Mapping Current Studies of tRNA Fragments onto Disease Landscape
Abstract
1. Introduction
Examples of Mechanisms and Processes Involving tRFs
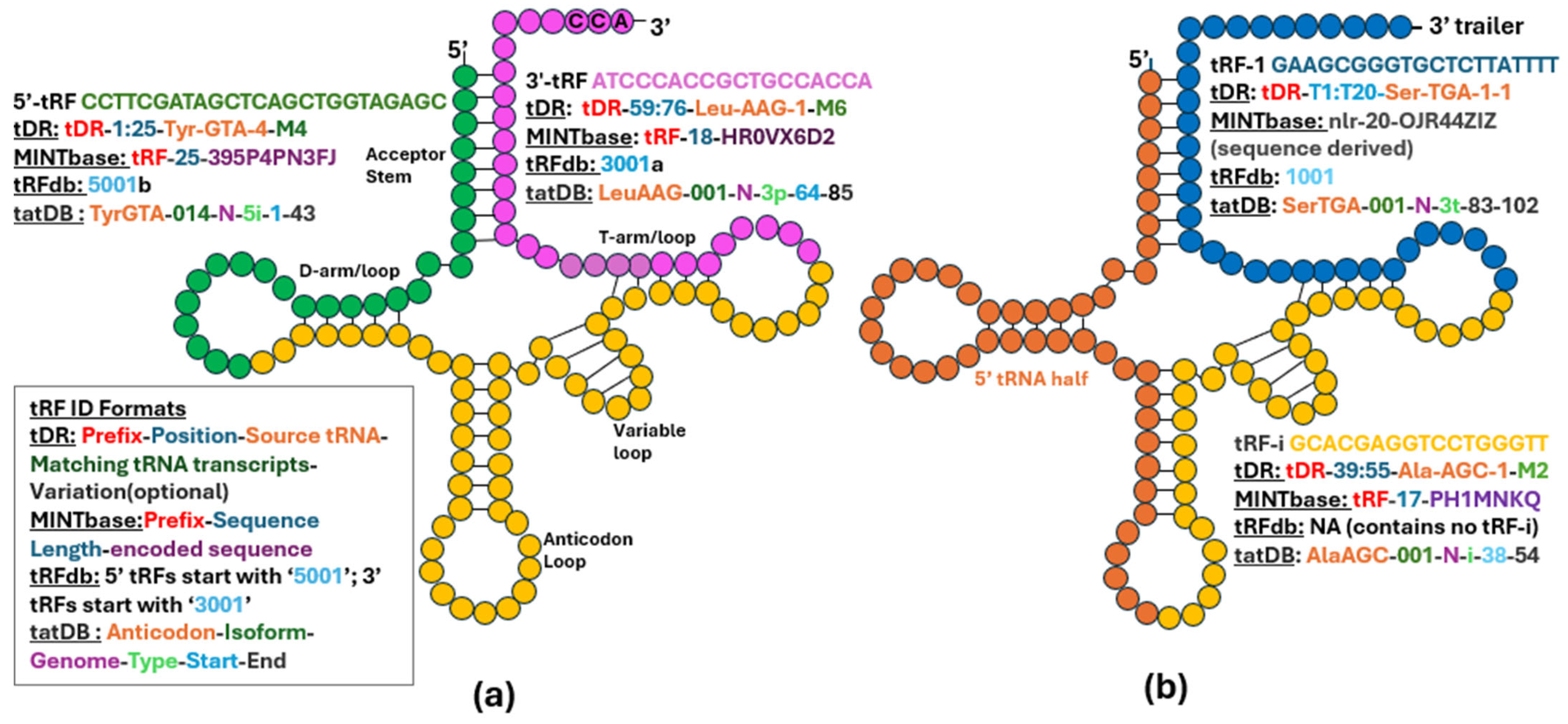
2. Naming tRFs and Classifying Paper/Study Types
Evolution of tRF Detection and Analysis
3. Cancer
3.1. tRFs in Pancreatic Cancer
3.2. tRFs in Lung Cancer
3.3. tRFs in Colorectal Cancer
3.4. tRFs in Gastric Cancer
3.5. tRFs in Nervous System Cancers
3.6. tRFs in Breast Cancer
3.7. tRFs in Ovarian Cancer
3.8. tRFs in Prostate Cancer
3.9. Leukemia
4. Neurological Diseases
4.1. Spinal Cord Injury and tRFs
4.2. MELAS and tRFs
4.3. Autism and tRFs
4.4. Traumatic Brain Injury and tRFs
4.5. Stroke and tRFs
4.6. Perioperative Neurocognitive Disorder and tRFs
4.7. Alzheimer’s Disease and tRFs
4.8. Huntington’s Disease and tRFs
4.9. Parkinsons and tRFs
4.10. Epilepsy and tRFs
4.11. Amyotrophic Lateral Sclerosis and tRFs
5. Cardiovascular Diseases
5.1. Cardiac Hypertrophy, Heart Failure and tRFs
5.2. Pulmonary Arterial Hypertension and tRFs
5.3. Atherosclerosis and tRFs
5.4. Rheumatic Heart Disease and tRFs
5.5. Aortic Dissection and tRFs
5.6. Myocardial Ischemia and tRFs
5.7. Intimal Hyperplasia and tRFs
6. Musculoskeletal Diseases
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sequence | ID | Reference |
|---|---|---|
| Cancer | ||
| TCCCTGGTGGTCTAGTGGTTAGGTT | T[33]a | [33] |
| ATCCCACCGCTGCCACCA | T[34]a | [34] |
| TCCCCGGCACCTCCACCA | T[35]a | [35] |
| CTTACACTTAGGAGATTTCAACTTAACTTGACCGCTCTGAC | T[36]a | [36] |
| CCCCGAAAATGTTGGTTATACCCTTCCCGTACTACCA | T[36]b | [36] |
| GGCCGGTTAGCTCAG | T[37]a | [37] |
| TTGTGGAAACAATGGTACGGCAAGGGCCTCTTT | T[38]a | [38] |
| AAGGGAGGTTATGATTAACTTT | T[38]b | [38] |
| GCTAAGGAAGTCCTGTGCTCAGTTT | T[38]c | [38,39] |
| CCGTGTTTCCCCCACGCTTT | T[38]d | [38,39] |
| GAAGCGGGTGCTCTTATTTTTT | T[40]a | [40] |
| TCGAGAGGGGCTGTGCTCGCAAGGTTTCTTTT | T[40]b | [40] |
| AAGTGGTTCCCGTTTT | T[40]c | [40] |
| TCCCACCGCTGCCACCC | T[41]a | [41] |
| GTTTCCGTAGTGTAGTGG | T[46]a | [46] |
| TAGGATGGGGTGTGATAGGTGGCA | T[47]a | [47,48] |
| GCATGGGTGGTTCAGTGGTAGAATTCTCGCCTG | T[27]a | [27] |
| GCTTCTGTAGTGTAGTGGTTATC | T[49]a | [49] |
| CAACTATCTTATTCTCCTTT | T[50]a | [50] |
| ATCCCCTTCTGACACCA | T[50]b | [50] |
| TCGAATCCCACTTCTGACACCA | T[50]c | [50] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTC | T[53]a | [53] |
| GCATGGGTGGTTCAGTGGTAGAATTCTCGCCTG | T[27]a | [53] |
| GCCGTGATCGTATAGTGGTTAGTACTCTGCG | T[53]b | [53] |
| CACTGTAAAGCTAACTTAGCATTAACCTTTTA | T[53]c | [53] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTG | T[53]d | [53] |
| GGCCGCGTGGCCTAATGGA | T[51]a | [51] |
| GGGCCAGTGGCGCAATGGA | T[51]b | [51] |
| AGAGGGTCTTTTTCACCCCGCTGTTGCTCTTT | T[54]a | [54] |
| TCGAGAGGGGCTGTGCTCGCAAGGTTTCTT | T[54]b | [54] |
| GCCGAAATAGCTCAGTTGGGAGAGCGTTAGACTG | T[54]c | [54] |
| AGGCGGCCCGGGTTCGACTCCCGGTGTGGGAA | T[55]a | [55] |
| AGTGGTTAGGATTCGGCGCTCTCACCGCCGCGGCCCGCGTTCGTTTCGCGGTCAGCCCA | T[56]a | [56] |
| AGTGGTTAGTATCCCCGCCTGTCACGCGGGAGACCGGCGTTCAATTCGGCGACGACCA | T[56]b | [56] |
| AGTGGTTAGCATAGCTGCCTTCCAAGCAGTTGACACGGGTTCGATTGGCGACCAACGCACCA | T[56]c | [56] |
| GTGAATCCTTCGATAGCTCAGTTGGTAGAGCGGAGGACTGTAGTTGGCTGTGTGCTTAGACATCCTTAGGTCGCTGGTTCGAATCCG | T[56]d | [56] |
| TCCCACCGCTGCCACCC | T[41]a | [57] |
| ACCTGGTGAACCGATGCAGGCG | T[58]a | [58] |
| Sequence not directly provided 1 | T[59]a | [59] |
| TCGATTCCCGGCCAATGCACCA | T[23]a | [23] |
| Neurological | ||
| GTCCGCTCTTAAGATGGTGACTTGGTGGGTACG | T[69]a | [69] |
| TTCCGGCTCGAAGGACCA | T[73]a | [73] |
| TCTCGCTGGGGCCTCC | T[73]b | [73] |
| ATCCTGCCGACTACGCCA | T[73]c | [73] |
| ATCACGTCGGGGTCACCA | T[73]d | [73] |
| TCGCGGGTTCGATCCCCGTACGGGCCAC | T[76]a | [76] |
| TCCAGGGTTCAAGTCCCTGTTCGGGCGC | T[76]b | [76] |
| TCGACTCCCGGTATGGGAACCA | T[77]a | [77] |
| CAAATCTCGGTGGAACCTCCA | T[77]b | [77] |
| TCGAGCCCCAGTGGAACCACCA | T[77]c | [77] |
| TCGAATCCCACCGCTGCCACCA | T[77]d | [77] |
| TCGATTCCCGGCCAATGCACC | T[79]a | [79] |
| TCGATCCCCGGCATCTCCACCA | T[79]b | [79] |
| TCCCCGGCACCTCCACCA | T[35]a | [79] |
| ATCCCACCGCTGCCACCA | T[34]a | [79] |
| TCCCCGGCATCTCCACCA | T[79]c | [79] |
| TCAATCCCCGGCACCTCCACCA | T[79]d | [79] |
| GGGGGATTAGCTCAAA | T[80]a | [80] |
| ATGGACTGCTAATCCATTGTGCTCT | T[80]b | [80] |
| TCCCACATGGTCTAGCGGTTAGGATTCCT | T[80]c | [80] |
| GCCCGGCTAGCTCAGTCGGTAGAGCATGGGAC | T[82]a | [82] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGCCT | T[83]a | [83] |
| TCCCTGGTGGTCTAGTGGTTAGGATTCGGCGCT | T[83]b | [83] |
| TCCCTGGTGGTCTAGTGGTTAGGATTCGGCGCTC | T[84]a | [84] |
| TAACCGAAAGGTTGGTGGTTCGAGCCCAACCCAGGGACGC | T[86]a | [86] |
| GGGGGTGTAGCTCAGTGGTAGAGCGCGTGC | T[87]a | [87] |
| TCCCTGGTGGTCTAGTGGTTAGGATTCGGCG | T[88]a | [88] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGC | T[88]b | [88] |
| GTGGTGTGCTAGTTAATTT | T[89]a | [89] |
| CCTGTCACGCGGGAGACCGGGGC | T[89]b | [89] |
| GCATGGGTGGTTCAGTGGTAGAAT | T[91]a | [91] |
| TCCCTGGTGGTCTAGTGGTTAGGATT | T[91]b | [91,92] |
| GGGGATGTAGCTCAGTGGTAGAGC | T[91]c | [91,92] |
| GTTTCCGTAGTGTAGTGGTTATCACGTTCGCCTC | T[93]a | [93] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTGC | T[94]a | [94] |
| Cardiovascular | ||
| GCAATGGTGGTTCAGTGGTAGAATTCTCGC | T[97]a | [97] |
| TCCCATATGGTCTAGCGGTTAGGATTCCTGGTTT | T[97]b | [97] |
| CTGAATCCAGCGATCCAGTT | T[98]a | [98] |
| TTCCCTGACGGGGAGCCA | T[101]a | [101] |
| CCTCACACGCGAAAGGTCCCCGGT | T[101]b | [101] |
| TAGTGTAGTGGTCATCACGTTCGCC | T[101]c | [101] |
| CCGTGATCGTATAGTGGTTAGTACTCTGC | T[101]d | [101] |
| GTCGGTAGAGCATGGGA | T[101]e | [101] |
| GCCGGGTACTTTCGTATTTT | T[102]a | [102] |
| Sequence not directly provided | T[103]a | [103] |
| TCCCTGGTGGTCTAGTGGTTAGGATTCGGCG | T[88]a | [105] |
| GCATTGGTGGTTCAGTGGTAGAATTCTC | T[105]a | [105] |
| GCCCGGCTAGCTCAGTCGGTAGAGCATGGGACTCT | T[107]a | [107] |
| GCGTTGGTGGTATAGTGGTGAGC | T[108]a | [108] |
| AGTAAGGTCAGCTAAATAAGCTATCGGGCC | T[109]a | [109] |
| GCAGTCAAATGCTCTACCACTGAGCTATACCCCC | T[110]a | [110] |
| TGAATCTAACAACAGGAAATCAAATTCCTTATTTACCCA | T[111]a | [111] |
| GCGTTGGTGGTATAGTGGTGAGCATAGCT | T[111]b | [111] |
| GTTTCCGTAGTGTAGTGGTTATCACGTTCGCCT | T[112]a | [112] |
| GCATTGGTGGTTCAGTGGTAGAATTCTCGCCT | T[83]a | [112] |
| ACTCTGGACTCTGAATCT | T[113]a | [113] |
| TCCCTGGTGGTCTAGTGGTTAGGATTTGGCG | T[114]a | [114] |
| Musculoskeletal | ||
| TCCCTGGTGGTCTAGTGGCTAGGATTCGGCGCTT | T[116]a | [116] |
| GTAGTCGTGGCCGAGTGGTTAAGGC | T[117]a | [117] |
| CGATTCCCGGCCAATGCACCA | T[119]a | [119] |
| ACCTCCCCCGTGGGCCT | T[120]a | [120] |
| Sequence not directly provided | T[121]a | [121] |
| GGCTCCATAGCTCAGGGGT | T[122]a | [122] |
| TGAATCTGACAACAGAGGCTTACGACCCCTTA | T[123]a | [123] |
| AGCCCCAGTGGAACCACCA | T[124]a | [124] |
| GGTTCCATGGTGTAATGGTTAGCACTCTGGACT | T[125]a | [125] |
| TCCCATATGGTCTAGCGGTTAGGATTCCTGGT | T[126]a | [126] |
| TCAATTCCTCTTCTTAACACCA | T[129]a | [129] |
| Sequence not directly provided | T[130]a | [130] |
References
- Yamasaki, S.; Ivanov, P.; Hu, G.-F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes. Dev. 2009, 23, 2639–2649. [Google Scholar]
- Cole, C.; Sobala, A.; Lu, C.; Thatcher, S.R.; Bowman, A.; Brown, J.W.; Green, P.J.; Barton, G.J.; Hutvagner, G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA 2009, 15, 2147–2160. [Google Scholar]
- Kuscu, C.; Kumar, P.; Kiran, M.; Su, Z.; Malik, A.; Dutta, A. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 2018, 24, 1093–1105. [Google Scholar]
- Hasler, D.; Lehmann, G.; Murakawa, Y.; Klironomos, F.; Jakob, L.; Grässer, F.A.; Rajewsky, N.; Landthaler, M.; Meister, G. The lupus autoantigen La prevents mis-channeling of tRNA fragments into the human microRNA pathway. Mol. Cell 2016, 63, 110–124. [Google Scholar]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar]
- Shigematsu, M.; Honda, S.; Kirino, Y. Transfer RNA as a source of small functional RNA. J. Mol. Biol. Mol. Imaging 2014, 1, 8. [Google Scholar]
- Karaiskos, S.; Naqvi, A.S.; Swanson, K.E.; Grigoriev, A. Age-driven modulation of tRNA-derived fragments in Drosophila and their potential targets. Biol. Direct 2015, 10, 51. [Google Scholar]
- Su, Z.; Kuscu, C.; Malik, A.; Shibata, E.; Dutta, A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J. Biol. Chem. 2019, 294, 16930–16941. [Google Scholar] [CrossRef]
- Honda, S.; Loher, P.; Shigematsu, M.; Palazzo, J.P.; Suzuki, R.; Imoto, I.; Rigoutsos, I.; Kirino, Y. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl. Acad. Sci. USA 2015, 112, E3816–E3825. [Google Scholar]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and Function of Transfer RNA-Related Fragments (tRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar]
- Holmes, A.D.; Chan, P.P.; Chen, Q.; Ivanov, P.; Drouard, L.; Polacek, N.; Kay, M.A.; Lowe, T.M. A standardized ontology for naming tRNA-derived RNAs based on molecular origin. Nat. Methods 2023, 20, 627–628. [Google Scholar]
- Pliatsika, V.; Loher, P.; Magee, R.; Telonis, A.G.; Londin, E.; Shigematsu, M.; Kirino, Y.; Rigoutsos, I. MINTbase v2.0: A comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic Acids Res. 2018, 46, D152–D159. [Google Scholar]
- Grigoriev, A. Transfer RNA and Origins of RNA Interference. Front. Mol. Biosci. 2021, 8, 708984. [Google Scholar]
- Guan, L.; Karaiskos, S.; Grigoriev, A. Inferring targeting modes of Argonaute-loaded tRNA fragments. RNA Biol. 2020, 17, 1070–1080. [Google Scholar]
- Guan, L.; Lam, V.; Grigoriev, A. Large-Scale Computational Discovery of Binding Motifs in tRNA Fragments. Front. Mol. Biosci. 2021, 8, 647449. [Google Scholar]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665. [Google Scholar]
- Guan, L.; Grigoriev, A. tatDB: A database of Ago1-mediated targets of transfer RNA fragments. Nucleic Acids Res. 2023, 51, D297–D305. [Google Scholar]
- Akiyama, Y.; Kharel, P.; Abe, T.; Anderson, P.; Ivanov, P. Isolation and initial structure-functional characterization of endogenous tRNA-derived stress-induced RNAs. RNA Biol. 2020, 17, 1116–1124. [Google Scholar]
- Kim, H.K.; Fuchs, G.; Wang, S.; Wei, W.; Zhang, Y.; Park, H.; Roy-Chaudhuri, B.; Li, P.; Xu, J.; Chu, K.; et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 2017, 552, 57–62. [Google Scholar] [PubMed]
- Saikia, M.; Jobava, R.; Parisien, M.; Putnam, A.; Krokowski, D.; Gao, X.-H.; Guan, B.-J.; Yuan, Y.; Jankowsky, E.; Feng, Z.; et al. Angiogenin-Cleaved tRNA Halves Interact with Cytochrome c, Protecting Cells from Apoptosis during Osmotic Stress. Mol. Cell Biol. 2014, 34, 2450–2463. [Google Scholar] [CrossRef]
- Maute, R.L.; Schneider, C.; Sumazin, P.; Holmes, A.; Califano, A.; Basso, K.; Dalla-Favera, R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1404–1409. [Google Scholar]
- Haussecker, D.; Huang, Y.; Lau, A.; Parameswaran, P.; Fire, A.Z.; Kay, M.A. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA 2010, 16, 673–695. [Google Scholar]
- Raina, M.; Ibba, M. tRNAs as regulators of biological processes. Front. Genet. 2014, 5, 171. [Google Scholar]
- Zhang, Y.; Zhou, H.; Chen, X.; Wang, N.; Zhan, Y.; Huang, Z.; Ruan, K.; Qi, Q.; Deng, M.; Jiang, Y. A novel tRNA-derived fragment tRF-3023b suppresses inflammation in RAW264.7 cells by targeting Cul4a through NF-κB signaling. Funct. Integr. Genom. 2024, 24, 9. [Google Scholar]
- Zhang, S.; Gu, Y.; Ge, J.; Xie, Y.; Yu, X.; Wu, X.; Sun, D.; Zhang, X.; Guo, J.; Guo, J. tRF-33-P4R8YP9LON4VDP inhibits gastric cancer progression via modulating STAT3 signaling pathway in an AGO2-dependent manner. Oncogene 2024, 43, 2160–2171. [Google Scholar]
- Nallagatla, S.R.; Jones, C.N.; Ghosh, S.K.B.; Sharma, S.D.; Cameron, C.E.; Spremulli, L.L.; Bevilacqua, P.C. Native tertiary structure and nucleoside modifications suppress tRNA’s intrinsic ability to activate the innate immune sensor PKR. PLoS ONE 2013, 8, e57905. [Google Scholar]
- Lee, S.; Kim, J.; Valdmanis, P.N.; Kim, H.K. Emerging roles of tRNA-derived small RNAs in cancer biology. Exp. Mol. Med. 2023, 55, 1293–1304. [Google Scholar]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar]
- Chen, W.; Peng, W.; Wang, R.; Bai, S.; Cao, M.; Xiong, S.; Li, Y.; Yang, Y.; Liang, J.; Liu, L.; et al. Exosome-derived tRNA fragments tRF-GluCTC-0005 promotes pancreatic cancer liver metastasis by activating hepatic stellate cells. Cell Death Dis. 2024, 15, 102. [Google Scholar] [CrossRef]
- Sui, S.; Wang, Z.; Cui, X.; Jin, L.; Zhu, C. The biological behavior of tRNA-derived fragment tRF-Leu-AAG in pancreatic cancer cells. Bioengineered 2022, 13, 10617–10628. [Google Scholar]
- Lan, S.; Liu, S.; Wang, K.; Chen, W.; Zheng, D.; Zhuang, Y.; Zhang, S. tRNA-derived RNA fragment, tRF-18-8R6546D2, promotes pancreatic adenocarcinoma progression by directly targeting ASCL2. Gene 2024, 927, 148739. [Google Scholar]
- Xue, M.; Shi, M.; Xie, J.; Zhang, J.; Jiang, L.; Deng, X.; Peng, C.; Shen, B.; Xu, H.; Chen, H. Serum tRNA-derived small RNAs as potential novel diagnostic biomarkers for pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 2021, 11, 837–848. [Google Scholar]
- Gao, Z.; Jijiwa, M.; Nasu, M.; Borgard, H.; Gong, T.; Xu, J.; Chen, S.; Fu, Y.; Chen, Y.; Hu, X.; et al. Comprehensive landscape of tRNA-derived fragments in lung cancer. Mol. Ther. Oncolytics 2022, 26, 207–225. [Google Scholar]
- Pekarsky, Y.; Balatti, V.; Palamarchuk, A.; Rizzotto, L.; Veneziano, D.; Nigita, G.; Rassenti, L.Z.; Pass, H.I.; Kipps, T.J.; Liu, C.-G.; et al. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5071–5076. [Google Scholar]
- Balatti, V.; Nigita, G.; Veneziano, D.; Drusco, A.; Stein, G.S.; Messier, T.L.; Farina, N.H.; Lian, J.B.; Tomasello, L.; Liu, C.-G.; et al. tsRNA signatures in cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 8071–8076. [Google Scholar]
- Huang, L.T.; Cui, M.; Silva, M.; Okuda, K.; Shimada, Y.; Wang, J.-H.; Wang, Y.-B. Expression profiles of tRNA-derived fragments and their potential roles in lung adenocarcinoma. Ann. Transl. Med. 2022, 10, 196. [Google Scholar]
- Huang, B.; Yang, H.; Cheng, X.; Wang, D.; Fu, S.; Shen, W.; Zhang, Q.; Zhang, L.; Xue, Z.; Li, Y.; et al. tRF/miR-1280 Suppresses Stem Cell-like Cells and Metastasis in Colorectal Cancer. Cancer Res. 2017, 77, 3194–3206. [Google Scholar]
- Cao, K.Y.; Pan, Y.; Yan, T.-M.; Tao, P.; Xiao, Y.; Jiang, Z.-H. Antitumor Activities of tRNA-Derived Fragments and tRNA Halves from Non-pathogenic Escherichia coli Strains on Colorectal Cancer and Their Structure-Activity Relationship. mSystems 2022, 7, e0016422. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Cai, H.; Peng, Y.; Yang, L.; Wang, Z. Identifying Differentially Expressed tRNA-Derived Small Fragments as a Biomarker for the Progression and Metastasis of Colorectal Cancer. Dis. Markers 2022, 2022, 2646173. [Google Scholar]
- Xiong, W.; Wang, X.; Cai, X.; Liu, Y.; Li, C.; Liu, Q.; Qin, J.; Li, Y. Identification of tRNA-derived fragments in colon cancer by comprehensive small RNA sequencing. Oncol. Rep. 2019, 42, 735–744. [Google Scholar]
- Ye, C.; Cheng, F.; Huang, L.; Wang, K.; Zhong, L.; Lu, Y.; Ouyang, M. New plasma diagnostic markers for colorectal cancer: Transporter fragments of glutamate tRNA origin. J. Cancer 2024, 15, 1299–1313. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Yu, X.; Ruan, Y.; Shen, Y.; Shao, Y.; Zhang, X.; Ye, G.; Guo, J. The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2021, 12, 418. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Fan, X.; He, X.; Chen, S.; Yu, S.; Zhang, Y. The tRNA-Derived Fragment tRF-24-V29K9UV3IU Functions as a miRNA-like RNA to Prevent Gastric Cancer Progression by Inhibiting GPR78 Expression. J. Oncol. 2022, 2022, 8777697. [Google Scholar] [CrossRef]
- Dong, X.; Fan, X.; He, X.; Chen, S.; Huang, W.; Gao, J.; Huang, Y.; Wang, H. Comprehensively Identifying the Key tRNA-Derived Fragments and Investigating Their Function in Gastric Cancer Processes. OncoTargets Ther. 2020, 13, 10931–10943. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Li, Y.; Li, X.; Huang, Y.; Ju, S. Transfer RNA-derived fragment tRF-23-Q99P9P9NDD promotes progression of gastric cancer by targeting ACADSB. J. Zhejiang Univ. Sci. B 2024, 25, 438–450. [Google Scholar]
- Xu, B.; Liang, J.; Zou, H.; Wang, J.; Xiong, Y.; Pei, J. Identification of Novel tRNA-Leu-CAA-Derived tsRNAs for the Diagnosis and Prognosis of Diffuse Gliomas. Cancer Manag. Res. 2022, 14, 2609–2623. [Google Scholar] [CrossRef]
- Wei, D.; Niu, B.; Zhai, B.; Liu, X.-B.; Yao, Y.-L.; Liang, C.-C.; Wang, P. Expression profiles and function prediction of tRNA-derived fragments in glioma. BMC Cancer 2023, 23, 1015. [Google Scholar]
- Emara, M.M.; Ivanov, P.; Hickman, T.; Dawra, N.; Tisdale, S.; Kedersha, N.; Hu, G.-F.; Anderson, P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 2010, 285, 10959–10968. [Google Scholar]
- Tu, M.; Zuo, Z.; Chen, C.; Zhang, X.; Wang, S.; Chen, C.; Sun, Y. Transfer RNA-derived small RNAs (tsRNAs) sequencing revealed a differential expression landscape of tsRNAs between glioblastoma and low-grade glioma. Gene 2023, 855, 147114. [Google Scholar]
- Wang, J.; Ma, G.; Ge, H.; Han, X.; Mao, X.; Wang, X.; Veeramootoo, J.S.; Xia, T.; Liu, X.; Wang, S. Circulating tRNA-derived small RNAs (tsRNAs) signature for the diagnosis and prognosis of breast cancer. NPJ Breast Cancer 2021, 7, 4. [Google Scholar]
- Falconi, M.; Giangrossi, M.; Zabaleta, M.E.; Wang, J.; Gambini, V.; Tilio, M.; Bencardino, D.; Occhipinti, S.; Belletti, B.; Laudadio, E.; et al. A novel 3′-tRNA(Glu)-derived fragment acts as a tumor suppressor in breast cancer by targeting nucleolin. FASEB J. 2019, 33, 13228–13240. [Google Scholar]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 2015, 161, 790–802. [Google Scholar]
- Wan, X.; Shi, W.; Ma, L.; Wang, L.; Zheng, R.; He, J.; Wang, Y.; Li, X.; Zha, X.; Wang, J.; et al. A 3′-pre-tRNA-derived small RNA tRF-1-Ser regulated by 25(OH)D promotes proliferation and stemness by inhibiting the function of MBNL1 in breast cancer. Clin. Transl. Med. 2024, 14, e1681. [Google Scholar]
- Cao, K.Y.; Yan, T.-M.; Zhang, J.-Z.; Chan, T.-F.; Li, J.; Li, C.; Leung, E.L.-H.; Gao, J.; Zhang, B.-X.; Jiang, Z.-H. A tRNA-derived fragment from Chinese yew suppresses ovarian cancer growth via targeting TRPA1. Mol. Ther. Nucleic Acids 2022, 27, 718–732. [Google Scholar]
- Zhang, M.; Li, F.; Wang, J.; He, W.; Li, Y.; Li, H.; Wei, Z.; Cao, Y. tRNA-derived fragment tRF-03357 promotes cell proliferation, migration and invasion in high-grade serous ovarian cancer. OncoTargets Ther. 2019, 12, 6371–6383. [Google Scholar]
- Garcia-Vilchez, R.; Añazco-Guenkova, A.M.; López, J.; Dietmann, S.; Tomé, M.; Jimeno, S.; Azkargorta, M.; Elortza, F.; Bárcena, L.; Gonzalez-Lopez, M.; et al. N7-methylguanosine methylation of tRNAs regulates survival to stress in cancer. Oncogene 2023, 42, 3169–3181. [Google Scholar]
- Cartlidge, R.A.; Knebel, A.; Peggie, M.; Alexandrov, A.; Phizicky, E.M.; Cohen, P. The tRNA methylase METTL1 is phosphorylated and inactivated by PKB and RSK in vitro and in cells. EMBO J. 2005, 24, 1696–1705. [Google Scholar]
- Liu, B.; Cao, J.; Wang, X.; Guo, C.; Liu, Y.; Wang, T. Deciphering the tRNA-derived small RNAs: Origin, development, and future. Cell Death Dis. 2021, 13, 24. [Google Scholar]
- Korver, W.; Guevara, C.; Chen, Y.; Neuteboom, S.; Bookstein, R.; Tavtigian, S.; Lees, E. The product of the candidate prostate cancer susceptibility gene ELAC2 interacts with the gamma-tubulin complex. Int. J. Cancer 2003, 104, 283–288. [Google Scholar]
- Takaku, H.; Minagawa, A.; Takagi, M.; Nashimoto, M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003, 31, 2272–2278. [Google Scholar]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar]
- Givalos, N.; Gakiopoulou, H.; Skliri, M.; Bousboukea, K.; E Konstantinidou, A.; Korkolopoulou, P.; Lelouda, M.; Kouraklis, G.; Patsouris, E.; Karatzas, G. Replication protein A is an independent prognostic indicator with potential therapeutic implications in colon cancer. Mod. Pathol. 2007, 20, 159–166. [Google Scholar]
- Cai, H.; Zhang, Y.; Meng, F.; Li, Y. Effects of spinal cord injury associated exosomes delivered tRF-41 on the progression of spinal cord injury progression. Genomics 2024, 116, 110885. [Google Scholar]
- Li, P.F.; Guo, S.-C.; Liu, T.; Cui, H.; Feng, D.; Yang, A.; Cheng, Z.; Luo, J.; Tang, T.; Wang, Y. Integrative analysis of transcriptomes highlights potential functions of transfer-RNA-derived small RNAs in experimental intracerebral hemorrhage. Aging 2020, 12, 22794–22813. [Google Scholar]
- Qin, C.; Feng, H.; Zhang, C.; Zhang, X.; Liu, Y.; Yang, D.-G.; Du, L.-J.; Sun, Y.-C.; Yang, M.-L.; Gao, F.; et al. Differential Expression Profiles and Functional Prediction of tRNA-Derived Small RNAs in Rats After Traumatic Spinal Cord Injury. Front. Mol. Neurosci. 2019, 12, 326. [Google Scholar]
- Meseguer, S.; Navarro-González, C.; Panadero, J.; Villarroya, M.; Boutoual, R.; Sánchez-Alcázar, J.A.; Armengod, M.-E. The MELAS mutation m.3243A>G alters the expression of mitochondrial tRNA fragments. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 1433–1449. [Google Scholar]
- Meseguer, S.; Rubio, M.-P. mt tRFs, New Players in MELAS Disease. Front. Physiol. 2022, 13, 800171. [Google Scholar]
- Ziats, C.A.; Patterson, W.G.; Friez, M. Syndromic Autism Revisited: Review of the Literature and Lessons Learned. Pediatr. Neurol. 2021, 114, 21–25. [Google Scholar]
- Su, Z.; Frost, E.L.; Lammert, C.R.; Przanowska, R.K.; Lukens, J.R.; Dutta, A. tRNA-derived fragments and microRNAs in the maternal-fetal interface of a mouse maternal-immune-activation autism model. RNA Biol. 2020, 17, 1183–1195. [Google Scholar]
- Albayram, O.; Kondo, A.; Mannix, R.; Smith, C.; Tsai, C.-Y.; Li, C.; Herbert, M.K.; Qiu, J.; Monuteaux, M.; Driver, J.; et al. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat. Commun. 2017, 8, 1000. [Google Scholar]
- Liu, B.-Y.; Xu, X.-J.; Yang, M.-S.; Zhang, B.; Ge, Q.-Q.; Niu, F.; Dong, J.-Q.; Zhuang, Y. Genome-wide interrogation of transfer RNA-derived small RNAs in a mouse model of traumatic brain injury. Neural Regen. Res. 2022, 17, 386–394. [Google Scholar]
- Puhakka, N.; Das Gupta, S.; Vuokila, N.; Pitkänen, A. Transfer RNA-Derived Fragments and isomiRs Are Novel Components of Chronic TBI-Induced Neuropathology. Biomedicines 2022, 10, 136. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Tang, T.; Li, P.-F.; Li, X.-X.; Wu, Y.; Feng, D.-D.; Hu, M.-R.; Dai, F.; Zheng, F.; Zhang, W.; et al. Systematic analysis of tRNA-derived small RNAs reveals therapeutic targets of Xuefu Zhuyu decoction in the cortexes of experimental traumatic brain injury. Phytomedicine 2022, 102, 154168. [Google Scholar]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar]
- Winek, K.; Lobentanzer, S.; Nadorp, B.; Dubnov, S.; Dames, C.; Jagdmann, S.; Moshitzky, G.; Hotter, B.; Meisel, C.; Greenberg, D.S.; et al. Transfer RNA fragments replace microRNA regulators of the cholinergic poststroke immune blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 32606–32616. [Google Scholar]
- Li, P.; Tang, T.; Liu, T.; Zhou, J.; Cui, H.; He, Z.; Zhong, Y.; Hu, E.; Yang, A.; Wei, G.; et al. Systematic Analysis of tRNA-Derived Small RNAs Reveals Novel Potential Therapeutic Targets of Traditional Chinese Medicine (Buyang-Huanwu-Decoction) on Intracerebral Hemorrhage. Int. J. Biol. Sci. 2019, 15, 895–908. [Google Scholar]
- Nguyen, T.T.; van der Bent, M.L.; Wermer, M.J.H.; Wijngaard, I.R.v.D.; van Zwet, E.W.; de Groot, B.; Quax, P.H.A.; Kruyt, N.D.; Nossent, A.Y. Circulating tRNA Fragments as a Novel Biomarker Class to Distinguish Acute Stroke Subtypes. Int. J. Mol. Sci. 2021, 22, 135. [Google Scholar] [CrossRef]
- Zhou, C.; Lian, F.; Li, H.; Deng, F. tsRNA-5006c regulates hippocampal neurons ferroptosis to ameliorate perioperative neurocognitive disorders in aged male mice. 3 Biotech 2024, 15, 16. [Google Scholar]
- Wu, W.; Lee, I.; Spratt, H.; Fang, X.; Bao, X. tRNA-Derived Fragments in Alzheimer’s Disease: Implications for New Disease Biomarkers and Neuropathological Mechanisms. J. Alzheimers Dis. 2021, 79, 793–806. [Google Scholar]
- Li, D.; Gao, X.; Ma, X.; Wang, M.; Cheng, C.; Xue, T.; Gao, F.; Shen, Y.; Zhang, J.; Liu, Q. Aging-induced tRNAGlu-derived fragment impairs glutamate biosynthesis by targeting mitochondrial translation-dependent cristae organization. Cell Metab. 2024, 36, 1059–1075.e9. [Google Scholar]
- Shulman, D.; Dubnov, S.; Zorbaz, T.; Madrer, N.; Paldor, I.; Bennett, D.A.; Seshadri, S.; Mufson, E.J.; Greenberg, D.S.; Loewenstein, Y.; et al. Sex-specific declines in cholinergic-targeting tRNA fragments in the nucleus accumbens in Alzheimer’s disease. Alzheimers Dement. 2023, 19, 5159–5172. [Google Scholar]
- Dubnov, S.; Bennett, E.R.; Yayon, N.; Yakov, O.; Bennett, D.A.; Seshadri, S.; Mufson, E.; Tzur, Y.; Greenberg, D.; Kuro-O, M.; et al. Knockout of the longevity gene Klotho perturbs aging and Alzheimer’s disease-linked brain microRNAs and tRNA fragments. Commun. Biol. 2024, 7, 720. [Google Scholar]
- Creus-Muncunill, J.; Guisado-Corcoll, A.; Venturi, V.; Pantano, L.; Escaramís, G.; de Herreros, M.G.; Solaguren-Beascoa, M.; Gámez-Valero, A.; Navarrete, C.; Masana, M.; et al. Huntington’s disease brain-derived small RNAs recapitulate associated neuropathology in mice. Acta Neuropathol. 2021, 141, 565–584. [Google Scholar]
- Herrero-Lorenzo, M.; Pérez-Pérez, J.; Escaramís, G.; Martínez-Horta, S.; Pérez-González, R.; Rivas-Asensio, E.; Kulisevsky, J.; Gámez-Valero, A.; Martí, E. Small RNAs in plasma extracellular vesicles define biomarkers of premanifest changes in Huntington’s disease. J. Extracell. Vesicles 2024, 13, e12522. [Google Scholar]
- Zhang, S.; Li, H.; Zheng, L.; Li, H.; Feng, C.; Zhang, W. Identification of functional tRNA-derived fragments in senescence-accelerated mouse prone 8 brain. Aging 2019, 11, 10485–10498. [Google Scholar]
- Magee, R.; Londin, E.; Rigoutsos, I. TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson’s disease. Park. Relat. Disord. 2019, 65, 203–209. [Google Scholar]
- Hogg, M.C.; Raoof, R.; El Naggar, H.; Monsefi, N.; Delanty, N.; O’brien, D.F.; Bauer, S.; Rosenow, F.; Henshall, D.C.; Prehn, J.H. Elevation in plasma tRNA fragments precede seizures in human epilepsy. J. Clin. Investig. 2019, 129, 2946–2951. [Google Scholar] [PubMed]
- McArdle, H.; Hogg, M.C.; Bauer, S.; Rosenow, F.; Prehn, J.H.M.; Adamson, K.; Henshall, D.C.; Spain, E. Quantification of tRNA fragments by electrochemical direct detection in small volume biofluid samples. Sci. Rep. 2020, 10, 7516. [Google Scholar]
- Hogg, M.C.; Rayner, M.; Susdalzew, S.; Monsefi, N.; Crivello, M.; Woods, I.; Resler, A.; Blackbourn, L.; Fabbrizio, P.; Trolese, M.C.; et al. 5′ValCAC tRNA fragment generated as part of a protective angiogenin response provides prognostic value in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, fcaa138. [Google Scholar]
- Jirström, E.; Matveeva, A.; Baindoor, S.; Donovan, P.; Ma, Q.; Morrissey, E.P.; Arijs, I.; Boeckx, B.; Lambrechts, D.; Garcia-Munoz, A.; et al. Effects of ALS-associated 5′tiRNAGly-GCC on the transcriptomic and proteomic profile of primary neurons in vitro. Exp. Neurol. 2025, 385, 115128. [Google Scholar]
- Ivanov, P.; O’day, E.; Emara, M.M.; Wagner, G.; Lieberman, J.; Anderson, P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA 2014, 111, 18201–18206. [Google Scholar]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar]
- Shen, L.; Gan, M.; Tan, Z.; Jiang, D.; Jiang, Y.; Li, M.; Wang, J.; Li, X.; Zhang, S.; Zhu, L. A Novel Class of tRNA-Derived Small Non-Coding RNAs Respond to Myocardial Hypertrophy and Contribute to Intergenerational Inheritance. Biomolecules 2018, 8, 54. [Google Scholar] [CrossRef]
- Xu, J.; Qian, B.; Wang, F.; Huang, Y.; Yan, X.; Li, P.; Zhang, Q.; Li, Y.; Sun, K. Global Profile of tRNA-Derived Small RNAs in Pathological Cardiac Hypertrophy Plasma and Identification of tRF-21-NB8PLML3E as a New Hypertrophy Marker. Diagnostics 2023, 13, 2065. [Google Scholar] [CrossRef]
- Wu, D.; Dasgupta, A.; Read, A.D.; Bentley, R.E.T.; Motamed, M.; Chen, K.-H.; Al-Qazazi, R.; Mewburn, J.D.; Dunham-Snary, K.J.; Alizadeh, E.; et al. Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer. Free Radic. Biol. Med. 2021, 170, 150–178. [Google Scholar]
- Li, D.; Shao, N.-Y.; Moonen, J.-R.; Zhao, Z.; Shi, M.; Otsuki, S.; Wang, L.; Nguyen, T.; Yan, E.; Marciano, D.P.; et al. ALDH1A3 Coordinates Metabolism With Gene Regulation in Pulmonary Arterial Hypertension. Circulation 2021, 143, 2074–2090. [Google Scholar]
- Chen, Y.; Tang, Y.; Hou, S.; Luo, J.; Chen, J.; Qiu, H.; Chen, W.; Li, K.; He, J.; Li, J. Differential expression spectrum and targeted gene prediction of tRNA-derived small RNAs in idiopathic pulmonary arterial hypertension. Front. Mol. Biosci. 2023, 10, 1204740. [Google Scholar]
- Zhang, J.; Li, Y.; Chen, Y.; Zhang, J.; Jia, Z.; He, M.; Liao, X.; He, S.; Bian, J.-S.; Nie, X.-W. o(8)G Site-Specifically Modified tRF-1-AspGTC: A Novel Therapeutic Target and Biomarker for Pulmonary Hypertension. Circ. Res. 2024, 135, 76–92. [Google Scholar] [PubMed]
- He, X.; Yang, Y.; Wang, Q.; Wang, J.; Li, S.; Li, C.; Zong, T.; Li, X.; Zhang, Y.; Zou, Y.; et al. Expression profiles and potential roles of transfer RNA-derived small RNAs in atherosclerosis. J. Cell Mol. Med. 2021, 25, 7052–7065. [Google Scholar] [PubMed]
- Bai, B.; Yang, Y.; Ji, S.; Wang, S.; Peng, X.; Tian, C.; Sun, R.-C.; Yu, T.; Chu, X.-M. MicroRNA-302c-3p inhibits endothelial cell pyroptosis via directly targeting NOD-, LRR- and pyrin domain-containing protein 3 in atherosclerosis. J. Cell Mol. Med. 2021, 25, 4373–4386. [Google Scholar]
- Chang, X.; Du, M.; Wei, J.; Zhang, Y.; Feng, X.; Deng, B.; Liu, P.; Wang, Y. Serum tsncRNAs reveals novel potential therapeutic targets of Salvianolic Acid B on atherosclerosis. Phytomedicine 2024, 134, 155994. [Google Scholar]
- Watkins, D.A.; Beaton, A.Z.; Carapetis, J.R.; Karthikeyan, G.; Mayosi, B.M.; Wyber, R.; Yacoub, M.H.; Zühlke, L.J. Rheumatic Heart Disease Worldwide: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2018, 72, 1397–1416. [Google Scholar]
- Yang, Z.Y.; Li, P.-F.; Li, Z.-Q.; Tang, T.; Liu, W.; Wang, Y. Altered Expression of Transfer-RNA-Derived Small RNAs in Human With Rheumatic Heart Disease. Front. Cardiovasc. Med. 2021, 8, 716716. [Google Scholar]
- Xie, L.; Zhao, Z.; Xia, H.; Su, S.; He, L.; Huang, Z.; Li, Y.; Gao, M.; Chen, J.; Peng, J.; et al. A novel tsRNA-5008a promotes ferroptosis in cardiomyocytes that causes atrial structural remodeling predisposed to atrial fibrillation. Exp. Cell Res. 2024, 435, 113923. [Google Scholar]
- Fu, X.; He, X.; Yang, Y.; Jiang, S.; Wang, S.; Peng, X.; Tang, G.; Zong, T.; Li, X.; Zhang, Y.; et al. Identification of transfer RNA-derived fragments and their potential roles in aortic dissection. Genomics 2021, 113, 3039–3049. [Google Scholar]
- Zong, T.; Yang, Y.; Lin, X.; Jiang, S.; Zhao, H.; Liu, M.; Meng, Y.; Li, Y.; Zhao, L.; Tang, G.; et al. 5′-tiRNA-Cys-GCA regulates VSMC proliferation and phenotypic transition by targeting STAT4 in aortic dissection. Mol. Ther. Nucleic Acids 2021, 26, 295–306. [Google Scholar]
- Liu, W.; Liu, Y.; Pan, Z.; Zhang, X.; Qin, Y.; Chen, X.; Li, M.; Chen, X.; Zheng, Q.; Liu, X.; et al. Systematic Analysis of tRNA-Derived Small RNAs Discloses New Therapeutic Targets of Caloric Restriction in Myocardial Ischemic Rats. Front. Cell Dev. Biol. 2020, 8, 568116. [Google Scholar]
- Li, Q.; Hu, B.; Hu, G.-W.; Chen, C.-Y.; Niu, X.; Liu, J.; Zhou, S.-M.; Zhang, C.-Q.; Wang, Y.; Deng, Z.-F. tRNA-Derived Small Non-Coding RNAs in Response to Ischemia Inhibit Angiogenesis. Sci. Rep. 2016, 6, 20850. [Google Scholar]
- Zhu, X.L.; Li, T.; Cao, Y.; Yao, Q.-P.; Liu, X.; Li, Y.; Guan, Y.-Y.; Deng, J.-J.; Jiang, R.; Jiang, J. tRNA-derived fragments tRF(GlnCTG) induced by arterial injury promote vascular smooth muscle cell proliferation. Mol. Ther. Nucleic Acids 2021, 23, 603–613. [Google Scholar] [PubMed]
- Liang, Y.; Kong, L.; Zhang, Y.; Zhang, Y.; Shi, M.; Huang, J.; Kong, H.; Qi, S.; Yang, Y.; Hong, J.; et al. Transfer RNA derived fragment, tRF-Glu-CTC, aggravates the development of neovascular age-related macular degeneration. Theranostics 2024, 14, 1500–1516. [Google Scholar]
- Burton, M.A.; Antoun, E.; Garratt, E.S.; Westbury, L.; Dennison, E.M.; Harvey, N.C.; Cooper, C.; Patel, H.P.; Godfrey, K.M.; Lillycrop, K.A. The serum small non-coding RNA (SncRNA) landscape as a molecular biomarker of age associated muscle dysregulation and insulin resistance in older adults. FASEB J. 2024, 38, e23423. [Google Scholar]
- Ling, Z.; Xiao, N.; Li, Y.; Xie, H.; Xiao, T.; Jiang, H.; Fu, Y. Differential expression profiles and function prediction of tRNA-derived fragments in fibrous dysplasia. Arch. Oral. Biol. 2022, 135, 105347. [Google Scholar]
- Ni, Y.; Wu, A.; Li, J.; Zhang, W.; Wang, Y. Evaluation of the serum tRNA-derived fragment tRF-5022B as a potential biomarker for the diagnosis of osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 800. [Google Scholar]
- Zhang, Y.; Cai, F.; Liu, J.; Chang, H.; Liu, L.; Yang, A.; Liu, X. Transfer RNA-derived fragments as potential exosome tRNA-derived fragment biomarkers for osteoporosis. Int. J. Rheum. Dis. 2018, 21, 1659–1669. [Google Scholar]
- Wang, P.; Fu, Z.; Liu, Y.; Huang, S.; Guo, Y.; Jin, J.; Fang, Y.; Pan, Y.; Fan, Z.; Yu, H. tRF-21-LNK8KEP1B as a potential novel diagnostic biomarker for enthesitis-related arthritis. Int. Immunopharmacol. 2023, 124 Pt A, 110820. [Google Scholar]
- Green, J.A.; Ansari, M.Y.; Ball, H.C.; Haqqi, T.M. tRNA-derived fragments (tRFs) regulate post-transcriptional gene expression via AGO-dependent mechanism in IL-1beta stimulated chondrocytes. Osteoarthr. Cartil. 2020, 28, 1102–1110. [Google Scholar]
- Deng, Z.; Long, D.; Liu, H.; Xu, Y.; Xin, R.; Liao, H.; Li, Z.; Li, R.; Mao, G.; Zhang, Z.; et al. tRNA-Derived Fragment tRF-5009A Regulates Autophagy and Degeneration of Cartilage in Osteoarthritis via Targeting mTOR. Oxid. Med. Cell Longev. 2022, 2022, 5781660. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Xu, Y.; Mao, G.; Xin, R.; Deng, Z.; Liao, H.; Li, Z.; Yang, Z.; Yu, B.; Yang, Z.; et al. tRNA-derived fragment TRF365 regulates the metabolism of anterior cruciate ligament cells by targeting IKBKB. Cell Death Discov. 2022, 8, 19. [Google Scholar] [PubMed]
- Soureas, K.; Papadimitriou, M.-A.; Malandrakis, P.; Papanota, A.-M.; Adamopoulos, P.G.; Ntanasis-Stathopoulos, I.; Liacos, C.-I.; Gavriatopoulou, M.; Sideris, D.C.; Kastritis, E.; et al. Small RNA-seq and clinical evaluation of tRNA-derived fragments in multiple myeloma: Loss of mitochondrial i-tRF(HisGTG) results in patients’ poor treatment outcome. Br. J. Haematol. 2024, 204, 1790–1800. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, W.; Qu, B.; Zhang, F.; Wu, Z.; Shi, J.; Chen, X.; Song, Y.; Wang, Z. The tRNA-Derived Fragment-3017A Promotes Metastasis by Inhibiting NELL2 in Human Gastric Cancer. Front. Oncol. 2021, 10, 570916. [Google Scholar]
- Krishna, S.; Yim, D.G.; Lakshmanan, V.; Tirumalai, V.; Koh, J.L.; Park, J.E.; Cheong, J.K.; Low, J.L.; Lim, M.J.; Sze, S.K.; et al. Dynamic expression of tRNA-derived small RNAs define cellular states. EMBO Rep. 2019, 20, e47789. [Google Scholar] [CrossRef]
- Gan, M.; Ma, J.; Chen, L.; Zhang, S.; Niu, L.; Zhao, Y.; Li, X.; Pan, H.; Zhu, L.; Shen, L. Identification of tRNA-derived small RNAs and their potential roles in porcine skeletal muscle with intrauterine growth restriction. Front. Physiol. 2022, 13, 962278. [Google Scholar] [CrossRef]
- Goldkamp, A.K.; Li, Y.; Rivera, R.M.; Hagen, D.E. Differentially expressed tRNA-derived fragments in bovine fetuses with assisted reproduction induced congenital overgrowth syndrome. Front. Genet. 2022, 13, 1055343. [Google Scholar]
- Tang, Z.; Zhang, S.; Ling, Z. Development of a tRNA-Derived Small RNA Prognostic Panel and Their Potential Functions in Osteosarcoma. Front. Oncol. 2021, 11, 652040. [Google Scholar] [CrossRef]
- Long, D.; Deng, Z.; Zhao, X.; Xu, Y.; Li, W.; Mo, X.; Zhong, Y.; Li, M.; He, A.; Zhang, Z.; et al. m(7)G-modified mt-tRF3b-LeuTAA regulates mitophagy and metabolic reprogramming via SUMOylation of SIRT3 in chondrocytes. Biomaterials 2025, 314, 122903. [Google Scholar]
- Bourgery, M.; Ekholm, E.; Hiltunen, A.; Heino, T.J.; Pursiheimo, J.-P.; Bendre, A.; Yatkin, E.; Laitala, T.; Määttä, J.; Säämänen, A.-M. Signature of circulating small non-coding RNAs during early fracture healing in mice. Bone Rep. 2022, 17, 101627. [Google Scholar] [CrossRef]
- Shen, L.; Liao, T.; Chen, Q.; Lei, Y.; Wang, L.; Gu, H.; Qiu, Y.; Zheng, T.; Yang, Y.; Wei, C.; et al. tRNA-derived small RNA, 5′tiRNA-Gly-CCC, promotes skeletal muscle regeneration through the inflammatory response. J. Cachexia Sarcopenia Muscle 2023, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaidhyanathan, S.; Durbin, M.; Adetowubo, A.A.; Do, L.H.; Kavehmoghaddam, S.; Jonnalagadda, S.A.; Aguilar, B.R.; Ortiz-Gomez, T.; Lin, Y.X.; Dave, A.; et al. Mapping Current Studies of tRNA Fragments onto Disease Landscape. Biomolecules 2025, 15, 512. https://doi.org/10.3390/biom15040512
Vaidhyanathan S, Durbin M, Adetowubo AA, Do LH, Kavehmoghaddam S, Jonnalagadda SA, Aguilar BR, Ortiz-Gomez T, Lin YX, Dave A, et al. Mapping Current Studies of tRNA Fragments onto Disease Landscape. Biomolecules. 2025; 15(4):512. https://doi.org/10.3390/biom15040512
Chicago/Turabian StyleVaidhyanathan, Sathyanarayanan, MacKenna Durbin, Adesupo A. Adetowubo, Lisa H. Do, Sheida Kavehmoghaddam, Sai Anusha Jonnalagadda, Bryan Ramirez Aguilar, Tamin Ortiz-Gomez, Yan X. Lin, Asim Dave, and et al. 2025. "Mapping Current Studies of tRNA Fragments onto Disease Landscape" Biomolecules 15, no. 4: 512. https://doi.org/10.3390/biom15040512
APA StyleVaidhyanathan, S., Durbin, M., Adetowubo, A. A., Do, L. H., Kavehmoghaddam, S., Jonnalagadda, S. A., Aguilar, B. R., Ortiz-Gomez, T., Lin, Y. X., Dave, A., Kiliç, F., Karp, A. R., Rahmah, M. I., Riaz, N. F., Mandava, N., Siner, A., & Grigoriev, A. (2025). Mapping Current Studies of tRNA Fragments onto Disease Landscape. Biomolecules, 15(4), 512. https://doi.org/10.3390/biom15040512







