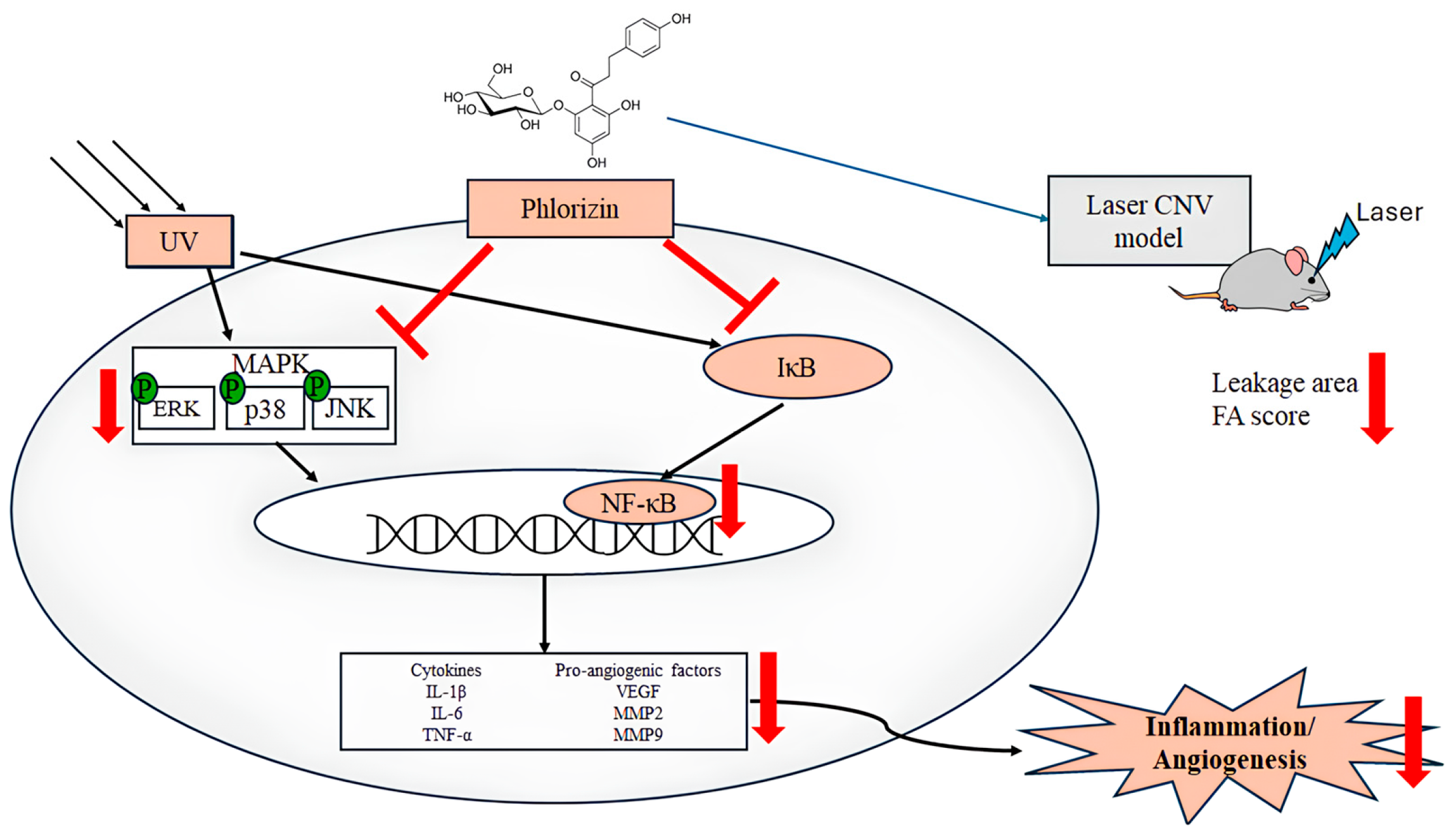
Phlorizin Protects Against Oxidative Stress and Inflammation in Age-Related Macular Degeneration Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Phlorizin
2.2. Cell Line Culture
2.3. UVA Source
2.4. MTT Assay
2.5. Measurement of ROS
2.6. Western Blot Analysis
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Animals
2.9. Laser-Induced CNV Model
2.10. Color Fundus Photography (CFP) and Fluorescence Angiography (FA)
2.11. Statistical Analysis
3. Results
3.1. Cell Viability Test
3.1.1. Cytotoxicity of Phlorizin on ARPE-19 Cells
3.1.2. UVA Exposure Effect on ARPE-19 Cell Viability
3.1.3. Phlorizin’s Protective Effect Against UVA-Induced Cytotoxicity
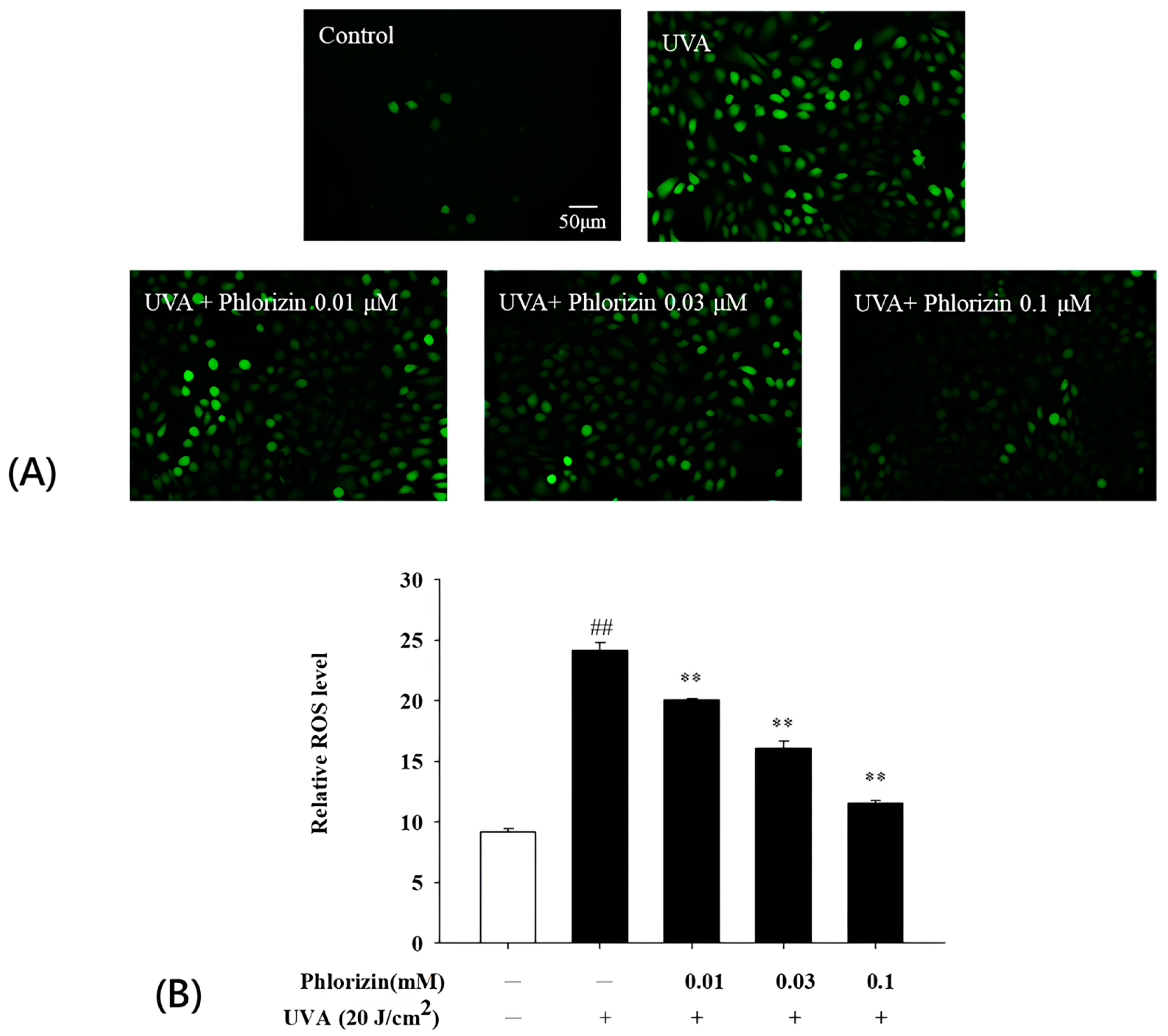
3.2. Phlorizin Inhibits UVA-Induced ROS Generation
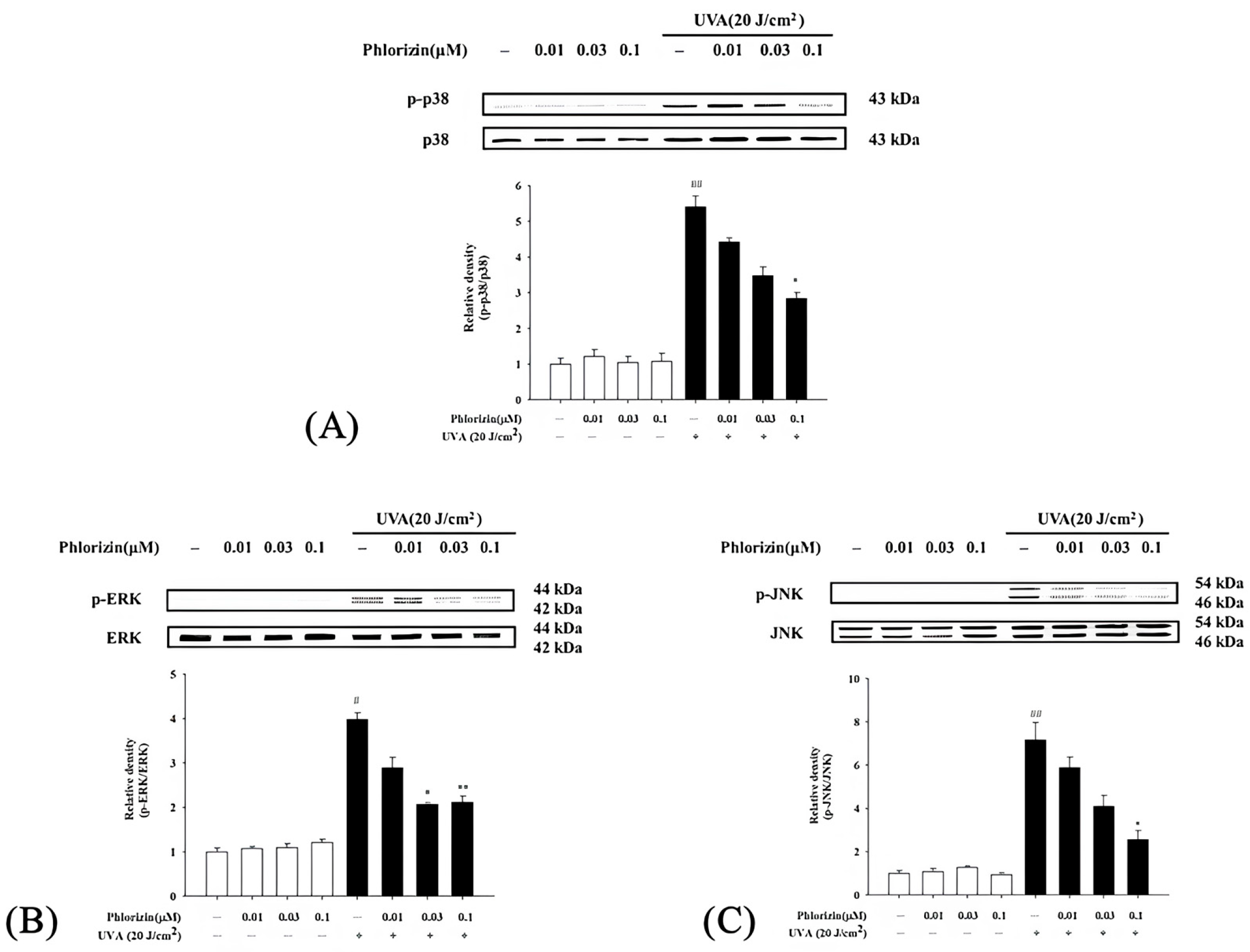
3.3. Reduction in UVA- and NaIO3-Induced MAPK Phosphorylation by Phlorizin
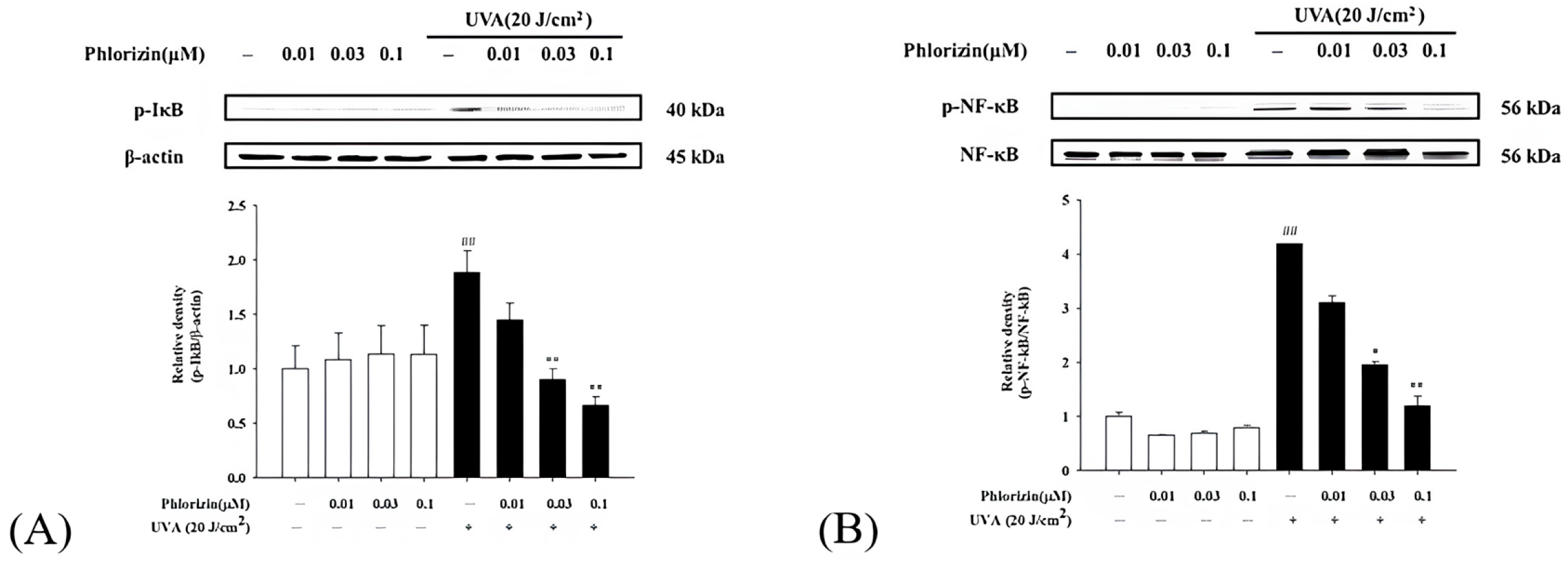
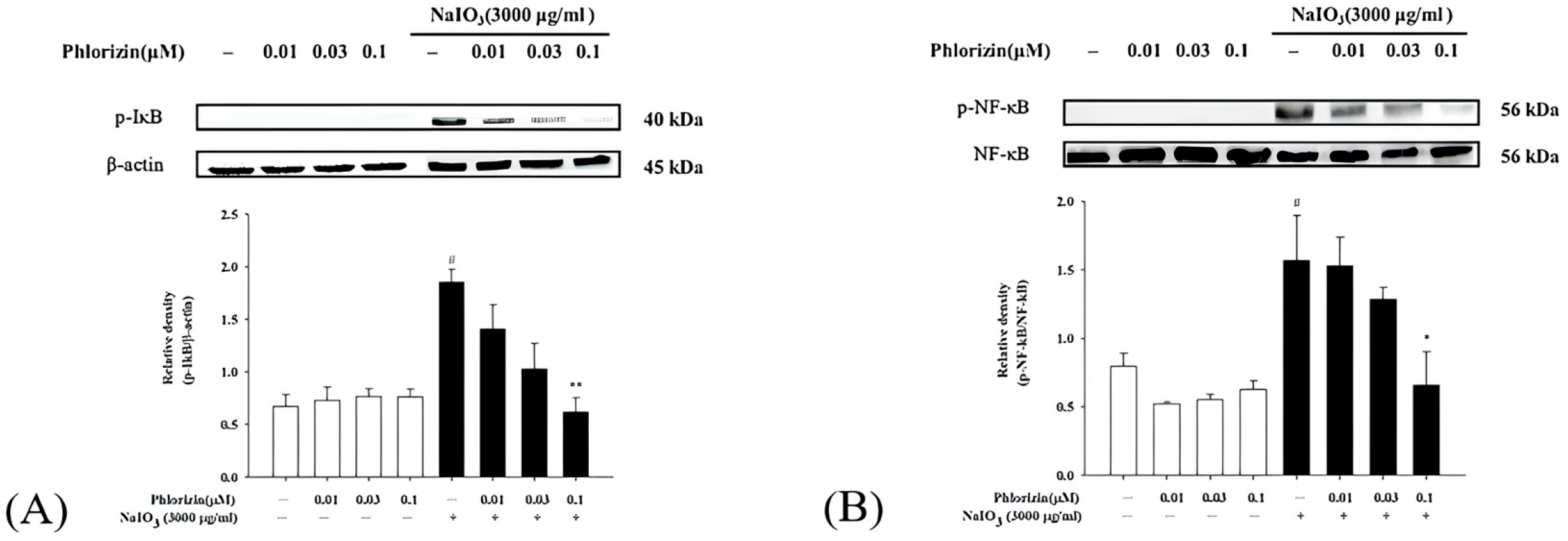
3.4. Phlorizin Activation Dampens UVA- and NaIO3-Induced NF-κB Signal Pathway
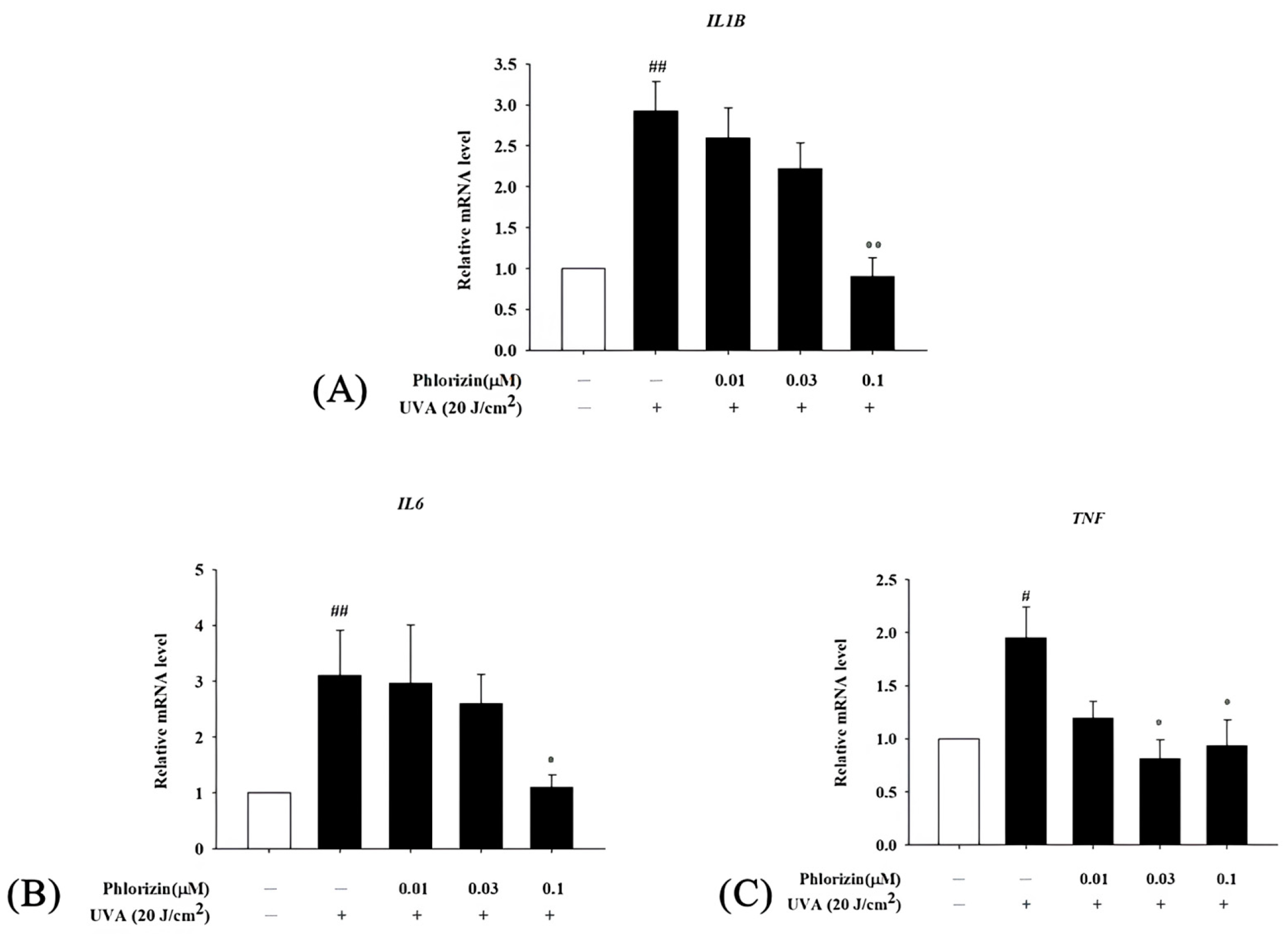
3.5. Phlorizin Downregulates Pro-Inflammatory Cytokine mRNA Expression Induced by UVA and NAIO3
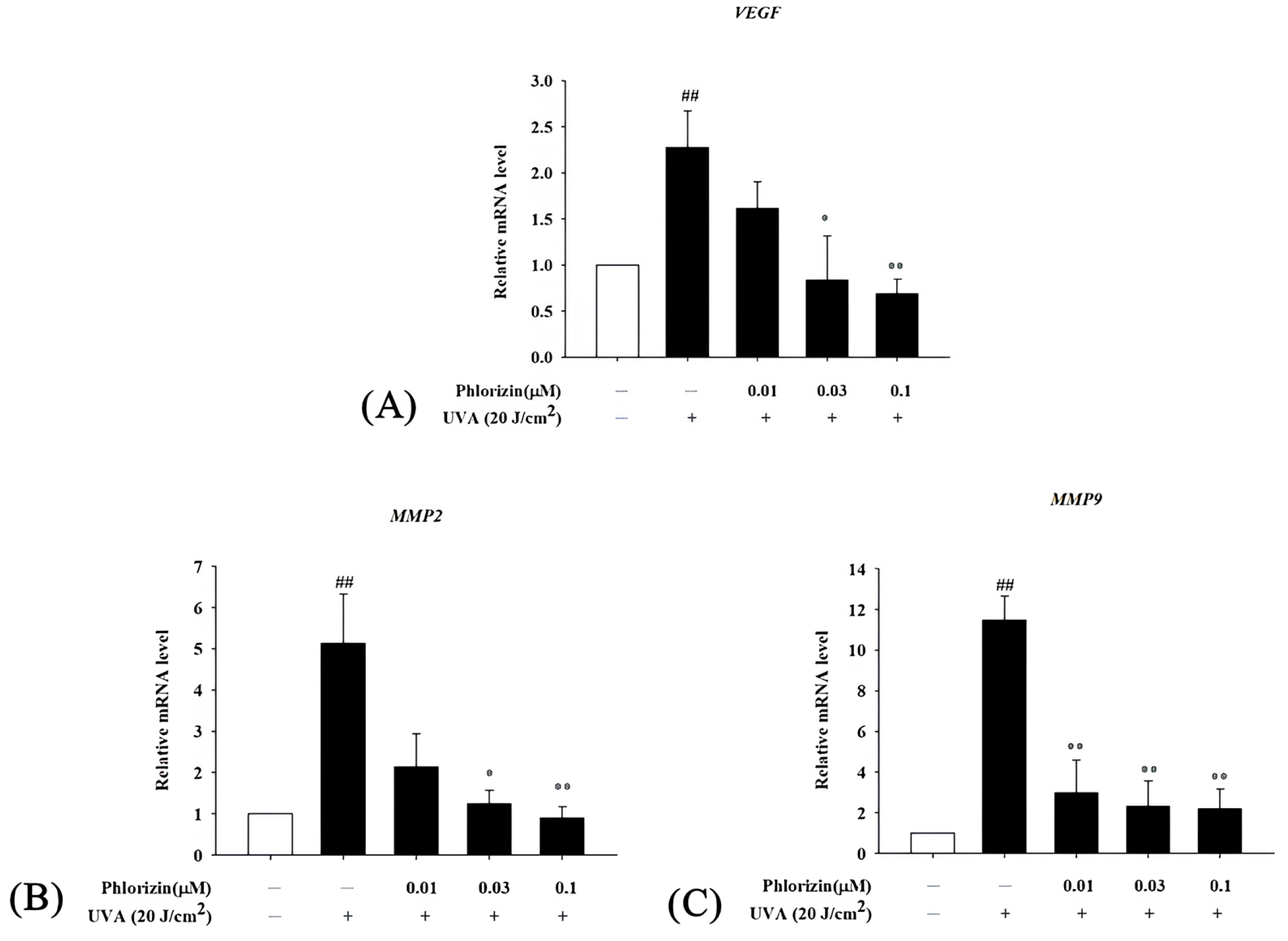
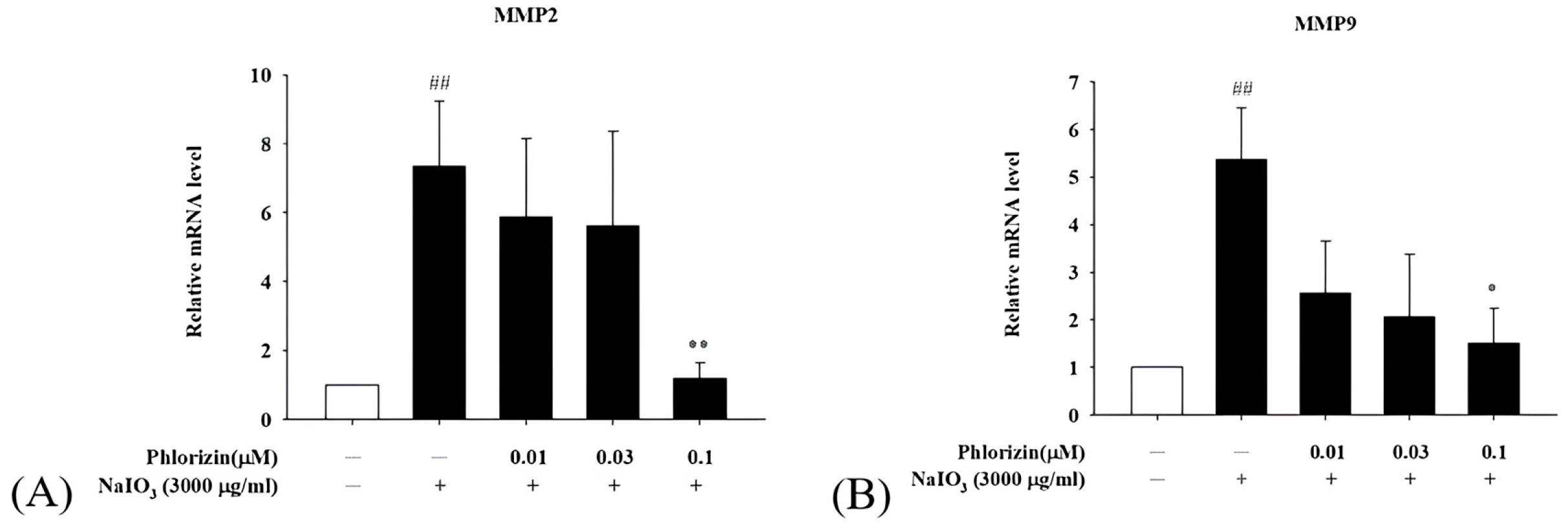
3.6. Phlorizin Downregulates Pro-Angiogenic Factor mRNA Expression Induced by UVA and NAIO3
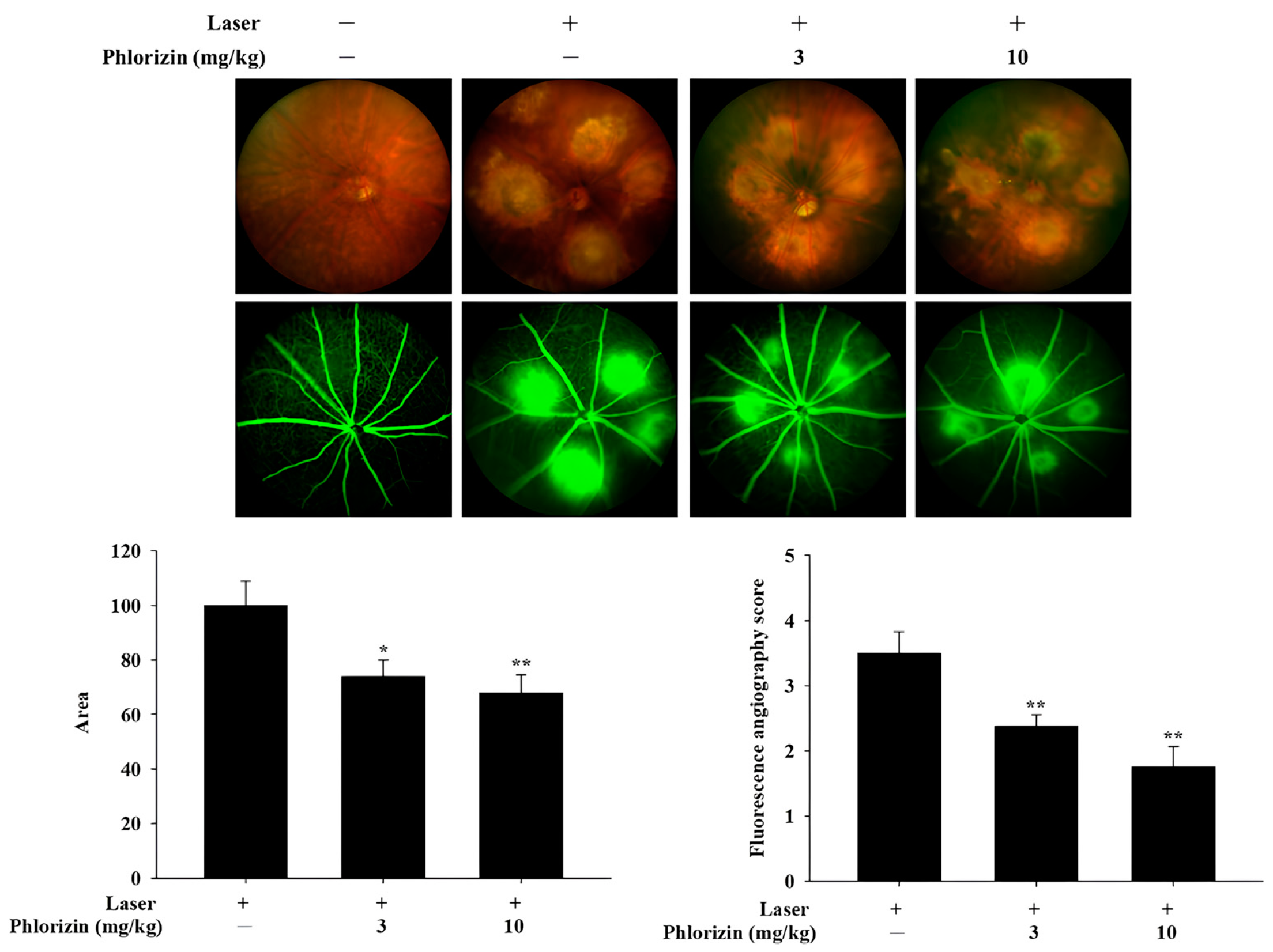
3.7. Preliminary In Vivo Study: Color Fundus Photography (CFP) and Fluorescence Angiography (FA) Analysis of Effects of In Vivo Study of Phlorizin on Laser-Induced CNV (n = 3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| ARPE-19 | Adult retinal pigmented epithelial -19 cells |
| UVA | Ultraviolet A |
| NaIO3 | Sodium Iodate |
| ROS | Reactive oxygen species |
| CFP | Color fundus photography |
| FA | Fluorescence angiography |
| CNV | Choroidal neovascularization |
| WHO | World Health Organization |
| GA | Geographic atrophy |
| RPE | Retinal pigment epithelium |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAPK | Mitogen-activated protein kinases |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor- α |
| VEGF | Vascular endothelial growth factor |
| RIPK | Receptor-interacting protein kinase |
| HMGB | High mobility group box |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| AREDS | Age-Related Eye Disease Study |
| BCRC | Biological Resource Collection and Research Center |
| DMEM | Dulbecco’s Modified Eagle Medium |
| AA | Antibiotic-antimycotic |
| DMSO | Dimethyl sulfoxide |
| SDS | Sodium dodecyl sulfate |
| ELISA | Enzyme-linked immunosorbent assay |
| PVDF | Polyvinylidene difluoride |
| RT-qPCR | Real-time Quantitative Polymerase Chain Reaction |
| IP | Intraperitoneal |
| SEM | Standard error of the mean |
| CREB | cAMP Response Element-Binding Protein |
| MCP | Monocyte Chemoattractant Protein |
References
- Jabbehdari, S.; Oganov, A.C.; Rezagholi, F.; Mohammadi, S.; Harandi, H.; Yazdanpanah, G.; Arevalo, J.F. Age-Related Macular Degeneration and Neurodegenerative Disorders: Shared Pathways in Complex Interactions. Surv. Ophthalmol. 2024, 69, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of Age-Related Macular Degeneration (AMD): Associations with Cardiovascular Disease Phenotypes and Lipid Factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Hassan, Y.; Bakka Vemana, P.P.S.; Bellary Pattanashetty, M.S.; Abdin, Z.U.; Siddiqui, H.F. Age-Related Macular Degeneration: An Exponentially Emerging Imminent Threat of Visual Impairment and Irreversible Blindness. Cureus 2023, 15, e39624. [Google Scholar] [CrossRef] [PubMed]
- Vision Loss Expert Group of the Global Burden of Disease Study; the GBD 2019 Blindness and Vision Impairment Collaborators. Global Estimates on the Number of People Blind or Visually Impaired by Age-Related Macular Degeneration: A Meta-Analysis from 2000 to 2020. Eye 2024, 38, 2070–2082. [Google Scholar] [CrossRef]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Ianchulev, S.; Adamis, A.P. Age-Related Macular Degeneration: Etiology, Pathogenesis, and Therapeutic Strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Wang, S. RPE Necroptosis in Response to Oxidative Stress and in AMD. Ageing Res. Rev. 2015, 24, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and Heterophagy Dysregulation Leads to Retinal Pigment Epithelium Dysfunction and Development of Age-Related Macular Degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-Related Macular Degeneration: Epidemiology, Genetics, Pathophysiology, Diagnosis, and Targeted Therapy. Genes. Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Nowak, J.Z. Age-Related Macular Degeneration (AMD): Pathogenesis and Therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar]
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of Age-Related Macular Degeneration: Implications for Treatment. Ophthalmic Res. 2022, 65, 615–636. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, E.; Obasanmi, G.; Zeglinski, M.R.; Granville, D.J.; Yeung, S.N.; Matsubara, J.A. Potential Role of Extracellular Granzyme B in Wet Age-Related Macular Degeneration and Fuchs Endothelial Corneal Dystrophy. Front. Pharmacol. 2022, 13, 980742. [Google Scholar] [CrossRef]
- Ke, K.M.; Chakravarthy, U.; O’Neill, C. Economic Cost of Age-Related Macular Degeneration: A Review of Recent Research. Drugs Aging 2006, 23, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-Related Macular Degeneration--Emerging Pathogenetic and Therapeutic Concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-Related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S. The Journey of “Geographic Atrophy” through Past, Present, and Future. Ophthalmologica 2017, 237, 11–20. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Arnold, J.J.; Sarks, J.P.; Killingsworth, M.C.; Kettle, E.K.; Sarks, S.H. Adult Vitelliform Macular Degeneration: A Clinicopathological Study. Eye 2003, 17, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through VEGF: Innate and Adaptive Immunity in Pathological Angiogenesis in the Eye. Trends Mol. Med. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Brijesh, T.; Shorya, A. Macular Atrophy Progression and 7-Year Vision Outcomes in Subjects From the ANCHOR, MARINA, and HORIZON Studies: The SEVEN-UP Study. Am. J. Ophthalmol. 2016, 162, 200. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxidative Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet Radiation Oxidative Stress Affects Eye Health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef]
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. A Review: Role of Ultraviolet Radiation in Age-Related Macular Degeneration. Eye Contact Lens 2011, 37, 225–232. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Sobczuk, A.; Szczepanska, J.; Kaarniranta, K. The Aging Stress Response and Its Implication for AMD Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8840. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Lu, L.; Hackett, S.F.; Mincey, A.; Lai, H.; Campochiaro, P.A. Effects of Different Types of Oxidative Stress in RPE Cells. J. Cell. Physiol. 2006, 206, 119–125. [Google Scholar] [CrossRef]
- Zarbin, M.A. Current Concepts in the Pathogenesis of Age-Related Macular Degeneration. Arch. Ophthalmol. 2004, 122, 598–614. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, E.L.; Kearns, J.D.; Hoffmann, A. UV as an Amplifier Rather than Inducer of NF-κB Activity. Mol. Cell 2008, 30, 632–641. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.M.C.; He, T.; Shao, Y.; Fonseca, M.J.V.; Verri, W.A.; Fisher, G.J.; Xu, Y. Quercetin Inhibits UV Irradiation-Induced Inflammatory Cytokine Production in Primary Human Keratinocytes by Suppressing NF-κB Pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Roduit, R.; Schorderet, D.F. MAP Kinase Pathways in UV-Induced Apoptosis of Retinal Pigment Epithelium ARPE19 Cells. Apoptosis 2008, 13, 343–353. [Google Scholar] [CrossRef]
- Chan, C.-M.; Huang, J.-H.; Lin, H.-H.; Chiang, H.-S.; Chen, B.-H.; Hong, J.-Y.; Hung, C.-F. Protective Effects of (-)-Epigallocatechin Gallate on UVA-Induced Damage in ARPE19 Cells. Mol. Vis. 2008, 14, 2528–2534. [Google Scholar]
- Glickman, R.D.; Natarajan, M.; Rockwell, B.A.; Denton, M.; Maswadi, S.; Kumar, N.; Nieves-Roldan, F. Intracellular Signaling Mechanisms Responsive to Laser-Induced Photochemical and Thermal Stress. In Proceedings of the Optical Interactions with Tissue and Cells XVI, San Jose, CA, USA, 22–27 January 2005. [Google Scholar]
- Basyal, D.; Lee, S.; Kim, H.J. Antioxidants and Mechanistic Insights for Managing Dry Age-Related Macular Degeneration. Antioxidants 2024, 13, 568. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.-M.; Rudolf, M.; Belyaeva, O.V.; Chung, B.H.; Messinger, J.D.; Kedishvili, N.Y.; Curcio, C.A. Lipoprotein Particles of Intraocular Origin in Human Bruch Membrane: An Unusual Lipid Profile. Invest. Ophthalmol. Vis. Sci. 2009, 50, 870–877. [Google Scholar] [CrossRef]
- Espitia-Arias, M.D.; de la Villa, P.; Paleo-García, V.; Germain, F.; Milla-Navarro, S. Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway. Antioxidants 2023, 12, 1594. [Google Scholar] [CrossRef]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct Effect of Sodium Iodate on Neurosensory Retina. Invest. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Invest. Ophthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Sorsby, A. Experimental pigmentary degeneration of the retina by sodium iodate. Br. J. Ophthalmol. 1941, 25, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Machalińska, A.; Lubiński, W.; Kłos, P.; Kawa, M.; Baumert, B.; Penkala, K.; Grzegrzółka, R.; Karczewicz, D.; Wiszniewska, B.; Machaliński, B. Sodium Iodate Selectively Injuries the Posterior Pole of the Retina in a Dose-Dependent Manner: Morphological and Electrophysiological Study. Neurochem. Res. 2010, 35, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Noell, W.K. Experimentally Induced Toxic Effects on Structure and Function of Visual Cells and Pigment Epithelium. Am. J. Ophthalmol. 1953, 36, 103–116. [Google Scholar] [CrossRef]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE Destruction Causes Choriocapillary Atrophy. Invest. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar]
- Ashburn, F.S.; Pilkerton, A.R.; Rao, N.A.; Marak, G.E. The Effects of Iodate and Iodoacetate on the Retinal Adhesion. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1427–1432. [Google Scholar]
- Baich, A.; Ziegler, M. The Effect of Sodium Iodate and Melanin on the Formation of Glyoxylate. Pigment. Cell Res. 1992, 5, 394–395. [Google Scholar] [CrossRef]
- Konda, B.R.; Pararajasegaram, G.; Wu, G.S.; Stanforth, D.; Rao, N.A. Role of Retinal Pigment Epithelium in the Development of Experimental Autoimmune Uveitis. Investig. Ophthalmol. Vis. Sci. 1994, 35, 40–47. [Google Scholar]
- Mao, X.; Pan, T.; Shen, H.; Xi, H.; Yuan, S.; Liu, Q. The Rescue Effect of Mesenchymal Stem Cell on Sodium Iodate-Induced Retinal Pigment Epithelial Cell Death through Deactivation of NF-κB-Mediated NLRP3 Inflammasome. Biomed. Pharmacother. 2018, 103, 517–523. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, S. Not All Stressors Are Equal: Mechanism of Stressors on RPE Cell Degeneration. Front. Cell Dev. Biol. 2020, 8, 591067. [Google Scholar] [CrossRef]
- Kannan, R.; Hinton, D.R. Sodium Iodate Induced Retinal Degeneration: New Insights from an Old Model. Neural Regen. Res. 2014, 9, 2044–2045. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Liu, H.-Y.; Luo, M.; Xia, Y.; Yang, X.; Li, H.-Y.; Wu, D.-T.; Sun, Q.; Geng, F.; Gan, R.-Y. Sweet Tea (Lithocarpus Polystachyus Rehd.) as a New Natural Source of Bioactive Dihydrochalcones with Multiple Health Benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 917–934. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A Review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Guo, Y.; Xu, L.; Wang, H. Phlorizin Exerts Potent Effects against Aging Induced by D-Galactose in Mice and PC12 Cells. Food Funct. 2021, 12, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Deng, Y.; Xiao, R.; Xu, F.; Li, M.; Gong, Q.; Gao, J. Anti-Fatigue Effect of Phlorizin on Exhaustive Exercise-Induced Oxidative Injury Mediated by Nrf2/ARE Signaling Pathway in Mice. Eur. J. Pharmacol. 2022, 918, 174563. [Google Scholar] [CrossRef]
- Cambeiro-Pérez, N.; González-Gómez, X.; González-Barreiro, C.; Pérez-Gregorio, M.R.; Fernandes, I.; Mateus, N.; de Freitas, V.; Sánchez, B.; Martínez-Carballo, E. Metabolomics Insights of the Immunomodulatory Activities of Phlorizin and Phloretin on Human THP-1 Macrophages. Molecules 2021, 26, 787. [Google Scholar] [CrossRef]
- Jia, Z.; Xie, Y.; Wu, H.; Wang, Z.; Li, A.; Li, Z.; Yang, Z.; Zhang, Z.; Xing, Z.; Zhang, X. Phlorizin from Sweet Tea Inhibits the Progress of Esophageal Cancer by Antagonizing the JAK2/STAT3 Signaling Pathway. Oncol. Rep. 2021, 46, 137. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Li, X.; Cheng, M.; Cai, Q.; Yu, F.; Wang, W.; Tan, M.; Yan, G.; Hu, S.; et al. Effects of Phlorizin on Diabetic Retinopathy According to Isobaric Tags for Relative and Absolute Quantification-Based Proteomics in Db/Db Mice. Mol. Vis. 2013, 19, 812–821. [Google Scholar]
- Cejková, J.; Ardan, T.; Cejka, C.; Luyckx, J. Favorable Effects of Trehalose on the Development of UVB-Mediated Antioxidant/pro-Oxidant Imbalance in the Corneal Epithelium, Proinflammatory Cytokine and Matrix Metalloproteinase Induction, and Heat Shock Protein 70 Expression. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1185–1194. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant Properties of Flavonoid Metal Complexes and Their Potential Inclusion in the Development of Novel Strategies for the Treatment against Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective Effects of Flavonoids against Alzheimer’s Disease-Related Neural Dysfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Park, J.R.; Choi, W.; Hong, H.K.; Kim, Y.; Jun Park, S.; Hwang, Y.; Kim, P.; Joon Woo, S.; Hyung Park, K.; Oh, W.-Y. Imaging Laser-Induced Choroidal Neovascularization in the Rodent Retina Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT331–OCT340. [Google Scholar] [CrossRef]
- Iwanishi, H.; Fujita, N.; Tomoyose, K.; Okada, Y.; Yamanaka, O.; Flanders, K.C.; Saika, S. Inhibition of Development of Laser-Induced Choroidal Neovascularization with Suppression of Infiltration of Macrophages in Smad3-Null Mice. Lab. Investig. 2016, 96, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Onami, H.; Nagai, N.; Kaji, H.; Nishizawa, M.; Sato, Y.; Osumi, N.; Nakazawa, T.; Abe, T. Transscleral Sustained Vasohibin-1 Delivery by a Novel Device Suppressed Experimentally-Induced Choroidal Neovascularization. PLoS ONE 2013, 8, e58580. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Makeen, H.A.; Albratty, M. Potential Role of Nutraceuticals via Targeting a Wnt/β-Catenin and NF-κB Pathway in Treatment of Osteoarthritis. J. Food Biochem. 2022, 46, e14427. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Wielockx, B.; Munaut, C.; Galopin, C.; Jost, M.; Itoh, T.; Werb, Z.; Baker, A.; Libert, C.; Krell, H.-W.; et al. MMP-2 and MMP-9 Synergize in Promoting Choroidal Neovascularization. FASEB J. 2003, 17, 2290–2292. [Google Scholar] [CrossRef]
- Steen, B.; Sejersen, S.; Berglin, L.; Seregard, S.; Kvanta, A. Matrix Metalloproteinases and Metalloproteinase Inhibitors in Choroidal Neovascular Membranes. Invest. Ophthalmol. Vis. Sci. 1998, 39, 2194–2200. [Google Scholar] [PubMed]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma Levels of Matrix Metalloproteinase-2 and -9 (MMP-2 and MMP-9) in Age-Related Macular Degeneration. Eye 2007, 21, 1511–1515. [Google Scholar] [CrossRef]
- Brodzka, S.; Baszyński, J.; Rektor, K.; Hołderna-Bona, K.; Stanek, E.; Kurhaluk, N.; Tkaczenko, H.; Malukiewicz, G.; Woźniak, A.; Kamiński, P. Immunogenetic and Environmental Factors in Age-Related Macular Disease. Int. J. Mol. Sci. 2024, 25, 6567. [Google Scholar] [CrossRef]
- Baek, S.-M.; Yu, S.-Y.; Son, Y.; Hong, H.S. Substance P Promotes the Recovery of Oxidative Stress-Damaged Retinal Pigmented Epithelial Cells by Modulating Akt/GSK-3β Signaling. Mol. Vis. 2016, 22, 1015–1023. [Google Scholar]
- Wang, H.; Cheng, J.; Wang, H.; Wang, M.; Zhao, J.; Wu, Z. Protective Effect of Apple Phlorizin on Hydrogen Peroxide-Induced Cell Damage in HepG2 Cells. J. Food Biochem. 2019, 43, e13052. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, S.-J.; Jung, U.J.; Ryu, R.; Choi, M.-S. Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Auner, B.G.; Wirth, M.; Valenta, C. Antioxidative Activity and Cytotoxicity of Four Different Flavonoids for Dermal Applications. J. Drug Deliv. Sci. Technol. 2005, 15, 227–232. [Google Scholar] [CrossRef]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Hanus, J.; Zhang, H.; Wang, Z.; Liu, Q.; Zhou, Q.; Wang, S. Induction of Necrotic Cell Death by Oxidative Stress in Retinal Pigment Epithelial Cells. Cell Death Dis. 2013, 4, e965. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating Mitochondria as a Target for Treating Age-Related Macular Degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef]
- Blasiak, J.; Glowacki, S.; Kauppinen, A.; Kaarniranta, K. Mitochondrial and Nuclear DNA Damage and Repair in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2013, 14, 2996–3010. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R.; et al. Altered Bioenergetics and Enhanced Resistance to Oxidative Stress in Human Retinal Pigment Epithelial Cells from Donors with Age-Related Macular Degeneration. Redox Biol. 2017, 13, 255–265. [Google Scholar] [CrossRef]
- Decanini, A.; Nordgaard, C.L.; Feng, X.; Ferrington, D.A.; Olsen, T.W. Changes in Select Redox Proteins of the Retinal Pigment Epithelium in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2007, 143, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, J.; Rowe, G.C.; Khadka, A.; Diaz-Aguilar, D.; Spencer, C.; Arany, Z.; Saint-Geniez, M. PGC-1α Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Elbay, A.; Ozer, O.F.; Akkan, J.C.U.; Celik, U.; Kutlutürk, I.; Koytak, A.; Ozdemir, H. Comparison of Serum Thiol-Disulphide Homeostasis and Total Antioxidant-Oxidant Levels between Exudative Age-Related Macular Degeneration Patients and Healthy Subjects. Int. Ophthalmol. 2017, 37, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, Z.K.; Sarı, İ.; Yıldırım, B.G.; Güntürk, İ.; Küçük, E.; Erşan, S.; Seydel, G.Ş. Evaluation of Oxidative Stress, 3-Nitrotyrosine, and HMGB-1 Levels in Patients with Wet Type Age-Related Macular Degeneration. J. Med. Biochem. 2022, 41, 275–281. [Google Scholar] [CrossRef]
- Zafrilla, P.; Losada, M.; Perez, A.; Caravaca, G.; Mulero, J. Biomarkers of Oxidative Stress in Patients with Wet Age Related Macular Degeneration. J. Nutr. Health Aging 2013, 17, 219–222. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Choi, C.-I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Zhuravlev, A.D.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 5371. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Shafi, V.; Khan, N.A.; Kazmi, J.; Siddiqui, I. Cytokine-Driven NF-κB Activation in Retinal Cells and Its Impact on the Pathogenesis of Age-Related Macular Degeneration: A Systematic Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Klettner, A. Oxidative Stress Induced Cellular Signaling in RPE Cells. Front. Biosci.-Sch. 2012, 4, 392–411. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, J.J. The Role of NF-kappaB in the Regulation of Cell Stress Responses. Int. Immunopharmacol. 2002, 2, 1509–1520. [Google Scholar] [CrossRef]
- Outtz, H.H.; Tattersall, I.W.; Kofler, N.M.; Steinbach, N.; Kitajewski, J. Notch1 Controls Macrophage Recruitment and Notch Signaling Is Activated at Sites of Endothelial Cell Anastomosis during Retinal Angiogenesis in Mice. Blood 2011, 118, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Richter, J.; Herndon, J.; Ferguson, T.A. Macrophages Inhibit Neovascularization in a Murine Model of Age-Related Macular Degeneration. PLoS Med. 2006, 3, e310. [Google Scholar] [CrossRef]
- Leibovich, S.J.; Polverini, P.J.; Shepard, H.M.; Wiseman, D.M.; Shively, V.; Nuseir, N. Macrophage-Induced Angiogenesis Is Mediated by Tumour Necrosis Factor-Alpha. Nature 1987, 329, 630–632. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P. Macrophage and Retinal Pigment Epithelium Expression of Angiogenic Cytokines in Choroidal Neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar]
- Ghasemi, H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul. Immunol. Inflamm. 2018, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Supanji; Perdamaian, A.B.I.; Dianratri, A.; Prayogo, M.E.; Sasongko, M.B.; Wardhana, F.S.; Widayanti, T.W.; Agni, A.N.; Oka, C. The Circulating Level of IL-1β in Patients with Age-Related Macular Degeneration (AMD) in Yogyakarta: Characteristics to Disease Activity; Atlantis Press: Dordrecht, The Netherlands, 2021; pp. 434–436. [Google Scholar]
- Qazi, Y.; Maddula, S.; Ambati, B.K. Mediators of Ocular Angiogenesis. J. Genet. 2009, 88, 495–515. [Google Scholar] [CrossRef]
- Campa, C.; Costagliola, C.; Incorvaia, C.; Sheridan, C.; Semeraro, F.; De Nadai, K.; Sebastiani, A.; Parmeggiani, F. Inflammatory Mediators and Angiogenic Factors in Choroidal Neovascularization: Pathogenetic Interactions and Therapeutic Implications. Mediat. Inflamm. 2010, 2010, 546826. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [CrossRef] [PubMed]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Age-Related Eye Disease Study 2 Research Group Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A Multitargeted Agent for Age-Associated Chronic Diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef]
- Khan, A.A.; Dace, D.S.; Ryazanov, A.G.; Kelly, J.; Apte, R.S. Resveratrol Regulates Pathologic Angiogenesis by a Eukaryotic Elongation Factor-2 Kinase-Regulated Pathway. Am. J. Pathol. 2010, 177, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A.; et al. Effects of Oxysterols on Cell Viability, Inflammatory Cytokines, VEGF, and Reactive Oxygen Species Production on Human Retinal Cells: Cytoprotective Effects and Prevention of VEGF Secretion by Resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-Related Macular Degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [CrossRef]
- Hara, R.; Inomata, Y.; Kawaji, T.; Sagara, N.; Inatani, M.; Fukushima, M.; Tanihara, H. Suppression of Choroidal Neovascularization by N-Acetyl-Cysteine in Mice. Curr. Eye Res. 2010, 35, 1012–1020. [Google Scholar] [CrossRef]
- Tisi, A.; Flati, V.; Delle Monache, S.; Lozzi, L.; Passacantando, M.; Maccarone, R. Nanoceria Particles Are an Eligible Candidate to Prevent Age-Related Macular Degeneration by Inhibiting Retinal Pigment Epithelium Cell Death and Autophagy Alterations. Cells 2020, 9, 1617. [Google Scholar] [CrossRef]
- Khoo, H.E.; Ng, H.S.; Yap, W.-S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef]
- Parmar, T.; Ortega, J.T.; Jastrzebska, B. Retinoid Analogs and Polyphenols as Potential Therapeutics for Age-Related Macular Degeneration. Exp. Biol. Med. 2020, 245, 1615–1625. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Kifley, A.; Flood, V.M.; Joachim, N.; Lewis, J.R.; Hodgson, J.M.; Mitchell, P. Dietary Flavonoids and the Prevalence and 15-y Incidence of Age-Related Macular Degeneration. Am. J. Clin. Nutr. 2018, 108, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, T.; Zhao, J.; Zhu, X.; Xin, W.; Zhang, F.; Zhang, L. Role of Flavonoids in Age-Related Macular Degeneration. Biomed. Pharmacother. 2023, 159, 114259. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C. Priming Plant Resistance by Activation of Redox-Sensitive Genes. Free. Radic. Biol. Med. 2018, 122, 171–180. [Google Scholar] [CrossRef]
- Ortega, J.T.; Jastrzebska, B. Neuroinflammation as a Therapeutic Target in Retinitis Pigmentosa and Quercetin as Its Potential Modulator. Pharmaceutics 2021, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Hytti, M.; Piippo, N.; Salminen, A.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Quercetin Alleviates 4-Hydroxynonenal-Induced Cytotoxicity and Inflammation in ARPE-19 Cells. Exp. Eye Res. 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsiao, Y.-P.; Lin, Y.-T.; Chen, C.; Lee, C.-M.; Liao, W.-C.; Tsou, S.-C.; Lin, H.-W.; Chang, Y.-Y. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1?-Sirt1 Pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liou, C.-J.; Shen, S.-C.; Hu, S.; Hsiao, C.-Y.; Wu, S.-J. Luteolin Attenuates IL-1β-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-κB and MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9421340. [Google Scholar] [CrossRef]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Chen, C.; Guo, D.; Lu, G. Wogonin Protects Human Retinal Pigment Epithelium Cells from LPS-Induced Barrier Dysfunction and Inflammatory Responses by Regulating the TLR4/NF-κB Signaling Pathway. Mol. Med. Rep. 2017, 15, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Zhang, S.; Rao, J.; Zhao, J.; Huang, H.; Liu, Y.; Ding, Y.; Liu, Y.; Ma, Y.; Zhang, S.; et al. Phlorizin, an Important Glucoside: Research Progress on Its Biological Activity and Mechanism. Molecules 2024, 29, 741. [Google Scholar] [CrossRef] [PubMed]



| Genes | Primers | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | GGGGCTGCCCAGAACATCAT |
| Reverse | GCCTGCTTCACCACCTTCTTG | |
| IL1-β | Forward | GCTGAGGAAGATGCTGGTTC |
| Reverse | TCCATATCCTGTCCCTGGAG | |
| IL-6 | Forward | CACAGACAGCCACTCACCTC |
| Reverse | TTTTCTGCCAGTGCCTCTTT | |
| IL-8 | Forward | GCAGAGGGTTGTGGAGAAGT |
| Reverse | TGGCATCTTCACTGATTCTTGG | |
| TNF-α | Forward | ATGCAGTTTGGCCAAGGAGA |
| Reverse | GCTTCTCAACAACCCTCTGA | |
| VEGF | Forward | AGTTCCACCACCAAACATGC |
| Reverse | TGAAGGGACACAACGACACA | |
| MMP-2 | Forward | TGGCAAGGTGTGGTGTGCGAC |
| Reverse | TCGGGGCCATCAGAGCTCCAG | |
| MMP-9 | Forward | GGTGTGCCCTGGAACTCACACG |
| Reverse | AGGGCACTGCAGGAGGTCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.-Y.; Hung, C.-Y.; Hsu, Y.-J.; Liang, I.-C.; Chen, Y.-C.; Sung, C.-H.; Hung, C.-F. Phlorizin Protects Against Oxidative Stress and Inflammation in Age-Related Macular Degeneration Model. Biomolecules 2025, 15, 523. https://doi.org/10.3390/biom15040523
Liao Z-Y, Hung C-Y, Hsu Y-J, Liang I-C, Chen Y-C, Sung C-H, Hung C-F. Phlorizin Protects Against Oxidative Stress and Inflammation in Age-Related Macular Degeneration Model. Biomolecules. 2025; 15(4):523. https://doi.org/10.3390/biom15040523
Chicago/Turabian StyleLiao, Zhen-Yu, Chih-Yu Hung, Yu-Jou Hsu, I-Chia Liang, Yi-Chun Chen, Chao-Hsien Sung, and Chi-Feng Hung. 2025. "Phlorizin Protects Against Oxidative Stress and Inflammation in Age-Related Macular Degeneration Model" Biomolecules 15, no. 4: 523. https://doi.org/10.3390/biom15040523
APA StyleLiao, Z.-Y., Hung, C.-Y., Hsu, Y.-J., Liang, I.-C., Chen, Y.-C., Sung, C.-H., & Hung, C.-F. (2025). Phlorizin Protects Against Oxidative Stress and Inflammation in Age-Related Macular Degeneration Model. Biomolecules, 15(4), 523. https://doi.org/10.3390/biom15040523








