Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Culture Conditions
2.2. Adipocyte Specific Manipulation of Gene Expression
2.3. Ovary Immunostaining and Fluorescence Microscopy
2.4. Adipocyte Immunostaining and Fluorescence Microscopy
2.5. Ovarian Analysis
2.6. Blocked Ovulation Analysis
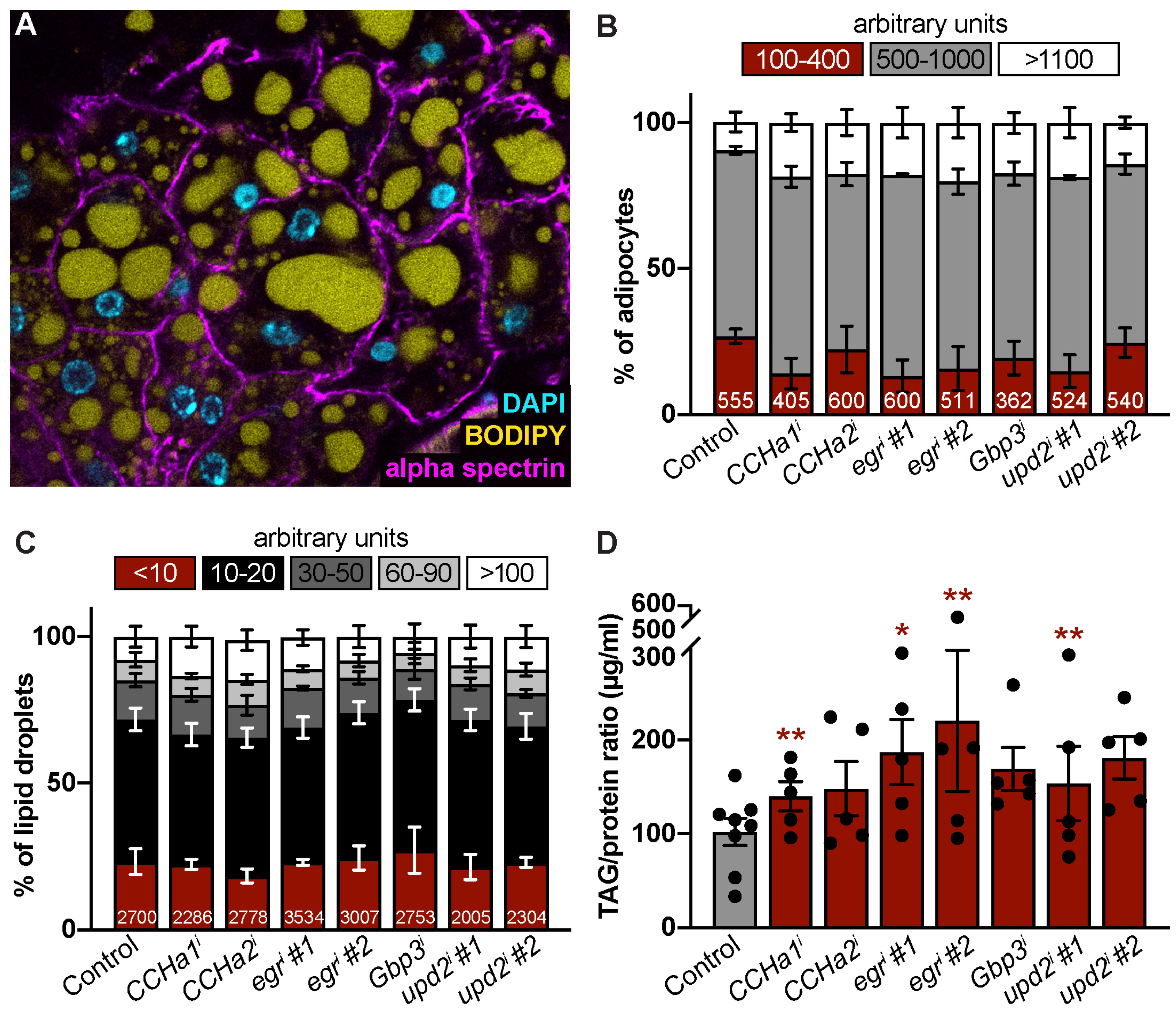
2.7. Measurement of Adipocyte and Lipid Droplet Size
2.8. Adipocyte Bradford and Triglyceride Assays
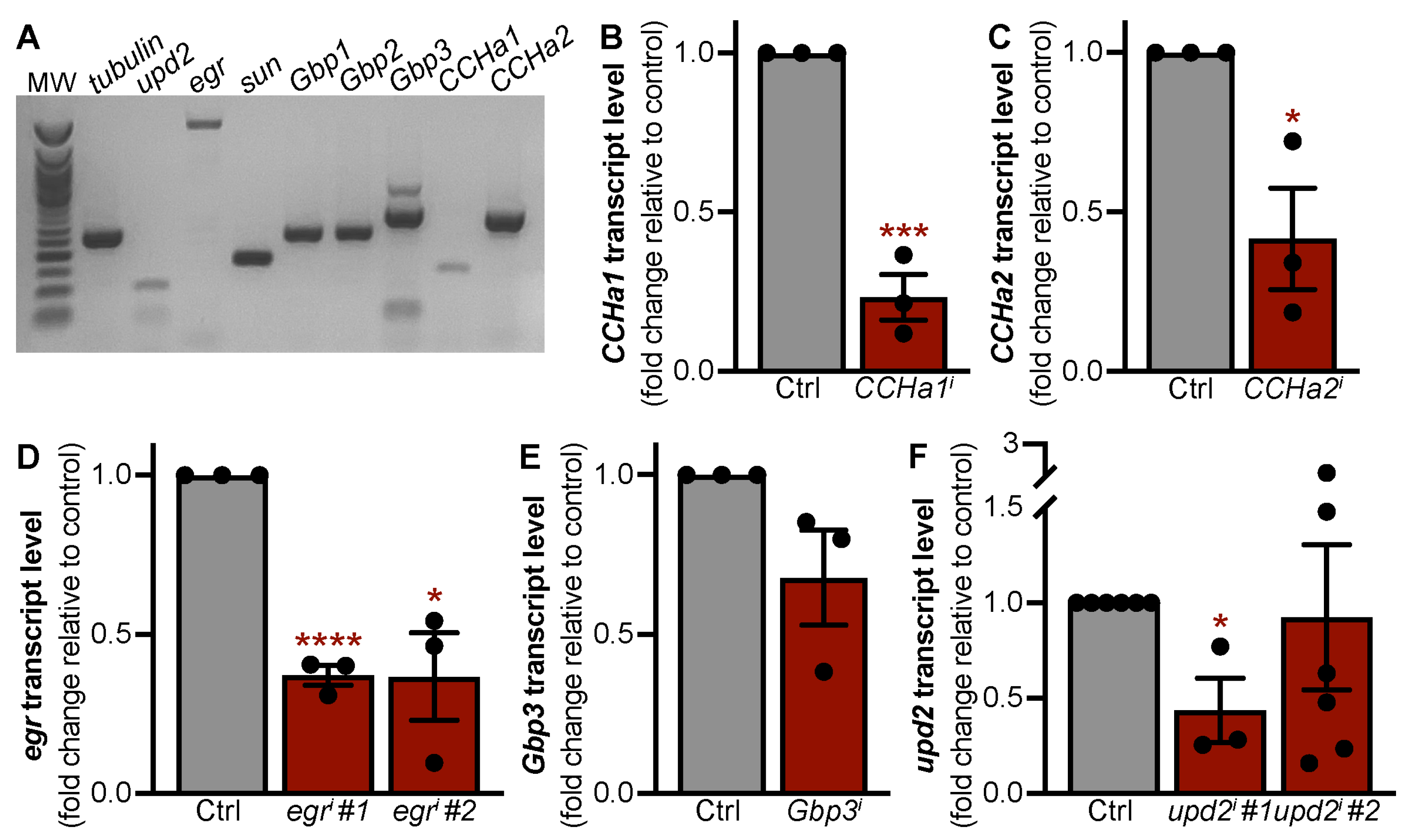
2.9. RNA Isolation, RT-PCR, and qPCR
3. Results
3.1. Adipokines with Roles in Larval Development Are Expressed in the Adult Fat Body
3.2. CCHa1, CCHa2, Egr, Gbp3, and Upd2 Do Not Cell-Autonomously Control Adipocyte or Lipid Droplet Size
3.3. Adipocyte-Derived CCHa1, CCHa2, Egr, Gbp3, and Upd2 Do Not Regulate Ovarian GSC Maintenance
3.4. Adipocyte-Derived CCHa1, CCHa2, Egr, Gbp3, and Upd2 Do Not Support Early Germline Survival or Progression Through Vitellogenesis
3.5. Adipocyte-Derived Gpb3 Promotes Ovulation of Mature Oocytes
4. Discussion
4.1. The Fat Body Utilizes Multiple Adipokines to Regulate the Ovarian Germline Stem Cell Lineage
4.2. Direct Communication from Adipocytes to the Ovary
4.3. Indirect Communication from Adipocytes to the Ovary
4.4. The Partial Knockdown of Target Adipokines from the Fat Body May Not Be Enough to Influence Ovarian Homeostasis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A Preventable, Treatable, but Relapsing Disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Restrepo, B.J. Obesity Prevalence among U.s. Adults during the COVID-19 Pandemic. Am. J. Prev. Med. 2022, 63, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Van Herpen, N.A.; Schrauwen-Hinderling, V.B. Lipid Accumulation in Non-Adipose Tissue and Lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Pérez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Gálvez, B.G. ‘Adipaging’: Ageing and Obesity Share Biological Hallmarks Related to a Dysfunctional Adipose Tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar]
- Kopelman, P.G. Obesity as a Medical Problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Ahima, R.S.; Lazar, M.A. Adipokines and the Peripheral and Neural Control of Energy Balance. Mol. Endocrinol. 2008, 22, 1023–1031. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as Disruptor of the Female Fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.P.; Kühnlein, R.P. Drosophila as a Model to Study Obesity and Metabolic Disease. J. Exp. Biol. 2018, 221, jeb163881. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamamoto, S. Role of Drosophila in Human Disease Research 2.0. Int. J. Mol. Sci. 2022, 23, 4203. [Google Scholar] [CrossRef]
- Droujinine, I.A.; Perrimon, N. Interorgan Communication Pathways in Physiology: Focus on Drosophila. Annu. Rev. Genet. 2016, 50, 539–570. [Google Scholar] [CrossRef]
- Beumer, K.J.; Carroll, D. Targeted Genome Engineering Techniques in Drosophila. Methods 2014, 68, 29–37. [Google Scholar] [CrossRef]
- Colombani, J.; Raisin, S.; Pantalacci, S.; Radimerski, T.; Montagne, J.; Léopold, P. A Nutrient Sensor Mechanism Controls Drosophila Growth. Cell 2003, 114, 739–749. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Bickmeyer, I.; Kühnlein, R.P. Reliable Drosophila Body Fat Quantification by a Coupled Colorimetric Assay. PLoS ONE 2011, 6, e23796. [Google Scholar] [CrossRef]
- Kühnlein, R.P. Energy Homeostasis Regulation in Drosophila: A Lipocentric Perspective. Results Probl. Cell Differ. 2010, 52, 159–173. [Google Scholar] [CrossRef]
- Meschi, E.; Delanoue, R. Adipokine and Fat Body in Flies: Connecting Organs. Mol. Cell. Endocrinol. 2021, 533, 111339. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Laws, K.M.; Drummond-Barbosa, D. Adipocyte Amino Acid Sensing Controls Adult Germline Stem Cell Number via the Amino Acid Response Pathway and Independently of Target of Rapamycin Signaling in Drosophila. Development 2014, 141, 4479–4488. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Armstrong, A.R.; Sampson, L.L.; Laws, K.M.; Drummond-Barbosa, D. Adipocyte Metabolic Pathways Regulated by Diet Control the Female Germline Stem Cell Lineage in Drosophila Melanogaster. Genetics 2017, 206, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Drummond-Barbosa, D. Insulin Signaling Acts in Adult Adipocytes via GSK-3β and Independently of FOXO to Control Drosophila Female Germline Stem Cell Numbers. Dev. Biol. 2018, 440, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.; Simmons, C.; Ott, R.K.; Armstrong, A.R. Ras/MAPK Signaling Mediates Adipose Tissue Control of Ovarian Germline Survival and Ovulation in Drosophila Melanogaster. Dev. Biol. 2024, 510, 17–28. [Google Scholar] [CrossRef]
- Grmai, L.; Michaca, M.; Lackner, E.; Nampoothiri, N.V.P.; Vasudevan, D. Integrated Stress Response Signaling Acts as a Metabolic Sensor in Fat Tissues to Regulate Oocyte Maturation and Ovulation. Cell Rep. 2024, 43, 113863. [Google Scholar] [CrossRef]
- Weaver, L.N.; Drummond-Barbosa, D. Maintenance of Proper Germline Stem Cell Number Requires Adipocyte Collagen in Adult Drosophila Females. Genetics 2018, 209, 1155–1166. [Google Scholar] [CrossRef]
- Weaver, L.N.; Drummond-Barbosa, D. The Nuclear Receptor Seven up Functions in Adipocytes and Oenocytes to Control Distinct Steps of Drosophila Oogenesis. Dev. Biol. 2019, 456, 179–189. [Google Scholar] [CrossRef]
- Carrera, P.; Odenthal, J.; Risse, K.S.; Jung, Y.; Kuerschner, L.; Bülow, M.H. The CD36 Scavenger Receptor Bez Regulates Lipid Redistribution from Fat Body to Ovaries in Drosophila. Development 2024, 151, dev202551. [Google Scholar] [CrossRef]
- Wieschaus, E.; Szabad, J. The Development and Function of the Female Germ Line in Drosophila Melanogaster: A Cell Lineage Study. Dev. Biol. 1979, 68, 29–46. [Google Scholar] [CrossRef]
- Lin, H.; Spradling, A.C. Germline Stem Cell Division and Egg Chamber Development in Transplanted Drosophila Germaria. Dev. Biol. 1993, 159, 140–152. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. The Control of Body Size in Insects. Dev. Biol. 2003, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Colombani, J.; Bianchini, L.; Layalle, S.; Pondeville, E.; Dauphin-Villemant, C.; Antoniewski, C.; Carré, C.; Noselli, S.; Léopold, P. Antagonistic Actions of Ecdysone and Insulins Determine Final Size in Drosophila. Science 2005, 310, 667–670. [Google Scholar] [CrossRef]
- Li, S.; Torre-Muruzabal, T.; Søgaard, K.C.; Ren, G.R.; Hauser, F.; Engelsen, S.M.; Pødenphanth, M.D.; Desjardins, A.; Grimmelikhuijzen, C.J.P. Expression Patterns of the Drosophila Neuropeptide CCHamide-2 and Its Receptor May Suggest Hormonal Signaling from the Gut to the Brain. PLoS ONE 2013, 8, e76131. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hermann-Luibl, C.; Katsura, M.; Sekiguchi, M.; Ida, T.; Helfrich-Förster, C.; Yoshii, T. The CCHamide1 Neuropeptide Expressed in the Anterior Dorsal Neuron 1 Conveys a Circadian Signal to the Ventral Lateral Neurons in Drosophila Melanogaster. Front. Physiol. 2018, 9, 1276. [Google Scholar] [CrossRef]
- Kuwano, R.; Katsura, M.; Iwata, M.; Yokosako, T.; Yoshii, T. Pigment-Dispersing Factor and CCHamide1 in the Drosophila Circadian Clock Network. Chronobiol. Int. 2023, 40, 284–299. [Google Scholar] [CrossRef]
- Sano, H.; Nakamura, A.; Texada, M.J.; Truman, J.W.; Ishimoto, H.; Kamikouchi, A.; Nibu, Y.; Kume, K.; Ida, T.; Kojima, M. The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of Drosophila Melanogaster. PLoS Genet. 2015, 11, e1005209. [Google Scholar] [CrossRef]
- Agrawal, N.; Delanoue, R.; Mauri, A.; Basco, D.; Pasco, M.; Thorens, B.; Léopold, P. The Drosophila TNF Eiger Is an Adipokine That Acts on Insulin-Producing Cells to Mediate Nutrient Response. Cell Metab. 2016, 23, 675–684. [Google Scholar] [CrossRef]
- Loudhaief, R.; Jneid, R.; Christensen, C.F.; Mackay, D.J.; Andersen, D.S.; Colombani, J. The Drosophila Tumor Necrosis Factor Receptor, Wengen, Couples Energy Expenditure with Gut Immunity. Sci. Adv. 2023, 9, eadd4977. [Google Scholar] [CrossRef]
- Koyama, T.; Mirth, C.K. Growth-Blocking Peptides As Nutrition-Sensitive Signals for Insulin Secretion and Body Size Regulation. PLoS Biol. 2016, 14, e1002392. [Google Scholar] [CrossRef]
- Rajan, A.; Perrimon, N. Drosophila Cytokine Unpaired 2 Regulates Physiological Homeostasis by Remotely Controlling Insulin Secretion. Cell 2012, 151, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Housden, B.E.; Wirtz-Peitz, F.; Holderbaum, L.; Perrimon, N. A Mechanism Coupling Systemic Energy Sensing to Adipokine Secretion. Dev. Cell 2017, 43, 83–98.e6. [Google Scholar] [CrossRef]
- Brent, A.E.; Rajan, A. Insulin and Leptin/Upd2 Exert Opposing Influences on Synapse Number in Fat-Sensing Neurons. Cell Metab. 2020, 32, 786–800.e7. [Google Scholar] [CrossRef]
- Flier, J.S.; Maratos-Flier, E. Leptin’s Physiologic Role: Does the Emperor of Energy Balance Have No Clothes? Cell Metab. 2017, 26, 24–26. [Google Scholar] [CrossRef]
- Abuhattum, S.; Kotzbeck, P.; Schlüßler, R.; Harger, A.; Ariza de Schellenberger, A.; Kim, K.; Escolano, J.-C.; Müller, T.; Braun, J.; Wabitsch, M.; et al. Adipose Cells and Tissues Soften with Lipid Accumulation While in Diabetes Adipose Tissue Stiffens. Sci. Rep. 2022, 12, 10325. [Google Scholar] [CrossRef]
- Pathak, H.; Varghese, J. Edem1 Activity in the Fat Body Regulates Insulin Signalling and Metabolic Homeostasis in Drosophila. Life Sci. Alliance 2021, 4, e202101079. [Google Scholar] [CrossRef]
- Ott, R.K.; Williams, I.H.; Armstrong, A.R. Improved Whole-Mount Immunofluorescence Protocol for Consistent and Robust Labeling of Adult Drosophila Melanogaster Adipose Tissue. Biol. Open 2024, 13, bio060491. [Google Scholar] [CrossRef]
- DiAngelo, J.R.; Birnbaum, M.J. Regulation of Fat Cell Mass by Insulin in Drosophila Melanogaster. Mol. Cell. Biol. 2009, 29, 6341–6352. [Google Scholar] [CrossRef]
- Drummond-Barbosa, D.; Spradling, A.C. Stem Cells and Their Progeny Respond to Nutritional Changes during Drosophila Oogenesis. Dev. Biol. 2001, 231, 265–278. [Google Scholar] [CrossRef]
- Droujinine, I.A.; Meyer, A.S.; Wang, D.; Udeshi, N.D.; Hu, Y.; Rocco, D.; McMahon, J.A.; Yang, R.; Guo, J.; Mu, L.; et al. Proteomics of Protein Trafficking by in Vivo Tissue-Specific Labeling. Nat. Commun. 2021, 12, 2382. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.D.; Drummond-Barbosa, D. A High-Sugar Diet, but Not Obesity, Reduces Female Fertility in Drosophila Melanogaster. Development 2023, 150, dev201769. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-Sensing Mechanisms and Pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-X.; Chen, D.; Deng, S.-L.; Li, J.; Li, Y.; Fu, Z.; Wang, X.-X.; Zhang, Y.; Chen, S.-R.; Liu, Y.-X. CRISPR/Cas9-Mediated Genome Editing Induces Gene Knockdown by Altering the Pre-MRNA Splicing in Mice. BMC Biotechnol. 2018, 18, 61. [Google Scholar] [CrossRef]
- Havula, E.; Ghazanfar, S.; Lamichane, N.; Francis, D.; Hasygar, K.; Liu, Y.; Alton, L.A.; Johnstone, J.; Needham, E.J.; Pulpitel, T.; et al. Genetic Variation of Macronutrient Tolerance in Drosophila Melanogaster. Nat. Commun. 2022, 13, 1637. [Google Scholar] [CrossRef]
- Palmerini, V.; Monzani, S.; Laurichesse, Q.; Loudhaief, R.; Mari, S.; Cecatiello, V.; Olieric, V.; Pasqualato, S.; Colombani, J.; Andersen, D.S.; et al. Drosophila TNFRs Grindelwald and Wengen Bind Eiger with Different Affinities and Promote Distinct Cellular Functions. Nat. Commun. 2021, 12, 2070. [Google Scholar] [CrossRef]
- Sung, E.J.; Ryuda, M.; Matsumoto, H.; Uryu, O.; Ochiai, M.; Cook, M.E.; Yi, N.Y.; Wang, H.; Putney, J.W.; Bird, G.S.; et al. Cytokine Signaling through Drosophila Mthl10 Ties Lifespan to Environmental Stress. Proc. Natl. Acad. Sci. USA 2017, 114, 13786–13791. [Google Scholar] [CrossRef]
- Ono, M.; Matsumura, T.; Sung, E.J.; Koyama, T.; Ochiai, M.; Shears, S.B.; Hayakawa, Y. Drosophila Cytokine GBP2 Exerts Immune Responses and Regulates GBP1 Expression through GPCR Receptor Mthl10. Insect Biochem. Mol. Biol. 2024, 167, 104086. [Google Scholar] [CrossRef]
- Brown, S.; Hu, N.; Hombría, J.C. Identification of the First Invertebrate Interleukin JAK/STAT Receptor, the Drosophila Gene Domeless. Curr. Biol. 2001, 11, 1700–1705. [Google Scholar] [CrossRef]
- Li, H.; Janssens, J.; De Waegeneer, M.; Kolluru, S.S.; Davie, K.; Gardeux, V.; Saelens, W.; David, F.P.A.; Brbić, M.; Spanier, K.; et al. Fly Cell Atlas: A Single-Nucleus Transcriptomic Atlas of the Adult Fruit Fly. Science 2022, 375, eabk2432. [Google Scholar] [CrossRef]
- Berg, C.; Sieber, M.; Sun, J. Finishing the Egg. Genetics 2024, 226, iyad183. [Google Scholar] [CrossRef] [PubMed]
- Ghiglione, C.; Devergne, O.; Georgenthum, E.; Carballès, F.; Médioni, C.; Cerezo, D.; Noselli, S. The Drosophila Cytokine Receptor Domeless Controls Border Cell Migration and Epithelial Polarization during Oogenesis. Development 2002, 129, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Beccari, S.; Teixeira, L.; Rørth, P. The JAK/STAT Pathway Is Required for Border Cell Migration during Drosophila Oogenesis. Mech. Dev. 2002, 111, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Hétié, P.; Matunis, E.L. Niche Signaling Promotes Stem Cell Survival in the Drosophila Testis via the JAK-STAT Target DIAP1. Dev. Biol. 2015, 404, 27–39. [Google Scholar] [CrossRef]
- Laws, K.M.; Drummond-Barbosa, D. Control of Germline Stem Cell Lineages by Diet and Physiology. Results Probl. Cell Differ. 2017, 59, 67–99. [Google Scholar] [CrossRef]
- Yamagata, N.; Imanishi, Y.; Wu, H.; Kondo, S.; Sano, H.; Tanimoto, H. Nutrient Responding Peptide Hormone CCHamide-2 Consolidates Appetitive Memory. Front. Behav. Neurosci. 2022, 16, 986064. [Google Scholar] [CrossRef]
- Drummond-Barbosa, D. Local and Physiological Control of Germline Stem Cell Lineages in Drosophila Melanogaster. Genetics 2019, 213, 9–26. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simmons, C.; Williams, I.H.; Bradshaw, T.W.; Armstrong, A.R. Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis. Biomolecules 2025, 15, 513. https://doi.org/10.3390/biom15040513
Simmons C, Williams IH, Bradshaw TW, Armstrong AR. Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis. Biomolecules. 2025; 15(4):513. https://doi.org/10.3390/biom15040513
Chicago/Turabian StyleSimmons, Chad, Isaiah H. Williams, Tancia W. Bradshaw, and Alissa Richmond Armstrong. 2025. "Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis" Biomolecules 15, no. 4: 513. https://doi.org/10.3390/biom15040513
APA StyleSimmons, C., Williams, I. H., Bradshaw, T. W., & Armstrong, A. R. (2025). Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis. Biomolecules, 15(4), 513. https://doi.org/10.3390/biom15040513





