Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by extracellular plaques containing amyloid β-protein (Aβ) and intracellular neurofibrillary tangles formed by tau. Cerebral Aβ accumulation initiates a noxious cascade that leads to irreversible neuronal degeneration and memory impairment in older adults. Recent advances in Aβ seeding studies offer a promising avenue for exploring the mechanisms underlying amyloid deposition and the complex pathological features of AD. However, the extent to which inoculated Aβ seeds can induce reproducible and reliable pathological manifestations remains unclear due to significant variability across studies. In this review, we will discuss several factors that contribute to the induction or acceleration of amyloid deposition and consequent pathologies. Specifically, we focus on the diversity of host animals, sources and recipe of Aβ seeds, and inoculating strategies. By integrating these key aspects, this review aims to offer a comprehensive perspective on Aβ seeding in AD and provide guidance for modeling AD pathogenesis through the exogenous introduction of Aβ seeds.
1. Introduction
Alzheimer’s disease (AD) is a widespread neurodegenerative disorder that is primarily characterized by progressive memory loss, notable changes in personality, and eventual severe cognitive decline and dementia [1,2]. Epidemiological studies indicate that AD can be categorized into a sporadic form (accounting for 95%) and a familial form (accounting for 1–5%), the latter of which is linked to specific genetic mutations [2,3,4]. Compelling data from genetic and clinical studies have suggested an initiating role of the amyloid β-protein (Aβ) in AD pathogenesis. The formation of extracellular amyloid plaques, primarily composed of aggregated Aβ, occurs years before disease onset and eventually induces neurofibrillary tangles and synaptic loss [5]. The amyloid hypothesis proposes that the deposition of Aβ in the brain parenchyma triggers a cascade of neurotoxic events and leads to widespread neurodegeneration [5,6,7]. However, recent studies have shown that there is a more complex AD etiology in which the role of Aβ in neurodegeneration is linked to multifactorial aspects [8].
Given the complexity and multifaceted nature of AD, as well as the difficulty of obtaining human resources, there is an urgent need for well-characterized animal models in the exploration of disease mechanisms and the development of early diagnostic and therapeutic strategies before translating to humans [9,10,11]. To date, hundreds of AD animal models have been developed. However, very few adequately replicate the complex neuropathological and behavioral phenotypes observed in AD patients [12,13,14]. For instance, transgenic mice that overexpress mutant amyloid precursor protein (APP) and/or APP/Presenilin 1 (PS1) recapitulate AD-like pathological features such as Aβ accumulation and neuroinflammation but also have artificial phenotypes due to excessive production of mislocalized APP/PS1 and their fragments [12,15,16,17]. To address these issues, single APP knock-in (KI) mouse models harboring the Swedish and Beyreuther/Iberian mutations were developed with a genome editing approach [18]. These mice exhibit Aβ pathology and neuroinflammation in an age-dependent manner and require up to 18 months to gain apparent cognitive impairment [18]. While introducing the Arctic mutation effectively accelerates the onset of memory decline, this model is not suitable for investigating Aβ metabolism and clearance, as the mutated Aβ is resistant to proteolytic degradation [18,19].
Emerging data from animal and human studies suggest that Aβ pathology can be induced by the injection of pre-formed Aβ seeds, which can initiate a cascade of downstream events, including neuroinflammation and memory loss [20,21,22,23]. Taking advantage of the Aβ seeding phenomenon and animal models, significant efforts have been made to develop inducible AD models that may recapitulate key disease features. This review aims to provide a comprehensive summary of the literature on in vivo Aβ seeding activity and its consequent disease manifestations. We will discuss three major factors that may influence this process: the host animals, the types of Aβ seeds, and the injection approaches (Figure 1). We also discuss the advantages of modeling AD pathology using the Aβ seeding approach, as well as the challenges that require further investigation.
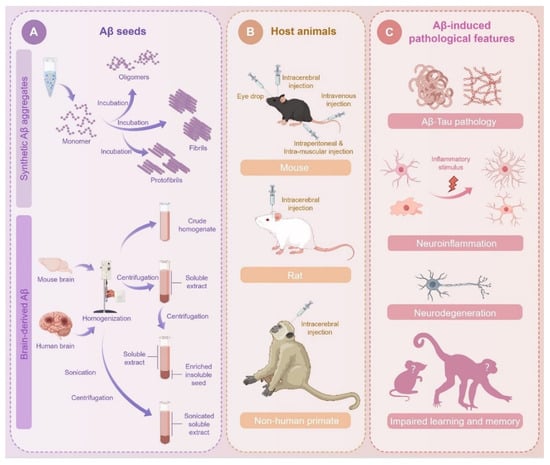
Figure 1.
Variables in seeds, host animals, and injection approaches for Aβ seeding. (A) Different types of synthetic Aβ aggregates are prepared by incubating monomer under certain conditions. Biological Aβ seeds are prepared from AD patient or mouse brain tissues through a series of processing steps, including mechanical homogenization, centrifugation, and sonication. (B) Aβ seeds can be introduced through different routines, including intracerebral and peripheral injection. (C) Aβ injection induces multiple AD-like pathological features in host animals, such as Aβ pathology, tau pathology, neuroinflammation, neuronal degeneration, and impaired learning and memory behaviors.
2. Rodents and Primates as Host Animals for Aβ Seeding
Exogenous seeding of Aβ pathology has been successfully achieved in various animal models, including rodents and non-human primates (NHPs) [20,21,24,25]. Commonly used rodents for seeding investigation include mice and rats that express multiple transgenes related to familial AD, such as mutant APP and PS1 [26]. These animals produce high levels of humanized Aβ and can develop pathology relatively early in life, making them valuable for studying the mechanisms of Aβ propagation and seeding.
2.1. Genetic Modified Mouse Models
Compared to human Aβ, murine Aβ is less prone to forming aggregates due to differences in three amino acids at the N-terminal positions 5, 10, and 13 [27]. Murine Aβ is also difficult to seed on its own or to cross-seed in the presence of pre-formed human Aβ aggregates [27,28]. Thus, nearly all investigations on the spreading of amyloid pathology rely on genetically modified murine models that carry humanized Aβ [29]. Genetic studies on human early-onset familial AD have identified various pathogenic mutations in the genes encoding APP, as well as PS1 and PS2, which influence the activity of β-secretase and γ-secretase during APP proteolytic processing [30]. The introduction of such mutations, along with the expression of human Aβ, can induce a range of AD-related phenotypes in mice [24,30,31].
The first generation of mice that recapitulate human AD pathology includes models that overexpress a single gene with one or more mutations, most commonly the human APP gene. The PDAPP line is the inaugural mouse model to mimic AD pathogenesis, which employs the platelet-derived growth factor (PDGF)-β promoter to drive the expression of the human APP gene carrying the Indiana mutation (V717F) [16,32,33]. The Tg2576 and APP23 mouse models overexpress the human APP gene with the Swedish mutation (KM670/671NL), leading to elevated levels of Aβ and age-related deposition of amyloid plaques [34,35,36,37]. TgCRND8 mice overexpress the human APP gene carrying both the Swedish mutation (KM670/671 NL) and the Indiana mutation (V717F) [38]. The introduction of highly pathogenic mutations in the APP, PS1, and PS2 genes causes advanced amyloid plaque deposition in the mouse brain, which progressively becomes more pronounced with age [34,39].
The second generation of transgenic mice takes advantage of multiple genes and mutations, which may contribute to the accelerated development of AD pathology. APP/PS1 is a double transgenic mouse model that overexpresses a chimeric mouse/human APP (Mo/HuAPP695swe) and a mutant human PS 1 (PS1-dE9) in neurons and provides an important tool for understanding the interaction between APP and PS1 in AD pathology during aging [40,41,42,43]. The 5×FAD mice overexpress human APP and PS1 genes with five mutations: the Swedish (K670N/M671L), Florida (I716V), and London (V717I) mutations in APP and the M146L and L286V mutations in PS1. This mouse model recapitulates many AD-related phenotypes and exhibits relatively early and aggressive progression. It has been one of the most widely used models over the past two decades [44,45,46]. The 3×Tg mice carry three mutations associated with familial AD (APP Swedish, MAPT P301L, and PS1 M146V) and develop age-related, progressive amyloid deposits, as well as synaptic dysfunction and cognitive impairment. This mouse model also produces hyperphosphorylated tau with age and develops extensive tau tangles due to the overexpression of mutant human tau, making it an ideal model for investigating both Aβ and tau pathology [47].
With the rapid advances in gene editing technology, a series of novel gene KI and knock-out (KO) murine models have been developed in recent years to better mimic AD pathogenesis. These gene-edited mouse lines eliminate several artificial effects and abnormal phenotypes caused by the random insertion of APP and/or PS1/2 genes, as well as the uncertain production of these proteins. The APP NL-F/NL-F mice express APP at wild-type levels but produce high levels of humanized Aβ due to two pathogenic mutations, the Swedish (K670N/M671L) and the Iberian (I716F) [18]. These mice recapitulate several AD-related pathologies, including increased hydrolysis of APP, cerebral amyloid deposition, synaptic loss, and microgliosis and astrocytosis, but generally lack tau protein-associated neurofibrillary tangles and cognitive dysfunction until very advanced ages [18,48]. To address these important issues, the same group developed a new mouse line that expresses human MAPT protein accompanied with elevated levels of human Aβ by crossing human MAPT-KI mice with APP-KI mice [49]. The double-KI mice display comparable levels of amyloid plaques, neuritic dystrophy, neuroinflammation, and memory deficits to APP KI mice but exhibit more pronounced tau phosphorylation. No evidence of tau pathology or neurodegeneration is observed before 24 months of age, unless accelerated by inoculating AD brain-derived tau seeds [50]. These studies demonstrate that the new generations of gene-edited mice exhibit only moderate progression of AD pathology and cognitive decline. They thus provide ideal models and a critical time window to monitor disease onset in the absence and presence of exogenous protein seeds and to dissect the role of the Aβ–tau axis in the etiology of AD.
2.2. Rat Models
Compared to mice, rats are closer to humans in terms of physiological structure and genetic characteristics. Their nervous system and cognitive functions are also significantly more complex than those of mice, making rats an important model for studying neurological diseases. The McGill-R Thy1-APP rat model, the most extensively studied APP transgenic rat strain, expresses human APP751 with the Swedish and Indiana mutations. It exhibits age-dependent accumulation of amyloid plaques, gliosis, loss of cholinergic synapses, and cognitive deficits [51,52]. The TgF344 rat model expresses human APP695 that carries the Swedish and PS1ΔE9 mutations, and develops region-wide amyloid accumulation [53]. Compared to McGill-R Thy1-APP rats, TgF344 rats exhibit significant tau phosphorylation and neurofibrillary tangles but lack synaptic loss and neurophysiological dysfunction [51,52,53]. Following the strategy used to develop APP KI mice, a research group has successfully developed an APP KI rat model that exhibits typical Aβ plaque deposition, microglial activation, gliosis, synaptic damage, and cognitive dysfunction. Most strikingly, these rats also develop AD-like phenotypes not commonly observed in other models, such as tau pathology, neuronal apoptosis, necrotic cell death, and cerebral atrophy [19]. However, the E22G mutation within the Aβ region in this model drives rapid formation of atypical Aβ conformations, restricting its relevance to sporadic AD.
Over the past few decades, a large number of genetically modified murine models have greatly enhanced our understanding of AD. However, they are still inadequate for fully reflecting the complexity and diversity of biological events in humans due to several differences between rodents and humans, such as brain structure and function, the aging process, and immune and metabolic systems. For instance, the prefrontal cortex of the human brain is crucial for maintaining cognitive functions, whereas this region is absent in the mouse brain [54,55]. Additionally, AD is a slow and progressive degenerative disease that may take ten to twenty years to develop detectable pathological changes and significant clinical symptoms. It is inevitable that the immune system and metabolic imbalances will affect disease onset during aging [56,57]. In the context of Aβ seeding, species-specific differences in immune responses and metabolic efficiency could also affect Aβ clearance, thereby shaping the development of AD pathology.
2.3. Non-Human Primates (NHPs)
In contrast to rodents, NHPs exhibit a high degree of similarity to humans in various biological characteristics, including genetic background, cognitive behavior, immune response, and anatomical structure. They possess advanced brain functions and neurological activities that are unparalleled in rodents, making them more suitable for studying human neuropsychiatric diseases and for drug discovery. Both naturally aging NHPs and artificially induced models have been reported in recent years. Apes, such as chimpanzees, gorillas, and orangutans, have Aβ sequences that are identical to those of humans [58]. These animals produce Aβ that gradually accumulates in the brain with age, eventually forming amyloid plaques and cerebrovascular amyloid lesions (cerebral amyloid angiopathy, CAA) in older individuals. However, NHPs tend to have more CAA and fewer cerebral plaques compared to humans [58,59,60]. Histological examination indicates that NHP plaques are mostly diffuse, while human plaques are more extensively compact [61].
Chimpanzees have been found to develop cortical tauopathies similar to those in humans, but their cognitive decline differs from the cognitive deficits observed in human AD patients [58,60,61,62]. These animals are suitable for studying human-like tau pathology to some extent but cannot be used as common research models due to their long lifespan, large size, and ethical constraints. In contrast, smaller NHPs, such as rhesus monkeys, baboons, and cynomolgus monkeys, are more convenient for use as model animals. Rhesus monkeys share structural homology with humans in their Aβ sequence, and aged individuals exhibit readily detectable Aβ plaques with distribution patterns similar to those observed in human AD patients [63,64,65,66]. The majority of Aβ plaques in the brains of rhesus monkeys are diffuse, with only about 20% being dense and associated with minor neuronal loss, suggesting a significant difference in how Aβ pathology manifests in NHPs compared to humans [67,68]. Despite the high degree of tau sequence homology between humans and rhesus monkeys, the latter develop very rare neurofibrillary tangles [67,69]. Nonetheless, aging rhesus macaques exhibit progressive cognitive decline, likely due to their close genetic similarity to humans [70,71,72]. In aged vervets, Aβ and tau burdens are associated with reduced brain volume and glucose metabolism, as well as with abnormalities of complex integrated behaviors such as gait speed. In addition, the observed Aβ plaque accumulation shares a similar distribution throughout the cerebral cortex as in humans with early AD neuropathologic changes [73]. While naturally aged NHPs can develop pathological changes and memory impairments resembling human AD to some extent, the variability in these manifestations among individuals and the long lifespan required to observe significant outcomes have substantially limited their use in the laboratory.
To accelerate AD-like progression in NHPs, recent research has focused on creating inducible models by introducing pathogenic protein seeds. For instance, inoculating Aβ oligomers into the brains of rhesus monkeys has been shown to impair synaptic integrity, induce neuroinflammation, and elevate AD biomarkers in the cerebrospinal fluid (CSF) to levels comparable to those observed in AD patients [74]. Rhesus monkeys injected with an adeno-associated virus (AAV) expressing a double tau mutation (P301L/S320F) exhibit misfolded tau propagation comparable to that observed in humans. This propagation is accompanied by a robust neuroinflammatory response and elevated biomarkers of inflammation and neuronal loss in CSF and plasma [75]. A recent study reported the generation of transgenic cynomolgus monkey models expressing tau (P301L) through lentiviral infection of monkey embryos and an inducible model that adult monkeys receive AAV-delivered tau (P301L). Both models develop severe tauopathy, leading to enhanced generation of Aβ oligomers in the spinal cord and resulting in age-dependent neurodegenerative lesions, abnormal glucose metabolism, and motor dysfunction [76]. These studies underscore the potential of developing more advanced NHP models that closely mimic human AD pathology by exogenously introducing pathogenic protein seeds, either through direct cerebral injection or a viral delivery strategy.
3. Resources of Aβ Seeds
The propagation of AD pathology and the subsequent molecular and cellular events are significantly influenced by the diversity of inoculated Aβ seeds, including their sources, species, and preparation methods [77]. Aβ seeds can be categorized into synthetic Aβ aggregates, brain homogenates, and extracts from transgenic mouse brains or human brain tissue [78]. Understanding the impact of different Aβ seeds is essential for accurately modeling AD pathology and evaluating potential therapeutic strategies.
3.1. Synthetic Aβ Aggregates
Over the past three decades, chemically synthesized Aβ peptides have been extensively utilized to study AD pathology. When these synthetic Aβ aggregates are injected into the brains of mice or NHPs, they can induce advanced amyloid deposition and various behavioral changes [75,77,79,80,81,82,83,84,85,86,87] (Table 1). Recent studies have shown that the human brain contains highly diverse Aβ primary structures encompassing a large number of N- and C-termini [88,89]. Synthetic Aβ40 and Aβ42 aggregates are the most widely used seeds and have been shown to efficiently induce amyloid pathology and accelerate disease progression in transgenic AD mouse models [79,90,91]. Aβ aggregates, despite originating from the same primary peptide, can adopt various conformations that differ in their toxicity mechanisms and pathological effects [92]. In the brain, certain Aβ strains exhibit a preference for incorporating specific Aβ isoforms into their aggregates. This strain-specific incorporation suggests that the seeding activity and the pathological changes induced may depend on both the Aβ peptides present in the host animal and the inoculated seeds [93,94].

Table 1.
Seeding of synthetic Aβ aggregates in different host animals.
In most genetic-modified AD models, the overexpression or more efficient cleavage of APP leads to elevated production of Aβ peptides, with Aβ42 levels significantly surpassing those of Aβ40. The aggregation propensity and toxicity of Aβ peptides are significantly influenced by their primary sequences and interplays. Aβ40 and Aβ42 interact strongly at the level of primary nucleation but self-assemble into separate homomolecular structures during fibril formation from mixed solutions of both peptides [95]. An early study has shown that Aβ40 monomers specifically require Aβ40 oligomers to induce growth of mature fibrils, whereas Aβ42 monomers are less selective and are stimulated by all types of seeds [96]; others report that Aβ40 aggregation can be accelerated by Aβ42 monomers but not Aβ42 fibrils by promoting primary nucleation through interaction between the different monomeric species in solution [95,97]. Controversially, other studies demonstrate that the fibrillization of the Aβ40 monomer can be accelerated by Aβ42 fibrils or high-molecular-weight oligomers by the association of Aβ40 monomer with the ends of Aβ42 fibrils and vice versa [98,99,100]. Monomeric Aβ40 alters the kinetic stability, solubility, and morphological properties of Aβ42 aggregates and prevents their conversion into mature fibrils [97,99]. In fact, rather than the morphology of the amyloid fibrils, the Aβ42:Aβ40 ratio modulates the aggregation behavior of each species [96]. A change in the Aβ42:Aβ40 ratio greatly induces the different structural types of aggregates such as different-sized oligomers or fibrils with their different morphologies and flexibilities [96,101]. These studies collectively demonstrate that cross-seeding between Aβ40 and Aβ42 results in heterogeneous aggregates, with structural outcomes dictated by seed type and concentration. Importantly, the cross-seeding behaviors of distinct Aβ species cannot be reduced to a simplistic interpretation where one species directly induces or delays the other’s aggregation.
Several studies have shown that AD patients have significantly increased levels of variable truncated Aβ peptides in the brain, which may be related to Aβ aggregation and toxicity. These truncated forms, such as N-terminally truncated peptides AβpE3–x and Aβ4–x, have been detected in high abundance in brain tissue and CSF from both sporadic and familial AD patients [88,89]. Other studies have shown that the middle region fragment Aβ25–35 is capable of inducing neuronal apoptosis, causing morphological changes and inflammatory responses in microglia, and eventually leading to memory deficits of mice and rats [102,103,104,105,106]. These findings suggest that truncated Aβ peptides may contribute to the progression of AD by modulating the aggregation of full-length Aβ, thereby affecting the formation of amyloid plaques and neurodegenerative processes.
To prepare Aβ seeds for inoculation, chemically synthesized Aβ peptides have to undergo a complex and cautious procedure that ensures reproducible generation of aggregates. Typically, Aβ monomer is incubated at a certain temperature for 3 days to a week to promote aggregation, and the resulting Aβ aggregates are snap-frozen and stored at −80 °C for subsequent injections [86,91,107,108]. The advantage of using highly pure synthetic Aβ aggregates as seeds is that the induced pathological changes are specifically attributed to Aβ itself. This allows for a more controlled and direct investigation of Aβ’s role in the development of amyloid pathology, without interference from other potential factors or contaminants. A standardized protocol enables the preparation of large-scale Aβ aggregates using the same batch of peptide stock, ensuring the reproducibility of both the inocula and their effects on disease progression. This consistency is crucial for comparing results across different studies and laboratories. It is also possible to investigate the seeding activity of a specific strain or species of Aβ, as well as the cross-seeding between different types of Aβ or between Aβ and other co-factors.
One limitation of using synthetic Aβ aggregates as seeds is the potential of structural differences between batches or vendors, which could affect the consistency and reproducibility of seeded pathology. In future studies, biophysical characterizations of synthetic Aβ seeds—using techniques such as circular dichroism (CD), atomic force microscopy (AFM), or size-exclusion chromatography (SEC)—may help standardize the properties of seed preparations and bioactivity. A second limitation is that synthetic Aβ may lack certain post-translational modifications as observed in humans. For example, isoAsp7-Aβ has been identified as a major Aβ variant in AD patients’ brains [109,110]. Repetitive intravenous administration of synthetic isoAsp7-Aβ(1–42) robustly accelerates formation of classic dense-core amyloid plaques in the brain of transgenic mice [111]. However, most studies have not incorporated such modifications into the seeds, and thus their ability to replicate key pathogenic properties observed in native Aβ aggregates remains uncertain.
3.2. Biological Aβ Seeds Derived from Mouse Brain
Unlike synthetic Aβ aggregates, brain-derived seeds may retain post-translational modifications, structural complexity, and biological cofactors, making them more physiologically relevant for investigating the seeding activity of Aβ. Aβ seeds derived from the brains of transgenic mice are capable of inducing amyloid deposition and tau pathology, exhibiting pathological characteristics similar to those observed in AD patients [5,22,77,112,113,114,115,116,117,118,119,120,121,122,123,124] (Table 2). For example, transgenic mouse models such as APP23, APP/PS1, 5×FAD, Tg2576, and TgCRND8 are often used as donors of Aβ seeds because of the high load of Aβ in their brains and their ability to cause cognitive dysfunction [5,77,113,114,115,116,117,118,119,120,122,123] (Table 2). When Aβ seeds were inoculated into an APP-deficient mouse model for six months and the latter was used in a secondary dissemination, β-amyloidosis was still induced in the APP transgenic mice. This suggests that Aβ seeds can persist in the brain for extended periods and, when host Aβ is present, resume their dissemination and pathogenic activity [123]. These mouse models provide a reliable source of Aβ seeds for studying the complex propagation of amyloid pathology and subsequent disease manifestations.

Table 2.
Seeding of mouse brain-derived Aβ seeds in different host animals.
However, the pathological features of widely used animal models exhibit significant heterogeneity in amyloid plaque morphology and biochemical composition. Donor animal brains may display a spectrum of Aβ deposits, including diffuse plaques, dense-core plaques, and vascular amyloid (e.g., CAA), which subsequently induce variable pathological phenotypes in host animal brains [5,22,77,112,114,121,125,126,127]. For instance, injection of Aβ seeds from APP23 mice into APP/PS1 mice leads to the appearance of diffuse filamentous Aβ, as well as dense plaques, which contrasts with the typical pathology observed in the APP/PS1 mice [77,112]. Thus, there is a strain-like behavior of Aβ seeds that gives rise to different pathological morphologies of cerebral amyloid deposition.
Human Aβs vary in primary structures (e.g., Aβ40, Aβ42, Aβ43) and post-translational modifications (e.g., pyroglutamate-modified Aβ, covalently cross-linked Aβ dimer), which are thought to play different roles in plaque formation. The variable Aβ peptides have their distinct aggregation kinetics and susceptibility to seeds. In certain transgenic mouse models such as APP/PS1, Aβ seeds are predominantly composed of Aβ42, which has strong propensity for aggregation and readily forms oligomers and fibrils [77,79,128,129]. Aβ42-rich seeds are more likely to induce the formation of diffuse plaques, which are characterized by their loosely structured morphology and extensive distribution [130,131]. In contrast, Aβ40 exhibits greater solubility in perivascular fluids, facilitating its accumulation within vascular walls [127]. When brain-derived Aβ seeds contain a higher proportion of Aβ40, the pathological manifestations in injected animal brains are more likely to favor vascular deposition, resulting in increased CAA and associated vascular dysfunction [132]. These findings indicate that the ratio of Aβ40 to Aβ42 varies across different mouse Aβ seeds, in which Aβ42 plays a more prominent role in plaque formation, while Aβ40 is more closely associated with vascular amyloid pathology [112,129].
In genetically modified animal models, the artificially introduced mutations in APP and/or PSEN1/2, or risk alleles like APOE4, are all contributing factors to Aβ production, aggregation, and clearance. APP23 and Tg2576 mice carry the APP Swedish mutation (K670N/M671L), which primarily enhances the cleavage efficiency of β-secretase (BACE) on APP, leading to age-related increase in total Aβ production and the Aβ40/42 ratio [36,37,133,134,135]. Other widely used animal models, such as APP/PS1, 5×FAD, 3×Tg, and APPNL-F/NL-F mice, harbor additional mutations (e.g., PS1 mutations or γ-secretase cleavage site mutations in APP) which significantly promote the generation of Aβ42 and increase the Aβ42/40 ratio [18,43,45,46,47,136,137,138]. The variability in Aβ profiles across different animal models, resulting from genetic modifications, plays a crucial role in both the activity of Aβ seeds and the resulting pathological manifestations when these models are used as hosts.
In addition to directly influencing the Aβ profile by genetic modifications, other genetic factors can contribute to inflammation, vascular dysfunction, and metabolic disorders, which may drive atypical amyloid deposition patterns. For instance, ApoE4 stabilizes soluble, cytotoxic, oligomeric Aβ and enhances fibrillogenesis and has been shown to accelerate early seeding of amyloid pathology by perturbing Aβ clearance and enhancing Aβ aggregation [139,140]. Thus, a consensus is that inoculating Aβ seeds from donor brains into animal models may yield variable results, and such pathological diversity underscores the importance of selecting appropriate donor Aβ seeds and host animals for a specific purpose.
3.3. Biological Aβ Seeds Derived from Human Brain
Compared to mouse Aβ, Aβ seeds derived from the brains of AD patients are more physiologically relevant and have been shown to be transmissible through experimental inoculation and certain medical or surgical procedures. Iatrogenic early-onset CAA has recently been identified in patients with a history of traumatic brain injury or other cerebral and extracerebral lesions requiring neurosurgery or other medical procedures, such as intravascular embolization with cadaveric dura mater extracts. In these patients, Aβ seed transmission was found to occur through exposure to cadaveric dura mater or contaminated neurosurgical instruments, often many years before the first intracerebral hemorrhage (ICH) event [141]. Human transmission of Aβ pathology and CAA has been reported in relatively young adults who died of iatrogenic Creutzfeldt–Jakob disease (CJD) following childhood treatment with cadaver-derived pituitary growth hormone contaminated with both CJD prions and Aβ seeds [23,142,143,144]. These clinical findings demonstrate that Aβ derived from AD patients can propagate between individuals through iatrogenic routes, highlighting the potent transmissibility of Aβ seeds in vivo.
Animal studies have provided strong experimental evidence supporting the prion-like propagation capacity of Aβ seeds derived from AD patients [21,22,77,93,125,145,146,147,148,149,150,151,152] (Table 3). Injection of Aβ seeds extracted from AD patient brains into mice has been shown to induce amyloid plaque formation and CAA in the recipient animals [22,23,93]. These findings collectively highlight the potent ability of human-derived Aβ seeds to drive amyloid pathology and offer critical insights into the mechanisms underlying Aβ propagation. However, human Aβ can exist in numerous forms, differing in primary structure, conformation, size, and disease-related bioactivity [88,153]. Therefore, the spreading capacity of human Aβ seeds and the resulting pathological changes in host brains may vary significantly upon inoculation. Furthermore, the genetic background, clinical history, and disease stage of brain donors may also influence the amyloid-inducing activity of Aβ seeds.

Table 3.
Seeding of human brain-derived Aβ seeds in different host animals.
Aβ derived from human brain tissue exists in multiple truncated forms, assembling into a wide range of structures, from dimers to high-ordered soluble and insoluble aggregates [88,154,155,156,157]. For example, Aβ40 and Aβ42 monomers can adopt distinct conformations, which may influence their aggregation behavior in the brains of AD patients [154,156,157]. The complex aggregation process of Aβ results in polymorphic aggregates with diverse structures and varying toxic effects [88,158,159]. Notably, the toxic effects induced by Aβ derived from AD brains are often hundreds to thousands of times more potent than those caused by synthetic Aβ aggregates [77]. Previous studies have shown that Aβ derived from the brains of AD patients can inhibit long-term potentiation, cause synaptic loss, induce neuronal excitotoxicity, and promote hyperphosphorylation of tau proteins [88,153,160]. Despite these findings, whether the neurotoxic effects and cerebral seeding activity of Aβ share a direct or mechanistic link remains unclear.
Several studies have demonstrated that while brain tissue from AD patients contains abundant diffuse and dense Aβ deposits, mice inoculated with human AD brain homogenates develop predominantly diffuse Aβ lesions and relatively few dense-core deposits [121,148,152,161,162]. Hippocampal injection of Aβ-rich brain homogenates from AD patients into APP/PS1 mice induces rapid Aβ deposition, neuronal damage, and intracellular Aβ aggregation in synaptic regions [149]. Similarly, injecting AD brain homogenates into the lateral ventricles of rats induces hippocampal microglial activation, triggers inflammatory responses, and reduces synaptic protein expression and brain volume [21]. In addition to rodent studies, several reports have described the induction of Aβ and tau pathology and cognitive decline in NHPs following inoculation of human brain-derived protein seeds [81,87,150,151,163,164]. These findings underscore the value of using human brain-derived Aβ seeds to inoculate mice and NHPs, providing biologically relevant models to study AD pathogenesis.
Islet amyloid polypeptide (IAPP) and pro-islet amyloid polypeptide (proIAPP) have been detected in cerebral and vascular Aβ deposits of AD patients, while Aβ reactivity is not present in islet amyloid extracts from patients with type 2 diabetes. Intravenous injection of preformed fibrils of synthetic IAPP, proIAPP, or Aβ in transgenic mice expressing human IAPP can act as a seed for IAPP amyloid in the islets of Langerhans. The heterologous seeding between IAPP and Aβ shown here may represent a molecular link between type 2 diabetes and AD [165]. Moreover, Aβ and α-synuclein have been shown to co-aggregate and form complexes in patient brains and transgenic models, providing clear evidence for their direct interaction [166]. Different structural forms of α-synuclein exert different effects on Aβ aggregation. Monomeric α-synuclein blocks the autocatalytic proliferation of Aβ42 fibrils, whereas fibrillar α-synuclein catalyzes the heterogeneous nucleation of Aβ42 aggregates [167]. Co-oligomer formation of α-synuclein and Aβ is generally more favorable than self-oligomer formation of α-synuclein at equilibrium [168]. These studies suggest that diabetes and other neurodegenerative disease may be risk factors for AD and that the cross-seeding of Aβ and other amyloidogenic proteins could play a critical role in AD pathology. Future studies investigating Aβ seeding and AD modeling should incorporate these cofactors to improve the relevance of disease models.
3.4. Methodologies for the Preparation of Brain-Derived Aβ Seeds
The methods for the preparation of biological Aβ seeds from animal or human brains vary widely between laboratories and in publications from the same group. In most cases, cortical tissue was mechanically homogenized in an aqueous buffer, diluted, and directly used as Aβ seeds. Only in a few cases were brain homogenates centrifuged, and the supernatant was used as the seeds [23,120,121]. Specifically, brain tissue was homogenized at 10% (w/v) in phosphate-buffered saline (PBS), then vortexed and sonicated three times for 5 s, and centrifuged at 3000× g for 5 min, and the supernatant was used as the Aβ seeds [77,79,112,114,123,125,148,152,161,169]. Alternatively, brain extracts can be further fractionated by ultracentrifugation to assess the seeding activity of both soluble and insoluble Aβ species. This distinction is important, as the morphological appearance of the induced Aβ deposits differs between the two types of seeds. Aβ deposits induced by insoluble seeds tend to be larger, often forming congophilic aggregates that appear nonuniformly distributed throughout the injected area. In contrast, soluble Aβ species from brain extracts typically induce smaller, Congo-red-negative patches of Aβ aggregates, which are more evenly distributed throughout the injected region [5].
The methodologies used to isolate biological Aβ seeds from brain tissue have several limitations. First, direct mechanical homogenization of brain tissue may disrupt the structural integrity of Aβ aggregates, potentially altering their pathological propagation effects [153,159,170]. Second, ultrasonic fragmentation may cause protein denaturation or disrupt the interactions between Aβ and other proteins or lipids, which might be critical in driving amyloid propagation [171]. Lastly, insufficient centrifugation of brain homogenates may fail to completely remove tissue or cell debris, potentially leading to artifacts in the induced pathology [120]. To address this issue, we previously developed a new gentle extraction protocol by soaking minced AD brain tissue in aqueous buffer. Next sequential centrifugation removes cellular debris and then large macromolecular assemblies. The resultant supernatant contains readily diffusible Aβ species, which make up a minority of total Aβ in AD brains but account for essentially all bioactivity present in extracts of homogenized brain [153,172]. Nonetheless, the variability in Aβ states in brain tissue and their neurotoxic effects support the notion that human Aβ is highly heterogeneous [172,173].
The amyloid-inducing activity of Aβ seeds can also be modified before injection. For example, pre-treating mouse brain-derived Aβ seeds with formic acid completely abolishes their amyloid-inducing activity, while heat treatment partially reduces but does not eliminate the seeding activity of mouse brain extracts [77]. Formaldehyde fixation alters the morphology of Aβ plaques in the donor brain and affects the subsequent seeding activity of the extracted seeds. Brain homogenates prepared from unfixed frozen tissue of APP23 mouse results in diffuse plaques in the host brain, whereas samples from formaldehyde-fixed tissue produce more punctate deposits [119]. Extended sonication of mouse brain extracts enhances the in vivo seeding activity of Aβ, possibly by generating smaller, fragmented aggregates from larger insoluble assemblies.
Collectively, prior studies have demonstrated that biological Aβ seeds isolated from mouse or human brains are a critical tool for investigating the molecular mechanisms underlying Aβ propagation in AD. Although Aβ seeds vary in their original amyloid morphology, molecular composition, and relative abundance, their ability to induce β-amyloidosis in host animals has provided valuable insights into the molecular pathology of AD. However, significant challenges remain in standardizing seed preparation methodologies, understanding strain-specific differences in Aβ seeding, and translating these pathological findings into accurate AD models. Conformation- and/or site-specific antibodies, such as A11, may be useful in characterizing the structural features and bioactivities of synthetic or brain-derived Aβ seeds.
3.5. Structural Features of Different Types of Aβ Seeds Acquired from in Vitro Synthesis, Mouse Brain, and Human Brain
Variations in Aβ fibril structure in vivo may correlate with differences in AD phenotype, in analogy to distinct prion strains that are associated with different clinical and pathological phenotypes. There have been many attempts to obtain high-resolution structural information about Aβ fibrils. Most of previously reported Aβ fibril structures are in-register, parallel, cross-β-sheets that mostly consist of two protofilaments twisted around each other. An atomic resolution structure of Aβ42 fibrils identified by magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy reveals parallel, in-register cross-β-sheets, with the fibril core consisting of a dimer of Aβ42 molecules, each containing four β-strands in an S-shaped amyloid fold and arranged in a manner that generates two hydrophobic cores that are capped at the end of the chain by a salt bridge [174]. Another study combining solid-state NMR spectroscopy and mass-per-length measurements from EM shows that the 3D structure of Aβ42 fibrils is composed of two molecules per fibril layer, with residues 15–42 forming a double-horseshoe-like cross-β-sheet entity with maximally buried hydrophobic side chains. Residues 1–14 are partially ordered and in a β-strand conformation but do not display unambiguous distance restraints to the remainder of the core structure [175]. As determined by cryo-electron microscopy (cryoEM) complemented by solid-state NMR, the structure of the Aβ42 fibril is composed of two intertwined protofilaments in which the N terminus serves as part of the cross-β structure, resulting in an overall “LS”-shaped topology of individual subunits [176]. Another study has reported that the Aβ42 fibril displays triple parallel β-sheet segments that differ from others. The Aβ40 fibril is incompatible with the triple-β-motif, because seeding with Aβ42 fibrils does not promote conversion of monomeric Aβ40 into fibrils via cross-replication [177]. The spherical amyloid assembly of the synthetic Aβ42 structure involves a β-loop-β motif, which significantly differed from the triple-β motif observed for the Aβ42 fibril [178]. Solid-state NMR analyses of soluble Aβ oligomers prepared from recombinant Aβ42 revealed a mixed parallel and antiparallel β-sheet structure that is different from fibrils which contain only parallel β-sheets [179].
Solid-state NMR analysis of tissue from two AD patients with distinct clinical histories identified a single predominant Aβ40 fibril structure in each patient, but different from one another and distinguishable from fibrils produced in vitro [156]. The predominant molecular structure in brain-seeded fibrils differs from the structures of purely synthetic Aβ40 fibrils [180]. Solid-state NMR measurements of Aβ40 and Aβ42 fibrils prepared by seeded growth from extracts of AD brain cortex demonstrate the existence of a specific predominant Aβ40 fibril structure in different AD clinical subtypes. There is also a qualitative difference between Aβ40 and Aβ42 aggregates in the brain tissue of patients with AD [154]. Further study involved a high-resolution cryoEM density map that showed that these fibrils have a four-layered cross-β structure, with twofold screw symmetry about the fibril growth direction and with fully extended conformations for Aβ40 molecules in the inner layers [181]. Structural analysis with cryoEM showed that brain-derived Aβ amyloid fibrils are right-hand twisted and their peptide fold differs sharply from previously analyzed Aβ fibrils that were formed in vitro [182,183]. CryoEM structures of Aβ42 filaments from human brain reveal two structurally related S-shaped protofilament folds that give rise to two types of filaments. Type I filaments are found mostly in sporadic AD and are made of two identical S-shaped protofilaments embracing each other with extended arms, and each protofilament comprises five β-strands. Type II filaments are mostly found in familial AD and APPNL-F mice and contain an ordered core that extends from 12 to 42 and comprises four β-strands [183].
Most Aβ filaments in AD patients with the Arctic mutation (Aβ E22G) consist of two pairs of non-identical protofilaments that comprise residues V12-V40 or E11-G37, whereas most filaments in APPNL-G-F mice are made of two identical mutant protofilaments that extend from D1 to G37 [184]. Parenchymal deposits of Aβ42 and blood vessel deposits of Aβ40 have distinct structures, supporting the view that AD and CAA are different Aβ proteinopathies [185]. Three types of Aβ40 filaments have been identified from the leptomeninges of individuals with AD and CAA, and each comprises one, two, or three protofilament pairs [185]. A recent study reports two types of Aβ40 filaments in adult individuals with Down syndrome (DS) that differ from those in sporadic AD and two types of Aβ42 filaments identical to those found in sporadic and familial AD [186]. The presence of structural variations among Aβ aggregates in the human and transgenic mouse brains, as well as environmental effects on the propagation of specific structures, should not be overlooked in the study of Aβ seeding. A central goal for future work is to determine whether structurally distinct Aβ aggregates can consistently seed different patterns of Aβ pathology in suitable animal models.
4. Experimental Strategies for Aβ Inoculation
The regional specificity of Aβ production and accumulation in each animal model or human donor may play a pivotal role in the development of AD pathology and associated cognitive impairment. For example, in 5×FAD mice, the deposition of Aβ plaques is positively associated with several brain regions, including the prefrontal cortex, somatosensory cortex, medial amygdala, thalamus, and hippocampus [187]. In humans, neuropathological studies indicate a spatiotemporal evolution of Aβ accumulation, beginning in cerebral regions with neuronal populations characterized by high metabolic and bioenergetic activity (such as the association cortices). This accumulation then spreads from the neocortex to the allocortex, brainstem, and eventually the cerebellum [188,189]. The varying vulnerability of distinct brain regions to Aβ accumulation is closely related to the specific molecular properties of the affected neural systems [190,191]. The injection site of Aβ seeds may play a crucial role in inducing amyloid deposition and could significantly influence subsequent pathological events.
4.1. Intracerebral Injection
In prior studies, Aβ seeds are typically administered via intracranial injection into the hippocampus and cortex—regions highly susceptible to Aβ deposition in AD [25,75,77,86,114,118,122,125,145,146,149,151,162,192,193]. Transgenic animals injected with synthetic Aβ aggregates or seed-containing brain extracts into the hippocampus exhibit early Aβ deposition, tau pathology, and memory impairment [5,77,118,145,146,150,193]. For example, both Tg2576 mice and APP21 rats developed senile plaques and CAA in the injected brain region following hippocampal injection of human AD brain extracts [22,162]. APPNL-F/NL-F mice injected with human and mouse brain extracts in the parietal lobe exhibit plaque deposition and CAA [194]. Although all injected brain regions (i.e., the olfactory bulb, parietal cortex, entorhinal cortex, striatum, and hippocampus) have the potential for the induction of Aβ deposition, there is significant difference between the amount and type of amyloid induced across these areas. For example, in the entorhinal cortex and hippocampus, amyloid induction was robust and predominantly congophilic, whereas in the striatum, far less amyloid was induced, and the deposits were primarily diffuse [113].
The induction of Aβ deposits is most pronounced in the vicinity of the injection site, yet it was also detectable throughout the entire injected brain area [113,125]. This spread likely involves both passive diffusion of soluble Aβ and active transport along axonal and synaptic connections. Other studies have shown that lateral ventricle injection of Aβ may distribute the seeds throughout the brain, thereby accelerating Aβ propagation [21,74,83]. For instance, rhesus monkeys injected with synthetic Aβ into the lateral ventricle develop age-related Aβ and tau pathology, accompanied by declines in learning and memory abilities [74]. Collectively, these studies suggest that the brain regions in which seeds are inoculated may affect the type of Aβ pathology that ultimately develops.
4.2. Peripheral Injection
It has been shown that an infectious prion particle can infect the host by various peripheral application routes. Cerebral Aβ amyloidosis can also be induced by peripheral injection of seeding-competent aggregates [114]. Intraperitoneally inoculated Aβ-containing brain extracts can induce the accumulation of cerebral Aβ in host animals at multiple loci, most efficiently in regions with high availability of soluble Aβ [114,115]. A recent study demonstrated that cerebral accumulation of Aβ can be accelerated after exposing mouse models of AD to Aβ seeds by different peripheral routes of administration, including intra-peritoneal and intra-muscular. Drops of brain homogenate laden with Aβ seeds in the eyes can efficiently induce amyloid deposition, while oral administration of large quantities of brain extracts does not have any effect [22]. Thus, cerebral β-amyloidosis can be seeded by homologous protein aggregates delivered into the peritoneal cavity following a similar pattern as prion disease, but the efficiency of peripheral administration routes may require more time and are less efficient than that of intracerebral injection [113,114]. The mechanism behind the peripheral induction of cerebral amyloidosis is not yet fully understood, but it is possible that certain Aβ seeds may either cross the blood–brain barrier or activate systemic pathways that promote cerebral amyloidosis.
5. Conclusions and Future Directions
Despite recent progress in drug discoveries, there are still needs in the field to develop more diverse animal models for the goals of studying heterogeneous mechanisms and searching for new targets of AD. Significant evidence has shown that inoculation of brain homogenate extracts containing certain Aβ seeds can induce AD-like pathology in transgenic murine models of amyloidosis or in NHPs [69,86,150,195,196]. Thus, modeling disease progression by Aβ seeding strategy may open a new path for specific research questions to be addressed in the AD field.
The advantage of the seeding strategy is that cerebral Aβ injection often results in rapid amyloid deposition in host animals, and the procedure can be easily controlled and manipulated. For instance, by injecting Aβ seeds into specific brain regions like the hippocampus or cortex, researchers can precisely investigate regional pathological changes and their projections to other brain areas [22]. Additionally, mouse models of amyloidosis typically do not develop robust tau pathology on their own but can be induced following intracerebral injection of Aβ-containing brain extracts. This approach provides a valuable tool for studying the synergistic effects of Aβ and tau in AD [197,198,199].
One major challenge in the field is the variability in the host animals used for Aβ seeding across different studies and laboratories. The vast majority of studies were performed on transgenic mouse or rat models that overexpress human APP and/or PS1 genes with genetic mutations associated with familial AD. While these models allow for efficient and rapid detection of pathological changes resembling familial AD, the drawbacks of aggressive gene manipulation cannot be overlooked. The most recently developed gene-edited animals may avoid some or all of these side effects. In the future, it will be essential to use newer generations of mouse models through KI/KO or CRISPR gene editing technologies or NHPs, which may more accurately model AD progression and avoid potential side effects caused by gene overexpression.
It remains unclear to what extent these animal models can capture the complexity and diversity of biological events observed in humans, given the significant differences between rodents and humans in brain structure, function, aging processes, and immune and metabolic systems. For example, the human prefrontal cortex plays a crucial role in maintaining cognitive functions, whereas this region is largely absent in the mouse brain. In addition, AD is a slow and progressive degenerative disorder that can take decades to develop significant pathological changes and clinical symptoms. A critical limitation of gene-edited murine models is their tendency to develop AD-like pathology at a very young age, raising an important question of whether aging should be more considered when modeling AD pathogenesis in these systems.
Another challenge is the variability in outcomes resulting from the diverse sources of Aβ seeds. Due to the limited availability of human brain tissue, many studies have relied on preformed fibrils generated from synthetic peptides that may not fully replicate the complex and heterogeneous nature of Aβ aggregates found in human brains. In contrast, brain-derived seeds may retain post-translational modifications, structural complexity, and associated biological cofactors, making them more physiologically relevant for investigating the prion-like propagation of Aβ pathology. However, a critical challenge in using brain-derived Aβ seeds is the potential modulation of their seeding potency by donor-specific factors, particularly genetic polymorphisms and disease stage. To develop a more accurate and consistent model, it is essential to better characterize the propensities of different host animals to develop Aβ deposition and carefully select the most suitable source of Aβ seeds.
The third challenge is the inconsistency in the methods used to prepare biological Aβ seeds from animal or human brains across different laboratories and publications. To better understand the mechanisms underlying seed-induced Aβ pathology and establish reliable models of AD pathogenesis, it is essential to systematically evaluate and standardize seed preparation and administration protocols. This includes, but is not limited to, brain tissue handling, seed isolation and purification, biochemical characterization, and the selection of appropriate inoculation sites. Standardizing these methodologies will help ensure consistency and reproducibility in experimental outcomes, ultimately advancing our understanding of Aβ propagation and AD progression.
Author Contributions
Conceptualization, W.H. and L.L.; literature search, Q.L.; writing—original draft preparation, Q.L. and S.S.; writing—review and editing, W.H. and Q.L.; visualization, Q.L. and S.S.; supervision, W.H. and L.L.; project administration, W.H.; funding acquisition, W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32471010, 32100800), the Key-Area Research and Development Program of Guangdong Province (2023B0303040004), the Guangdong Science and Technology Program (2021QN02Y925), the Shenzhen Science and Technology Program (JCYJ20220818100802005, KQTD20210811090117032), and the Shenzhen Key Laboratory of Neuroimmunomodulation for Neurological Diseases (ZDSYS20220304163558001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Figure support was provided by Figdraw.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eshraghi, M.; Ahmadi, M.; Afshar, S.; Lorzadeh, S.; Adlimoghaddam, A.; Rezvani Jalal, N.; West, R.; Dastghaib, S.; Igder, S.; Torshizi, S.R.N.; et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol. Ther. 2022, 237, 108171. [Google Scholar] [CrossRef]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Eisele, Y.S.; Fritschi, S.K.; Staufenbiel, M.; Walker, L.C.; Jucker, M. Soluble Aβ Seeds Are Potent Inducers of Cerebral β-Amyloid Deposition. J. Neurosci. 2011, 31, 14488–14495. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Rijal Upadhaya, A.; Kosterin, I.; Kumar, S.; Von Arnim, C.A.F.; Yamaguchi, H.; Fändrich, M.; Walter, J.; Thal, D.R. Biochemical stages of amyloid-β peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain 2014, 137, 887–903. [Google Scholar] [CrossRef]
- Carreiras, M.; Mendes, E.; Perry, M.; Francisco, A.; Marco-Contelles, J. The Multifactorial Nature of Alzheimer’s Disease for Developing Potential Therapeutics. Curr. Top. Med. Chem. 2013, 13, 1745–1770. [Google Scholar] [CrossRef]
- Hirtz, D.; Thurman, D.J.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A.R.; Zalutsky, R. How common are the “common” neurologic disorders? Neurology 2007, 68, 326–337. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood–Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef]
- Lukiw, W.J. MicroRNA (miRNA) Complexity in Alzheimer’s Disease (AD). Biology 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 89. [Google Scholar] [CrossRef]
- Banik, A.; Brown, R.E.; Bamburg, J.; Lahiri, D.K.; Khurana, D.; Friedland, R.P.; Chen, W.; Ding, Y.; Mudher, A.; Padjen, A.L.; et al. Translation of Pre-Clinical Studies into Successful Clinical Trials for Alzheimer’s Disease: What are the Roadblocks and How Can They Be Overcome? J. Alzheimer’s Dis. 2015, 47, 815–843. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Borthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Bales, K.R.; Tenkova, T.; Fagan, A.M.; Parsadanian, M.; Sartorius, L.J.; Mackey, B.; Olney, J.; McKeel, D.; Wozniak, D.; et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2892–2897. [Google Scholar] [CrossRef]
- Saito, T.; Matsuba, Y.; Mihira, N.; Takano, J.; Nilsson, P.; Itohara, S.; Iwata, N.; Saido, T.C. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 2014, 17, 661–663. [Google Scholar] [CrossRef]
- Pang, K.; Jiang, R.; Zhang, W.; Yang, Z.; Li, L.L.; Shimozawa, M.; Tambaro, S.; Mayer, J.; Zhang, B.; Li, M.; et al. An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2022, 32, 157–175. [Google Scholar] [CrossRef]
- He, Z.; Zhang, W.; Chen, P.; Li, S.; Tao, M.; Yue, F.; Hong, W.; Feng, S.; Jing, N. Amyloid-β oligomers drive amyloid deposit and cascaded tau pathology of Alzheimer’s disease in aged brains of non-human primates. J. Genet. Genom. 2025. [Google Scholar] [CrossRef]
- Baerends, E.; Soud, K.; Folke, J.; Pedersen, A.K.; Henmar, S.; Konrad, L.; Lycas, M.D.; Mori, Y.; Pakkenberg, B.; Woldbye, D.P.D.; et al. Modeling the early stages of Alzheimer’s disease by administering intracerebroventricular injections of human native Aβ oligomers to rats. Acta Neuropathol. Commun. 2022, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Bravo-Alegria, J.; Moreno-Gonzalez, I.; Duran-Aniotz, C.; Gamez, N.; Edwards Iii, G.; Soto, C. Transmission of cerebral amyloid pathology by peripheral administration of misfolded Aβ aggregates. Mol. Psychiatry 2021, 26, 5690–5701. [Google Scholar] [CrossRef]
- Purro, S.A.; Farrow, M.A.; Linehan, J.; Nazari, T.; Thomas, D.X.; Chen, Z.; Mengel, D.; Saito, T.; Saido, T.; Rudge, P.; et al. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature 2018, 564, 415–419. [Google Scholar] [CrossRef]
- Trujillo-Estrada, L.; Sanchez-Mejias, E.; Sanchez-Varo, R.; Garcia-Leon, J.A.; Nuñez-Diaz, C.; Davila, J.C.; Vitorica, J.; Laferla, F.M.; Moreno-Gonzalez, I.; Gutierrez, A.; et al. Animal and Cellular Models of Alzheimer’s Disease: Progress, Promise, and Future Approaches. Neurosci. 2022, 28, 572–593. [Google Scholar] [CrossRef] [PubMed]
- Herard, A.S.; Petit, F.; Gary, C.; Guillermier, M.; Boluda, S.; Garin, C.M.; The Brainbank Neuro-CEB Neuropathology Network; Lam, S.; Dhenain, M. Induction of amyloid-β deposits from serially transmitted, histologically silent, Aβ seeds issued from human brains. Acta Neuropathol. Commun. 2020, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.A.; Kechko, O.I.; Adzhubei, A.A.; Makarov, A.A.; Mitkevich, V.A. Switching On/Off Amyloid Plaque Formation in Transgenic Animal Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 25, 72. [Google Scholar] [CrossRef]
- Xu, G.; Ran, Y.; Fromholt, S.E.; Fu, C.; Yachnis, A.T.; Golde, T.E.; Borchelt, D.R. Murine Aβ over-production produces diffuse and compact Alzheimer-type amyloid deposits. Acta Neuropathol. Commun. 2015, 3, 72. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Younkin, L.H.; Gonzales, V.; Fadale, D.J.; Slunt, H.H.; Lester, H.A.; Younkin, S.G.; Borchelt, D.R. Rodent Aβ Modulates the Solubility and Distribution of Amyloid Deposits in Transgenic Mice. J. Biol. Chem. 2007, 282, 22707–22720. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Ye, K. Advancements and challenges in mouse models of Alzheimer’s disease. Trends Mol. Med. 2024, 30, 1152–1164. [Google Scholar] [CrossRef]
- Zhong, M.Z.; Peng, T.; Duarte, M.L.; Wang, M.; Cai, D. Updates on mouse models of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 23. [Google Scholar] [CrossRef]
- Dodart, J.-C.; Meziane, H.; Mathis, C.; Bales, K.R.; Paul, S.M.; Ungerer, A. Behavioral disturbances in transgenic mice overexpressing the V717F Β-amyloid precursor protein. Behav. Neurosci. 1999, 113, 982–990. [Google Scholar] [CrossRef]
- Hartman, R.E.; Izumi, Y.; Bales, K.R.; Paul, S.M.; Wozniak, D.F.; Holtzman, D.M. Treatment with an Amyloid-β Antibody Ameliorates Plaque Load, Learning Deficits, and Hippocampal Long-Term Potentiation in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 6213–6220. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, A.; Vickers, J.C.; Adlard, P.A.; Dickson, T.C. Dystrophic neurites in TgCRND8 and Tg2576 mice mimic human pathological brain aging. Neurobiol. Aging 2009, 30, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.H.; Bondolfi, L.; Hunziker, D.; Schlecht, H.P.; Carver, K.; Maguire, E.; Abramowski, D.; Wiederhold, K.H.; Sturchler-Pierrat, C.; Jucker, M.; et al. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol. Aging 2003, 24, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.-H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Bürki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative Memory Deficits, Aβ Elevation, and Amyloid Plaques in Transgenic Mice. Science 1996, 274, 99–103. [Google Scholar] [CrossRef]
- Chishti, M.A.; Yang, D.-S.; Janus, C.; Phinney, A.L.; Horne, P.; Pearson, J.; Strome, R.; Zuker, N.; Loukides, J.; French, J.; et al. Early-onset Amyloid Deposition and Cognitive Deficits in Transgenic Mice Expressing a Double Mutant Form of Amyloid Precursor Protein 695. J. Biol. Chem. 2001, 276, 21562–21570. [Google Scholar] [CrossRef]
- Dudal, S.; Krzywkowski, P.; Paquette, J.; Morissette, C.; Lacombe, D.; Tremblay, P.; Gervais, F. Inflammation occurs early during the Aβ deposition process in TgCRND8 mice. Neurobiol. Aging 2004, 25, 861–871. [Google Scholar] [CrossRef]
- Janus, C.; Flores, A.Y.; Xu, G.; Borchelt, D.R. Behavioral abnormalities in APPSwe/PS1dE9 mouse model of AD-like pathology: Comparative analysis across multiple behavioral domains. Neurobiol. Aging 2015, 36, 2519–2532. [Google Scholar] [CrossRef]
- Maia, L.F.; Kaeser, S.A.; Reichwald, J.; Hruscha, M.; Martus, P.; Staufenbiel, M.; Jucker, M. Changes in Amyloid-β and Tau in the Cerebrospinal Fluid of Transgenic Mice Overexpressing Amyloid Precursor Protein. Sci. Transl. Med. 2013, 5, 194re2. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Kang, Z.; Pei, G.; Le, Y. Amyloid Deposition and Inflammation in APPswe/PS1dE9 Mouse Model of Alzheimers Disease. Curr. Alzheimer Res. 2009, 6, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef]
- Richard, B.C.; Kurdakova, A.; Baches, S.; Bayer, T.A.; Weggen, S.; Wirths, O. Gene Dosage Dependent Aggravation of the Neurological Phenotype in the 5XFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; Laferla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Masuda, A.; Kobayashi, Y.; Kogo, N.; Saito, T.; Saido, T.C.; Itohara, S. Cognitive deficits in single App knock-in mouse models. Neurobiol. Learn. Mem. 2016, 135, 73–82. [Google Scholar] [CrossRef]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef]
- Saito, T.; Mihira, N.; Matsuba, Y.; Sasaguri, H.; Hashimoto, S.; Narasimhan, S.; Zhang, B.; Murayama, S.; Higuchi, M.; Lee, V.M.Y.; et al. Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J. Biol. Chem. 2019, 294, 12754–12765. [Google Scholar] [CrossRef]
- Hanzel, C.E.; Pichet-Binette, A.; Pimentel, L.S.; Iulita, M.F.; Allard, S.; Ducatenzeiler, A.; Do Carmo, S.; Cuello, A.C. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Leon, W.C.; Canneva, F.; Partridge, V.; Allard, S.; Ferretti, M.T.; DeWilde, A.; Vercauteren, F.; Atifeh, R.; Ducatenzeiler, A.; Klein, W.; et al. A novel transgenic rat model with a full Alzheimer’s-like amyloid pathology displays pre-plaque intracellular amyloid-β-associated cognitive impairment. J. Alzheimers Dis. 2010, 20, 113–126. [Google Scholar] [CrossRef]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef]
- Le Merre, P.; Ahrlund-Richter, S.; Carlen, M. The mouse prefrontal cortex: Unity in diversity. Neuron 2021, 109, 1925–1944. [Google Scholar] [CrossRef]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef]
- Lutshumba, J.; Nikolajczyk, B.S.; Bachstetter, A.D. Dysregulation of Systemic Immunity in Aging and Dementia. Front. Cell. Neurosci. 2021, 15, 652111. [Google Scholar] [CrossRef]
- Ennerfelt, H.E.; Lukens, J.R. The role of innate immunity in Alzheimer’s disease. Immunol. Rev. 2020, 297, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.F.; Farberg, A.S.; Gearing, M.; Dooyema, J.; Long, P.M.; Anderson, D.C.; Davis-Turak, J.; Coppola, G.; Geschwind, D.H.; Pare, J.F.; et al. Tauopathy with paired helical filaments in an aged chimpanzee. J. Comp. Neurol. 2008, 509, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Gearing, M.; Tigges, J.; Mori, H.; Mirra, S.S. β-Amyloid (Aβ) Deposition in the Brains of Aged Orangutans. Neurobiol. Aging 1997, 18, 139–146. [Google Scholar] [CrossRef]
- Edler, M.K.; Sherwood, C.C.; Meindl, R.S.; Hopkins, W.D.; Ely, J.J.; Erwin, J.M.; Mufson, E.J.; Hof, P.R.; Raghanti, M.A. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol. Aging 2017, 59, 107–120. [Google Scholar] [CrossRef]
- Heuer, E.; Rosen, R.F.; Cintron, A.; Walker, L.C. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr. Pharm. Des. 2012, 18, 1159–1169. [Google Scholar] [CrossRef]
- Edler, M.K.; Munger, E.L.; Meindl, R.S.; Hopkins, W.D.; Ely, J.J.; Erwin, J.M.; Mufson, E.J.; Hof, P.R.; Sherwood, C.C.; Raghanti, M.A. Neuron loss associated with age but not Alzheimer’s disease pathology in the chimpanzee brain. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190619. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; He, X.-P.; Li, H.; He, R.-Q.; Hu, X.-T. Age-associated changes in amyloid-β and formaldehyde concentrations in cerebrospinal fluid of rhesus monkeys. Zool. Res. 2020, 41, 444–448. [Google Scholar] [CrossRef]
- Stonebarger, G.; Urbanski, H.; Woltjer, R.; Vaughan, K.; Ingram, D.; Schultz, P.; Calderazzo, S.; Siedeman, J.; Mattison, J.; Rosene, D.; et al. Amyloidosis increase is not attenuated by long-term calorie restriction or related to neuron density in the prefrontal cortex of extremely aged rhesus macaques. GeroScience 2020, 42, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, B.; Lu, J.; Wu, Y.; Wang, S.; Yao, Z.; Zhu, L.; Qiao, Y.; Sun, Q.; Qin, W.; et al. Brains of rhesus monkeys display Aβ deposits and glial pathology while lacking Aβ dimers and other Alzheimer’s pathologies. Aging Cell 2019, 18, e12978. [Google Scholar] [CrossRef]
- Rhesus Macaque Genome Sequencing and Analysis Consortium; Gibbs, R.A.; Rogers, J.; Katze, M.G.; Bumgarner, R.; Weinstock, G.M.; Mardis, E.R.; Remington, K.A.; Strausberg, R.L.; Venter, J.C.; et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science 2007, 316, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Wibom, M.; Pawlik, D.; Englund, E.; Hansson, O. Correlation of In Vivo [18F]Flortaucipir With Postmortem Alzheimer Disease Tau Pathology. JAMA Neurol. 2019, 76, 310–317. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Leslie, S.; Yang, S.T.; Wang, M.; Nairn, A.C. Alzheimer’s-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2019, 116, 26230–26238. [Google Scholar] [CrossRef]
- Moore, T.L.; Killiany, R.J.; Herndon, J.G.; Rosene, D.L.; Moss, M.B. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol. Aging 2006, 27, 1484–1493. [Google Scholar] [CrossRef]
- Moore, T.L.; Killiany, R.J.; Herndon, J.G.; Rosene, D.L.; Moss, M.B. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol. Aging 2003, 24, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.G.; Moss, M.B.; Rosene, D.L.; Killiany, R.J. Patterns of cognitive decline in aged rhesus monkeys. Behav. Brain Res. 1997, 87, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.S.; Shively, C.A.; Keene, C.D.; Jorgensen, M.J.; Andrews, R.N.; Register, T.C.; Montine, T.J.; Wilson, A.M.; Neth, B.J.; Mintz, A.; et al. A nonhuman primate model of early Alzheimer’s disease pathologic change: Implications for disease pathogenesis. Alzheimer’s Dement. 2019, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Beckman, D.; Ott, S.; Donis-Cox, K.; Janssen, W.G.; Bliss-Moreau, E.; Rudebeck, P.H.; Baxter, M.G.; Morrison, J.H. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc. Natl. Acad. Sci. USA 2019, 116, 26239–26246. [Google Scholar] [CrossRef]
- Beckman, D.; Chakrabarty, P.; Ott, S.; Dao, A.; Zhou, E.; Janssen, W.G.; Donis-Cox, K.; Muller, S.; Kordower, J.H.; Morrison, J.H. A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimer’s Dement. 2021, 17, 933–945. [Google Scholar] [CrossRef]
- Tu, Z.; Yan, S.; Han, B.; Li, C.; Liang, W.; Lin, Y.; Ding, Y.; Wei, H.; Wang, L.; Xu, H.; et al. Tauopathy promotes spinal cord-dependent production of toxic amyloid-beta in transgenic monkeys. Signal Transduct. Target. Ther. 2023, 8, 358. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Coomaraswamy, J.; Bolmont, T.; Kaeser, S.; Schaefer, C.; Kilger, E.; Neuenschwander, A.; Abramowski, D.; Frey, P.; Jaton, A.L.; et al. Exogenous Induction of Cerebral ß-Amyloidogenesis Is Governed by Agent and Host. Science 2006, 313, 1781–1784. [Google Scholar] [CrossRef]
- Ulm, B.S.; Borchelt, D.R.; Moore, B.D. Remodeling Alzheimer-amyloidosis models by seeding. Mol. Neurodegener. 2021, 16, 8. [Google Scholar] [CrossRef]
- Stohr, J.; Watts, J.C.; Mensinger, Z.L.; Oehler, A.; Grillo, S.K.; DeArmond, S.J.; Prusiner, S.B.; Giles, K. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. USA 2012, 109, 11025–11030. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Min, F.; Li, Z.; Huang, J.; Huang, R. A nonhuman primate model of Alzheimer’s disease generated by intracranial injection of amyloid-beta42 and thiorphan. Metab. Brain Dis. 2010, 25, 277–284. [Google Scholar] [CrossRef]
- Baker, H.F.; Ridley, R.M.; Duchen, L.W.; Crow, T.J.; Bruton, C.J. Induction of β(A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol. Neurobiol. 1994, 8, 25–39. [Google Scholar] [CrossRef]
- Parvin, F.; Larsson, J.N.K.; Jackson, W.S.; Nyström, S.; Hammarström, P. Efficient Seeding of Cerebral Vascular Aβ-Amyloidosis by Recombinant AβM1-42 Amyloid Fibrils. J. Mol. Biol. 2025, 437, 168923. [Google Scholar] [CrossRef]
- Nagao, M.; Hatae, A.; Mine, K.; Tsutsumi, S.; Omori, H.; Hirata, M.; Arimatsu, M.; Taniguchi, C.; Watanabe, T.; Kubota, K.; et al. The Effects of Ninjinyoeito on Impaired Spatial Memory and Prefrontal Cortical Synaptic Plasticity through α-Amino-3-hydroxy-5-4-isoxazole Propionic Acid Receptor Subunit in a Rat Model with Cerebral Ischemia and β-Amyloid Injection. Evid.-Based Complement. Altern. Med. 2023, 2023, 6035589. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Yamano, S.; Imagawa, N.; Igami, K.; Miyazaki, T.; Ito, H.; Watanabe, T.; Kubota, K.; Katsurabayashi, S.; Iwasaki, K. Effect of Lactobacillus paracasei A221-fermented ginseng on impaired spatial memory in a rat model with cerebral ischemia and β-amyloid injection. Tradit. Kampo Med. 2019, 6, 96–104. [Google Scholar] [CrossRef]
- Forny-Germano, L.; Silva, N.M.L.E.; Batista, A.F.; Brito-Moreira, J.; Gralle, M.; Boehnke, S.E.; Coe, B.C.; Lablans, A.; Marques, S.A.; Martinez, A.M.B.; et al. Alzheimer’s Disease-Like Pathology Induced by Amyloid-β Oligomers in Nonhuman Primates. J. Neurosci. 2014, 34, 13629–13643. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Feng, S.; Lu, C.; Zhang, T.; Tao, G.; Liu, J.; Yue, C.; Jing, N. Synthetic amyloid-β oligomers drive early pathological progression of Alzheimer’s disease in nonhuman primates. iScience 2021, 24, 103207. [Google Scholar] [CrossRef] [PubMed]
- Ridley, R.M.; Baker, H.F.; Windle, C.P.; Cummings, R.M. Very long term studies of the seeding of β-amyloidosis in primates. J. Neural Transm. 2006, 113, 1243–1251. [Google Scholar] [CrossRef]
- Brinkmalm, G.; Hong, W.; Wang, Z.; Liu, W.; O’Malley, T.T.; Sun, X.; Frosch, M.P.; Selkoe, D.; Portelius, E.; Zetterberg, H.; et al. Identification of neurotoxic cross-linked amyloid-β dimers in Alzheimer brain. Brain 2019, 142, 1441–1457. [Google Scholar] [CrossRef]
- Mc Donald, J.M.; O’Malley, T.T.; Liu, W.; Mably, A.J.; Brinkmalm, G.; Portelius, E.; Wittbold, W.M., 3rd; Frosch, M.P.; Walsh, D.M. The aqueous phase of Alzheimer’s disease brain contains assemblies built from ~4 and ~7 kDa Aβ species. Alzheimers Dement. 2015, 11, 1286–1305. [Google Scholar] [CrossRef]
- Ahmed, M.; Davis, J.; Aucoin, D.; Sato, T.; Ahuja, S.; Aimoto, S.; Elliott, J.I.; Van Nostrand, W.E.; Smith, S.O. Structural conversion of neurotoxic amyloid-β1–42 oligomers to fibrils. Nat. Struct. Mol. Biol. 2010, 17, 561–567. [Google Scholar] [CrossRef]
- Stohr, J.; Condello, C.; Watts, J.C.; Bloch, L.; Oehler, A.; Nick, M.; DeArmond, S.J.; Giles, K.; DeGrado, W.F.; Prusiner, S.B. Distinct synthetic Aβ prion strains producing different amyloid deposits in bigenic mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10329–10334. [Google Scholar] [CrossRef]
- De, S.; Wirthensohn, D.C.; Flagmeier, P.; Hughes, C.; Aprile, F.A.; Ruggeri, F.S.; Whiten, D.R.; Emin, D.; Xia, Z.; Varela, J.A.; et al. Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms. Nat. Commun. 2019, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.C.; Condello, C.; Stohr, J.; Oehler, A.; Lee, J.; DeArmond, S.J.; Lannfelt, L.; Ingelsson, M.; Giles, K.; Prusiner, S.B. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2014, 111, 10323–10328. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.H.C.; Ingelsson, M.; Watts, J.C. The existence of Aβ strains and their potential for driving phenotypic heterogeneity in Alzheimer’s disease. Acta Neuropathol. 2021, 142, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Cukalevski, R.; Yang, X.; Meisl, G.; Weininger, U.; Bernfur, K.; Frohm, B.; Knowles, T.P.J.; Linse, S. The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation. Chem. Sci. 2015, 6, 4215–4233. [Google Scholar] [CrossRef]
- Pauwels, K.; Williams, T.L.; Morris, K.L.; Jonckheere, W.; Vandersteen, A.; Kelly, G.; Schymkowitz, J.; Rousseau, F.; Pastore, A.; Serpell, L.C.; et al. Structural basis for increased toxicity of pathological Aβ42:Aβ40 ratios in Alzheimer disease. J. Biol. Chem. 2012, 287, 5650–5660. [Google Scholar] [CrossRef]
- Meng, F.; Kim, J.Y.; Louis, J.M.; Chung, H.S. Single-Molecule Characterization of Heterogeneous Oligomer Formation during Co-Aggregation of 40- and 42-Residue Amyloid-β. J. Am. Chem. Soc. 2024, 146, 24426–24439. [Google Scholar] [CrossRef]
- Chang, H.W.; Ma, H.I.; Wu, Y.S.; Lee, M.C.; Chung-Yueh Yuan, E.; Huang, S.J.; Cheng, Y.S.; Wu, M.H.; Tu, L.H.; Chan, J.C.C. Site specific NMR characterization of abeta-40 oligomers cross seeded by abeta-42 oligomers. Chem. Sci. 2022, 13, 8526–8535. [Google Scholar] [CrossRef]
- Jan, A.; Gokce, O.; Luthi-Carter, R.; Lashuel, H.A. The ratio of monomeric to aggregated forms of Aβ40 and Aβ42 is an important determinant of amyloid-β aggregation, fibrillogenesis, and toxicity. J. Biol. Chem. 2008, 283, 28176–28189. [Google Scholar] [CrossRef]
- Hasegawa, K.; Yamaguchi, I.; Omata, S.; Gejyo, F.; Naiki, H. Interaction between Aβ(1-42) and Aβ(1-40) in Alzheimer’s β-amyloid fibril formation in vitro. Biochemistry 1999, 38, 15514–15521. [Google Scholar] [CrossRef]
- Wang, L.; Park, S.; Choi, J.H.; Lee, C.Y.; Eom, K.; Kwon, T. Molecular insight into cross-interaction between amyloid β isoforms and its effect on aggregation pathways. J. Biomol. Struct. Dyn. 2025. [Google Scholar] [CrossRef]
- Shen, Y.X.; Wei, W.; Xu, S.Y. Protective effects of melatonin on cortico-hippocampal neurotoxicity induced by amyloid beta-peptide 25-35. Acta Pharmacol. Sin. 2002, 23, 71–76. [Google Scholar]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; De Simone, A.; Andrisano, V. Amyloid β-peptide 25–35 self-assembly and its inhibition: A model undecapeptide system to gain atomistic and secondary structure details of the Alzheimer’s disease process and treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Zheng, Q.; Song, Y.; Huang, W.; Yang, L.; Xiong, Y.; Cai, Z.; Chen, Y.; Yuan, J. Kai-xin-san improves cognitive impairment in D-gal and Aβ25-35 induced ad rats by regulating gut microbiota and reducing neuronal damage. J. Ethnopharmacol. 2024, 329, 118161. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Russo, R.; Avagliano, C.; Cristiano, C.; Meli, R.; Calignano, A. Palmitoylethanolamide protects against the amyloid-β25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 2012, 37, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Konishi, M.; Higashi, Y.; Saito, M.; Akizawa, T. Five-mer peptides prevent short-term spatial memory deficits in Aβ25-35-induced Alzheimer’s model mouse by suppressing Aβ25-35 aggregation and resolving its aggregate form. Alzheimer’s Res. Ther. 2023, 15, 83. [Google Scholar] [CrossRef]
- Stine, W.B., Jr.; Dahlgren, K.N.; Krafft, G.A.; LaDu, M.J. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J. Biol. Chem. 2003, 278, 11612–11622. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y.; Feng, Y.; Liu, H.; Shen, X.; Chen, K.; Ma, J.; Jiang, H. Inhibitor discovery targeting the intermediate structure of β-amyloid peptide on the conformational transition pathway: Implications in the aggregation mechanism of β-amyloid peptide. Biochemistry 2006, 45, 10963–10972. [Google Scholar] [CrossRef]
- Schrempel, S.; Kottwitz, A.K.; Piechotta, A.; Gnoth, K.; Büschgens, L.; Hartlage-Rübsamen, M.; Morawski, M.; Schenk, M.; Kleinschmidt, M.; Serrano, G.E.; et al. Identification of isoAsp7-Aβ as a major Aβ variant in Alzheimer’s disease, dementia with Lewy bodies and vascular dementia. Acta Neuropathol. 2024, 148, 78. [Google Scholar] [CrossRef]
- Kozin, S.A.; Mitkevich, V.A.; Makarov, A.A. Amyloid-β containing isoaspartate 7 as potential biomarker and drug target in Alzheimer’s disease. Mendeleev Commun. 2016, 26, 269–275. [Google Scholar] [CrossRef]
- Kozin, S.A.; Cheglakov, I.B.; Ovsepyan, A.A.; Telegin, G.B.; Tsvetkov, P.O.; Lisitsa, A.V.; Makarov, A.A. Peripherally Applied Synthetic Peptide isoAsp7-Aβ(1-42) Triggers Cerebral β-Amyloidosis. Neurotox. Res. 2013, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, G.; Eisele, Y.S.; Langer, F.; Kaeser, S.A.; Novotny, R.; Nagarathinam, A.; Åslund, A.; Hammarström, P.; Nilsson, K.P.R.; Jucker, M. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 2013, 14, 1017–1022. [Google Scholar] [CrossRef]
- Eisele, Y.S.; Bolmont, T.; Heikenwalder, M.; Langer, F.; Jacobson, L.H.; Yan, Z.X.; Roth, K.; Aguzzi, A.; Staufenbiel, M.; Walker, L.C.; et al. Induction of cerebral β-amyloidosis: Intracerebral versus systemic Aβ inoculation. Proc. Natl. Acad. Sci. USA 2009, 106, 12926–12931. [Google Scholar] [CrossRef] [PubMed]
- Eisele, Y.S.; Obermuller, U.; Heilbronner, G.; Baumann, F.; Kaeser, S.A.; Wolburg, H.; Walker, L.C.; Staufenbiel, M.; Heikenwalder, M.; Jucker, M. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 2010, 330, 980–982. [Google Scholar] [CrossRef]
- Eisele, Y.S.; Fritschi, S.K.; Hamaguchi, T.; Obermuller, U.; Fuger, P.; Skodras, A.; Schafer, C.; Odenthal, J.; Heikenwalder, M.; Staufenbiel, M.; et al. Multiple Factors Contribute to the Peripheral Induction of Cerebral β-Amyloidosis. J. Neurosci. 2014, 34, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Katzmarski, N.; Ziegler-Waldkirch, S.; Scheffler, N.; Witt, C.; Abou-Ajram, C.; Nuscher, B.; Prinz, M.; Haass, C.; Meyer-Luehmann, M. Aβ oligomers trigger and accelerate Aβ seeding. Brain Pathol. 2020, 30, 36–45. [Google Scholar] [CrossRef]
- Roos, T.T.; Garcia, M.G.; Martinsson, I.; Mabrouk, R.; Israelsson, B.; Deierborg, T.; Kobro-Flatmoen, A.; Tanila, H.; Gouras, G.K. Neuronal spreading and plaque induction of intracellular Aβ and its disruption of Aβ homeostasis. Acta Neuropathol. 2021, 142, 669–687. [Google Scholar] [CrossRef]
- Aires, V.; Ziegler-Waldkirch, S.; Friesen, M.; Reichardt, W.; Erny, D.; Loreth, D.; Harborne, A.; Kretz, O.; Von Elverfeldt, D.; Meyer-Luehmann, M. Seed-induced Aβ deposits in the corpus callosum disrupt white matter integrity in a mouse model of Alzheimer’s disease. Front. Cell. Neurosci. 2022, 16, 862918. [Google Scholar] [CrossRef]
- Fritschi, S.K.; Cintron, A.; Ye, L.; Mahler, J.; Buhler, A.; Baumann, F.; Neumann, M.; Nilsson, K.P.; Hammarstrom, P.; Walker, L.C.; et al. Aβ seeds resist inactivation by formaldehyde. Acta Neuropathol. 2014, 128, 477–484. [Google Scholar] [CrossRef]
- Ye, L.; Rasmussen, J.; Kaeser, S.A.; Marzesco, A.M.; Obermuller, U.; Mahler, J.; Schelle, J.; Odenthal, J.; Kruger, C.; Fritschi, S.K.; et al. Aβ seeding potency peaks in the early stages of cerebral β-amyloidosis. EMBO Rep. 2017, 18, 1536–1544. [Google Scholar] [CrossRef]
- Ruiz-Riquelme, A.; Lau, H.H.C.; Stuart, E.; Goczi, A.N.; Wang, Z.; Schmitt-Ulms, G.; Watts, J.C. Prion-like propagation of β-amyloid aggregates in the absence of APP overexpression. Acta Neuropathol. Commun. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Burwinkel, M.; Lutzenberger, M.; Heppner, F.L.; Schulz-Schaeffer, W.; Baier, M. Intravenous injection of beta-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol. Commun. 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Fritschi, S.K.; Schelle, J.; Obermuller, U.; Degenhardt, K.; Kaeser, S.A.; Eisele, Y.S.; Walker, L.C.; Baumann, F.; Staufenbiel, M.; et al. Persistence of Aβ seeds in APP null mouse brain. Nat. Neurosci. 2015, 18, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Eisele, Y.S.; Varvel, N.H.; Lamb, B.T.; Walker, L.C.; Jucker, M. The presence of Aβ seeds, and not age per se, is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol. 2012, 123, 31–37. [Google Scholar] [CrossRef]
- Walker, L.C.; Callahan, M.J.; Bian, F.; Durham, R.A.; Roher, A.E.; Lipinski, W.J. Exogenous induction of cerebral β-amyloidosis in βAPP-transgenic mice. Peptides 2002, 23, 1241–1247. [Google Scholar] [CrossRef]
- Watts, J.C.; Giles, K.; Grillo, S.K.; Lemus, A.; DeArmond, S.J.; Prusiner, S.B. Bioluminescence imaging of Aβ deposition in bigenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2011, 108, 2528–2533. [Google Scholar] [CrossRef]
- Herzig, M.C.; Winkler, D.T.; Burgermeister, P.; Pfeifer, M.; Kohler, E.; Schmidt, S.D.; Danner, S.; Abramowski, D.; Stürchler-Pierrat, C.; Bürki, K.; et al. Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci. 2004, 7, 954–960. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Neurodegeneration: Amyloid-β pathology induced in humans. Nature 2015, 525, 193–194. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Walker, L.C.; Jucker, M. Seeds of Dementia. Sci. Am. 2013, 308, 52–57. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.; Thal, D.R.; Van Nostrand, W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2011, 37, 75–93. [Google Scholar] [CrossRef]
- Kawarabayashi, T.; Younkin, L.H.; Saido, T.C.; Shoji, M.; Ashe, K.H.; Younkin, S.G. Age-Dependent Changes in Brain, CSF, and Plasma Amyloid β Protein in the Tg2576 Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2001, 21, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kokjohn, T.A.; Roher, A.E. Amyloid precursor protein transgenic mouse models and Alzheimer’s disease: Understanding the paradigms, limitations, and contributions. Alzheimer’s Dement. 2009, 5, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.; Oltersdorf, T.; Haass, C.; McConlogue, L.; Hung, A.Y.; Seubert, P.; Vigo-Pelfrey, C.; Lieberburg, I.; Selkoe, D.J. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 1992, 360, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Tanzi, R.E. Presenilins and Alzheimer’s disease. Curr. Opin. Neurobiol. 1997, 7, 683–688. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef]
- Andersson, E.; Schultz, N.; Saito, T.; Saido, T.C.; Blennow, K.; Gouras, G.K.; Zetterberg, H.; Hansson, O. Cerebral Aβ deposition precedes reduced cerebrospinal fluid and serum Aβ42/Aβ40 ratios in the AppNL−F/NL−F knock-in mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2023, 15, 64. [Google Scholar] [CrossRef]
- Garai, K.; Verghese, P.B.; Baban, B.; Holtzman, D.M.; Frieden, C. The binding of apolipoprotein E to oligomers and fibrils of amyloid-β alters the kinetics of amyloid aggregation. Biochemistry 2014, 53, 6323–6331. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhao, N.; Fu, Y.; Wang, N.; Linares, C.; Tsai, C.W.; Bu, G. ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 2017, 96, 1024–1032.e1023. [Google Scholar] [CrossRef]
- Milani, R.; Mazzeo, L.A.; Vismara, D.; Salemi, I.; Dainese, E.; Maderna, E.; Pellencin, E.; Catania, M.; Campanella, N.; Di Fede, G.; et al. Spontaneous intracerebral haemorrhage associated with early-onset cerebral amyloid angiopathy and Alzheimer’s disease neuropathological changes five decades after cadaveric dura mater graft. Acta Neuropathol. Commun. 2023, 11, 30. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Barria, M.A.; Peden, A.H.; Yull, H.M.; Kirkpatrick, J.; Adlard, P.; Ironside, J.W.; Head, M.W. UK Iatrogenic Creutzfeldt–Jakob disease: Investigating human prion transmission across genotypic barriers using human tissue-based and molecular approaches. Acta Neuropathol. 2017, 133, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, J.D.F.; Nicoll, A.J.; Kenny, J.; Launchbury, F.; Linehan, J.; Richard-Loendt, A.; Walker, A.S.; et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 2015, 525, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Farmer, S.F.; Hyare, H.; Jaunmuktane, Z.; Mead, S.; Ryan, N.S.; Schott, J.M.; Werring, D.J.; Rudge, P.; Collinge, J. Iatrogenic Alzheimer’s disease in recipients of cadaveric pituitary-derived growth hormone. Nat. Med. 2024, 30, 394–402. [Google Scholar] [CrossRef]
- Li, X.; Ospitalieri, S.; Robberechts, T.; Hofmann, L.; Schmid, C.; Rijal Upadhaya, A.; Koper, M.J.; Von Arnim, C.A.F.; Kumar, S.; Willem, M.; et al. Seeding, maturation and propagation of amyloid β-peptide aggregates in Alzheimer’s disease. Brain 2022, 145, 3558–3570. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Mahler, J.; Beschorner, N.; Kaeser, S.A.; Häsler, L.M.; Baumann, F.; Nyström, S.; Portelius, E.; Blennow, K.; Lashley, T.; et al. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 13018–13023. [Google Scholar] [CrossRef]
- Walker, L.C.; Levine, H. Corruption and Spread of Pathogenic Proteins in Neurodegenerative Diseases. J. Biol. Chem. 2012, 287, 33109–33115. [Google Scholar] [CrossRef]
- Kane, M.D.; Lipinski, W.J.; Callahan, M.J.; Bian, F.; Durham, R.A.; Schwarz, R.D.; Roher, A.E.; Walker, L.C. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 2000, 20, 3606–3611. [Google Scholar] [CrossRef]
- Lam, S.; Hérard, A.-S.; Boluda, S.; Petit, F.; Eddarkaoui, S.; Cambon, K.; Letournel, F.; Martin-Négrier, M.-L.; Faisant, M.; Godfraind, C.; et al. Pathological changes induced by Alzheimer’s brain inoculation in amyloid-beta plaque-bearing mice. Acta Neuropathol. Commun. 2022, 10, 112. [Google Scholar] [CrossRef]
- Lam, S.; Petit, F.; Herard, A.S.; Boluda, S.; Eddarkaoui, S.; Guillermier, M.; The Brain Bank Neuro–Cognitive and Experimental Biology Neuropathology Network; Buee, L.; Duyckaerts, C.; Haik, S.; et al. Transmission of amyloid-beta and tau pathologies is associated with cognitive impairments in a primate. Acta Neuropathol. Commun. 2021, 9, 165. [Google Scholar] [CrossRef]
- Gary, C.; Lam, S.; Hérard, A.-S.; Koch, J.E.; Petit, F.; Gipchtein, P.; Sawiak, S.J.; Caillierez, R.; Eddarkaoui, S.; Colin, M.; et al. Encephalopathy induced by Alzheimer brain inoculation in a non-human primate. Acta Neuropathol. Commun. 2019, 7, 126. [Google Scholar] [CrossRef]
- Fritschi, S.K.; Langer, F.; Kaeser, S.A.; Maia, L.F.; Portelius, E.; Pinotsi, D.; Kaminski, C.F.; Winkler, D.T.; Maetzler, W.; Keyvani, K.; et al. Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 2014, 137, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wang, Z.; Liu, W.; O’Malley, T.T.; Jin, M.; Willem, M.; Haass, C.; Frosch, M.P.; Walsh, D.M. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer’s disease brain. Acta Neuropathol. 2018, 136, 19–40. [Google Scholar] [CrossRef]
- Qiang, W.; Yau, W.-M.; Lu, J.-X.; Collinge, J.; Tycko, R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Esparza, T.J.; Leduc, R.D.; Fellers, R.T.; Thomas, P.M.; Cairns, N.J.; Kelleher, N.L.; Bateman, R.J.; Brody, D.L. Diversity of Amyloid-beta Proteoforms in the Alzheimer’s Disease Brain. Sci. Rep. 2017, 7, 9520. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-X.; Qiang, W.; Yau, W.-M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular Structure of β-Amyloid Fibrils in Alzheimer’s Disease Brain Tissue. Cell 2013, 154, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Yagi-Utsumi, M.; Kato, K. Conformational Variability of Amyloid-β and the Morphological Diversity of Its Aggregates. Molecules 2022, 27, 4787. [Google Scholar] [CrossRef]
- Niu, Z.; Gui, X.; Feng, S.; Reif, B. Aggregation Mechanisms and Molecular Structures of Amyloid-β in Alzheimer’s Disease. Chem. A Eur. J. 2024, 30, e202400277. [Google Scholar] [CrossRef]
- Askenazi, M.; Kavanagh, T.; Pires, G.; Ueberheide, B.; Wisniewski, T.; Drummond, E. Compilation of reported protein changes in the brain in Alzheimer’s disease. Nat. Commun. 2023, 14, 4466. [Google Scholar] [CrossRef]
- Corbett, G.T.; Wang, Z.; Hong, W.; Colom-Cadena, M.; Rose, J.; Liao, M.; Asfaw, A.; Hall, T.C.; Ding, L.; Desousa, A.; et al. PrP is a central player in toxicity mediated by soluble aggregates of neurodegeneration-causing proteins. Acta Neuropathol. 2020, 139, 503–526. [Google Scholar] [CrossRef]
- Morales, R.; Duran-Aniotz, C.; Castilla, J.; Estrada, L.D.; Soto, C. De novo induction of amyloid-β deposition in vivo. Mol. Psychiatry 2012, 17, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.F.; Fritz, J.J.; Dooyema, J.; Cintron, A.F.; Hamaguchi, T.; Lah, J.J.; LeVine, H., 3rd; Jucker, M.; Walker, L.C. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J. Neurochem. 2012, 120, 660–666. [Google Scholar] [CrossRef]
- Baker, H.F.; Ridley, R.M.; Duchen, L.W.; Crow, T.J.; Bruton, C.J. Evidence for the experimental transmission of cerebral beta-amyloidosis to primates. Int. J. Exp. Pathol. 1993, 74, 441–454. [Google Scholar] [PubMed]
- Maclean, C.J.; Baker, H.F.; Ridley, R.M.; Mori, H. Naturally occurring and experimentally induced β-amyloid deposits in the brains of marmosets (Callithrix jacchus). J. Neural Transm. 2000, 107, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, M.E.; Paulsson, J.F.; Schultz, S.W.; Ingelsson, M.; Westermark, P.; Westermark, G.T. In vivo seeding and cross-seeding of localized amyloidosis: A molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol. 2015, 185, 834–846. [Google Scholar] [CrossRef]
- Mandal, P.K.; Pettegrew, J.W.; Masliah, E.; Hamilton, R.L.; Mandal, R. Interaction between Aβ peptide and α synuclein: Molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem. Res. 2006, 31, 1153–1162. [Google Scholar] [CrossRef]
- Chia, S.; Flagmeier, P.; Habchi, J.; Lattanzi, V.; Linse, S.; Dobson, C.M.; Knowles, T.P.J.; Vendruscolo, M. Monomeric and fibrillar α-synuclein exert opposite effects on the catalytic cycle that promotes the proliferation of Aβ42 aggregates. Proc. Natl. Acad. Sci. USA 2017, 114, 8005–8010. [Google Scholar] [CrossRef]
- Iljina, M.; Dear, A.J.; Garcia, G.A.; De, S.; Tosatto, L.; Flagmeier, P.; Whiten, D.R.; Michaels, T.C.T.; Frenkel, D.; Dobson, C.M.; et al. Quantifying Co-Oligomer Formation by α-Synuclein. ACS Nano 2018, 12, 10855–10866. [Google Scholar] [CrossRef]
- Ziegler-Waldkirch, S.; D′Errico, P.; Sauer, J.F.; Erny, D.; Savanthrapadian, S.; Loreth, D.; Katzmarski, N.; Blank, T.; Bartos, M.; Prinz, M.; et al. Seed-induced Aβ deposition is modulated by microglia under environmental enrichment in a mouse model of Alzheimer’s disease. EMBO J. 2018, 37, 167–182. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fa, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2019, 68, 415. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Xiao, L.; Huang, Y.; Yang, Z.; Wu, Z.; Tu, Z.; Qin, W.; Chen, H.; Wu, D.; et al. Effects of ultrasound on functional properties, structure and glycation properties of proteins: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2471–2481. [Google Scholar] [CrossRef]
- Hong, W.; Liu, W.; Desousa, A.O.; Young-Pearse, T.; Walsh, D.M. Methods for the isolation and analysis of Aβ from postmortem brain. Front. Neurosci. 2023, 17, 1108715. [Google Scholar] [CrossRef] [PubMed]
- Esparza, T.J.; Wildburger, N.C.; Jiang, H.; Gangolli, M.; Cairns, N.J.; Bateman, R.J.; Brody, D.L. Soluble Amyloid-beta Aggregates from Human Alzheimer’s Disease Brains. Sci. Rep. 2016, 6, 38187. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.T.; Silvers, R.; Ni, Q.Z.; Can, T.V.; Sergeyev, I.; Rosay, M.; Donovan, K.J.; Michael, B.; Wall, J.; Linse, S.; et al. Atomic Resolution Structure of Monomorphic Aβ42 Amyloid Fibrils. J. Am. Chem. Soc. 2016, 138, 9663–9674. [Google Scholar] [CrossRef] [PubMed]
- Walti, M.A.; Ravotti, F.; Arai, H.; Glabe, C.G.; Wall, J.S.; Bockmann, A.; Guntert, P.; Meier, B.H.; Riek, R. Atomic-resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc. Natl. Acad. Sci. USA 2016, 113, E4976–E4984. [Google Scholar] [CrossRef]
- Gremer, L.; Scholzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1–42) by cryo-electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef]
- Xiao, Y.; Matsuda, I.; Inoue, M.; Sasahara, T.; Hoshi, M.; Ishii, Y. NMR-based site-resolved profiling of β-amyloid misfolding reveals structural transitions from pathologically relevant spherical oligomer to fibril. J. Biol. Chem. 2020, 295, 458–467. [Google Scholar] [CrossRef]
- Yu, L.; Edalji, R.; Harlan, J.E.; Holzman, T.F.; Lopez, A.P.; Labkovsky, B.; Hillen, H.; Barghorn, S.; Ebert, U.; Richardson, P.L.; et al. Structural characterization of a soluble amyloid β-peptide oligomer. Biochemistry 2009, 48, 1870–1877. [Google Scholar] [CrossRef]
- Paravastu, A.K.; Qahwash, I.; Leapman, R.D.; Meredith, S.C.; Tycko, R. Seeded growth of β-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc. Natl. Acad. Sci. USA 2009, 106, 7443–7448. [Google Scholar] [CrossRef]
- Ghosh, U.; Thurber, K.R.; Yau, W.M.; Tycko, R. Molecular structure of a prevalent amyloid-β fibril polymorph from Alzheimer’s disease brain tissue. Proc. Natl. Acad. Sci. USA 2021, 118, e2023089118. [Google Scholar] [CrossRef] [PubMed]
- Kollmer, M.; Close, W.; Funk, L.; Rasmussen, J.; Bsoul, A.; Schierhorn, A.; Schmidt, M.; Sigurdson, C.J.; Jucker, M.; Fandrich, M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 2019, 10, 4760. [Google Scholar] [CrossRef]
- Yang, Y.; Arseni, D.; Zhang, W.; Huang, M.; Lovestam, S.; Schweighauser, M.; Kotecha, A.; Murzin, A.G.; Peak-Chew, S.Y.; Macdonald, J.; et al. Cryo-EM structures of amyloid-β 42 filaments from human brains. Science 2022, 375, 167–172. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Murzin, A.G.; Schweighauser, M.; Huang, M.; Lovestam, S.; Peak-Chew, S.Y.; Saito, T.; Saido, T.C.; Macdonald, J.; et al. Cryo-EM structures of amyloid-β filaments with the Arctic mutation (E22G) from human and mouse brains. Acta Neuropathol. 2023, 145, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Murzin, A.G.; Peak-Chew, S.; Franco, C.; Garringer, H.J.; Newell, K.L.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of Aβ40 filaments from the leptomeninges of individuals with Alzheimer’s disease and cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2023, 11, 191. [Google Scholar] [CrossRef]
- Fernandez, A.; Hoq, M.R.; Hallinan, G.I.; Li, D.; Bharath, S.R.; Vago, F.S.; Zhang, X.; Ozcan, K.A.; Newell, K.L.; Garringer, H.J.; et al. Cryo-EM structures of amyloid-β and tau filaments in Down syndrome. Nat. Struct. Mol. Biol. 2024, 31, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.C.; Roy, J.; Chau, S.C.; Wong, K.H.; Shi, L.; Poon, C.H.; Wang, Y.; Strekalova, T.; Aquili, L.; Chang, R.C.; et al. Distribution and inter-regional relationship of amyloid-beta plaque deposition in a 5xFAD mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 964336. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A β-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Yao, J.; Li, Z.; Zhou, Z.; Bao, A.; Wang, Z.; Wei, H.; He, H. Distinct regional vulnerability to Aβ and iron accumulation in post mortem AD brains. Alzheimer’s Dement. 2024, 20, 6984–6997. [Google Scholar] [CrossRef]
- Grothe, M.J.; Sepulcre, J.; Gonzalez-Escamilla, G.; Jelistratova, I.; Scholl, M.; Hansson, O.; Teipel, S.J.; Alzheimer’s Disease Neuroimaging Initiative. Molecular properties underlying regional vulnerability to Alzheimer’s disease pathology. Brain 2018, 141, 2755–2771. [Google Scholar] [CrossRef] [PubMed]
- Célestine, M.; Jacquier-Sarlin, M.; Borel, E.; Petit, F.; Perot, J.-B.; Hérard, A.-S.; Bousset, L.; Buisson, A.; Dhenain, M. Long term worsening of amyloid pathology, cerebral function, and cognition after a single inoculation of beta-amyloid seeds with Osaka mutation. Acta Neuropathol. Commun. 2023, 11, 66. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Kim, J.H.; Hasegawa, A.; Goto, R.; Sakai, K.; Ono, K.; Itoh, Y.; Yamada, M. Exogenous Aβ seeds induce Aβ depositions in the blood vessels rather than the brain parenchyma, independently of Aβ strain-specific information. Acta Neuropathol. Commun. 2021, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Riquelme, A.; Mao, A.; Barghash, M.M.; Lau, H.H.C.; Stuart, E.; Kovacs, G.G.; Nilsson, K.P.R.; Fraser, P.E.; Schmitt-Ulms, G.; Watts, J.C. Aβ43 aggregates exhibit enhanced prion-like seeding activity in mice. Acta Neuropathol. Commun. 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Gotz, J.; Bodea, L.G.; Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 583–598. [Google Scholar] [CrossRef]
- Stonebarger, G.A.; Bimonte-Nelson, H.A.; Urbanski, H.F. The Rhesus Macaque as a Translational Model for Neurodegeneration and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 734173. [Google Scholar] [CrossRef]
- Purro, S.A.; Farmer, M.; Quarterman, E.; Ravey, J.; Thomas, D.X.; Noble, E.; Turnbull, C.; Linehan, J.; Nazari, T.; Brandner, S.; et al. Induction and characterisation of Aβ and tau pathology in AppNL-F/NL-F mice following inoculation with Alzheimer’s disease brain homogenate. BioRxiv 2024. preprint. [Google Scholar]
- Noda-Saita, K.; Terai, K.; Iwai, A.; Tsukamoto, M.; Shitaka, Y.; Kawabata, S.; Okada, M.; Yamaguchi, T. Exclusive association and simultaneous appearance of congophilic plaques and AT8-positive dystrophic neurites in Tg2576 mice suggest a mechanism of senile plaque formation and progression of neuritic dystrophy in Alzheimer’s disease. Acta Neuropathol. 2004, 108, 435–442. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).