepsy Res. 2016, 120, 1–6. [Google Scholar] [CrossRef]
- Lorigados-Pedre, L.; Morales-Chacón, L.; Pavón-Fuentes, N.; Serrano-Sánchez, T.; Robinson-Agramonte, M.A.; García-Navarro, M.E.; Bender-del Busto, J.E. Immunological disorders in epileptic patients are associated to the epileptogenic focus localization. Rev. Neurol. 2004, 39, 101–104. [Google Scholar]
- Calderon-Ospina, C.A.; Galvez, J.M.; López-Cabra, C.; Morales, N.; Restrepo, C.M.; Rodríguez, J.; Aristizábal-Gutiérrez, F.A.; Velez-van-Meerbeke, A.; Laissue, P.; Fonseca-Mendoza, D.J. Possible Genetic Determinants of Response to Phenytoin in a Group of Colombian Patients With Epilepsy. Front. Pharmacol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Toledo, S.; Barreto-Acevedo, E.; Zavaleta, A.I.; Izaguirre, V.; Zúñiga-Gamarra, M. Ausencia de Asociación Entre Polimorfismos de Los Genes ABCB1 c.C3435T y ABCC2 c. -24C > T con Epilepsia Farmacorresistente en Pacientes Peruanos. Rev. Neuropsiquiatr. 2019, 82, 234–241. [Google Scholar] [CrossRef]
- López-García, M.A.; Feria-Romero, I.A.; Serrano, H.; Rayo-Mares, D.; Fagiolino, P.; Vázquez, M.; Escamilla-Núñez, C.; Grijalva, I.; Escalante-Santiago, D.; Orozco-Suarez, S. Influence of Genetic Variants of CYP2D6, CYP2C9, CYP2C19 and CYP3A4 on Antiepileptic Drug Metabolism in Pediatric Patients with Refractory Epilepsy. Pharmacol. Rep. 2017, 69, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Maradei-Anaya, S.J.; Espinosa, E.; Izquierdo, A.; Velasco-Parra, H.M. Detection of Subtelomeric Rearrangements Due to MLPA in Paediatric Patients with Refractory Epilepsy in Colombia: The Role of the CHL1 Gene in Pharmacoresistance. Rev. Neurol. 2013, 57, 444–450. [Google Scholar] [CrossRef]
- Kauffman, M.A.; Consalvo, D.; Gonzalez-Morón, D.; Aguirre, F.; D’Alessio, L.; Kochen, S. Serotonin Transporter Gene Variation and Refractory Mesial Temporal Epilepsy with Hippocampal Sclerosis. Epilepsy Res. 2009, 85, 231–234. [Google Scholar] [CrossRef]
- Kravetz, M.C.; Viola, M.S.; Prenz, J.; Curi, M.; Bramuglia, G.F.; Tenembaum, S. Case Report of Novel Genetic Variant in KCNT1 Channel and Pharmacological Treatment With Quinidine. Precision Medicine in Refractory Epilepsy. Front. Pharmacol. 2021, 12, 648519. [Google Scholar] [CrossRef]
- Lazarowski, A.; Massaro, M.; Schteinschnaider, A.; Intruvini, S.; Sevlever, G.; Rabinowicz, A. Neuronal MDR-1 Gene Expression and Persistent Low Levels of Anticonvulsants in a Child with Refractory Epilepsy. Ther. Drug Monit. 2004, 26, 44–46. [Google Scholar] [CrossRef]
- Lazarowski, A.; Sevlever, G.; Taratuto, A.; Massaro, M.; Rabinowicz, A. Tuberous Sclerosis Associated with MDR1 Gene Expression and Drug- Resistant Epilepsy. Pediatr. Neurol. 1999, 21, 731–734. [Google Scholar] [CrossRef]
- Martínez-Levy, G.A.; Rocha, L.; Lubin, F.D.; Alonso-Vanegas, M.A.; Nani, A.; Buentello-García, R.M.; Pérez-Molina, R.; Briones-Velasco, M.; Recillas-Targa, F.; Pérez-Molina, A.; et al. Increased Expression of BDNF Transcript with Exon VI in Hippocampi of Patients with Pharmaco-Resistant Temporal Lobe Epilepsy. Neuroscience 2016, 314, 12–21. [Google Scholar] [CrossRef]
- Navarrete-Modesto, V.; Orozco-Suárez, S.; Alonso-Vanegas, M.; Feria-Romero, I.A.; Rocha, L. REST/NRSF Transcription Factor Is Overexpressed in Hippocampus of Patients with Drug-Resistant Mesial Temporal Lobe Epilepsy. Epilepsy Behav. 2019, 94, 118–123. [Google Scholar] [CrossRef]
- Nuñez-Lumbreras, M.d.L.Á.; Castañeda-Cabral, J.L.; Valle-Dorado, M.G.; Sánchez-Valle, V.; Orozco-Suárez, S.; Guevara-Guzmán, R.; Martínez-Juárez, I.; Alonso-Vanegas, M.; Walter, F.; Deli, M.A.; et al. Drug-Resistant Temporal Lobe Epilepsy Alters the Expression and Functional Coupling to Gαi/o Proteins of CB1 and CB2 Receptors in the Microvasculature of the Human Brain. Front. Behav. Neurosci. 2021, 14, 611780. [Google Scholar] [CrossRef]
- Torres, C.M.; Siebert, M.; Bock, H.; Mota, S.M.; Krammer, B.R.; Duarte, J.Á.; Bragatti, J.A.; Castan, J.U.; de Castro, L.A.; Saraiva-Pereira, M.L.; et al. NTRK2 (TrkB Gene) Variants and Temporal Lobe Epilepsy: A Genetic Association Study. Epilepsy Res. 2017, 137, 1–8. [Google Scholar] [CrossRef]
- Horta, W.G.; Paradela, E.; Figueiredo, A.; Meira, I.D.; Pereira, V.C.S.R.; Rego, C.C.; Oliveira, R.; Andraus, M.E.C.; de Lacerda, G.C.B.; Moura, P.; et al. Genetic Association Study of the HLA Class II Alleles DRB1, DQA1, and DQB1 in Patients with Pharmacoresistant Temporal Lobe Epilepsy Associated with Mesial Hippocampal Sclerosis. Seizure 2015, 31, 7–11. [Google Scholar] [CrossRef]
- Vega-García, A.; Orozco-Suárez, S.; Villa, A.; Rocha, L.; Feria-Romero, I.; Alonso Vanegas, M.A.; Guevara-Guzmán, R. Cortical Expression of IL1-β, Bcl-2, Caspase-3 and 9, SEMA-3a, NT-3 and P-Glycoprotein as Biological Markers of Intrinsic Severity in Drug-Resistant Temporal Lobe Epilepsy. Brain Res. 2021, 1758, 147303. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Morelli, C.V.; de Vasconcellos, J.F.; Bruxel, E.M.; Rocha, C.S.; do Canto, A.M.; Tedeschi, H.; Yasuda, C.L.; Cendes, F.; Lopes-Cendes, I. Gene Expression Profile Suggests Different Mechanisms Underlying Sporadic and Familial Mesial Temporal Lobe Epilepsy. Exp. Biol. Med. 2022, 247, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Pereira Dalio, M.T.R.; Velasco, T.R.; Feitosa, I.D.F.; Assirati Junior, J.A.; Carlotti Junior, C.G.; Leite, J.P.; Dos Santos, A.C.; Alexandre, V.; Nakano, F.N.; Saute, R.L.; et al. Long-Term Outcome of Temporal Lobe Epilepsy Surgery in 621 Patients With Hippocampal Sclerosis: Clinical and Surgical Prognostic Factors. Front. Neurol. 2022, 13, 833293. [Google Scholar] [CrossRef] [PubMed]
- Placencia, M.; Sander, J.W.; Roman, M.; Madera, A.; Crespo, F.; Cascante, S.; Shorvon, S.D. The Characteristics of Epilepsy in a Largely Untreated Population in Rural Ecuador. J. Neurol. Neurosurg. Psychiatry 1994, 57, 320–325. [Google Scholar] [CrossRef]
- de Araújo Filho, G.M.; Martins, D.P.; Lopes, A.M.; de Jesus Brait, B.; Furlan, A.E.R.; Oliveira, C.I.F.; Marques, L.H.N.; Souza, D.R.S.; de Almeida, E.A. Oxidative Stress in Patients with Refractory Temporal Lobe Epilepsy and Mesial Temporal Sclerosis: Possible Association with Major Depressive Disorder? Epilepsy Behav. 2018, 80, 191–196. [Google Scholar] [CrossRef]
- Godoi, A.B.; Do Canto, A.M.; Donatti, A.; Rosa, D.C.; Bruno, D.C.F.; Alvim, M.K.; Yasuda, C.L.; Martins, L.G.; Quintero, M.; Tasic, L.; et al. Circulating Metabolites as Biomarkers of Disease in Patients with Mesial Temporal Lobe Epilepsy. Metabolites 2022, 12, 446. [Google Scholar] [CrossRef]
- Simões, P.S.R.; Zanelatto, A.O.; Assis, M.C.; Varella, P.P.V.; Yacubian, E.M.; Carrete, H.; Centeno, R.; Araujo, M.S.; Cavalheiro, E.A.; Tersariol, I.L.S.; et al. Plasma Kallikrein-Kinin System Contributes to Peripheral Inflammation in Temporal Lobe Epilepsy. J. Neurochem. 2019, 150, 296–311. [Google Scholar] [CrossRef]
- Vincentiis, S.; Alcantara, J.; Rzezak, P.; Kerr, D.; dos Santos, B.; Alessi, R.; van der Linden, H.; Arruda, F.; Chaim-Avancini, T.; Serpa, M.; et al. Higher Transcription Alleles of the MAOA-UVNTR Polymorphism Are Associated with Higher Seizure Frequency in Temporal Lobe Epilepsy. Epilepsy Res. 2019, 149, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.J.; Shao, X.Q.; Cui, T.; Wang, Q. Significance of MDR1 Gene C3435T Polymorphism in Predicting Childhood Refractory Epilepsy. Epilepsy Res. 2017, 132, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.C.F.; Wong, A.; Wong, H.C.; Man, B.L.; Au-Yeung, K.M.; Wong, K.S. Refractory Epilepsy in a Chinese Population. Clin. Neurol. Neurosurg. 2007, 109, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Makowska, M.; Romanowicz, H. Pharmacogenetics of Drug-Resistant Epilepsy (Review of Literature). Int. J. Mol. Sci. 2021, 22, 11696. [Google Scholar] [CrossRef]
- Hung, C.C.; Tai, J.J.; Kao, P.J.; Lin, M.S.; Liou, H.H. Association of Polymorphisms in NR1I2 and ABCB1 Genes with Epilepsy Treatment Responses. Pharmacogenomics 2007, 8, 1151–1158. [Google Scholar] [CrossRef]
- Soranzo, N.; Cavalleri, G.L.; Weale, M.E.; Wood, N.W.; Depondt, C.; Marguerie, R.; Sisodiya, S.M.; Goldstein, D.B. Identifying Candidate Causal Variants Responsible for Altered Activity of the ABCB1 Multidrug Resistance Gene. Genome Res. 2004, 14, 1333–1344. [Google Scholar] [CrossRef]
- Tan, N.C.K.; Heron, S.E.; Scheffer, I.E.; Pelekanos, J.T.; McMahon, J.M.; Vears, D.F.; Mulley, J.C.; Berkovic, S.F. Failure to Confirm Association of a Polymorphism in ABCB1 with Multidrug-Resistant Epilepsy. Neurology 2004, 63, 1090–1092. [Google Scholar] [CrossRef]
- Sills, G.J.; Mohanraj, R.; Butler, E.; McCrindle, S.; Collier, L.; Wilson, E.A.; Brodie, M.J. Lack of Association between the C3435T Polymorphism in the Human Multidrug Resistance (MDR1) Gene and Response to Antiepileptic Drug Treatment. Epilepsia 2005, 46, 643–647. [Google Scholar] [CrossRef]
- Maqbool, H.; Saleem, T.; Sheikh, N.; Ashfaq, A. Genetic Analysis of CYP2C9 with Reference to Drug Response in Epilepsy Patients of Pakistan. Genet. Res. 2022, 2022, 1451007. [Google Scholar] [CrossRef]
- Lakshmi, G.S.R.; Mohiuddin, M.K.; Mounika, K.; Reddy, P.S.; Devulapalli, K. Genetic Analysis of Cytochrome P450 Polymorphisms in Drug-Responsive and Drug-Refractory Epileptic Patients in Telangana. Open Neurol. J. 2024, 17, 1–13. [Google Scholar] [CrossRef]
- Chen, M.; Wu, X.; Zhang, B.; Shen, S.; He, L.; Zhou, D. Associations of Overweight and Obesity with Drug-Resistant Epilepsy. Seizure 2021, 92, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in Epileptogenesis: From Pathophysiology to Therapeutic Strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Pietrafusa, N. New-Onset Refractory Status Epilepticus and Febrile Infection-Related Epilepsy Syndrome. Dev. Med. Child. Neurol. 2020, 62, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, R.; Poyuran, R.; Arimappamagan, A.; Sinha, S.; Kulanthaivelu, K.; Kenchaiah, R.; Ajay, A.; Chowdary, R.M.; Saini, J.; Bharath, R.D.; et al. Dual/Double Pathology in Neurocysticercosis Causing Drug Resistant Epilepsy—Chance Association or Causal? Epilepsy Res. 2020, 168, 106472. [Google Scholar] [CrossRef]
- Rathore, C.; Thomas, B.; Kesavadas, C.; Abraham, M.; Radhakrishnan, K. Calcified Neurocysticercosis Lesions and Antiepileptic Drug-Resistant Epilepsy: A Surgically Remediable Syndrome? Epilepsia 2013, 54, 1815–1822. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and Epilepsy: Excitability and Inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]

| Author (Year) | Study Type | Level of Bias |
|---|---|---|
| Aberastury, M. (2016) [5] | Cross-sectional | High |
| Alves, S. (2013) [6] | Cohort | High |
| Bilevicius E. (2010) [7] | Case–control | Moderately low |
| Calderon-Ospina, C. (2020) [21] | Cohort | Moderately low |
| Calderón-Toledo, S. (2019) [22] | Case–control | Moderately low |
| de Araújo Filho GM. (2018) [38] | Cross-sectional | Moderately low |
| Duarte, J. (2018) [8] | Cohort | High |
| Garcia (2017) [9] | Cohort | High |
| Godoi AB. (2022) [39] | Cross-sectional | High |
| Horta WG. (2015) [33] | Cross-sectional | High |
| Kauffman MA. (2009) [25] | Cross-sectional | Moderately low |
| Kobayashi (2001) [10] | Case–control | Moderately low |
| Kravetz (2021) [26] | Case report | Moderately low |
| Ladino (2015) [11] | Case report | Moderately low |
| Lara-Giron (2021) [12] | Case report | Moderately low |
| Latini (2022) [13] | Clinical trial | Moderately low |
| Lazarowski (2004) [27] | Case report | Moderately low |
| Lazarowski (1999) [28] | Case report | Moderately low |
| Lopez-Garcia (2017) [23] | Cross-sectional | Moderately low |
| Lorigados-Pedre (2018) [17] | Case–control | Moderately low |
| Lorigados-Pedre (2004) [20] | Cross-sectional | Moderately low |
| Maradei-Anaya (2013) [24] | Cross-sectional | Moderately low |
| Martinez-Levy (2016) [29] | Case–control | Moderately low |
| Maurer-Morelli (2022) [35] | Case–control | Moderately low |
| Navarrete-Modesto (2019) [30] | Case–control | Moderately low |
| Nunez-Lumbreras (2020) [31] | Case–control | Moderately low |
| Pereira, M. (2022) [36] | Cohort | Moderately low |
| Pinheiro, A. (2012) [14] | Cross-sectional | Moderately low |
| Placencia, M. (1994) [37] | Case–control | Moderately low |
| Quiroga, P. (2022) [15] | Cohort | Moderately low |
| Rosa, D. (2016) [19] | Case–control | Moderately low |
| Sanabria C. (2016) [16] | Cross-sectional | Moderately low |
| Santos, R. (2021) [18] | Case–control | Moderately low |
| Simões, P. (2011) [40] | Case–control | Moderately low |
| Torres, C. (2017) [32] | Case–control | Moderately low |
| Vega-García, A. (2021) [34] | Case–control | Moderately low |
| Vincentiis, S. (2019) [41] | Case–control | Moderately low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojosa-Figueroa, M.S.; Cruz-Caraguay, M.; Torres Pasquel, A.; Puga Rosero, V.; Eguiguren Chavez, C.B.; Rodas, J.A.; Leon-Rojas, J.E. Etiologies of Multidrug-Resistant Epilepsy in Latin America: A Comprehensive Review of Structural, Genetic, Metabolic, Inflammatory, and Infectious Origins: A Systematic Review. Biomolecules 2025, 15, 576. https://doi.org/10.3390/biom15040576
Hinojosa-Figueroa MS, Cruz-Caraguay M, Torres Pasquel A, Puga Rosero V, Eguiguren Chavez CB, Rodas JA, Leon-Rojas JE. Etiologies of Multidrug-Resistant Epilepsy in Latin America: A Comprehensive Review of Structural, Genetic, Metabolic, Inflammatory, and Infectious Origins: A Systematic Review. Biomolecules. 2025; 15(4):576. https://doi.org/10.3390/biom15040576
Chicago/Turabian StyleHinojosa-Figueroa, Mario S., Mishell Cruz-Caraguay, Alejandro Torres Pasquel, Vanesa Puga Rosero, Camila Belen Eguiguren Chavez, Jose A. Rodas, and Jose E. Leon-Rojas. 2025. "Etiologies of Multidrug-Resistant Epilepsy in Latin America: A Comprehensive Review of Structural, Genetic, Metabolic, Inflammatory, and Infectious Origins: A Systematic Review" Biomolecules 15, no. 4: 576. https://doi.org/10.3390/biom15040576
APA StyleHinojosa-Figueroa, M. S., Cruz-Caraguay, M., Torres Pasquel, A., Puga Rosero, V., Eguiguren Chavez, C. B., Rodas, J. A., & Leon-Rojas, J. E. (2025). Etiologies of Multidrug-Resistant Epilepsy in Latin America: A Comprehensive Review of Structural, Genetic, Metabolic, Inflammatory, and Infectious Origins: A Systematic Review. Biomolecules, 15(4), 576. https://doi.org/10.3390/biom15040576








