STAT Signature Dish: Serving Immunity with a Side of Dietary Control
Abstract
1. Introduction
2. Overview of Immunity and Immunotolerance
3. The Economics of Immunity
3.1. Energy Costs
- Mounting Costs: The energy expenditure associated with activating immune cells during an infection. This includes pathogen recognition, inflammatory signaling, and the activation and proliferation of immune cells [54,56,57]. Fever, a common immune response, exemplifies the systemic energetic demand, as it raises body temperature to inhibit pathogen replication but significantly increases metabolic rates. If unregulated, these demands can deplete vital resources, threatening survival and emphasizing the importance of precise control mechanisms to balance immune activity with metabolic costs [60,61,62].
3.2. Choice Costs
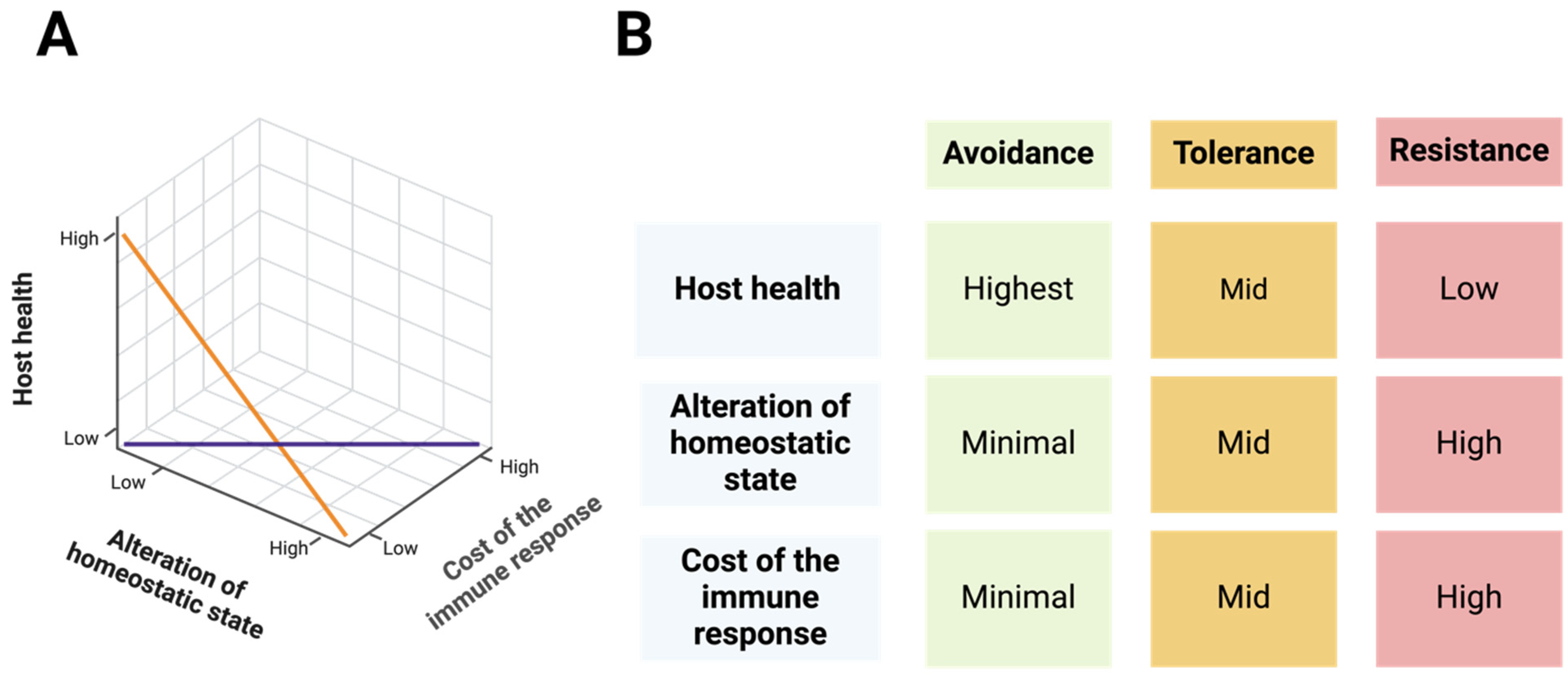
4. The “ART” of Immunity Decision-Making
4.1. Avoidance: The First Line of Defense
4.2. Resistance: Active Pathogen Clearance
4.3. Tolerance: Minimizing Damage
5. STAT Signaling: The Mastermind of Immune Cell Decision-Making and Communication
5.1. STAT Signaling in Barrier Defense
5.2. STAT Signaling in Immune Responses and Tolerance
6. The Role of STAT Signaling in Dietary Control and Metabolism
6.1. STAT Proteins and Immune Cell Metabolism
6.2. The Crosstalk Between STAT Proteins and Dietary Regulation
7. The “Theory of Relativity” in STAT Signaling
8. The JAK Tip of the STAT Iceberg
8.1. Overview of the JAK-STAT Pathway
8.2. Tracing Evolutionary Origins
9. STAT Signatures: Building a Toolkit for Complex Biological Responses
9.1. Key Parameters for STAT-Driven Cellular Functionality
- Expression levels: The relative expression of STAT proteins and the presence of their isoforms play a crucial role in shaping downstream cellular functions. For instance, IL-21 primarily activates STAT3, which suppresses the expression of the TBX21 and IFNG genes. However, in the absence of STAT3, IL-21 signaling shifts toward STAT1 activation, leading to the upregulation of these genes [187]. This regulatory balance is evident in patients with autosomal dominant hyper-IgE syndrome (AD-HIES, also known as Job syndrome), where STAT3 deficiency due to autosomal dominant mutations results in impaired STAT3 activation, altering cellular responses to IL-21 [216,217]. Beyond overall expression levels, differentially spliced isoforms of STAT proteins further diversify their functions. These isoforms, often characterized by truncations in the C-terminal domain, promote distinct biological outcomes. For example, STAT1α (full-length) and STAT1β (truncated) regulate different subsets of antiviral defense genes, control cell cycle and apoptosis in B cells, and influence NK cell activity and antitumor surveillance [117,218,219,220]. Similarly, STAT3α and STAT3β play distinct roles in cancer progression and inflammation. While STAT3α is widely recognized for its oncogenic properties, STAT3β has emerged as a potential tumor suppressor, underscoring the functional complexity introduced by STAT isoforms [221,222,223].
- PTMs: PTMs play a crucial role in shaping STAT functionality, with phosphorylation being the most extensively studied, particularly JAK-mediated tyrosine phosphorylation [10,87]. Different phosphorylation events can have synergistic or opposing effects on STAT-driven cellular functions. For instance, STAT1 serine 727 phosphorylation is essential for maximal IFN-mediated STAT1 activity, working in synergy with JAK-mediated tyrosine phosphorylation to enhance antiviral defense [224,225,226,227]. Conversely, STAT1 threonine 748 phosphorylation limits its JAK-mediated tyrosine phosphorylation, thereby promoting inflammatory responses in macrophages [228,229,230]. The interplay between tyrosine and threonine phosphorylation plays a crucial role in the phosphorylation-dependent modulation of STAT-driven immune responses and autoimmunity [229,231]. Additionally, other PTMs—including acetylation, methylation, and ubiquitination—have been shown to fine-tune STAT-mediated cellular functions, further expanding the regulatory landscape of STAT signaling [10,87].
- Cellular localization: Although STAT proteins primarily function as transcriptional activators in the nucleus, they also exert distinct roles in other cellular compartments, such as the cytoplasm and mitochondria [232]. For instance, STAT3 and STAT5A localize to mitochondria, where they regulate gene expression and remodel cellular metabolism [233,234,235,236]. Additionally, cytoplasmic STAT3 modulates the microtubule network by binding to the C-terminal tubulin-interacting domain of stathmin, thereby counteracting its microtubule-destabilizing activity [237].
- Interactome: The interactions between STAT proteins and other cellular factors play a crucial role in shaping their functional outcomes. For example, IFN-α and IFN-β promote the formation of the STAT1-STAT2-IRF9 complex, which drives the expression of specific IFN-induced genes like OAS1. In contrast, IFN-γ induces STAT1 homodimerization, leading to the activation of distinct IFN-responsive genes such as IRF1 [238]. Additionally, STAT1’s interaction with NF-κB is critical for cytokine gene expression [201,239,240,241], while the crosstalk between WNT, STAT, and TGF-β pathways (via β-catenin, STAT3, and SMAD3, respectively) shapes context-specific cellular responses [242]. Moreover, STAT proteins interact with the transcriptional coactivators p300/CBP to enhance gene expression by promoting chromatin remodeling and transcriptional activation [243,244,245].
9.2. STAT Signature Model
10. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatton, I.A.; Galbraith, E.D.; Merleau, N.S.C.; Miettinen, T.P.; Smith, B.M.D.; Shander, J.A. The human cell count and size distribution. Proc. Natl. Acad. Sci. USA 2023, 120, e2303077120. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Medzhitov, R.; Iwasaki, A. Exploring new perspectives in immunology. Cell 2024, 187, 2079–2094. [Google Scholar] [CrossRef]
- Okin, D.; Medzhitov, R. Evolution of inflammatory diseases. Curr. Biol. 2012, 22, R733–R740. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.; Bhalla, U.S.; Iyengar, R. Complexity in Biological Signaling Systems A Signaling Wire. Science 1999, 284, 92–96. [Google Scholar]
- Göpfrich, K.; Platzman, I.; Spatz, J.P. Mastering complexity: Towards bottom-up construction of multifunctional eukaryotic synthetic cells. Trends Biotechnol. 2018, 36, 938–951. [Google Scholar] [CrossRef]
- Bhattacharyya, R.P.; Reményi, A.; Yeh, B.J.; Lim, W.A. Domains, motifs, and scaffolds: The role of modular interactions in the evolution and wiring of cell signaling circuits. Annu. Rev. Biochem. 2006, 75, 655–680. [Google Scholar] [CrossRef]
- Grecco, H.E.; Schmick, M.; Bastiaens, P.I.H. Signaling from the living plasma membrane. Cell 2011, 144, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Philips, R.L.; Wang, Y.; Cheon, H.; Kanno, Y.; Gadina, M.; Sartorelli, V.; Horvath, C.M.; Darnell, J.E., Jr.; Stark, G.R.; O’Shea, J.J. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 2022, 185, 3857–3876. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Leonard, W.J.; O’Shea, J.J. Jaks and STATs: Biological implications. Annu Rev Immunol. 1998, 16, 293–322. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Sun, J.F.; Nie, P.; Herdewijn, P.; Wang, Y.T. Synthesis and clinical application of small-molecule inhibitors of janus kinase. Eur. J. Med. Chem. 2023, 261, 115848. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; Peterson, D.; Ramseier, J.; Al-Bawardy, B.; Chun, H.; Proctor, D.; Strand, V.; Flavell, R.A.; King, B. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J. Allergy Clin. Immunol. 2021, 147, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Barrangou, R.; Marraffini, L.A. CRISPR-cas systems: Prokaryotes upgrade to adaptive immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Metwally, H.; Kishimoto, T. The Yin and the Yang of STAT1 Downstream of TLR4 Endocytosis: STAT1 beyond Interferon Signaling. J. Cell. Immunol. Comment. 2020, 2, 205–210. [Google Scholar]
- Kawai, T.; Akira, S. Pathogen recognition with toll-like receptors. Curr. Opin. Immunol. 2005, 17, 338–344. [Google Scholar] [CrossRef]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Pepper, M.; Thomas, P.G. Principles and therapeutic applications of adaptive Immunity. Cell 2024, 187, 2052–2078. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.; Lee, Y.S.; Farber, D.L. A guide to adaptive immune memory. Nat. Rev. Immunol. 2024, 24, 810–829. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Locksley, R.M. The ins and outs of innate and adaptive type 2 Immunity. Immunity 2023, 56, 704–722. [Google Scholar] [CrossRef]
- Kaushik, M.S.; Chakraborty, S.; Veleri, S.; Kateriya, S. Mucociliary Respiratory Epithelium Integrity in Molecular Defense and Susceptibility to Pulmonary Viral Infections. Biology 2021, 10, 476. [Google Scholar] [CrossRef]
- Pradeu, T.; Thomma, B.P.H.J.; Girardin, S.E.; Lemaitre, B. The conceptual foundations of innate immunity: Taking stock 30 years later. Immunity 2024, 57, 613–631. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef]
- Allen, J.E.; Maizels, R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Friberg, I.M.; Bradley, J.E.; Jackson, J.A. Macroparasites, innate immunity and immunoregulation: Developing natural models. Trends Parasitol. 2010, 26, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Grencis, R.K. Immunity to helminths: Resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu. Rev. Immunol. 2015, 33, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Vacca, F.; Le Gros, G. Tissue-specific immunity in helminth infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Parasite immune evasion: A momentous molecular war. Curr. Opin. Immunol. 2008, 23, 318–326. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S41–S52. [Google Scholar] [CrossRef]
- Abboud, N.; Chow, S.K.; Saylor, C.; Janda, A.; Ravetch, J.V.; Scharff, M.D.; Casadevall, A. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010, 207, 2395–2405. [Google Scholar] [CrossRef]
- Wen, Y.; Mu, L.; Shi, Y. Immunoregulatory functions of immune complexes in vaccine and therapy. EMBO Mol. Med. 2016, 8, 1120–1133. [Google Scholar] [CrossRef]
- Breinig, F.; Sendzik, T.; Eisfeld, K.; Schmitt, M.J. Dissecting Toxin Immunity in Virus-Infected Killer Yeast Uncovers an Intrinsic Strategy of Self-Protection. Proc. Natl. Acad. Sci. USA 2006, 103, 3810–3815. [Google Scholar] [CrossRef]
- Plum, T.; Feyerabend, T.B.; Rodewald, H.R. Beyond classical immunity: Mast cells as signal converters between tissues and neurons. Immunity 2024, 57, 2723–2736. [Google Scholar] [CrossRef]
- Florsheim, E.B.; Bachtel, N.D.; Cullen, J.L.; Lima, B.G.C.; Godazgar, M.; Carvalho, F.; Chatain, C.P.; Zimmer, M.R.; Zhang, C.; Gautier, G.; et al. Immune sensing of food allergens promotes avoidance behaviour. Nature 2023, 620, 643–650. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Scott, D.W. Tolerance Is Different in T and B Cells. J. Immunol. 2013, 191, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nature 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Hooper, L.V.; MacPherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Traxinger, B.R.; Richert-Spuhler, L.E.; Lund, J.M. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2022, 15, 398–407. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Viney, M.E.; Riley, E.M.; Buchanan, K.L. Optimal immune responses: Immunocompetence revisited. Trends Ecol. Evol. 2005, 20, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y. Explaining variable costs of the immune response: Selection for specific versus non-specific immunity and facultative life history change. Oikos 2003, 102, 213–216. [Google Scholar] [CrossRef]
- Rauw, W.M. Immune response from a resource allocation perspective. Front. Genet. 2012, 3, 267. [Google Scholar] [CrossRef]
- Wegner, K.M.; Kalbe, M.; Reusch, T.B.H. Innate versus adaptive immunity in sticklebacks: Evidence for trade-offs from a selection experiment. Evol. Ecol. 2007, 21, 473–483. [Google Scholar] [CrossRef]
- Sandland, G.J.; Minchella, D.J. Costs of immune defense: An enigma wrapped in an environmental cloak? Trends Parasitol. 2003, 19, 571–574. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Szi, M. Fever and hypothermia: Two adaptive thermoregulatory responses to systemic inflammation. Med. Hypotheses 1998, 50, 219–226. [Google Scholar]
- Haddad, F.; Soliman, A.M.; Wong, M.E.; Albers, E.H.; Semple, S.L.; Torrealba, D.; Heimroth, R.D.; Nashiry, A.; Tierney, K.B.; Barreda, D.R. Fever integrates antimicrobial defences, inflammation control, and tissue repair in a cold-blooded vertebrate. Elife 2023, 12, e83644. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the Thermal Regulation of Immunity: The Immune System Feels the Heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W.; Georgiev, A.V.; Kuzawa, C.W. Trade-offs between acquired and innate immune defenses in humans. Evol. Med. Public Health 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Sadd, B.M.; Schmid-Hempel, P. Principles of ecological immunology. Evol. Appl. 2009, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.S.; Ayres, J.S. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008, 8, 889–895. [Google Scholar] [CrossRef]
- Plum, T.; Binzberger, R.; Thiele, R.; Shang, F.; Postrach, D.; Fung, C.; Fortea, M.; Stakenborg, N.; Wang, Z.; Tappe-Theodor, A.; et al. Mast cells link immune sensing to antigen-avoidance behaviour. Nature 2023, 620, 634–642. [Google Scholar] [CrossRef]
- Florsheim, E.B.; Sullivan, Z.A.; Khoury-Hanold, W.; Medzhitov, R. Food allergy as a biological food quality control system. Cell 2021, 184, 1440–1454. [Google Scholar] [CrossRef]
- Ayres, J.S.; Schneider, D.S. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009, 7, e1000150. [Google Scholar] [CrossRef]
- Sperandio, B.; Fischer, N.; Sansonetti, P.J. Mucosal physical and chemical innate barriers: Lessons from microbial evasion strategies. Semin. Immunol. 2015, 27, 111–118. [Google Scholar] [CrossRef]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J. War and peace at mucosal surfaces. Nat. Rev. Immunol. 2004, 4, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Gutner, M.; Chaushu, S.; Balter, D.; Bachrach, G. Saliva enables the antimicrobial activity of LL-37 in the presence of proteases of Porphyromonas gingivalis. Infect. Immun. 2009, 77, 5558–5563. [Google Scholar] [CrossRef]
- Lee, E.Y.; Zhang, C.; Di Domizio, J.; Jin, F.; Connell, W.; Hung, M.; Malkoff, N.; Veksler, V.; Gilliet, M.; Ren, P.; et al. Helical antimicrobial peptides assemble into protofibril scaffolds that present ordered dsDNA to TLR9. Nat. Commun. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Rosenstein, R.K.; Medzhitov, R. Allergic host defences. Nature 2012, 484, 465–472. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Kayongo, A.; Nyiro, B.; Siddharthan, T.; Kirenga, B.; Checkley, W.; Lutaakome Joloba, M.; Ellner, J.; Salgame, P. Mechanisms of lung damage in tuberculosis: Implications for chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2023, 13, 1146571. [Google Scholar] [CrossRef]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease tolerance as a defense strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of immune tolerance to allergens: Role of IL-10 and Tregs. J. Clin. Investig. 2014, 124, 4678–4680. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, T.; Groom, Z.; Christophides, G.K. Immune resistance and tolerance strategies in malaria vector and non-vector mosquitoes. Parasit. Vectors 2017, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, W.; Ivens, A.; Spence, P.J. Inducible mechanisms of disease tolerance provide an alternative strategy of acquired immunity to malaria. Elife 2021, 10, e63838. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef]
- Fortelny, N.; Farlik, M.; Fife, V.; Gorki, A.D.; Lassnig, C.; Maurer, B.; Meissl, K.; Dolezal, M.; Boccuni, L.; Ravi Sundar Jose Geetha, A.; et al. JAK-STAT signaling maintains homeostasis in T cells and macrophages. Nat. Immunol. 2024, 25, 847–859. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E. The JAK-STAT Pathway at Twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar]
- McGee, H.M.; Schmidt, B.A.; Booth, C.J.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A.; Horsley, V. IL-22 promotes fibroblast-mediated wound repair in the skin. J. Investig. Dermatol. 2013, 133, 1321–1329. [Google Scholar] [CrossRef]
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 Receptor-Dependent STAT5 Activation Is Required for the Development of Foxp3 Regulatory T Cells. J. Immunol. 2007, 178, 280–290. [Google Scholar]
- Mahmud, S.A.; Manlove, L.S.; Farrar, M.A. Interleukin-2 and STAT5 in regulatory T cell development and function. JAK-STAT 2013, 2, e23154. [Google Scholar] [CrossRef] [PubMed]
- Walford, H.H.; Doherty, T.A. STAT6 and lung inflammation. JAK-STAT 2013, 2, e25301. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827–2833. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; McLeod, L.; Miller, A.; Jones, J.; Vlahos, R.; Ramm, G.; Longano, A.; Bardin, P.G.; Bozinovski, S.; Anderson, G.P.; et al. Deregulated Stat3 signaling dissociates pulmonary inflammation from emphysema in gp130 mutant mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, 627–639. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Fielding, C.A.; McLoughlin, R.M.; McLeod, L.; Colmont, C.S.; Najdovska, M.; Grail, D.; Ernst, M.; Jones, S.A.; Topley, N.; Jenkins, B.J. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008, 181, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Ruwanpura, S.M.; McLeod, L.; Brooks, G.D.; Bozinovski, S.; Vlahos, R.; Longano, A.; Bardin, P.G.; Anderson, G.P.; Jenkins, B.J. IL-6/Stat3-driven pulmonary inflammation, but not emphysema, is dependent on interleukin-17A in mice. Respirology 2014, 19, 419–427. [Google Scholar] [CrossRef]
- Gilbert, S.; Nivarthi, H.; Mayhew, C.N.; Lo, Y.H.; Noah, T.K.; Vallance, J.; Rülicke, T.; Müller, M.; Jegga, A.G.; Tang, W.; et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015, 4, 209–225. [Google Scholar] [CrossRef]
- Bauché, D.; Joyce-Shaikh, B.; Fong, J.; Villarino, A.V.; Ku, K.S.; Jain, R.; Lee, Y.C.; Annamalai, L.; Yearley, J.H.; Cua, D.J. IL-23 and IL-2 activation of STAT5 is required for optimal IL-22 production in ILC3s during colitis. Sci. Immunol. 2020, 5, eaav1080. [Google Scholar]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Zindl, C.L.; Lai, J.F.; Lee, Y.K.; Maynard, C.L.; Harbour, S.N.; Ouyang, W.; Chaplin, D.D.; Weaver, C.T. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 12768–12773. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, N.; Pickert, G.; Billmeier, U.; Mahapatro, M.; Wirtz, S.; Martini, E.; Leppkes, M.; Neurath, M.F.; Becker, C. Activation of intestinal epithelial stat3 orchestrates tissue defense during gastrointestinal infection. PLoS ONE 2015, 10, e0118401. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hruz, P.; Dann, S.M.; Eckmann, L. STAT3 and its activators in intestinal defense and mucosal homeostasis. Curr. Opin. Gastroenterol. 2010, 26, 109–115. [Google Scholar] [CrossRef]
- Paul, W.E.; Zhu, J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; He, W.Q.; Yu, J.; Xin, Y.; Zhang, X.; Huang, R.; Ma, H.; Xu, S.; Li, Z.; et al. Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity. Nat. Commun. 2022, 13, 5192. [Google Scholar] [CrossRef]
- Lin, Y.; Li, B.; Yang, X.; Liu, T.; Shi, T.; Deng, B.; Zhang, Y.; Jia, L.; Jiang, Z.; He, R. Non-hematopoietic STAT6 induces epithelial tight junction dysfunction and promotes intestinal inflammation and tumorigenesis. Mucosal Immunol. 2019, 12, 1304–1315. [Google Scholar] [CrossRef]
- Westermann, S.; Radtke, D.; Kramer, L.; Wirtz, S.; Voehringer, D. Activation of STAT6 in Intestinal Epithelial Cells Predisposes to Gut Inflammation. Eur. J. Immunol. 2024, 55, e202451394. [Google Scholar] [CrossRef]
- Darnell, J.E. Interferon-Dependent Tyrosine Phosphorylation of. Science 1992, 257, 809–813. [Google Scholar]
- Schindler, C.; Shuai, K.; Prezioso, V.R.; Darnell, J.E., Jr. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science 1992, 257, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Kessler, D.S.; Pine, R.; Darnell, J.E. Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes. Dev. 1989, 3, 1362–1371. [Google Scholar] [CrossRef]
- Kim, T.K.; Maniatis, T.; Chua, M.A.; McWhirter, S.M.; García-Sastre, A.; Maniatis, T. Regulation of Interferon-gamma -Activated STAT1 by the Ubiquitin-Proteasome Pathway. Science 1996, 273, 1717–1719. [Google Scholar] [CrossRef]
- Shuai, K.; Stark, G.R.; Kerr, I.M.; Darnell, J.E. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-γ. Science 1993, 261, 1744–1746. [Google Scholar] [CrossRef]
- Levy, D.; Larner, A.; Chaudhuri, A.; Babiss, L.E.; Darnell, J.E. Interferon-stimulated transcription: Isolation of an inducible gene and identification of its regulatory region. Proc. Natl. Acad. Sci. USA 1986, 83, 8929–8933. [Google Scholar] [CrossRef] [PubMed]
- Ramana, C.V.; Gil, M.P.; Han, Y.; Ransohoff, R.M.; Schreiber, R.D.; Stark, G.R. Stat1-Independent Regulation of Gene Expression in Response to IFN. Proc. Natl. Acad. Sci. USA 2001, 98, 6674–6679. [Google Scholar]
- Meissl, K.; Simonović, N.; Amenitsch, L.; Witalisz-Siepracka, A.; Klein, K.; Lassnig, C.; Puga, A.; Vogl, C.; Poelzl, A.; Bosmann, M.; et al. STAT1 Isoforms Differentially Regulate NK Cell Maturation and Anti-tumor Activity. Front. Immunol. 2020, 11, 2189. [Google Scholar] [CrossRef]
- Lee, C.K.; Rao, D.T.; Gertner, R.; Gimeno, R.; Frey, A.B.; Levy, D.E. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 2000, 165, 3571–3577. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Kishimoto, T. IL-6 and NF-IL6 in Acute-Phase Response and Viral Infection. Immunol. Rev. 1992, 127, 25–50. [Google Scholar] [CrossRef]
- Kishimoto, T. INTERLEUKIN-6: From Basic Science to Medicine—40 Years in Immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S2. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Immunotherapy of Tocilizumab for Rheumatoid Arthritis. J. Clin. Cell Immunol. 2013, 1 (Suppl. S6), 1. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2023, 6, a016295. [Google Scholar] [CrossRef]
- Kishimoto, T.; Taga, T.; Akira, S. Cytokine signal transduction. Immunity 1994, 76, 253–262. [Google Scholar] [CrossRef]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 2018, 54, 1–78. [Google Scholar]
- Gotthardt, D.; Putz, E.M.; Grundschober, E.; Prchal-Murphy, M.; Straka, E.; Kudweis, P.; Heller, G.; Bago-Horvath, Z.; Witalisz-Siepracka, A.; Cumaraswamy, A.A.; et al. STAT5 is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov. 2016, 6, 414–429. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Grassmann, S.; Lau, C.M.; Rapp, M.; Villarino, A.V.; Friedrich, C.; Gasteiger, G.; O’Shea, J.J.; Sun, J.C. Divergent Role for STAT5 in the Adaptive Responses of Natural Killer Cells. Cell Rep. 2020, 33, 108498. [Google Scholar] [CrossRef]
- Gotthardt, D.; Sexl, V. STATs in NK-cells: The good, the bad, and the ugly. Front. Immunol. 2017, 7, 694. [Google Scholar] [CrossRef]
- Jacobson, N.G.; Szabo, S.J.; Weber-Nordt, R.M.; Zhong, Z.; Schreiber, R.D.; Darnell, J.E., Jr.; Murphy, K.M. Interleukin 12 Signaling in T Helper Type 1 (Thl) Cells Involves Tyrosine Phosphorylation of Signal Transducer and Activator of Transcription (Stat)3 and Stat4. J. Exp. Med. 1995, 181, 1755–1762. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in T reg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Wojno, E.D.T.; Hunter, C.A.; Stumhofer, J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Ouyang, W.; Löhning, M.; Gao, Z.; Assenmacher, M.; Ranganath, S.; Radbruch, A.; Murphy, K.M. Stat6-Independent GATA-3 Autoactivation Directs IL-4-Independent Th2 Development and Commitment. Immunity 2000, 12, 27–37. [Google Scholar] [CrossRef]
- Dent, A.L.; Hu-Li, J.; Paul, W.E.; Staudt, L.M. T Helper Type 2 Inflammatory Disease in the Absence of Interleukin 4 and Transcription Factor STAT6. Proc. Natl. Acad. Sci. USA 1998, 95, 13823–13828. [Google Scholar] [PubMed]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 Is Required for Mediating Responses to IL-4 and for the Development of Th2 Cells. Immunity 1996, 4, 313–319. [Google Scholar] [PubMed]
- Qin, Z.; Wang, R.; Hou, P.; Zhang, Y.; Yuan, Q.; Wang, Y.; Yang, Y.; Xu, T. TCR signaling induces STAT3 phosphorylation to promote TH17 cell differentiation. J. Exp. Med. 2024, 221, e20230683. [Google Scholar] [CrossRef]
- Chalmin, F.; Mignot, G.; Bruchard, M.; Chevriaux, A.; Végran, F.; Hichami, A.; Ladoire, S.; Derangère, V.; Vincent, J.; Masson, D.; et al. Stat3 and Gfi-1 Transcription Factors Control Th17 Cell Immunosuppressive Activity via the Regulation of Ectonucleotidase Expression. Immunity 2012, 36, 362–373. [Google Scholar] [CrossRef]
- Purvis, H.A.; Anderson, A.E.; Young, D.A.; Isaacs, J.D.; Hilkens, C.M.U. A Negative Feedback Loop Mediated by STAT3 Limits Human Th17 Responses. J. Immunol. 2014, 193, 1142–1150. [Google Scholar] [CrossRef]
- Dikiy, S.; Li, J.; Bai, L.; Jiang, M.; Janke, L.; Zong, X.; Hao, X.; Hoyos, B.; Wang, Z.M.; Xu, B.; et al. A distal Foxp3 enhancer enables interleukin-2 dependent thymic Treg cell lineage commitment for robust immune tolerance. Immunity 2021, 54, 931–946.e11. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, O.C.; Singh, S.; Hippen, K.L.; Maynard, J.C.; Burlingame, A.L.; Ball, L.E.; Blazar, B.R.; Farrar, M.A.; Hogquist, K.A.; et al. The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat. Commun. 2019, 10, 354. [Google Scholar] [CrossRef]
- Minami, Y.; Kono, T.; Yamada, K.; Taniguchi, T. The Interleukin-2 receptors: Insights into a complex signalling mechanism. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 1992, 1114, 163–177. [Google Scholar]
- Passerini, L.; Allan, S.E.; Battaglia, M.; Di Nunzio, S.; Alstad, A.N.; Levings, M.K.; Roncarolo, M.G.; Bacchetta, R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int. Immunol. 2008, 20, 421–431. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type i interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed]
- Langston, P.K.; Shibata, M.; Horng, T. Metabolism supports macrophage activation. Front. Immunol. 2017, 8, 61. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, C.; Herrmann, A.; Song, J.; Egelston, C.; Wang, T.; Zhang, Z.; Li, W.; Lee, H.; Aftabizadeh, M.; et al. STAT3 Activation-Induced Fatty Acid Oxidation in CD8+ T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth. Cell Metab. 2020, 31, 148–161.e5. [Google Scholar] [CrossRef]
- Pallandre, J.R.; Brillard, E.; Créhange, G.; Radlovic, A.; Remy-Martin, J.P.; Saas, P.; Rohrlich, P.S.; Pivot, X.; Ling, X.; Tiberghien, P.; et al. Role of STAT3 in CD4 CD25 FOXP3 Regulatory Lymphocyte Generation: Implications in Graft-versus-Host Disease and Antitumor Immunity. J. Immunol. 2007, 179, 7593–7604. [Google Scholar]
- Deswal, B.; Bagchi, U.; Santra, M.K.; Garg, M.; Kapoor, S. Inhibition of STAT3 by 2-Methoxyestradiol suppresses M2 polarization and protumoral functions of macrophages in breast cancer. BMC Cancer 2024, 24, 1129. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, M.; Lei, W.; Yang, R.; Fu, S.; Fan, Z.; Yang, Y.; Zhang, T. Advances in the role of STAT3 in macrophage polarization. Front. Immunol. 2023, 14, 1160719. [Google Scholar] [CrossRef]
- Newton, R.; Priyadharshini, B.; Turka, L.A. Immunometabolism of regulatory T cells. Nat. Immunol. 2016, 17, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Galván-Peña, S.; O’Neill, L.A.J. Metabolic reprogramming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar] [CrossRef]
- Lacy-Hulbert, A.; Moore, K.J. Designer macrophages: Oxidative metabolism fuels inflammation repair. Cell Metab. 2006, 4, 7–8. [Google Scholar] [CrossRef]
- Mackie, J.; Ma, C.S.; Tangye, S.G.; Guerin, A. The ups and downs of STAT3 function: Too much, too little and human immune dysregulation. Clin. Exp. Immunol. 2023, 212, 107–116. [Google Scholar] [CrossRef]
- Gubser, P.M.; Bantug, G.R.; Razik, L.; Fischer, M.; Dimeloe, S.; Hoenger, G.; Durovic, B.; Jauch, A.; Hess, C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 2013, 14, 1064–1072. [Google Scholar] [CrossRef]
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008, 111, 2101–2111. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, K.; Cloer, C.; Neale, G.; Vogel, P.; Chi, H. MTORC1 couples immune signals and metabolic programming to establish T reg-cell function. Nature 2013, 499, 485–490. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L.; et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A.J. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Kujawski, M.; Wang, T.; Wei, S.; Zhang, S.; Pilon-Thomas, S.; Niu, G.; Kay, H.; Mulé, J.; Kerr, W.G.; et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005, 11, 1314–1321. [Google Scholar] [CrossRef]
- Rah, B.; Rather, R.A.; Bhat, G.R.; Baba, A.B.; Mushtaq, I.; Farooq, M.; Yousuf, T.; Dar, S.B.; Parveen, S.; Hassan, R.; et al. JAK/STAT Signaling: Molecular Targets, Therapeutic Opportunities, and Limitations of Targeted Inhibitions in Solid Malignancies. Front. Pharmacol. 2022, 13, 821344. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Poret, J.M.; Souza-Smith, F.; Marcell, S.J.; Gaudet, D.A.; Tzeng, T.H.; Braymer, H.D.; Harrison-Bernard, L.M.; Primeaux, S.D. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int. J. Obes. 2018, 42, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Metwally, H.; Kishimoto, T. Gut Microbiome Metabolites and the Intestinal Homeostasis. J. Clin. Res. Rep. 2024, 15, 1–6. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and ‘Western-Lifestyle’ Inflammatory Diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Potla, R.; Chwae, Y.J.; Sepuri, N.B.; Zhang, Q.; Koeck, T.; Derecka, M.; Szczepanek, K.; Szelag, M.; Gornicka, A.; et al. Function of mitochondrial Stat3 in cellular respiration. Science 2009, 323, 793–797. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span-From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Tran, M.; Fernandez-Rojo, M.A.; Merry, T.L.; Zhang, X.; Xu, Y.; Fukushima, A.; Waters, M.J.; Watt, M.J.; Andrikopoulos, S.; et al. Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab. 2014, 20, 85–102. [Google Scholar] [CrossRef]
- Lin, J.X.; Leonard, W.J. Fine-Tuning Cytokine Signals. Annu. Rev. Immunol. 2019, 37, 295–324. [Google Scholar] [CrossRef]
- Lin, J.X.; Migone, T.S.; Tsang, M.; Friedmann, M.; Weatherbee, J.A.; Zhou, L.; Yamauchi, A.; Bloom, E.T.; Mietz, J.; John, S.; et al. The Role of Shared Receptor Motifs and Common Stat Proteins in the Generation of Cytokine Pleiotropy and Redundancy by IL-2, IL-4, 11-7, 11-13, and IL-15. Immunity 1995, 2, 331–339. [Google Scholar] [CrossRef]
- Weber-Nordt, R.M.; Riley, J.K.; Greenlund, A.C.; Moore, K.W.; Darnell, J.E.; Schreiber, R.D. Stat3 Recruitment by Two Distinct Ligand-induced, Tyrosine-phosphorylated Docking Sites in the Interleukin-10 Receptor Intracellular Domain. J. Biol. Chem. 1996, 271, 27954–27961. [Google Scholar] [CrossRef] [PubMed]
- Greenlund, A.C.; Morales, M.O.; Viviano, B.L.; Yan, H.; Krolewski, J.; Schreiber, R.D. Stat Recruitment by Tyrosine-Phosphorylated Cytokine Receptors: An Ordered Reversible Affinity-Driven Process. Immunity 1995, 2, 677–687. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Casas, E.; Zhu, W.; Levy, D.E.; Leonard, W.J. The molecular basis of IL-21-mediated proliferation. Blood 2007, 109, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Leonard, W.J. The common cytokine receptor γ chain family of cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028449. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.K.; Andraski, A.B.; Spolski, R.; Li, P.; Kazemian, M.; Oh, J.; Samsel, L.; Swanson, P.A., 2nd; McGavern, D.B.; Sampaio, E.P.; et al. Opposing roles of STAT1 and STAT3 in IL-21 function in CD4+ T cells. Proc. Natl. Acad. Sci. USA 2015, 112, 9394–9399. [Google Scholar] [CrossRef]
- Meyer Zu Horste, G.; Przybylski, D.; Schramm, M.A.; Wang, C.; Schnell, A.; Lee, Y.; Sobel, R.; Regev, A.; Kuchroo, V.K. Fas Promotes T Helper 17 Cell Differentiation and Inhibits T Helper 1 Cell Development by Binding and Sequestering Transcription Factor STAT1. Immunity 2018, 48, 556–569.e7. [Google Scholar] [CrossRef]
- Dai, H.; He, F.; Tsokos, G.C.; Kyttaris, V.C. IL-23 Limits the Production of IL-2 and Promotes Autoimmunity in Lupus. J. Immunol. 2017, 199, 903–910. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Wang, L.; Li, P.; Leonard, W.J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 2011, 12, 551–559. [Google Scholar] [CrossRef]
- Lazarevic, V.; Chen, X.; Shim, J.H.; Hwang, E.S.; Jang, E.; Bolm, A.N.; Oukka, M.; Kuchroo, V.K.; Glimcher, L.H. T-bet represses TH 17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 2011, 12, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Schones, D.E.; Oh, J.; Cui, Y.; Cui, K.; Roh, T.Y.; Zhao, K.; Leonard, W.J. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 2008, 9, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Cote-Sierra, J.; Foucras, G.; Guo, L.; Chiodetti, L.; Young, H.A.; Hu-Li, J.; Zhu, J.; Paul, W.E. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 3880–3885. [Google Scholar] [CrossRef]
- Liongue, C.; Ward, A.C. Evolution of the JAK-STAT pathway. JAK-STAT 2013, 2, e22756. [Google Scholar] [CrossRef]
- Liongue, C.; O’Sullivan, L.A.; Trengove, M.C.; Ward, A.C. Evolution of JAK-STAT pathway components: Mechanisms and role in immune system development. PLoS ONE 2012, 7, e32777. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Wang, Y.; Levy, D.E. Comparative evolutionary genomics of the STAT family of transcription factors. JAK-STAT 2012, 1, 23–36. [Google Scholar] [CrossRef]
- Cheon, H.; Stark, G.R. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9373–9378. [Google Scholar] [CrossRef]
- Nan, J.; Wang, Y.; Yang, J.; Stark, G.R. IRF9 and unphosphorylated STAT2 cooperate with NF-κB to drive IL6 expression. Proc. Natl. Acad. Sci. USA 2018, 115, 3906–3911. [Google Scholar] [CrossRef]
- Majoros, A.; Platanitis, E.; Szappanos, D.; Cheon, H.; Vogl, C.; Shukla, P.; Stark, G.R.; Sexl, V.; Schreiber, R.; Schindler, C.; et al. Response to interferons and antibacterial innate immunity in the absence of tyrosine-phosphorylated STAT 1. EMBO Rep. 2016, 17, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Liongue, C.; Sertori, R.; Ward, A.C. Evolution of Cytokine Receptor Signaling. J. Immunol. 2016, 197, 11–18. [Google Scholar] [CrossRef]
- Hou, X.S.; Melnick, M.B. marelle Acts Downstream of the Drosophila HOP/JAK Kinase and Encodes a Protein Similar to the Mammalian STATs. Cell 1996, 84, 411–419. [Google Scholar]
- Yan, R.; Small, S.; Desplan, C.; Dearolf, C.R.; Darnell, J.E. Identification of a Stat Gene That Functions in Drosophila Development The receptors involved include both those that lack and those that possess intrinsic tyrosine kinase activity. Cell 1996, 84, 421–430. [Google Scholar] [PubMed]
- Zhang, Y.; Li, W.; Li, L.; Li, Y.; Fu, R.; Zhu, Y.; Li, J.; Zhou, Y.; Xiong, S.; Zhang, H. Structural Damage in the C. elegans Epidermis Causes Release of STA-2 and Induction of an Innate Immune Response. Immunity 2015, 42, 309–320. [Google Scholar] [CrossRef]
- Kawata, T. STAT signaling in Dictyostelium development. Dev. Growth Differ. 2011, 53, 548–557. [Google Scholar] [CrossRef]
- Araki, T.; Tsujioka, M.; Abe, T.; Fukuzawa, M.; Meima, M.; Schaap, P.; Morio, T.; Urushihara, H.; Katoh, M.; Maeda, M.; et al. A STAT-regulated, stress-induced signalling pathway in Dictyostelium. J. Cell Sci. 2003, 116, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Oldroyd, G.E.D. GRAS-domain transcription factors that regulate plant development. Plant Signal. Behav. 2009, 4, 698–700. [Google Scholar] [CrossRef]
- Shi, S.; Calhoun, H.C.; Xia, F.; Li, J.; Le, L.; Li, W.X. JAK signaling globally counteracts heterochromatic gene silencing. Nat. Genet. 2006, 38, 1071–1076. [Google Scholar] [CrossRef]
- Tanguy, M.; Véron, L.; Stempor, P.; Ahringer, J.; Sarkies, P.; Miska, E.A. An alternative STAT signaling pathway acts in viral immunity in caenorhabditis elegans. mBio 2017, 8, e00924-17. [Google Scholar] [CrossRef]
- Dierking, K.; Polanowska, J.; Omi, S.; Engelmann, I.; Gut, M.; Lembo, F.; Ewbank, J.J.; Pujol, N. Unusual regulation of a STAT protein by an SLC6 family transporter in C. elegans epidermal innate immunity. Cell Host Microbe 2011, 9, 425–435. [Google Scholar] [CrossRef]
- Tsurumi, A.; Zhao, C.; Li, W.X. Canonical and non-canonical JAK/STAT transcriptional targets may be involved in distinct and overlapping cellular processes. BMC Genom. 2017, 18, 718. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, M.; Araki, T.; Adrian, I.; Williams, J.G. Tyrosine Phosphorylation-Independent Nuclear Translocation of a Dictyostelium STAT in Response to DIF Signaling. Immunity 2001, 7, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Rauch, I.; Müller, M.; Decker, T. The regulation of inflammation by interferons and their STATs. JAK-STAT 2013, 2, e23820. [Google Scholar] [CrossRef]
- Minegishi, Y.; Saito, M.; Tsuchiya, S.; Tsuge, I.; Takada, H.; Hara, T.; Kawamura, N.; Ariga, T.; Pasic, S.; Stojkovic, O.; et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007, 448, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.; et al. STAT3 Mutations in the Hyper-IgE Syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar] [CrossRef]
- Baran-Marszak, F.; Feuillard, J.; Najjar, I.; Le Clorennec, C.; Béchet, J.M.; Dusanter-Fourt, I.; Bornkamm, G.W.; Raphaël, M.; Fagard, R. Differential roles of STAT1α and STAT1β in fludarabine-induced cell cycle arrest and apoptosis in human B cells. Blood 2004, 104, 2475–2483. [Google Scholar] [CrossRef]
- Semper, C.; Leitner, N.R.; Lassnig, C.; Parrini, M.; Mahlakõiv, T.; Rammerstorfer, M.; Lorenz, K.; Rigler, D.; Müller, S.; Kolbe, T.; et al. STAT1β Is Not Dominant Negative and Is Capable of Contributing to Gamma Interferon-Dependent Innate Immunity. Mol. Cell. Biol. 2014, 34, 2235–2248. [Google Scholar] [CrossRef]
- Parrini, M.; Meissl, K.; Ola, M.J.; Lederer, T.; Puga, A.; Wienerroither, S.; Kovarik, P.; Decker, T.; Müller, M.; Strobl, B. The C-Terminal Transactivation Domain of STAT1 Has a Gene-Specific Role in Transactivation and Cofactor Recruitment. Front. Immunol. 2018, 9, 2879. [Google Scholar] [CrossRef]
- Maritano, D.; Sugrue, M.L.; Tininini, S.; Dewilde, S.; Strobl, B.; Fu, X.; Murray-Tait, V.; Chiarle, R.; Poli, V. The STAT3 isoforms α and β have unique and specific functions. Nat. Immunol. 2004, 5, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Aigner, P.; Just, V.; Stoiber, D. STAT3 isoforms: Alternative fates in cancer? Cytokine 2019, 118, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, J.; Dong, S.; Redell, M.S.; Poli, V.; Mancini, M.A.; Tweardy, D.J. Stat3 isoforms, α and β, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3β mapping to its unique C-terminal end. J. Biol. Chem. 2007, 282, 34958–34967. [Google Scholar] [CrossRef]
- Sadzak, I.; Schiff, M.; Gattermeier, I.; Glinitzer, R.; Sauer, I.; Saalmüller, A.; Yang, E.; Schaljo, B.; Kovarik, P. Recruitment of Stat1 to Chromatin Is Required for Interferon-Induced Serine Phosphorylation of Stat1 Transactivation Domain. Proc. Natl. Acad. Sci. USA 2008, 105, 8944–8949. [Google Scholar] [PubMed]
- Zhu, X.; Wen, Z.; Xu, L.Z.; Darnell, J.E. Stat1 serine phosphorylation occurs independently of tyrosine phosphorylation and requires an activated Jak2 kinase. Mol. Cell. Biol. 1997, 17, 6618–6623. [Google Scholar] [CrossRef]
- Wen, Z.; Zhong, Z.; Darnell, J.E. Maximal activation of transcription by statl and stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Rhee, S.H.; Jones, B.W.; Toshchakov, V.; Vogel, S.N.; Fenton, M.J. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J. Biol. Chem. 2003, 278, 22506–22512. [Google Scholar] [CrossRef]
- Metwally, H.; Kishimoto, T. Distinct Phosphorylation of STAT1 Confers Distinct DNA Binding and Gene-regulatory Properties. J. Cell. Signal. Comment. 2020, 1, 50–55. [Google Scholar]
- Metwally, H.; Elbrashy, M.M.; Ozawa, T.; Okuyama, K.; White, J.T.; Tulyeu, J.; Søndergaard, J.N.; Wing, J.B.; Muratsu, A.; Matsumoto, H.; et al. Threonine phosphorylation of STAT1 restricts interferon signaling and promotes innate inflammatory responses. Proc. Natl. Acad. Sci. USA 2024, 121, e2402226121. [Google Scholar] [CrossRef]
- Metwally, H.; Tanaka, T.; Li, S.; Parajuli, G.; Kang, S.; Hanieh, H.; Hashimoto, S.; Chalise, J.P.; Gemechu, Y.; Standley, D.M.; et al. Noncanonical STAT1 Phosphorylation expands its transcriptional activity into promoting LPS-induced IL-6 and IL-12p40 production. Sci. Signal. 2020, 13, eaay0574. [Google Scholar]
- Elbrashy, M.M.; Metwally, H.; Sakakibara, S.; Kishimoto, T. Threonine Phosphorylation and the Yin and Yang of STAT1: Phosphorylation-Dependent Spectrum of STAT1 Functionality in Inflammatory Contexts. Cells 2024, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Liongue, C.; Ward, A.C. STAT proteins: A kaleidoscope of canonical and non-canonical functions in immunity and cancer. J. Hematol. Oncol. 2021, 14, 198. [Google Scholar] [CrossRef]
- Fernando, C.D.; Jayasekara, W.S.N.; Inampudi, C.; Kohonen-Corish, M.R.J.; Cooper, W.A.; Beilharz, T.H.; Josephs, T.M.; Garama, D.J.; Gough, D.J. A STAT3 protein complex required for mitochondrial mRNA stability and cancer. Cell Rep. 2023, 42, 113033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Liu, Y.; Zhang, P.; Nie, J.; Zhao, R.; Shi, Q.; Sun, H.; Jiao, D.; Chen, Y.; et al. Mitochondrial STAT5A promotes metabolic remodeling and the Warburg effect by inactivating the pyruvate dehydrogenase complex. Cell Death Dis. 2021, 12, 634. [Google Scholar] [CrossRef]
- Macias, E.; Rao, D.; Carbajal, S.; Kiguchi, K.; Digiovanni, J. Stat3 binds to mtDNA and regulates mitochondrial gene expression in keratinocytes. J. Investig. Dermatol. 2014, 134, 1971–1980. [Google Scholar] [CrossRef]
- Meier, J.A.; Larner, A.C. Toward a new STATe: The role of STATs in mitochondrial function. Semin. Immunol. 2014, 26, 20–28. [Google Scholar] [CrossRef]
- Ng, D.C.; Lin, B.H.; Lim, C.P.; Huang, G.; Zhang, T.; Poli, V.; Cao, X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 2006, 172, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Begitt, A.; Droescher, M.; Meyer, T.; Schmid, C.D.; Baker, M.; Antunes, F.; Knobeloch, K.P.; Owen, M.R.; Naumann, R.; Decker, T.; et al. STAT1-cooperative DNA binding distinguishes type 1 from type 2 interferon signaling. Nat. Immunol. 2014, 15, 168–176. [Google Scholar] [CrossRef]
- Tahk, S.; Liu, B.; Chernishof, V.; Wong, K.A.; Wu, H.; Shuai, K. Control of specificity and magnitude of NF-κB and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl. Acad. Sci. USA 2007, 104, 11643–11648. [Google Scholar] [CrossRef]
- Wienerroither, S.; Shukla, P.; Farlik, M.; Majoros, A.; Stych, B.; Vogl, C.; Cheon, H.; Stark, G.R.; Strobl, B.; Müller, M.; et al. Cooperative Transcriptional Activation of Antimicrobial Genes by STAT and NF-κB Pathways by Concerted Recruitment of the Mediator Complex. Cell Rep. 2015, 12, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, A.V.; Dall’Agnese, A.; Henninger, J.E.; Manteiga, J.C.; Afeyan, L.K.; Hannett, N.M.; Coffey, E.L.; Li, C.H.; Oksuz, O.; Sabari, B.R.; et al. Mediator Condensates Localize Signaling Factors to Key Cell Identity Genes. Mol. Cell 2019, 76, 753–766.e6. [Google Scholar] [CrossRef]
- Vahedi, G.; Takahashi, H.; Nakayamada, S.; Sun, H.W.; Sartorelli, V.; Kanno, Y.; O’Shea, J.J. STATs shape the active enhancer landscape of T cell populations. Cell 2012, 151, 981–993. [Google Scholar] [CrossRef]
- Wojciak, J.M.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J. 2009, 28, 948–958. [Google Scholar] [CrossRef]
- Paulson, M.; Pisharody, S.; Pan, L.; Guadagno, S.; Mui, A.L.; Levy, D.E. Stat protein transactivation domains recruit p300/CBP through widely divergent sequences. J. Biol. Chem. 1999, 274, 25343–25349. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Wang, X.; Almet, A.A.; Nie, Q. Detecting global and local hierarchical structures in cell-cell communication using CrossChat. Nat. Commun. 2024, 15, 10542. [Google Scholar] [CrossRef] [PubMed]
- Di-Bella, J.P.; Colman-Lerner, A.; Ventura, A.C. Properties of cell signaling pathways and gene expression systems operating far from steady-state. Sci. Rep. 2018, 8, 17035. [Google Scholar] [CrossRef]
- Pryciak, P.M. Designing New Cellular Signaling Pathways. Chem. Biol. 2009, 16, 249–254. [Google Scholar] [CrossRef][Green Version]
- Perrimon, N.; Pitsouli, C.; Shilo, B.Z. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb. Perspect. Biol. 2012, 4, a005975. [Google Scholar] [CrossRef]
- Lim, W.A. The emerging era of cell engineering: Harnessing the modularity of cells to program complex biological function. Science 2022, 378, 848–852. [Google Scholar] [PubMed]
- Lim, W.A. Designing customized cell signalling circuits. Nat. Rev. Mol. Cell Biol. 2010, 11, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gordley, R.M.; Bugaj, L.J.; Lim, W.A. Modular engineering of cellular signaling proteins and networks. Curr. Opin. Struct. Biol. 2016, 39, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.A.; Del Castillo, J.S.; Pelkmans, L. Systems biology multimodal perception links cellular state to decision-making in single cells. Science 2022, 377, 642–648. [Google Scholar]
- Meizlish, M.L.; Franklin, R.A.; Zhou, X.; Medzhitov, R. Tissue Homeostasis and Inflammation. Annu. Rev. Immunol. 2025, 43, 7. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwally, H. STAT Signature Dish: Serving Immunity with a Side of Dietary Control. Biomolecules 2025, 15, 487. https://doi.org/10.3390/biom15040487
Metwally H. STAT Signature Dish: Serving Immunity with a Side of Dietary Control. Biomolecules. 2025; 15(4):487. https://doi.org/10.3390/biom15040487
Chicago/Turabian StyleMetwally, Hozaifa. 2025. "STAT Signature Dish: Serving Immunity with a Side of Dietary Control" Biomolecules 15, no. 4: 487. https://doi.org/10.3390/biom15040487
APA StyleMetwally, H. (2025). STAT Signature Dish: Serving Immunity with a Side of Dietary Control. Biomolecules, 15(4), 487. https://doi.org/10.3390/biom15040487





