Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Model of PGR
2.2. DHA Supplementation
2.3. Serum Fatty Acids
2.4. Lung Mechanics
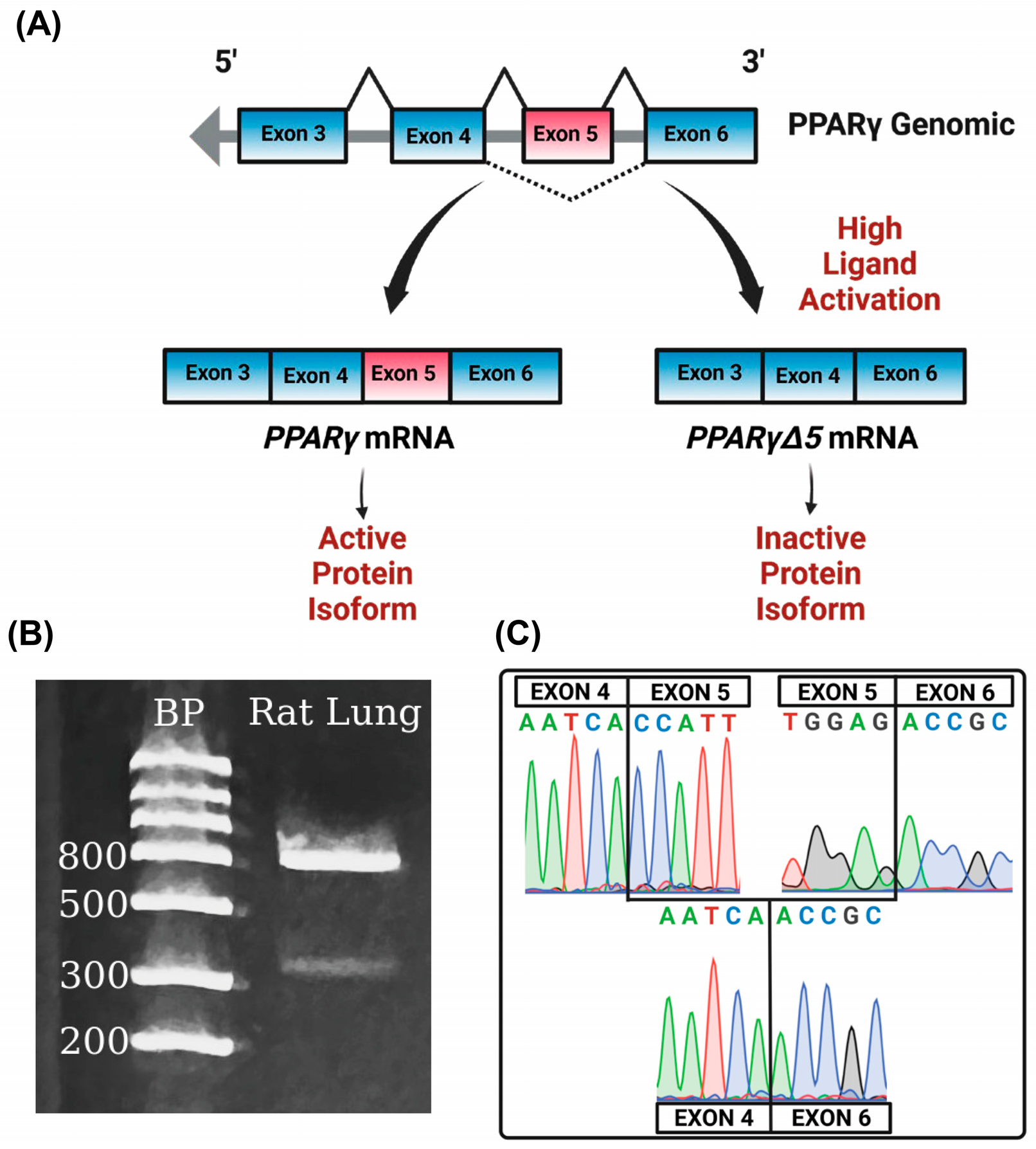
2.5. Identification of PPARγΔ5 in the Lung
2.6. mRNA Transcript Levels
2.7. Protein Abundance
2.8. Statistics
3. Results
3.1. Rat Model of PGR and DHA Supplementation
3.2. Serum Fatty Acids
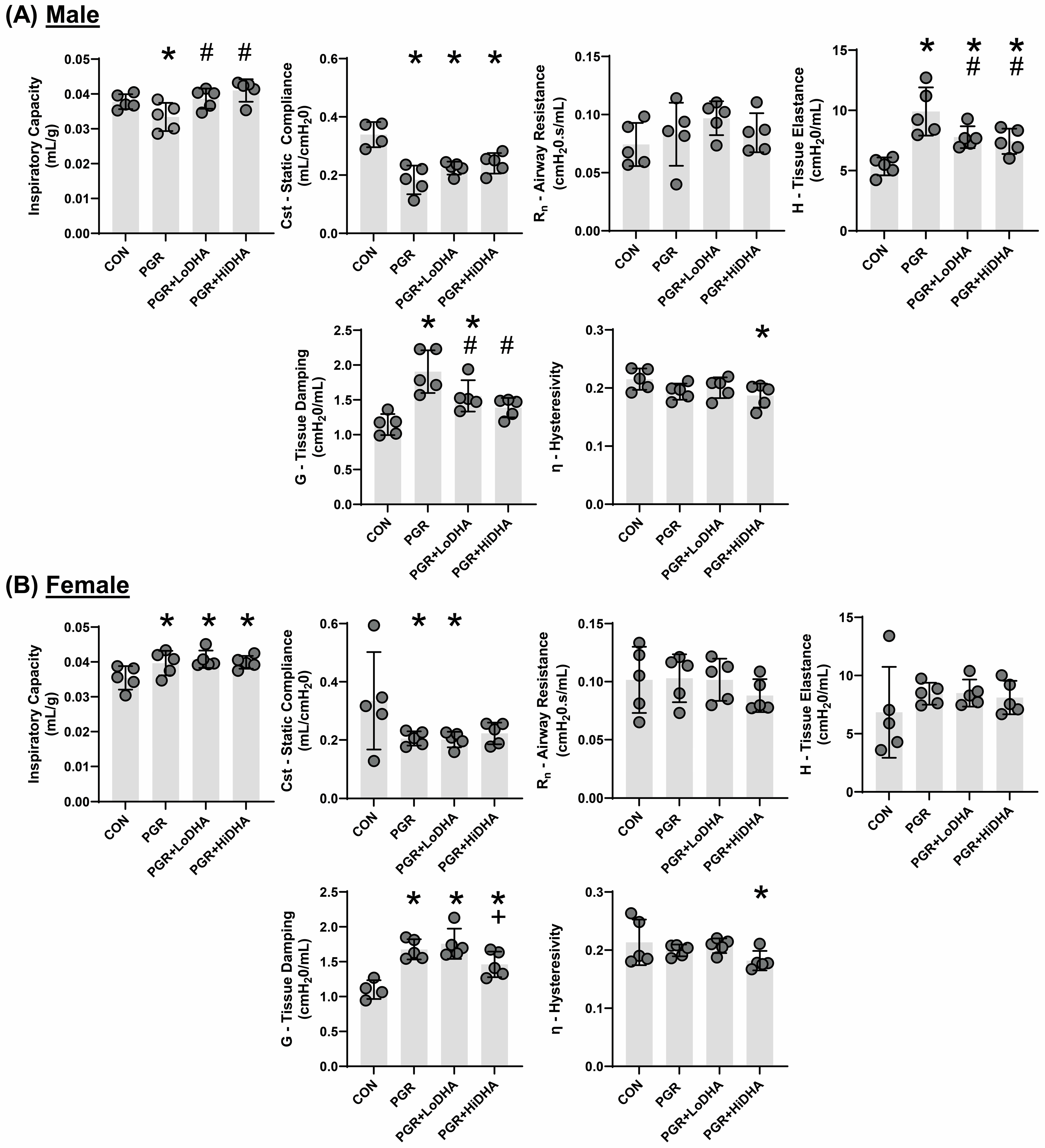
3.3. Lung Mechanics
3.4. PPARγΔ5 in the Lung
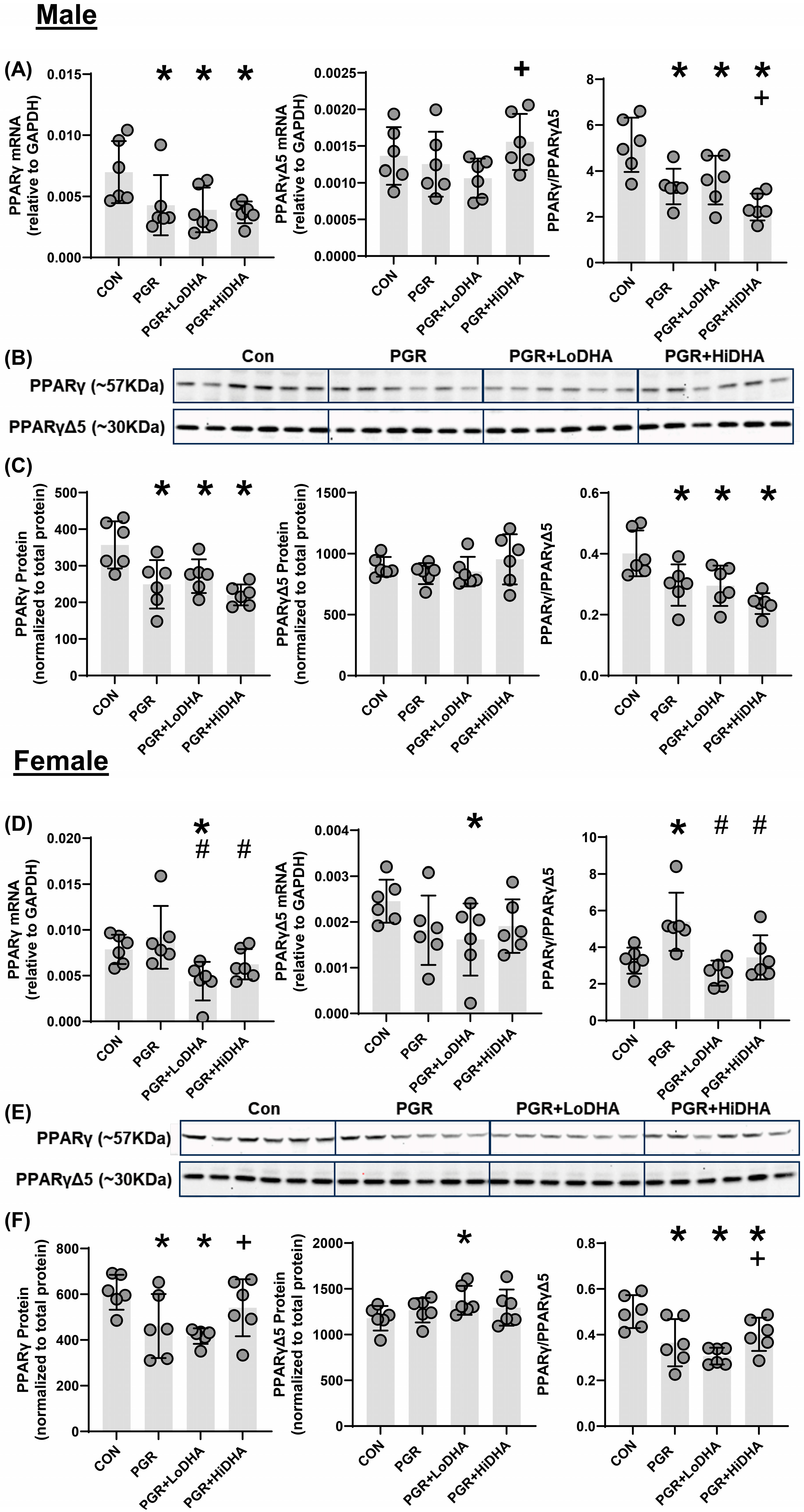
3.5. PPARγ mRNA and Protein Quantification
3.6. mRNA of Downstream PPARγ Target Gene Plin2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD | Bronchopulmonary Dysplasia |
| DHA | Docosahexaenoic Acid |
| PPARγ | Peroxisome Proliferator Activated Receptor gamma |
| PGR | Postnatal Growth Restriction |
| ARA | Arachidonic Acid |
| PLIN2 | Perilipin 2 |
References
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopmental Outcomes of Preterm Infants Fed High-Dose Docosahexaenoic Acid. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Joss-Moore, L.A.; Wang, Y.; Baack, M.L.; Yao, J.; Norris, A.W.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Albertine, K.H.; Lane, R.H. IUGR decreases PPARγ and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum. Dev. 2010, 86, 785–791. [Google Scholar] [CrossRef]
- Rogers, L.K.; Valentine, C.J.; Pennell, M.; Velten, M.; Britt, R.D.; Dingess, K.; Zhao, X.; Welty, S.E.; Tipple, T.E. Maternal docosahexaenoic acid supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. J. Nutr. 2011, 141, 214–222. [Google Scholar] [CrossRef]
- Ali, M.; Heyob, K.M.; Velten, M.; Tipple, T.E.; Rogers, L.K. DHA suppresses chronic apoptosis in the lung caused by perinatal inflammation. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L441–L448. [Google Scholar] [CrossRef]
- Velten, M.; Britt, R.D.; Heyob, K.M.; Tipple, T.E.; Rogers, L.K. Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J. Nutr. 2014, 144, 258–266. [Google Scholar] [CrossRef]
- Collins, C.T.; Makrides, M.; McPhee, A.J.; Sullivan, T.R.; Davis, P.G.; Thio, M.; Simmer, K.; Rajadurai, V.S.; Travadi, J.; Berry, M.J.; et al. Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N. Engl. J. Med. 2017, 376, 1245–1255. [Google Scholar] [CrossRef]
- Marc, I.; Piedboeuf, B.; Lacaze-Masmonteil, T.; Fraser, W.; Mâsse, B.; Mohamed, I.; Qureshi, M.; Afifi, J.; Lemyre, B.; Caouette, G.; et al. Effect of Maternal Docosahexaenoic Acid Supplementation on Bronchopulmonary Dysplasia-Free Survival in Breastfed Preterm Infants: A Randomized Clinical Trial. JAMA 2020, 324, 157–167. [Google Scholar] [CrossRef]
- Simon, D.M.; Arikan, M.C.; Srisuma, S.; Bhattacharya, S.; Tsai, L.W.; Ingenito, E.P.; Gonzalez, F.; Shapiro, S.D.; Mariani, T.J. Epithelial cell PPARγ contributes to normal lung maturation. FASEB J. 2006, 20, 1507–1509. [Google Scholar] [CrossRef]
- Cerny, L.; Torday, J.S.; Rehan, V.K. Prevention and treatment of bronchopulmonary dysplasia: Contemporary status and future outlook. Lung 2008, 186, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Gien, J.; Tseng, N.; Seedorf, G.; Roe, G.; Abman, S.H. Abman Peroxisome proliferator activated receptor-gamma-Rho-kinase interactions contribute to vascular remodeling after chronic intrauterine pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L299–L308. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Woeller, C.F.; Thatcher, T.H.; Ramon, S.; Phipps, R.P.; Sime, P.J. Sime Emerging PPARgamma-Independent Role of PPARgamma Ligands in Lung Diseases. PPAR Res. 2012, 2012, 705352. [Google Scholar] [CrossRef]
- Hagood, J.S. Beyond the genome: Epigenetic mechanisms in lung remodeling. Physiology 2014, 29, 177–185. [Google Scholar] [CrossRef]
- Morales, E.; Sakurai, R.; Husain, S.; Paek, D.; Gong, M.; Ibe, B.; Li, Y.; Husain, M.; Torday, J.S.; Rehan, V.K. Nebulized PPARγ agonists: A novel approach to augment neonatal lung maturation and injury repair in rats. Pediatr. Res. 2014, 75, 631–640. [Google Scholar] [CrossRef]
- Rehan, V.K.; Wang, Y.; Patel, S.; Santos, J.; Torday, J.S. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr. Pulmonol. 2006, 41, 558–569. [Google Scholar] [CrossRef]
- Aprile, M.; Cataldi, S.; Ambrosio, M.R.; D’esposito, V.; Lim, K.; Dietrich, A.; Blüher, M.; Savage, D.B.; Formisano, P.; Ciccodicola, A.; et al. PPARγΔ5, a Naturally Occurring Dominant-Negative Splice Isoform, Impairs PPARγ Function and Adipocyte Differentiation. Cell Rep. 2018, 25, 1577–1592.e6. [Google Scholar] [CrossRef]
- Shi, C.-Y.; Xu, J.-J.; Li, C.; Yu, J.-L.; Wu, Y.-T.; Huang, H.-F. A PPARG Splice Variant in Granulosa Cells Is Associated with Polycystic Ovary Syndrome. J. Clin. Med. 2022, 11, 7285. [Google Scholar] [CrossRef]
- Zhao, J.; Ballard, C.; Cohen, A.J.; Ringham, B.; Zhao, B.; Wang, H.; Zuspan, K.; Rebentisch, A.; Locklear, B.A.; Dahl, M.; et al. Postnatal growth restriction impairs rat lung structure and function. Anat. Rec. 2025, 308, 1051–1065. [Google Scholar] [CrossRef]
- Weinheimer, C.; Wang, H.; Comstock, J.M.; Singh, P.; Wang, Z.; Locklear, B.A.; Goodwin, K.L.; Maschek, J.A.; Cox, J.E.; Baack, M.L.; et al. Maternal Tobacco Smoke Exposure Causes Sex-Divergent Changes in Placental Lipid Metabolism in the Rat. Reprod. Sci. 2020, 27, 631–643. [Google Scholar] [CrossRef]
- McGovern, T.K.; Robichaud, A.; Fereydoonzad, L.; Schuessler, T.F.; Martin, J.G. Evaluation of Respiratory System Mechanics in Mice using the Forced Oscillation Technique. J. Vis. Exp. 2013, 75, e50172. [Google Scholar] [CrossRef]
- Joss-Moore, L.; Carroll, T.; Yang, Y.; Fitzhugh, M.; Metcalfe, D.; Oman, J.; Hale, M.; Dong, L.; Wang, Z.-M.; Yu, X.; et al. Intrauterine growth restriction transiently delays alveolar formation and disrupts retinoic acid receptor expression in the lung of female rat pups. Pediatr. Res. 2013, 73, 612–620. [Google Scholar] [CrossRef]
- Frank, L.; Sosenko, I.R. Undernutrition as a major contributing factor in the pathogenesis of bronchopulmonary dysplasia. Am. Rev. Respir. Dis. 1988, 138, 725–729. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Das, A.; Wrage, L.A.; Poindexter, B.B.; Higgins, R.D.; Stoll, B.J.; Oh, W. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr. Res. 2011, 69, 522–529. [Google Scholar] [CrossRef]
- Morrow, L.A.; Wagner, B.D.; Ingram, D.A.; Poindexter, B.B.; Schibler, K.; Cotten, C.M.; Dagle, J.; Sontag, M.K.; Mourani, P.M.; Abman, S.H. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am. J. Respir. Crit. Care Med. 2017, 196, 364–374. [Google Scholar] [CrossRef]
- Reiss, I.; Landmann, E.; Heckmann, M.; Misselwitz, B.; Gortner, L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch. Gynecol. Obstet. 2003, 269, 40–44. [Google Scholar] [CrossRef]
- Bose, C.; Van Marter, L.J.; Laughon, M.; O’Shea, T.M.; Allred, E.N.; Karna, P.; Ehrenkranz, R.A.; Boggess, K.; Leviton, A. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 2009, 124, e450–e458. [Google Scholar] [CrossRef]
- Ambalavanan, N.; Van Meurs, K.P.; Perritt, R.; Carlo, W.A.; Ehrenkranz, R.A.; Stevenson, D.K.; Lemons, J.A.; Poole, W.K.; Higgins, R.D. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J. Perinatol. 2008, 28, 420–426. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- Khan, M.A.; Kuzma-O’Reilly, B.; Brodsky, N.L.; Bhandari, V. Site-specific characteristics of infants developing bronchopulmonary dysplasia. J. Perinatol. 2006, 26, 428–435. [Google Scholar] [CrossRef][Green Version]
- Figueras-Aloy, J.; Palet-Trujols, C.; Matas-Barceló, I.; Botet-Mussons, F.; Carbonell-Estrany, X. Extrauterine growth restriction in very preterm infant: Etiology, diagnosis, and 2-year follow-up. Eur. J. Pediatr. 2020, 179, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Bartolák-Suki, E.; Majumdar, A.; Suki, B. Changes in respiratory elastance after deep inspirations reflect surface film functionality in mice with acute lung injury. J. Appl. Physiol. 2015, 119, 258–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, L.C.; Gow, A.J.; Abramova, E.; Vayas, K.; Guo, C.; Noto, J.; Lyman, J.; Rodriquez, J.; Gelfand-Titiyevskiy, B.; Malcolm, C.; et al. Role of PPARγ in dyslipidemia and altered pulmonary functioning in mice following ozone exposure. Toxicol. Sci. 2023, 194, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Albertine, K.H. Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Semin. Perinatol. 2013, 37, 60–68. [Google Scholar] [CrossRef]
- Cantu, A.; Gutierrez, M.C.; Dong, X.; Leek, C.; Sajti, E.; Lingappan, K. Remarkable sex-specific differences at single-cell resolution in neonatal hyperoxic lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L5–L31. [Google Scholar] [CrossRef]
- Grimm, S.L.; Dong, X.; Zhang, Y.; Carisey, A.F.; Arnold, A.P.; Moorthy, B.; Coarfa, C.; Lingappan, K. Effect of sex chromosomes versus hormones in neonatal lung injury. JCI Insight. 2021, 6, e146863. [Google Scholar] [CrossRef]
- Fougère, H.; Bilodeau, J.-F.; Lavoie, P.M.; Mohamed, I.; Rudkowska, I.; Pronovost, E.; Simonyan, D.; Berthiaume, L.; Guillot, M.; Piedboeuf, B.; et al. Docosahexaenoic acid-rich algae oil supplementation on breast milk fatty acid profile of mothers who delivered prematurely: A randomized clinical trial. Sci. Rep. 2021, 11, 21492. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef]
- Wendel, K.; Aas, M.F.; Gunnarsdottir, G.; Rossholt, M.E.; Bratlie, M.; Nordvik, T.; Landsend, E.C.S.; Fugelseth, D.; Domellöf, M.; Pripp, A.H.; et al. Effect of arachidonic and docosahexaenoic acid supplementation on respiratory outcomes and neonatal morbidities in preterm infants. Clin. Nutr. 2023, 42, 22–28. [Google Scholar] [CrossRef]
- McCulley, D.; Wienhold, M.; Sun, X. The pulmonary mesenchyme directs lung development. Curr. Opin. Genet. Dev. 2015, 32, 98–105. [Google Scholar] [CrossRef]
- Kyle, J.E.; Clair, G.; Bandyopadhyay, G.; Misra, R.S.; Zink, E.M.; Bloodsworth, K.J.; Shukla, A.K.; Du, Y.; Lillis, J.; Myers, J.R.; et al. Cell type-resolved human lung lipidome reveals cellular cooperation in lung function. Sci. Rep. 2018, 8, 13455. [Google Scholar] [CrossRef]
- Varisco, B.M.; Ambalavanan, N.; Whitsett, J.A.; Hagood, J.S. Hagood Thy-1 signals through PPARgamma to promote lipofibroblast differentiation in the developing lung. Am. J. Respir. Cell Mol. Biol. 2012, 46, 765–772. [Google Scholar] [CrossRef]
- Scarpato, M.; Federico, A.; Ciccodicola, A.; Costa, V. Novel transcription factor variants through RNA-sequencing: The importance of being “alternative”. Int. J. Mol. Sci. 2015, 16, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, G.E.; Zhang, X.; Floyd, Z.E. PPAR-gamma AF-2 domain functions as a component of a ubiquitin-dependent degradation signal. Obesity 2009, 17, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Torday, J.S.; Rehan, V.K. Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene regulatory network. Pediatr. Res. 2006, 60, 382–388. [Google Scholar] [CrossRef]

| Control | PGR | PGR + LoDHA | PGR + HiDHA | |
|---|---|---|---|---|
| Weight, g ± SD | ||||
| Male | 53.4 ± 3.3 | 35.7 ± 4.5 * | 33.0 ± 2.7 * | 39.2 ± 3.5 * |
| Female | 52.2 ± 3.8 | 34.6 ± 4.6 * | 31.2 ± 3.6 * | 37.6 ± 2.8 * |
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid (µg/mL ± SD) | Control | PGR | PGR +LoDHA | PGR +HiDHA | Control | PGR | PGR +LoDHA | PGR +HiDHA | |
| Saturated | |||||||||
| Palmitic Acid (16:0) | 537 ± 36 | 485 ± 142 | 775 ± 192 *† | 699 ± 91 † | 633 ± 117 | 674 ± 133 | 676 ± 51 | 700 ± 81 | |
| Stearic Acid (18:0) | 458 ± 162 | 268 ± 106 * | 436 ± 113 | 352 ± 28 | 402 ± 158 | 477 ± 139 | 376 ± 60 | 380 ± 65 | |
| Monounsaturated | |||||||||
| Palmitoleic Acid (16:1) | 34 ± 8 | 28 ± 7 | 14 ± 5 *† | 18 ± 3 *† | 44 ± 19 | 45 ± 20 | 11 ± 2 *† | 21 ± 2 *† | |
| Oleic Acid (18:1) | 421 ± 171 | 240 ± 89 * | 356 ± 94 | 392 ± 89 | 475 ± 182 | 561 ± 198 | 321 ± 62 † | 386 ± 46 | |
| ω-6 | |||||||||
| Linoleic Acid (18:2n6) | 1068 ± 320 | 642 ± 228 * | 1261 ± 192 † | 1346 ± 261 † | 1047 ± 350 | 1258 ± 224 | 1135 ± 181 | 1191 ± 226 | |
| Arachidonic Acid (20:4n6) | 1335 ± 439 | 1187 ± 972 | 2153 ± 445 † | 2367 ± 226 *† | 1337 ± 498 | 1631± 658 | 1835 ± 542 | 2239 ± 508 | |
| ω-3 | |||||||||
| Docosahexaenoic Acid (22:6n3) | 142 ± 31 | 95 ± 44 * | 228 ± 70 † | 589 ± 123 *†‡ | 145 ± 50 | 143 ± 62.0 | 175 ± 46 | 524 ± 96 *†‡ | |
| LA/DHA | 8 ± 3 | 10 ± 6 | 6 ± 2 | 2 ± 0.1 *†‡ | 8 ± 2 | 10 ± 4 | 7 ± 2 | 2 ± 0.2 *†‡ | |
| ARA/DHA | 9 ± 1 | 12 ± 2 * | 10 ± 1 † | 4 ± 1 *†‡ | 9 ± 1 | 12 ± 4 | 10 ± 1 | 4 ± 0.3 *†‡ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.J.; Chidester, W.R.; Wray, D.T.; Jessen, N.; Jones, A.; Bitsui, C.; Zhao, J.; Maschek, J.A.; Cox, J.E.; Martin, C.R.; et al. Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity. Biomolecules 2025, 15, 551. https://doi.org/10.3390/biom15040551
Cohen AJ, Chidester WR, Wray DT, Jessen N, Jones A, Bitsui C, Zhao J, Maschek JA, Cox JE, Martin CR, et al. Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity. Biomolecules. 2025; 15(4):551. https://doi.org/10.3390/biom15040551
Chicago/Turabian StyleCohen, Adrienne J., Wesley R. Chidester, Daniel T. Wray, Nicolette Jessen, Aimee Jones, Cheylah Bitsui, James Zhao, J. Alan Maschek, James E. Cox, Camilia R. Martin, and et al. 2025. "Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity" Biomolecules 15, no. 4: 551. https://doi.org/10.3390/biom15040551
APA StyleCohen, A. J., Chidester, W. R., Wray, D. T., Jessen, N., Jones, A., Bitsui, C., Zhao, J., Maschek, J. A., Cox, J. E., Martin, C. R., & Joss-Moore, L. A. (2025). Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity. Biomolecules, 15(4), 551. https://doi.org/10.3390/biom15040551







