The Flavonoids and Monoterpenes from Citrus unshiu Peel Contained in Ninjinyoeito Synergistically Activate Orexin 1 Receptor: A Possible Mechanism of the Orexigenic Effects of Ninjinyoeito
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Establishment of Stable Cell Lines
2.3. Cell Culture
2.4. Measurement of OX1R and OX2R Activity, and GPCR Families Using the CellKeyTM System
2.5. Statistical Assessment
3. Results
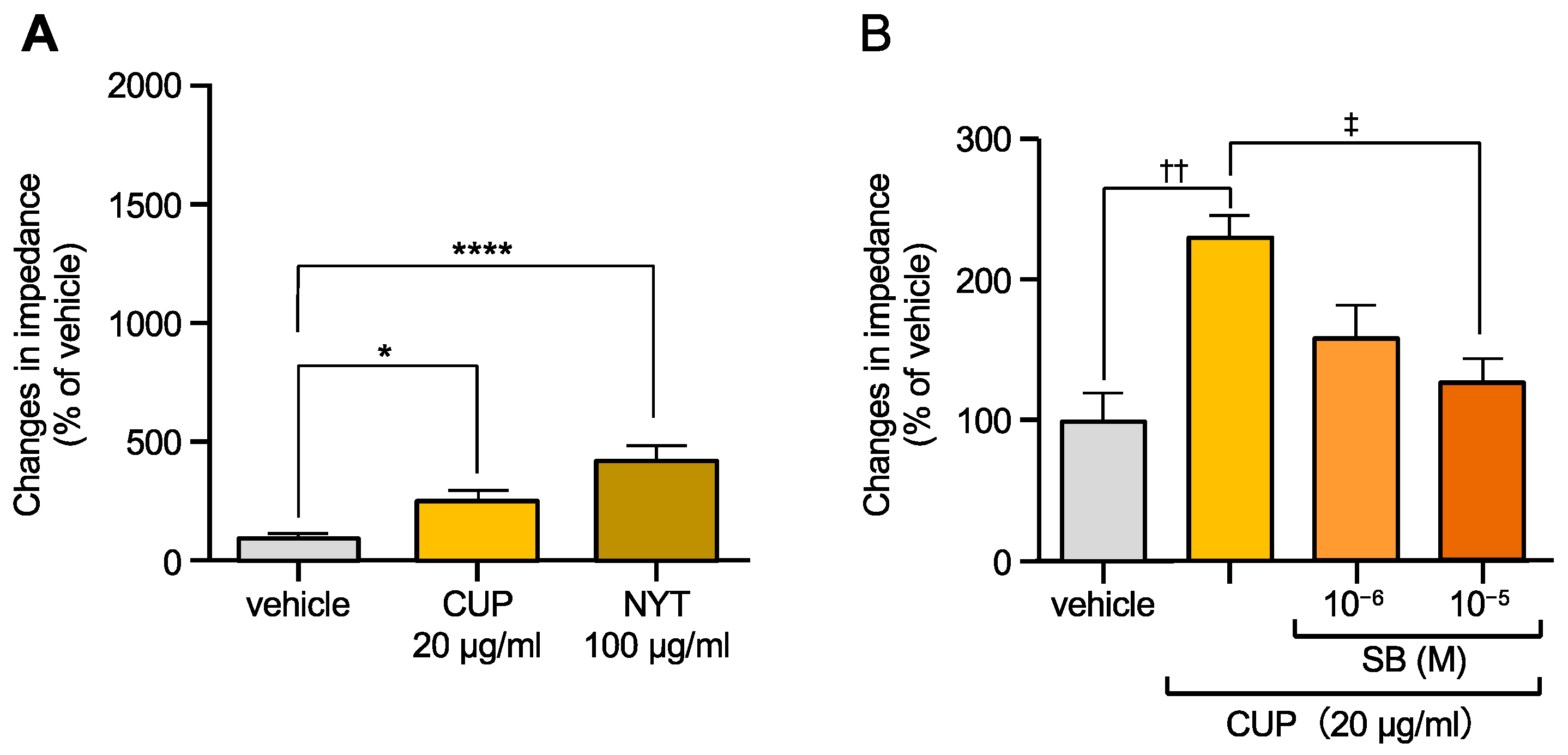
3.1. NYT and CUP Activated OX1R
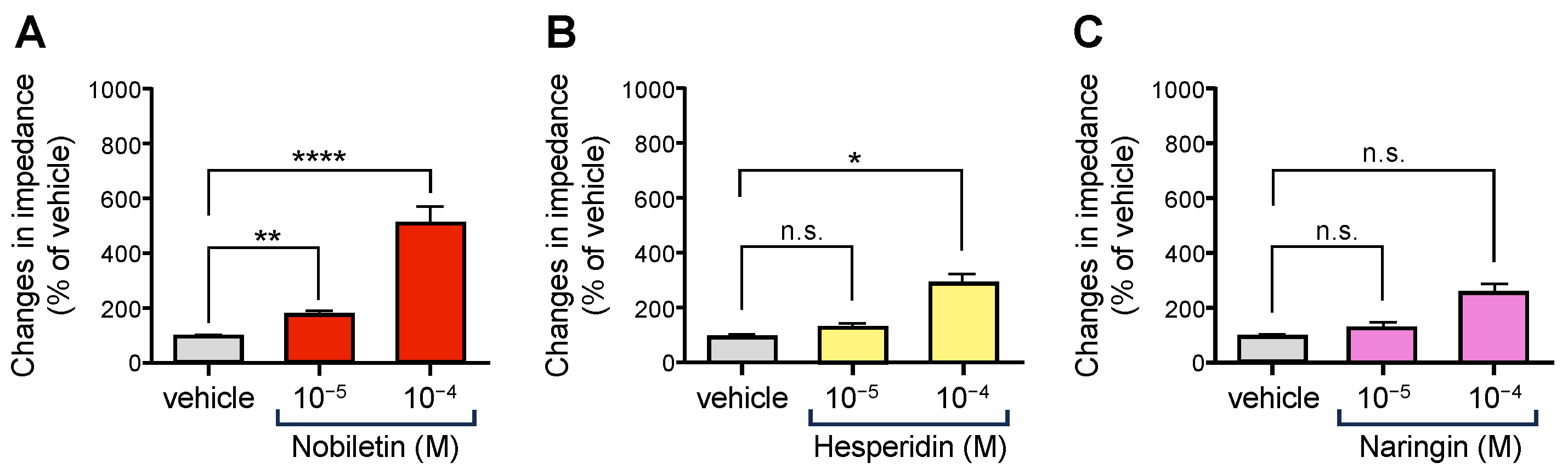
3.2. Flavonoids Contained in CUP of NYT Activated OX1R
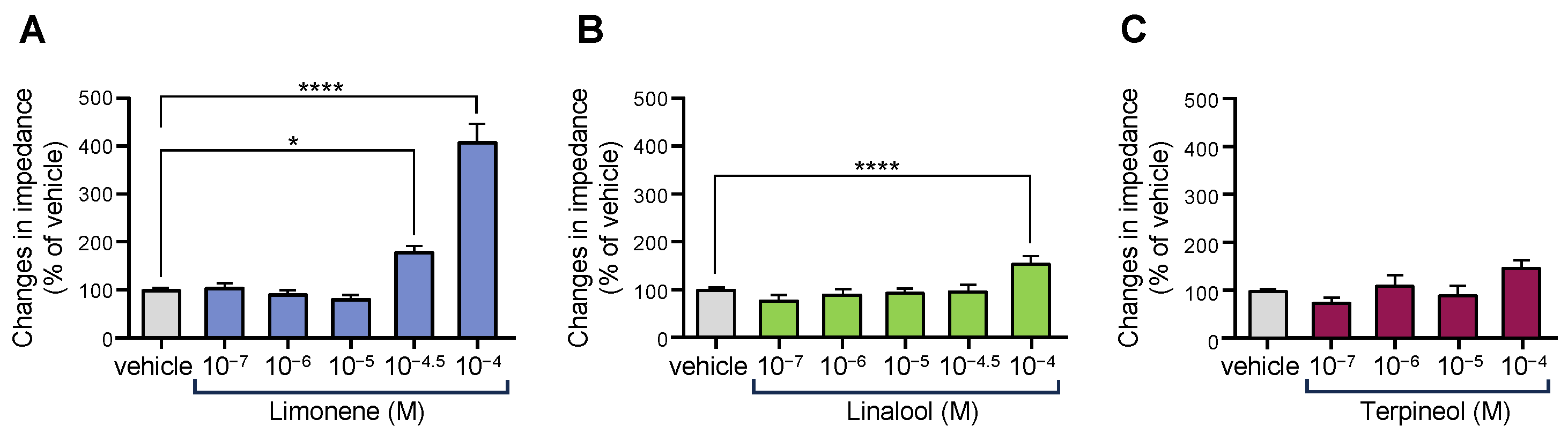
3.3. Some Monoterpenes Activated OX1R
3.4. Neither NYT nor CUP Activated OX2R
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi-Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome—When all you can eat is yourself. Nat. Clin. Pr. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Sudo, Y.; Otsuka, H.; Miyakawa, R.; Goto, A.; Kashiwase, Y.; Terawaki, K.; Miyano, K.; Hirao, Y.; Taki, K.; Tagawa, R.; et al. Differential metabolic responses to adipose atrophy associated with cancer cachexia and caloric restriction in rats and the effect of rikkunshito in cancer cachexia. Int. J. Mol. Sci. 2018, 19, 3852. [Google Scholar] [CrossRef]
- Ramos, E.J.; Suzuki, S.; Marks, D.; Inui, A.; Asakawa, A.; Meguid, M.M. Cancer anorexia-cachexia syndrome: Cytokines and neuropeptides. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 427–434. [Google Scholar] [CrossRef]
- Uezono, Y.; Miyano, K.; Sudo, Y.; Suzuki, M.; Shiraishi, S.; Terawaki, K. A review of traditional Japanese medicines and their potential mechanism of action. Curr. Pharm. Des. 2012, 18, 4839–4853. [Google Scholar] [CrossRef]
- Motoo, Y.; Mouri, H.; Ohtsubo, K.; Yamaguchi, Y.; Watanabe, H.; Sawabu, N. Herbal medicine Ninjinyoeito ameliorates ribavirin-induced anemia in chronic hepatitis C: A randomized controlled trial. World J. Gastroenterol. 2005, 11, 4013–4017. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, A.; Ohsawa, M.; Motoo, Y.; Mizukami, H.; Makino, T. Effect of ninjin’yoeito and ginseng extracts on oxaliplatin-induced neuropathies in mice. J. Nat. Med. 2017, 71, 757–764. [Google Scholar] [CrossRef]
- Miyano, K.; Nonaka, M.; Uzu, M.; Ohshima, K.; Uezono, Y. Multifunctional actions of ninjinyoeito, a Japanese kampo medicine: Accumulated scientific evidence based on experiments with cells and animal models, and clinical studies. Front. Nutr. 2018, 5, 93. [Google Scholar] [CrossRef]
- Miyano, K.; Ohshima, K.; Suzuki, N.; Furuya, S.; Yoshida, Y.; Nonaka, M.; Higami, Y.; Yoshizawa, K.; Fujii, H.; Uezono, Y. Japanese herbal medicine ninjinyoeito mediates its orexigenic properties partially by activating orexin 1 receptors. Font. Nutr. 2020, 7, 5. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shen, R.F.; Bi, J.; Tian, X.S.; Hinchliffe, T.; Xia, Y. Catalpol: A potential therapeutic for neurodegenerative diseases. Curr. Med. Chem. 2015, 22, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Ma, T.; Tian, Y.; Li, C.; Li, H. Catalpol inhibits ischemia-induced premyelinating oligodendrocyte damage through regulation of intercellular calcium homeostasis via Na+/Ca2+ exchanger 3. Int. J. Mol. Sci. 2018, 19, 1925. [Google Scholar] [CrossRef]
- Zhao, L.X.; Jiang, B.C.; Wu, X.B.; Cao, D.L.; Gao, Y.J. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spina astrocytes. Eur. J. Neurosci. 2014, 39, 1391–1402. [Google Scholar] [CrossRef]
- Qian, B.; Li, F.; Zhao, L.X.; Dong, Y.L.; Gao, Y.J.; Zhang, Z.J. Ligustilide ameliorates inflammatory pain and inhibits TLR4 upregulation in spinal astrocytes following complete Freund’s adjuvant peripheral injection. Cell Mol. Neurobiol. 2016, 36, 143–149. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Z.; Dong, L.; Wang, R.; Qiu, G. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid. Based Complement. Altern. Med. 2008, 5, 337–344. [Google Scholar] [CrossRef]
- Shimato, Y.; Ota, M.; Asai, K.; Atsumi, T.; Tabuchi, Y.; Makino, T. Comparison of byakujutsu (Atractylodes rhizome) and sojutsu (Atractylodes lancea rhizome) on anti-inflammatory and immunostimulative effects in vitro. J. Nat. Med. 2018, 72, 192–201. [Google Scholar] [CrossRef]
- Yang, L.; Yu, H.; Hou, A.; Man, W.; Wang, S.; Zhang, J.; Wang, X.; Zheng, S.; Jiang, H.; Kuang, H. A review of the ethnopharmacology, phytochemistry, pharmacology, application, quality control, processing, toxicology, and pharmacokinetics of the dried rhizome of Atractylodes macrocephala. Front. Pharmacol. 2021, 12, 727154. [Google Scholar] [CrossRef]
- Sadakane, C.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Saegusa, Y.; Nahata, M.; Hattori, T.; Asaka, M.; Takeda, H. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem. Biophys. Res. Commun. 2011, 412, 506–511. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, P.; Liu, Y.; Xu, K. Pachymic acid inhibits tumorigenesis in gallbladder carcinoma cells. Int. J. Clin. Exp. Med. 2015, 8, 17781–17788. [Google Scholar] [PubMed]
- Wen, H.; Wu, Z.; Hu, H.; Wu, Y.; Yang, G.; Lu, J.; Yang, G.; Guo, G.; Dong, Q. The anti-tumor effect of pachymic acid on osteosarcoma cells by inducing PTEN and Caspase 3/7-dependent apoptosis. J. Nat. Med. 2018, 72, 57–63. [Google Scholar] [CrossRef]
- Zhong, Y.-M.; Nishijo, H.; Uwano, T.; Tamura, R.; Kawanishi, K.; Ono, T. Red ginseng ameliorated place navigation deficits in young rats with hippocampal lesions and aged rats. Physiol. Behav. 2000, 69, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A potential neuroprotective agent. Biomed. Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Delampasona, M.P.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]
- Kwon, H.K.; Jeon, W.K.; Hwang, J.S.; Lee, C.G.; So, J.S.; Park, J.A.; Ko, B.S.; Im, S.H. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009, 278, 174–182. [Google Scholar] [CrossRef]
- Lv, C.; Yuan, X.; Zeng, H.W.; Liu, R.H.; Zhang, W.D. Protective effect of cinnamaldehyde against glutamate-induced oxidative stress and apoptosis in PC12 cells. Eur. J. Pharmacol. 2017, 815, 487–494. [Google Scholar] [CrossRef]
- Sato, N.; Seiwa, C.; Uruse, M.; Yamamoto, M.; Tanaka, K.; Kawakita, T.; Komatsu, Y.; Yasukawa, A.; Takao, M.; Kudo, C.; et al. Administration of chinpi, a component of the herbal medicine ninjin-youei-to, reverses age-induced demyelination. Evid. Based Complement. Altern. Med. 2011, 2011, 617438. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef]
- Justin Thenmozhi, A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, osidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Chen, W.; Wang, N.; Li, L. Tenuigenin promotes proliferation and differentiation of hippocampal neural stem cells. Neurochem. Res. 2012, 37, 771–777. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Cao, Y.; Wang, J.; Zhang, H.; Zhou, X.; Li, Q.; Wang, L. Tenuigenin inhibits LPS-induced inflammatory responses in microglia via activating the Nrf2-mediated HO-1 signaling pathway. Eur. J. Pharmacol. 2017, 809, 196–202. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Wu, S.N.; Liu, Y.C.; Wu, A.Z.; Tsai, Y.C. Inhibitory action of L-type Ca2+ current by paeoniflorin, a major constituent of peony root, in NG108-15 neuronal cells. Eur. J. Pharmacol. 2005, 523, 16–24. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Li, X.; Shu, X.; Shan, B.; Zhang, L.; Gong, Y.; Dong, Z. Pain-relieving effect of a compound isolated from white peony root oral liquid on acute radiation-induced esophagitis. Mol. Med. Rep. 2013, 7, 1950–1954. [Google Scholar] [CrossRef][Green Version]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Xu, A.; Wang, H.; Hoo, R.L.; Sweeney, G.; Vanhoutte, P.M.; Wang, Y.; Wu, D.; Chu, W.; Qin, G.; Lam, K.S.L. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology 2009, 150, 625–633. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological effects of glycyrrhiza spp. and its bioactive constituents: Update and review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef]
- Dastagir, G.; Rizvi, M.A. Glycyrrhiza glabra L. (Liquorice). Pak. J. Pharm. Sci. 2016, 29, 1727–1733. [Google Scholar]
- Kim, Y.J.; Yoo, S.R.; Chae, C.K.; Jung, U.J.; Choi, M.S. Omija fruit extract improves endurance and energy metabolism by upregulating PGC-1α expression in the skeletal muscle of exercised rats. J. Med. Food 2014, 17, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Hiraki, Y.; Nishida, S.; Inatomi, Y.; Yabe, T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J. Pharmacol. Sci. 2016, 132, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Lobina, C.; Carai, M.A.; Loi, B.; Gessa, G.L.; Riva, A.; Cabri, W.; Petrangolini, G.; Morazzoni, P.; Colombo, G. Protective effect of Panax ginseng in cisplatin-induced cachexia in rats. Future Oncol. 2014, 10, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Tahaghoghi-Hajghorbani, S.; Ebrahimzadeh, M.A.; Rafiei, A.; Golpour, M.; Hosseini-Khah, Z.; Akhtari, J. Improvement of chemotherapy through reducing of cachexia by using Citrus unshiu peel extract. J. Ethnopharmacol. 2019, 242, 111929. [Google Scholar] [CrossRef]
- Han, Y.; Kim, H.I.; Park, J. The Role of Natural Products in the Improvement of Cancer-Associated Cachexia. Int. J. Mol. Sci. 2023, 24, 8772. [Google Scholar] [CrossRef]
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer induced weight loss in mice bearing CT-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214. [Google Scholar] [CrossRef]
- Meguro, Y.; Miyano, K.; Hirayama, S.; Yoshida, Y.; Ishibashi, N.; Ogino, T.; Fujii, Y.; Manabe, S.; Eto, M.; Nonaka, M.; et al. Neuropeptide oxytocin enhances mu opioid receptor signaling as a positive allosteric modulator. J. Pharmacol. Sci. 2018, 137, 67–75. [Google Scholar] [CrossRef]
- Manabe, S.; Miyano, K.; Fujii, Y.; Ohshima, K.; Yoshida, Y.; Nonaka, M.; Uzu, M.; Matsuoka, Y.; Sato, T.; Uezono, Y.; et al. Possible biased analgesic of hydromorphone through the G protein-over beta-arrestin mediated pathway: cAMP, CellKey, and receptor internalization analyses. J. Pharmacol. Sci. 2019, 140, 171–177. [Google Scholar] [CrossRef]
- Miyano, K.; Sudo, Y.; Yokoyama, A.; Hisaoka-Nakashima, K.; Morioka, N.; Takebayashi, M.; Nakata, Y.; Higami, Y.; Uezono, Y. History of the Gprotein-coupled receptor (GPCR) assays from traditional to a state-of-the-art biosensor assay. J. Pharmacol. Sci. 2014, 126, 302–309. [Google Scholar] [CrossRef]
- Scott, C.W.; Peters, M.F. Label-free whole-cell assays: Expanding the scope of GPCR screening. Drug Discov. Today 2010, 15, 704–716. [Google Scholar] [CrossRef]
- Peters, M.F.; Vaillancourt, F.; Heroux, M.; Valiquette, M.; Scott, C.W. Comparing label-free biosensors for pharmacological screening with cell-based functional assays. Assay Drug Dev. Technol. 2010, 8, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Abusnana, S.; Sunter, D.; Murphy, K.G.; Ghatei, M.A.; Bloom, S.R. The effect of the orexins on food intake: Comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol. 1999, 160, R7–R12. [Google Scholar] [CrossRef]
- Haynes, A.C.; Jackson, B.; Chapman, H.; Tadayyon, M.; Johns, A.; Porter, R.A.; Arch, J.R. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 2000, 96, 45–51. [Google Scholar] [CrossRef]
- Yamada, H.; Okumura, T.; Motomura, W.; Kobayashi, Y.; Kohgo, Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem. Biophys. Res. Commun. 2000, 267, 527–531. [Google Scholar] [CrossRef]
- Haynes, A.C.; Chapman, H.; Taylor, C.; Moore, G.B.; Cawthorne, M.A.; Tadayyon, M.; Clapham, J.C.; Arch, J.R. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul. Pept. 2002, 104, 153–159. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J. Enzym. Inhib. Med. Chem. 2007, 22, 83–90. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, K.; Jung, Y.R.; Bian, Y.; Ngo, T.; Bae, O.N.; Lim, K.-M.; Chung, J.-H. Co-existence of hypertensive and anti-hypertensive constituents, synephrine, and nobiletin in Citrus unshiu Peel. Molecules 2019, 24, 1197. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef]
- Kitagawa, H.; Munekage, M.; Matsumoto, T.; Sadakane, C.; Fukutake, M.; Aoki, K.; Watanabe, J.; Maemura, K.; Hattori, T.; Kase, Y.; et al. Pharmacokinetic profiles of active ingredients and its metabolites derived from rikkunshito, a ghrelin enhancer, in healthy Japanese volunteers: A cross-over, randomized study. PLoS ONE 2015, 10, e0133159. [Google Scholar] [CrossRef]
- Singh, S.P.; Wahajuddin, M.; Tewari, D.; Patel, K.; Jain, G.K. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia 2011, 82, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of polymethoxyflavones into the mouse brain and their effect on MK-801-induced locomotive hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of blood-brain barrier permeability of polyphenols, anthocyanins, and their metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- Nishi, K.; Nakatani, Y.; Ishida, M.; Kadota, A.; Sugahara, T. Anti-inflammatory activity of the combination of nobiletin and docosahexaenoic acid in lipopolysaccharide-stimulated RAW 264.7 cells: A potential synergistic anti-inflammatory effect. Nutrients 2024, 16, 2080. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Couvineau, A.; Voisin, T.; Nicole, P.; Gratio, V.; Abad, C.; Tan, Y.V. Orexins as novel therapeutic targets in inflammatory and neurodegenerative diseases. Front. Endocrinol. 2019, 10, 709. [Google Scholar] [CrossRef]
- Ten-Blanco, M.; Flores, Á.; Cristino, L.; Pereda-Pérez, I.; Berrendero, F. Targeting the orexin/hypocretin system for the treatment of neuropsychiatric and neurodegenerative diseases: From animal to clinical studies. Front. Neuroendocr. 2023, 69, 101066. [Google Scholar] [CrossRef]
- Cluderay, J.E.; Harrison, D.C.; Hervieu, G.J. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul. Pept. 2002, 104, 131–144. [Google Scholar] [CrossRef]
- RayatSanati, K.; Jamali, S.; Hassanlou, A.A.; Haghparast, A. Blockade of orexin receptors in the hippocampal dentate gyrus reduced the extinction latency of morphine-induced place preference in male rats. Neurosci. Lett. 2021, 756, 135946. [Google Scholar] [CrossRef]
- Panahi, P.S.; Esmaili, S.; Ghalandari-Shamami, M.; Mousavi, Z.; Haghparast, A. Similar functional roles of the Orexin-1 and Orexin-2 receptors within the dentate gyrus area of the hippocampus in the stress-induced antinociceptive responses in the acute pain model in the rat. Physiol. Behav. 2023, 270, 114311. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Uezono, Y.; Minami, K.; Yamaguchi, T.; Niijima, A.; Yada, T.; Maejima, Y.; Sedbazar, U.; Sakai, T.; et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl. Psychiatry 2011, 1, e23. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Uezono, Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Front. Pharmacol. 2014, 5, 271. [Google Scholar] [CrossRef]
- Terawaki, K.; Sawada, Y.; Kashiwase, Y.; Hashimoto, H.; Yoshimura, M.; Suzuki, M.; Miyano, K.; Sudo, Y.; Shiraishi, S.; Higami, Y.; et al. New cancer cachexia rat model generated by implantation of a peritoneal dissemination-derived human stomach cancer cell line. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E373–E387. [Google Scholar] [CrossRef]
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLoS ONE 2017, 12, e0173113. [Google Scholar] [CrossRef]
- Terawaki, K.; Kashiwase, Y.; Uzu, M.; Nonaka, M.; Sawada, Y.; Miyano, K.; Higami, Y.; Yanagihara, K.; Yamamoto, M.; Uezono, Y. Leukemia inhibitory factor via the Toll-like receptor 5 signaling pathway involves aggravation of cachexia induced by human gastric cancer-derived 85As2 cells in rats. Oncotarget 2018, 9, 34748–34764. [Google Scholar] [CrossRef]


| Herbal Components | Formula in NYT | Main Ingredients | Functions or Sites of Action | References | |
|---|---|---|---|---|---|
| 1 | Rehmannia Root | 4.0 g | catalpol | • Antineurodegenerative • Anti-ischemia-induced oligodentrocyte damage by Na+/Ca2+ exchanger 3 | [12,13,14] |
| 2 | Japanese Angelica Root | 4.0 g | ligustilide | • Anti-inflammatory | [15,16] |
| 3 | Atractylodes Rhizome | 4.0 g | atractylenolide | • Improve symptom of cancer patients • Anti-inflammatory | [17,18,19] |
| 4 | Poria Sclerotium | 4.0 g | pachymic acid | • Antitumor • Inhibition of enzymes from active acyl ghrelin to inactive des-acyl ghrelin | [20,21,22] |
| 5 | Ginseng | 3.0 g | ginsenoside | • Antitumor • Anti-inflammatory • Antioxidative | [9,23,24,25] |
| 6 | Cinnamon Bark | 2.5 g | cinnamaldehyde | • Anti-inflammatory • Antioxidative • Antitumor • Neuroprotective | [26,27,28] |
| 7 | Citrus unshiu Peel * * | 2.0 g | flavonoidsmonoterpenes | • Orexin 1 receptor activation • Neuroprotective • Antioxidant • Anti-inflammatory | [11,29,30,31,32] |
| 8 | Polygala Root | 2.0 g | tenuigenin | • Neuroprotective • Anti-inflammatory | [33,34] |
| 9 | Peony Root | 2.0 g | paeoniflorin | • Pain relief • Ca2+ channel inhibition | [35,36] |
| 10 | Astragalus Root | 1.5 g | astragaloside, isoastragaloside | • Elevation of adiponectin production • Antitumor | [37,38] |
| 11 | Glycyrrhiza | 1.0 g | glycyrrhizinglycycoumarin | • Ant-inflammatory • Antioxidative • Neuroprotective • Keep ghrelin levels as pachymic acid | [20,39,40] |
| 12 | Schisandra Fruit | 1.0 g | schizandrin | • Ant-inflammatory • Enhancement of skeletal muscle endurance | [41,42] |
| Sites of Function | Experimental Modes | Mechanisms | References | |
|---|---|---|---|---|
| 1 | Improve muscle atrophy | Mice cachexia model | Decreased levels of TNF-α, IL-6, IL-1β | [46] |
| 2 | Improve adipose tissue atrophy | Mice cachexia model | Decreased levels of TNF-α, IL-6, IL-1β | [46] |
| 3 | Ameliorate chemotherapy-induced atrophy | Mice cachexia model | Decreased levels of IL-6, TNF-α, IL-1β, malondialdehyde-thiobarbituric acid (MDA) | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohshima, K.; Miyano, K.; Nonaka, M.; Aiso, S.; Fukuda, M.; Furuya, S.; Fujii, H.; Uezono, Y. The Flavonoids and Monoterpenes from Citrus unshiu Peel Contained in Ninjinyoeito Synergistically Activate Orexin 1 Receptor: A Possible Mechanism of the Orexigenic Effects of Ninjinyoeito. Biomolecules 2025, 15, 533. https://doi.org/10.3390/biom15040533
Ohshima K, Miyano K, Nonaka M, Aiso S, Fukuda M, Furuya S, Fujii H, Uezono Y. The Flavonoids and Monoterpenes from Citrus unshiu Peel Contained in Ninjinyoeito Synergistically Activate Orexin 1 Receptor: A Possible Mechanism of the Orexigenic Effects of Ninjinyoeito. Biomolecules. 2025; 15(4):533. https://doi.org/10.3390/biom15040533
Chicago/Turabian StyleOhshima, Kaori, Kanako Miyano, Miki Nonaka, Sayaka Aiso, Mao Fukuda, Saho Furuya, Hideaki Fujii, and Yasuhito Uezono. 2025. "The Flavonoids and Monoterpenes from Citrus unshiu Peel Contained in Ninjinyoeito Synergistically Activate Orexin 1 Receptor: A Possible Mechanism of the Orexigenic Effects of Ninjinyoeito" Biomolecules 15, no. 4: 533. https://doi.org/10.3390/biom15040533
APA StyleOhshima, K., Miyano, K., Nonaka, M., Aiso, S., Fukuda, M., Furuya, S., Fujii, H., & Uezono, Y. (2025). The Flavonoids and Monoterpenes from Citrus unshiu Peel Contained in Ninjinyoeito Synergistically Activate Orexin 1 Receptor: A Possible Mechanism of the Orexigenic Effects of Ninjinyoeito. Biomolecules, 15(4), 533. https://doi.org/10.3390/biom15040533








