Microcomputed Tomography in the Adriamycin Rat Model of Malformations—Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
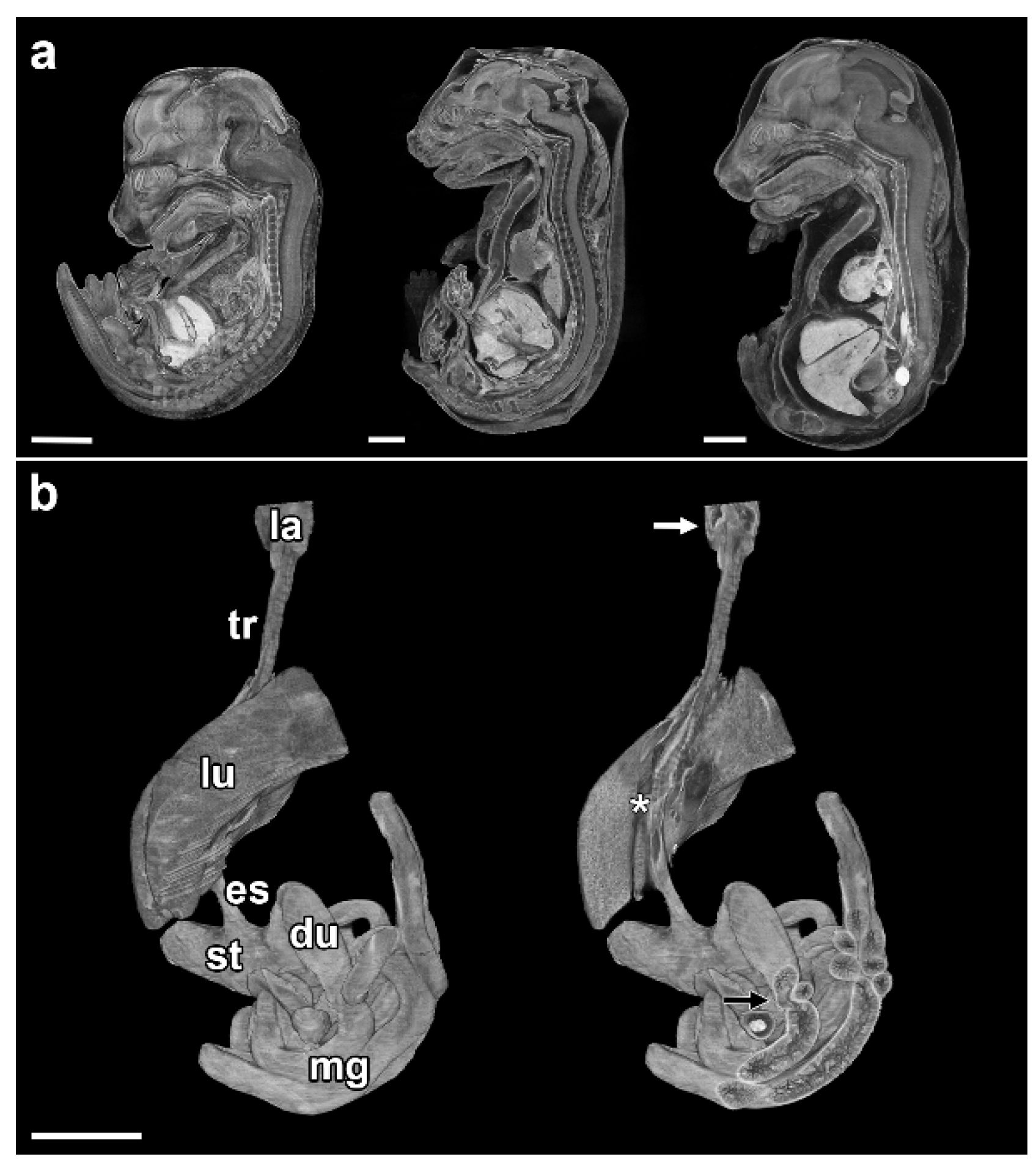
3. Results
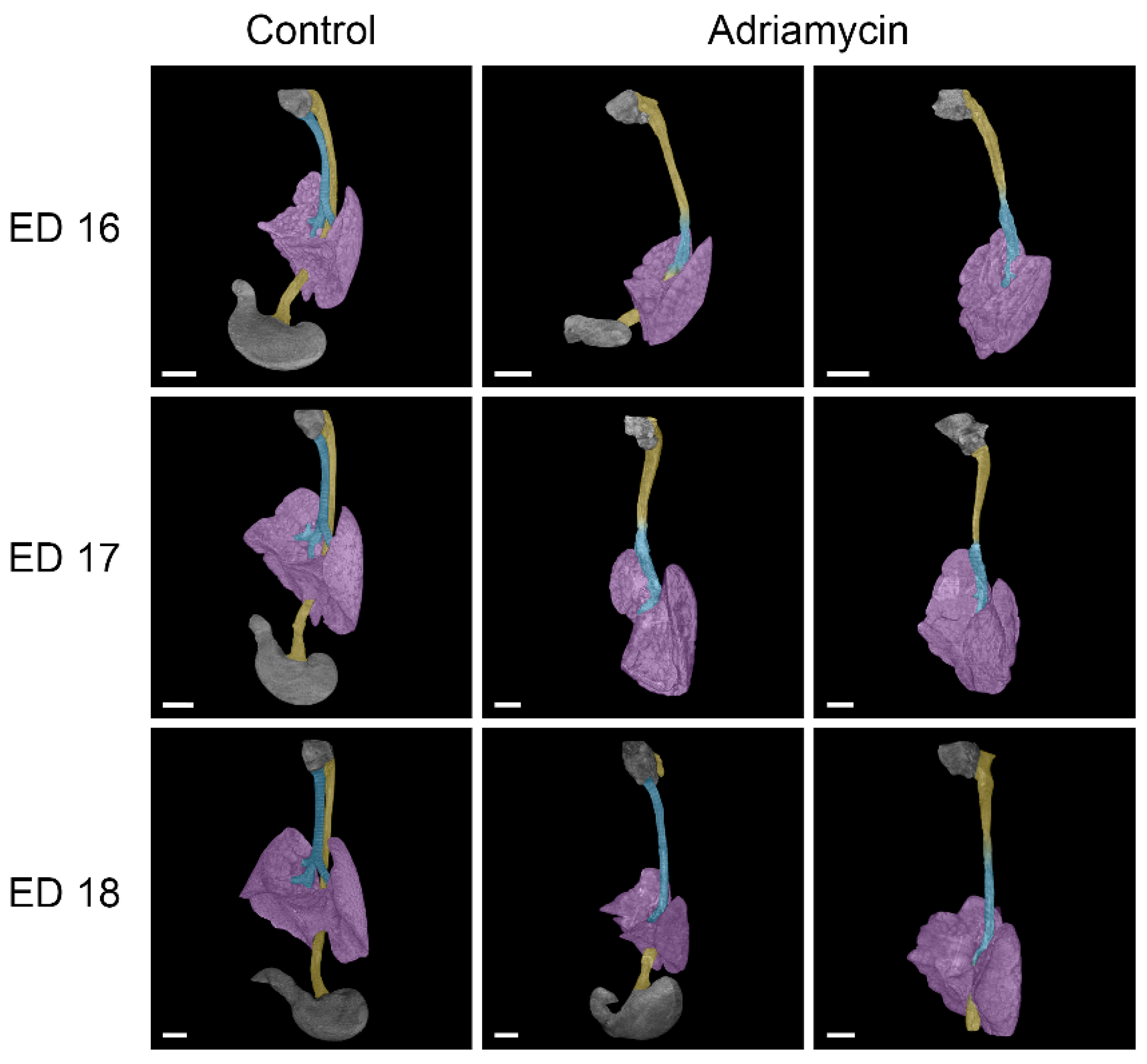
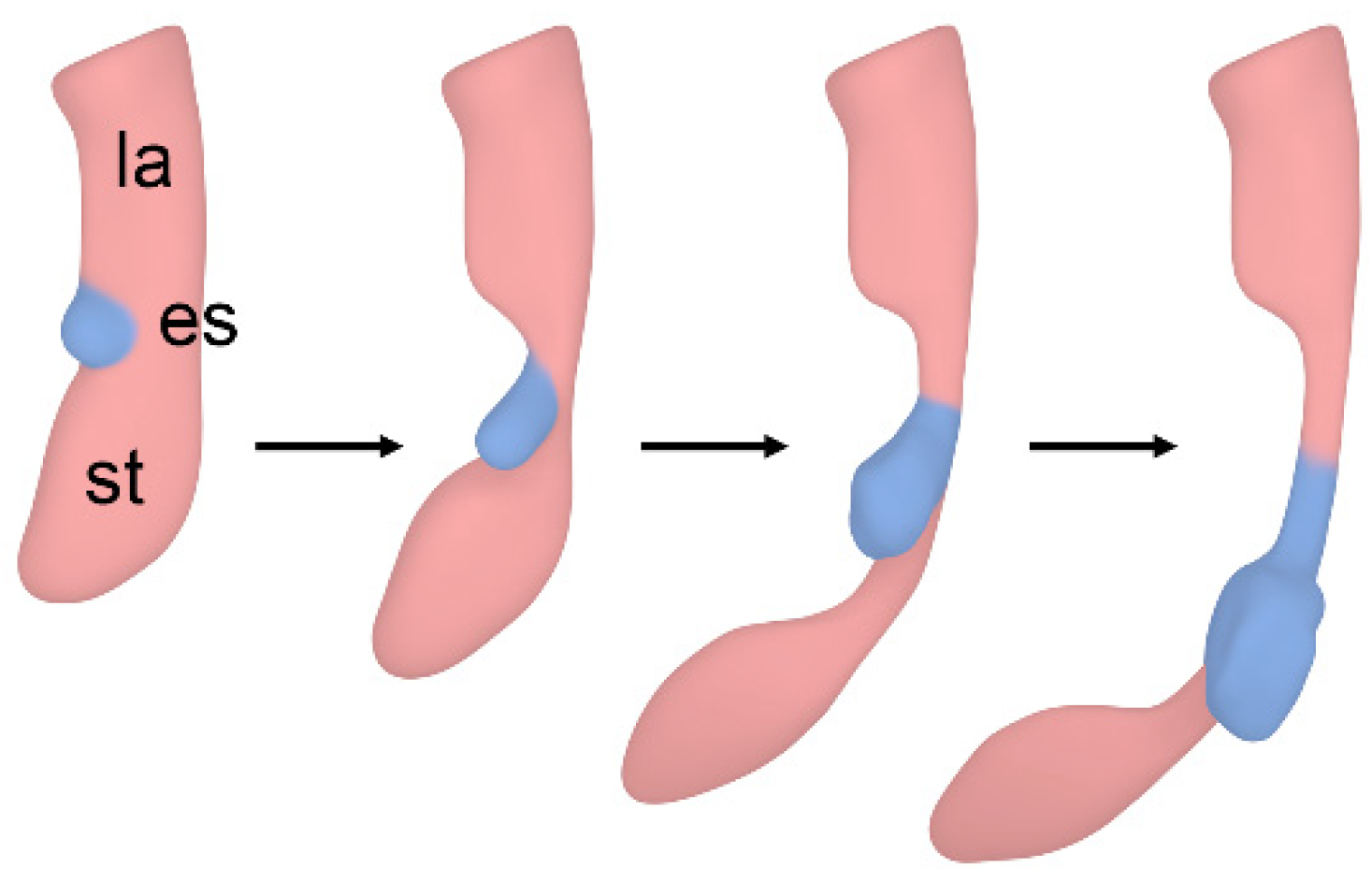
3.1. Esophageal Atresia
- -
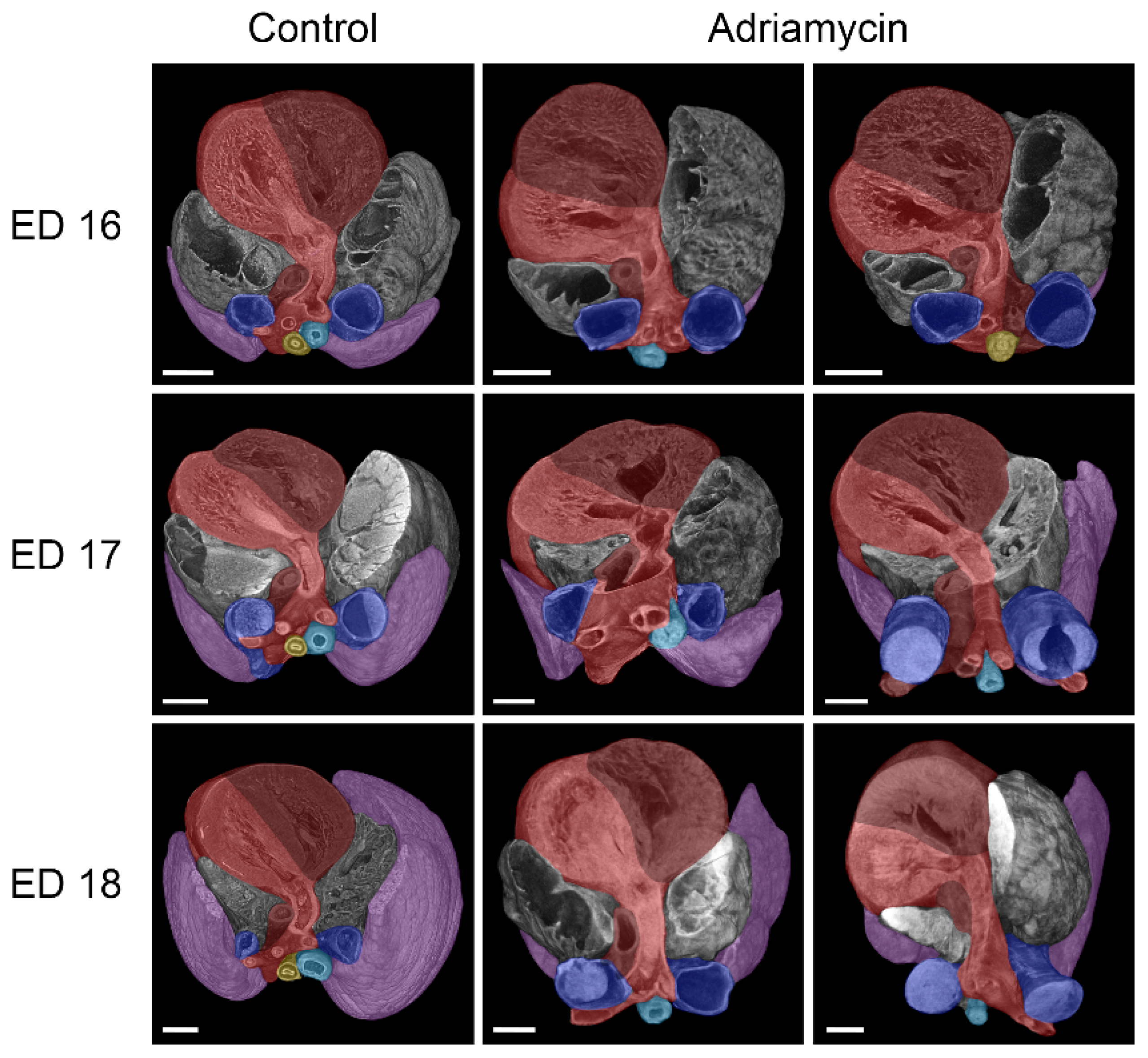
- Anomalies of the heart and great vessels.
- -
- Duodenal stenosis.
- -
- Malformations of the kidneys (hydronephrosis).
- -
- Additional malformations of the gut (anorectal atresia/agenesis).
3.2. Tracheal Agenesis
3.3. Cardiovascular Anomalies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, D.J.; Molello, J.A.; Strebing, R.J.; Dyke, I.L. Teratogenicity of adriamycin and daunomycin in the rat and rabbit. Teratology 1978, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Beasley, S.W.; Diez Pardo, J.; Qi, B.Q.; Tovar, J.A.; Xia, H.M. The contribution of the adriamycin-induced rat model of the VATER association to our understanding of congenital abnormalities and their embryogenesis. Pediatr. Surg. Int. 2000, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pardo, J.A.; Baoquan, Q.; Navarro, C.; Tovar, J.A. A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: Preliminary report. J. Pediatr. Surg. 1996, 31, 498–502. [Google Scholar] [CrossRef]
- Qi, B.Q.; Merei, J.; Farmer, P.; Hasthorpe, S.; Myers, N.A.; Beasley, S.W.; Hutson, J.M. The vagus and recurrent laryngeal nerves in the rodent experimental model of esophageal atresia. J. Pediatr. Surg. 1997, 32, 1580–1586. [Google Scholar] [CrossRef]
- Merei, J.M.; Farmer, P.; Hasthorpe, S.; Qi, B.Q.; Beasley, S.W.; Myers, N.A.; Hutson, J.M. Timing and embryology of esophageal atresia and tracheo-esophageal fistula. Anat. Rec. 1997, 249, 240–248. [Google Scholar] [CrossRef]
- Merei, J.M.; Hasthorpe, S.; Farmer, P.; Hutson, J.M. Embryogenesis of tracheal atresia. Anat. Rec. 1998, 252, 271–275. [Google Scholar] [CrossRef]
- Possögel, A.K.; Diez-Pardo, J.A.; Morales, C.; Navarro, C.; Tovar, J.A. Embryology of esophageal atresia in the adriamycin rat model. J. Pediatr. Surg. 1998, 33, 606–612. [Google Scholar] [CrossRef]
- Qi, B.Q.; Beasley, S.W. Pathohistological study of adriamycin-induced tracheal agenesis in the fetal rat. Pediatr. Surg. Int. 1999, 15, 17–20. [Google Scholar] [CrossRef]
- Ioannides, A.S.; Massa, V.; Ferraro, E.; Cecconi, F.; Spitz, L.; Henderson, D.J.; Copp, A.J. Foregut separation and tracheo-oesophageal malformations: The role of tracheal outgrowth, dorso-ventral patterning and programmed cell death. Dev. Biol. 2010, 337, 351–362. [Google Scholar] [CrossRef]
- Ginzel, M.; Huber, N.; Bauer, L.; Kluth, D.; Metzger, R. Development of the foregut and the formation of the trachea and esophagus in rat embryos. A symphony of confusion. Front. Cell Dev. Biol. 2023, 11, 1092753. [Google Scholar] [CrossRef]
- Zaw-Tun, H.A. The tracheo-esophageal septum--fact or fantasy? Origin and development of the respiratory primordium and esophagus. Acta Anat. 1982, 114, 1–21. [Google Scholar] [CrossRef]
- Kluth, D. Atlas of esophageal atresia. J. Pediatr. Surg. 1976, 11, 901–919. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Javan-Farazmand, N.; Motamedi, A.; Monajemzadeh, M.; Amini, E. The optimal dose of Adriamycin to create a viable rat model potentially applicable to congenital obstructive uropathy. J. Pediatr. Surg. 2011, 46, 1544–1549. [Google Scholar] [CrossRef]
- Ginzel, M.; Martynov, I.; Haak, R.; Lacher, M.; Kluth, D. Midgut development in rat embryos using microcomputed tomography. Commun. Biol. 2021, 4, 190. [Google Scholar] [CrossRef]
- Brosig, S.; Peukert, N.; Metzger, R.; Schneider, H.; Haak, R.; Gosemann, J.; Lacher, M.; Kluth, D. X-ray micro-computed-tomography in pediatric surgery: A new tool for studying embryos. Pediatr. Surg. Int. 2018, 34, 297–305. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Vogt, E.C. Congenital esophageal atresia. Am. J. Roenigenol. 1929, 22, 463. [Google Scholar]
- Floyd, J.; Campbell, D.C., Jr.; Dominy, D.E. Agenesis of the trachea. Am. Rev. Respir. Dis. 1962, 86, 557–560. [Google Scholar] [CrossRef]
- Happel, C.M.; Klose, C.; Witton, G.; Angrisani, G.L.; Wienecke, S.; Groos, S.; Bach, F.W.; Bormann, D.; Männer, J.; Yelbuz, T.M. Non-destructive, high-resolution 3-dimensional visualization of a cardiac defect in the chick embryo resembling complex heart defect in humans using micro-computed tomography: Double outlet right ventricle with left juxtaposition of atrial appendages. Circulation 2010, 122, e561–e564. [Google Scholar] [CrossRef]
- Berg, C.; Geipel, A.; Gembruch, U. The four-chamber view in fetal echocardiography. Ultraschall Med. 2007, 28, 132–155. [Google Scholar] [CrossRef]
- Metscher, B.D. MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009, 9, 11. [Google Scholar] [CrossRef]
- Degenhardt, K.; Wright, A.C.; Horng, D.; Padmanabhan, A.; Epstein, J.A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ. Cardiovasc. Imaging 2010, 3, 314–322. [Google Scholar] [CrossRef]
- Wong, M.D.; Dorr, A.E.; Walls, J.R.; Lerch, J.P.; Henkelman, R.M. A novel 3D mouse embryo atlas based on micro-CT. Development 2012, 139, 3248–3256. [Google Scholar] [CrossRef]
- Wong, M.D.; Maezawa, Y.; Lerch, J.P.; Henkelman, R.M. Automated pipeline for anatomical phenotyping of mouse embryos using micro-CT. Development 2014, 141, 2533–2541. [Google Scholar] [CrossRef]
- Dunmore-Buyze, P.J.; Tate, E.; Xiang, F.L.; Detombe, S.A.; Nong, Z.; Pickering, J.G.; Drangova, M. Three-dimensional imaging of the mouse heart and vasculature using micro-CT and whole-body perfusion of iodine or phosphotungstic acid. Contrast Media Mol. Imaging 2014, 9, 383–390. [Google Scholar] [CrossRef]
- Steding, G. The Anatomy of the Human Embryo—A Scanning Electron-Microscopic Atlas; Karger: Basel, Switzerland, 2009; pp. 204–253. [Google Scholar] [CrossRef]
- Steding, G.; Seidl, W. Contribution to the development of the heart. Part 1: Normal development. Thorac. Cardiovasc. Surg. 1980, 28, 386–409. [Google Scholar] [CrossRef]
- Butcher, J.T.; Sedmera, D.; Guldberg, R.E.; Markwald, R.R. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev. Dyn. 2007, 236, 802–809. [Google Scholar] [CrossRef]
- Naïja, A.; Mutlu, O.; Khan, T.; Seers, T.D.; Yalcin, H.C. An optimized CT-dense agent perfusion and micro-CT imaging protocol for chick embryo developmental stages. BMC Biomed. Eng. 2024, 6, 3. [Google Scholar] [CrossRef]
- Thalidomide Victims Association of Canada. Available online: https://www.thalidomide.ca/wp-content/uploads/2017/12/Dr-Lenz-history-of-thalidomide-1992.pdf (accessed on 28 February 2025).


| Gestational Age: | Embryos Harvested (Used): | Embryos Resorbed: |
|---|---|---|
| ED 16 | 12 (3) | 1 |
| ED 17 | 3 (3) | 6 |
| ED 18 | 6 (3) | 4 |
| ED 21 | 10 (1) | 2 |
| ED 16 | ED 17 | ED 18 | ED 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #1 | #2 | #3 | #1 | #2 | #3 | #1 | |
| Esophageal atresia | - | - | - | - | - | - | - | X | - | X |
| Tracheal agenesis | X | X | X | X | X | X | X | - | X | - |
| Duod. sten./atresia | - | - | - | - | - | - | - | X | - | X |
| Missing bladder | X | X | X | X | X | X | X | X | X | X |
| Missing thymus | X | X | X | X | X | X | X | X | X | X |
| Missing stomach | X | - | X | X | X | - | - | - | X | - |
| Stomach atresia | - | X | - | - | - | X | X | - | - | - |
| Midgut atresia | X | X | X | X | X | X | - | - | X | - |
| Anal atresia | - | X | - | X | X | X | - | X | - | X |
| Cardiov. mal. | X | X | X | X | X | X | X | X | X | X |
| Kidney mal. | - | - | - | X | - | - | - | - | - | X |
| Lung mal. | X | X | X | X | X | X | X | X | X | X |
| Limb mal. | - | - | - | - | - | - | - | - | - | X |
| Total | 7 | 8 | 7 | 9 | 8 | 8 | 6 | 7 | 7 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherberich, J.; Bauer, L.; Huber-Liu, N.; Krombach, G.A.; Metzger, R.; Kluth, D.; Ginzel, M. Microcomputed Tomography in the Adriamycin Rat Model of Malformations—Preliminary Results. Biomolecules 2025, 15, 569. https://doi.org/10.3390/biom15040569
Scherberich J, Bauer L, Huber-Liu N, Krombach GA, Metzger R, Kluth D, Ginzel M. Microcomputed Tomography in the Adriamycin Rat Model of Malformations—Preliminary Results. Biomolecules. 2025; 15(4):569. https://doi.org/10.3390/biom15040569
Chicago/Turabian StyleScherberich, Jan, Leopold Bauer, Nana Huber-Liu, Gabriele Anja Krombach, Roman Metzger, Dietrich Kluth, and Marco Ginzel. 2025. "Microcomputed Tomography in the Adriamycin Rat Model of Malformations—Preliminary Results" Biomolecules 15, no. 4: 569. https://doi.org/10.3390/biom15040569
APA StyleScherberich, J., Bauer, L., Huber-Liu, N., Krombach, G. A., Metzger, R., Kluth, D., & Ginzel, M. (2025). Microcomputed Tomography in the Adriamycin Rat Model of Malformations—Preliminary Results. Biomolecules, 15(4), 569. https://doi.org/10.3390/biom15040569







