Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Production and Purification of rBanLec
2.3. Experimental Design
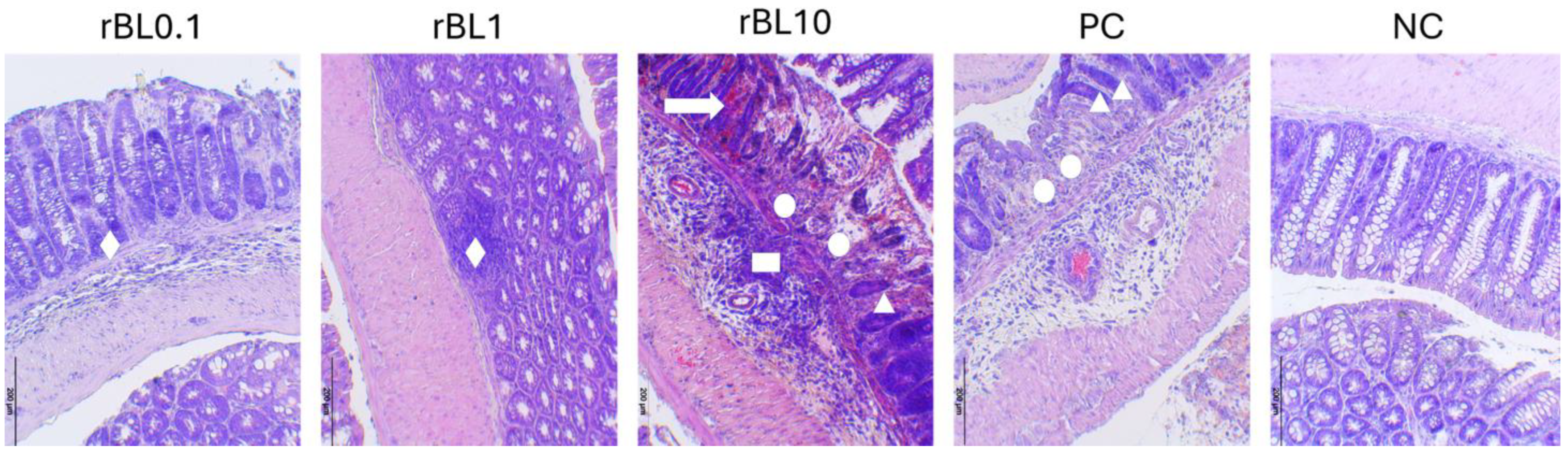
2.4. Histopathological Assessment of Severity of Experimental Colitis
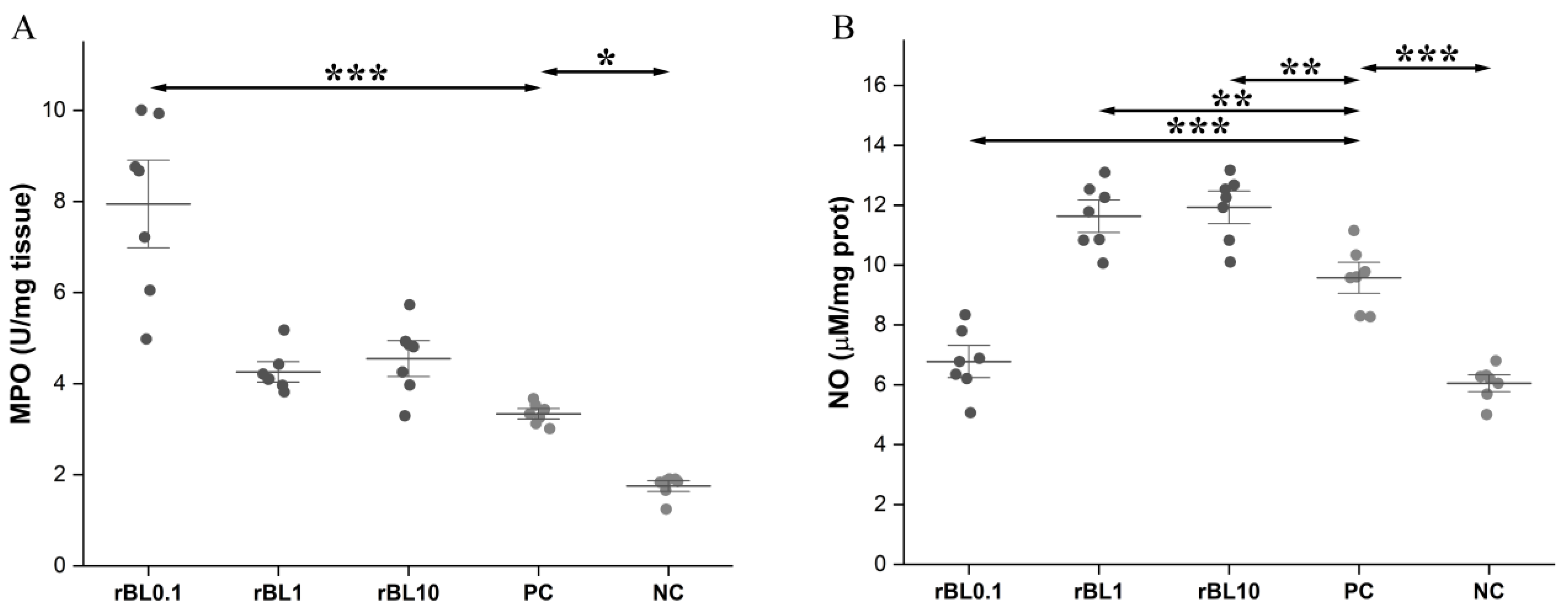
2.5. Measurement of MPO Activity in Colon Tissue
2.6. Homogenisation of Colonic Tissue
2.7. Assessment of Local NO Production
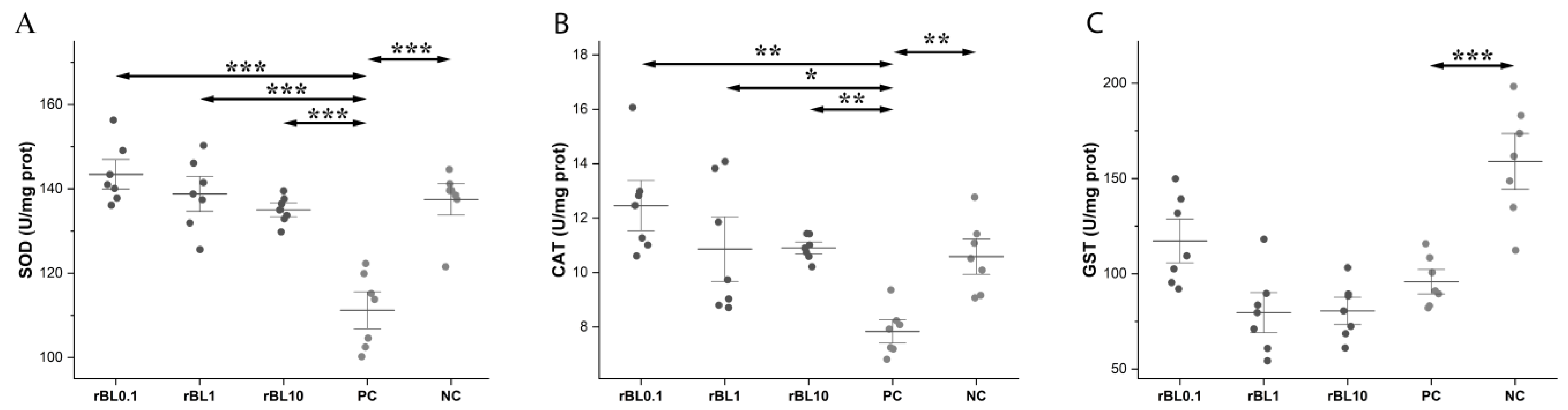
2.8. Determination of the Activity of the Antioxidative Enzymes
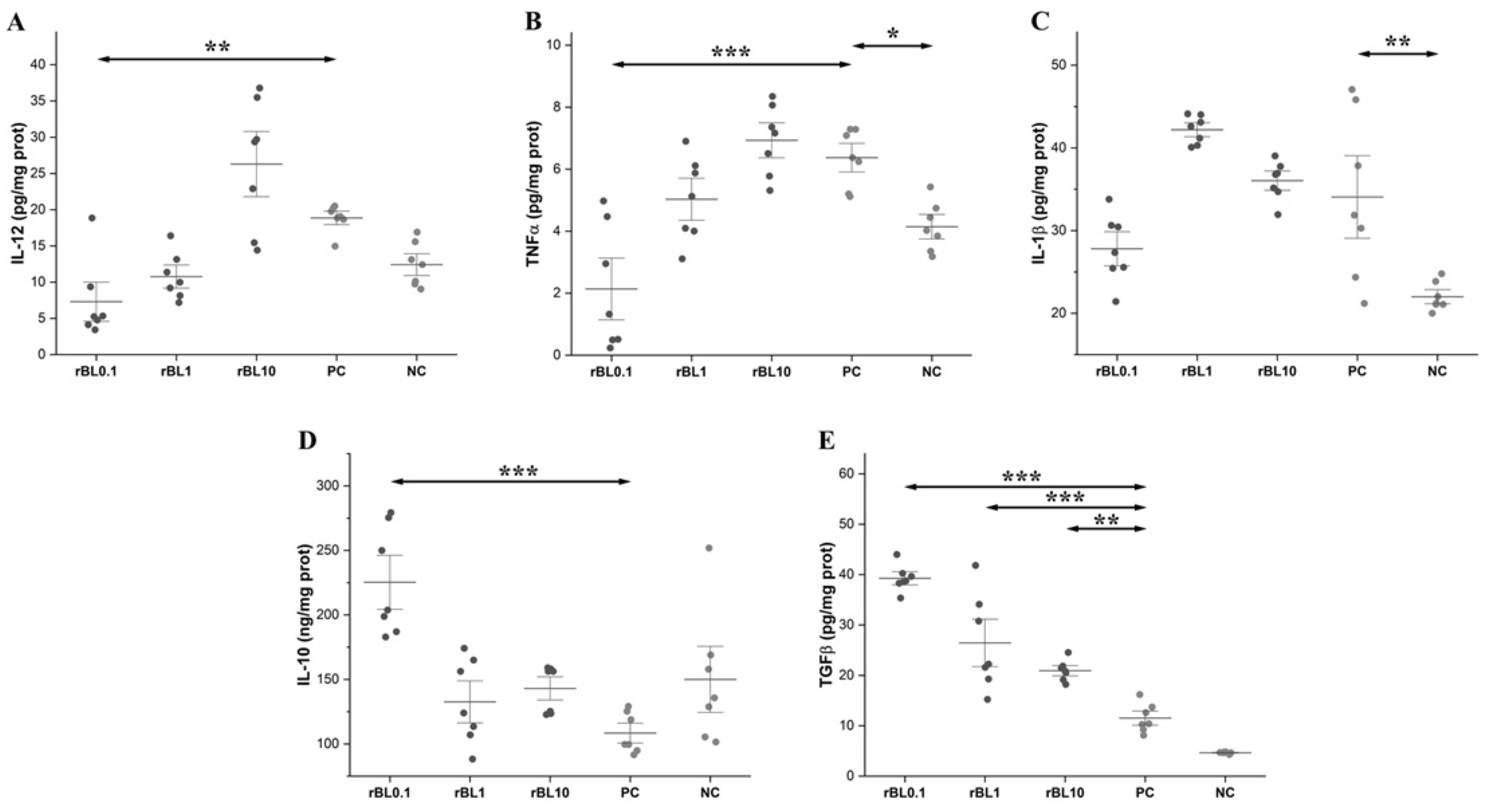
2.9. Analyses of Cytokines
2.10. Statistical Analysis
3. Results
3.1. Low-Dose rBanLec Prophylactic Treatment Reduces the Severity of TNBS-Induced Colitis at the Peak of the Disease
3.2. Prophylactic Treatment with rBanLec Is Associated with Modulation of MPO Activity and NO Production in the Colon at the Peak of TNBS-Induced Colitis
3.3. Prophylactic Treatment with rBanLec Is Associated with Enhancement in the Activity of Antioxidative Enzymes in the Colon at the Peak of TNBS-Induced Colitis
3.4. Prophylactic Treatment with rBanLec Shapes Characteristics of the Cytokine Milieu in the Colon at the Peak of TNBS-Induced Colitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BW | body weight |
| CAT | catalase |
| CHI | colitis histology index |
| GI | gastrointestinal tract |
| GSH | glutathione |
| GST | glutathione S-transferase |
| IBD | inflammatory bowel diseases |
| IFNγ | interferon γ |
| IL | interleukin |
| rBanLec | recombinant banana lectin |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RT | room temperature |
| SOD | superoxide dismutase |
| TGFβ | transforming growth factor beta |
| TLR2 | Toll-like receptor 2 |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Bain, C.C.; Fenton, T.M.; Kelly, A.; Brown, S.L.; Ivens, A.C.; Travis, M.A.; Cook, P.C.; MacDonald, A.S. Dynamics of Colon Monocyte and Macrophage Activation During Colitis. Front. Immunol. 2018, 9, 2764. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Sundrud, M.S. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Yang, G.Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef]
- Kruidenier, L.; Verspaget, H.W. Review article: Oxidative stress as a pathogenic factor in inflammatory bowel disease—Radicals or ridiculous? Aliment. Pharmacol. Ther. 2002, 16, 1997–2015. [Google Scholar] [CrossRef]
- Gomez, A.; Serrano, A.; Salero, E.; Tovar, A.; Amescua, G.; Galor, A.; Keane, R.W.; de Rivero Vaccari, J.P.; Sabater, A.L. Tumor necrosis factor-alpha and interferon-gamma induce inflammasome-mediated corneal endothelial cell death. Exp. Eye Res. 2021, 207, 108574. [Google Scholar] [CrossRef]
- Morikawa, A.; Koide, N.; Kato, Y.; Sugiyama, T.; Chakravortty, D.; Yoshida, T.; Yokochi, T. Augmentation of nitric oxide production by gamma interferon in a mouse vascular endothelial cell line and its modulation by tumor necrosis factor alpha and lipopolysaccharide. Infect. Immun. 2000, 68, 6209–6214. [Google Scholar] [CrossRef]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Gunnett, C.A.; Heistad, D.D.; Berg, D.J.; Faraci, F.M. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1555–H1562. [Google Scholar] [CrossRef]
- Pezzilli, R.; Billi, P.; Miniero, R.; Barakat, B. Serum interleukin-10 in human acute pancreatitis. Dig. Dis. Sci. 1997, 42, 1469–1472. [Google Scholar] [CrossRef]
- Fink, M.; Wrana, J.L. Regulation of homeostasis and regeneration in the adult intestinal epithelium by the TGF-β superfamily. Dev. Dyn. 2023, 252, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Borges, C.V.; de Sousa Cardoso, S.M.; de Almeida Pereira da Rocha, M.F.; Maraschin, M. Banana (Musa spp.) as a Source of Bioactive Compounds for Health Promotion. In Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition; Wiley Online Library: New York, NY, USA, 2020; pp. 227–244. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Liu, H.; Jiang, Y.; Yang, B. Immunomodulatory mechanism of alpha-d-(1→6)-glucan isolated from banana. RSC Adv. 2019, 9, 6995–7003. [Google Scholar] [CrossRef]
- Koshte, V.L.; Aalbers, M.; Calkhoven, P.G.; Aalberse, R.C. The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int. Arch. Allergy Immunol. 1992, 97, 17–24. [Google Scholar] [CrossRef]
- Gavrovic-Jankulovic, M.; Poulsen, K.; Brckalo, T.; Bobic, S.; Lindner, B.; Petersen, A. A novel recombinantly produced banana lectin isoform is a valuable tool for glycoproteomics and a potent modulator of the proliferation response in CD3+, CD4+, and CD8+ populations of human PBMCs. Int. J. Biochem. Cell Biol. 2008, 40, 929–941. [Google Scholar] [CrossRef]
- Degroote, R.L.; Korbonits, L.; Stetter, F.; Kleinwort, K.J.H.; Schilloks, M.-C.; Amann, B.; Hirmer, S.; Hauck, S.M.; Deeg, C.A. Banana Lectin from Musa paradisiaca Is Mitogenic for Cow and Pig PBMC via IL-2 Pathway and ELF1. Immuno 2021, 1, 264–276. [Google Scholar] [CrossRef]
- Cheung, A.H.; Wong, J.H.; Ng, T.B. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine 2009, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, M.M.; Živković, I.P.; Petrušić, V.Ž.; Kosec, D.J.; Dimitrijević, R.D.; Jankov, R.M.; Dimitrijević, L.A.; Gavrović-Jankulović, M.Đ. In vitro stimulation of Balb/c and C57 BL/6 splenocytes by a recombinantly produced banana lectin isoform results in both a proliferation of T cells and an increased secretion of interferon-gamma. Int. Immunopharmacol. 2010, 10, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, R.; Stojanovic, M.; Micic, M.; Dimitrijevic, L.; Gavrovic-Jankulovic, M. Recombinant banana lectin as mucosal immunostimulator. J. Funct. Foods 2012, 4, 636–641. [Google Scholar] [CrossRef]
- Dimitrijevic, R.; Jadranin, M.; Burazer, L.; Ostojic, S.; Gavrovic-Jankulovic, M. Evaluation of the thermal stability and digestibility of heterologously produced banana lectin. Food Chem. 2010, 120, 1113–1118. [Google Scholar] [CrossRef]
- Marinković, E.; Lukić, I.; Kosanović, D.; Inić-Kanada, A.; Gavrović-Jankulović, M.; Stojanović, M. Recombinantly produced banana lectin isoform promotes balanced pro-inflammatory response in the colon. J. Funct. Foods 2016, 20, 68–78. [Google Scholar] [CrossRef]
- Marinkovic, E.; Djokic, R.; Lukic, I.; Filipovic, A.; Inic-Kanada, A.; Kosanovic, D.; Gavrovic-Jankulovic, M.; Stojanovic, M. Modulation of functional characteristics of resident and thioglycollate-elicited peritoneal murine macrophages by a recombinant banana lectin. PLoS ONE 2017, 12, e0172469. [Google Scholar] [CrossRef]
- Miljkovic, R.; Marinkovic, E.; Lukic, I.; Kovacevic, A.; Lopandic, Z.; Popovic, M.; Gavrovic-Jankulovic, M.; Schabussova, I.; Inic-Kanada, A.; Stojanovic, M. Banana Lectin: A Novel Immunomodulatory Strategy for Mitigating Inflammatory Bowel Disease. Nutrients 2024, 16, 1705. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Baydi, Z.; Limami, Y.; Khalki, L.; Zaid, N.; Naya, A.; Mtairag, E.M.; Oudghiri, M.; Zaid, Y. An Update of Research Animal Models of Inflammatory Bowel Disease. Sci. World J. 2021, 2021, 7479540. [Google Scholar] [CrossRef]
- Silva, I.; Pinto, R.; Mateus, V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. J. Clin. Med. 2019, 8, 1574. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Wildenberg, M.E.; Stitt, L.W.; Feagan, B.G.; Koldijk, M.; van’t Wout, A.B.; Atreya, R.; Vieth, M.; Brandse, J.F.; Duijst, S.; et al. Development of Reliable, Valid and Responsive Scoring Systems for Endoscopy and Histology in Animal Models for Inflammatory Bowel Disease. J. Crohns Colitis 2018, 12, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Dodda, D.; Chhajed, R.; Mishra, J.; Padhy, M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: Role of quercetin. Indian J. Pharmacol. 2014, 46, 286–291. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Weissman, S.M. Red Cell Metabolism. A Manual of Biochemical Methods. 2nd Edition. Yale J. Biol. Med. 1976, 49, 310–311. [Google Scholar]
- Nebert, D.W.; Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004, 1, 460–464. [Google Scholar] [CrossRef]
- Arafa, H.M.; Hemeida, R.A.; El-Bahrawy, A.I.; Hamada, F.M. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food Chem. Toxicol. 2009, 47, 1311–1317. [Google Scholar] [CrossRef]
- Chami, B.; Martin, N.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef]
- Villegas, I.; de la Lastra, C.A.; Orjales, A.; La Casa, C. A new flavonoid derivative, dosmalfate, attenuates the development of dextran sulphate sodium-induced colitis in mice. Int. Immunopharmacol. 2003, 3, 1731–1741. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Muljadi, R.C.; O’Sullivan, K.M.; Kettle, A.J.; Dickerhof, N.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Suppression of Autoimmunity and Renal Disease in Pristane-Induced Lupus by Myeloperoxidase. Arthritis Rheumatol. 2015, 67, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Bouali, H.; Nietert, P.; Nowling, T.M.; Pandey, J.; Dooley, M.A.; Cooper, G.; Harley, J.; Kamen, D.L.; Oates, J.; Gilkeson, G. Association of the G-463A myeloperoxidase gene polymorphism with renal disease in African Americans with systemic lupus erythematosus. J. Rheumatol. 2007, 34, 2028–2034. [Google Scholar] [PubMed]
- Endo, D.; Saito, T.; Umeki, Y.; Suzuki, K.; Aratani, Y. Myeloperoxidase negatively regulates the expression of proinflammatory cytokines and chemokines by zymosan-induced mouse neutrophils. Inflamm. Res. 2016, 65, 151–159. [Google Scholar] [CrossRef]
- Homme, M.; Tateno, N.; Miura, N.; Ohno, N.; Aratani, Y. Myeloperoxidase deficiency in mice exacerbates lung inflammation induced by nonviable Candida albicans. Inflamm. Res. 2013, 62, 981–990. [Google Scholar] [CrossRef]
- Kremserova, S.; Perecko, T.; Soucek, K.; Klinke, A.; Baldus, S.; Eiserich, J.P.; Kubala, L. Lung Neutrophilia in Myeloperoxidase Deficient Mice during the Course of Acute Pulmonary Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5219056. [Google Scholar] [CrossRef]
- Reber, L.L.; Gillis, C.M.; Starkl, P.; Jönsson, F.; Sibilano, R.; Marichal, T.; Gaudenzio, N.; Bérard, M.; Rogalla, S.; Contag, C.H.; et al. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J. Exp. Med. 2017, 214, 1249–1258. [Google Scholar] [CrossRef]
- Kettle, A.J.; Anderson, R.F.; Hampton, M.B.; Winterbourn, C.C. Reactions of superoxide with myeloperoxidase. Biochemistry 2007, 46, 4888–4897. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B.; Livesey, J.H.; Kettle, A.J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: Implications for microbial killing. J. Biol. Chem. 2006, 281, 39860–39869. [Google Scholar] [CrossRef]
- Wéra, O.; Lancellotti, P.; Oury, C. The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Stables, M.J.; Shah, S.; Camon, E.B.; Lovering, R.C.; Newson, J.; Bystrom, J.; Farrow, S.; Gilroy, D.W. Transcriptomic analyses of murine resolution-phase macrophages. Blood 2011, 118, e192–e208. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef]
- Ishihara, T.; Tanaka, K.; Tasaka, Y.; Namba, T.; Suzuki, J.; Ishihara, T.; Okamoto, S.; Hibi, T.; Takenaga, M.; Igarashi, R.; et al. Therapeutic effect of lecithinized superoxide dismutase against colitis. J. Pharmacol. Exp. Ther. 2009, 328, 152–164. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Caputi, A.P.; Riley, D.P.; Salvemini, D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur. J. Pharmacol. 2001, 432, 79–89. [Google Scholar] [CrossRef]
- Miller Jr, F.J.; Chu, X.; Stanic, B.; Tian, X.; Sharma, R.V.; Davisson, R.L.; Lamb, F.S. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid. Redox Signal. 2010, 12, 583–593. [Google Scholar] [CrossRef]
- Oakley, F.D.; Abbott, D.; Li, Q.; Engelhardt, J.F. Signaling components of redox active endosomes: The redoxosomes. Antioxid. Redox Signal. 2009, 11, 1313–1333. [Google Scholar] [CrossRef]
- Haddad, J.J.; Fahlman, C.S. Redox- and oxidant-mediated regulation of interleukin-10: An anti-inflammatory, antioxidant cytokine? Biochem. Biophys. Res. Commun. 2002, 297, 163–176. [Google Scholar] [CrossRef]
- Kamizato, M.; Nishida, K.; Masuda, K.; Takeo, K.; Yamamoto, Y.; Kawai, T.; Teshima-Kondo, S.; Tanahashi, T.; Rokutan, K. Interleukin 10 inhibits interferon γ- and tumor necrosis factor α-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J. Gastroenterol. 2009, 44, 1172–1184. [Google Scholar] [CrossRef]
- Tréton, X.; Pedruzzi, E.; Guichard, C.; Ladeiro, Y.; Sedghi, S.; Vallée, M.; Fernandez, N.; Bruyère, E.; Woerther, P.L.; Ducroc, R.; et al. Combined NADPH oxidase 1 and interleukin 10 deficiency induces chronic endoplasmic reticulum stress and causes ulcerative colitis-like disease in mice. PLoS ONE 2014, 9, e101669. [Google Scholar] [CrossRef] [PubMed]
- Hahm, K.; Im, Y.; Parks, T.; Park, S.; Markowitz, S.; Jung, H.; Green, J.; Kim, S. Loss of transforming growth factor β signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut 2001, 49, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Kaplan, G.G. The role of TNFα in ulcerative colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Silva, F.A.; Rodrigues, B.L.; Ayrizono, M.d.L.S.; Leal, R.F. The immunological basis of inflammatory bowel disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.; Koenders, M.I.; Devesa, I.; Roelofs, M.F.; Radstake, T.R.; Heuvelmans-Jacobs, M.; Akira, S.; Nicklin, M.J.; Ribeiro-Dias, F.; et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Investig. 2008, 118, 205–216. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Takayama, Y.; Sugawara, K.; Ohno, H.; Ishii, H.; Tsuda, S.; Tsuji, T.; Komatsu, M.; Sugawara, T. Development and Application of a Plant-Based Diet Scoring System for Japanese Patients with Inflammatory Bowel Disease. Perm. J. 2016, 20, 16–019. [Google Scholar] [CrossRef]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210–220. [Google Scholar]
- Konozy, E.H.E.; Osman, M.E.M. From inflammation to immune regulation: The dual nature of dietary lectins in health and disease. Heliyon 2024, 10, e39471. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, X.; Yang, Y.; Yan, Y.; Yu, C.; Zhou, R.; Jiang, W. Plant Lectins Activate the NLRP3 Inflammasome To Promote Inflammatory Disorders. J. Immunol. 2017, 198, 2082–2092. [Google Scholar] [CrossRef]
- Radovani, B.; Gudelj, I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e310. [Google Scholar] [CrossRef]
- Reich, J.; Wasan, S.; Farraye, F.A. Vaccinating Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 540–546. [Google Scholar]
- Beaugerie, L.; Kirchgesner, J. Balancing Benefit vs Risk of Immunosuppressive Therapy for Individual Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 370–379. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljković, R.; Marinković, E.; Prodić, I.; Kovačević, A.; Protić-Rosić, I.; Vasić, M.; Lukić, I.; Gavrović-Jankulović, M.; Stojanović, M. Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon. Biomolecules 2025, 15, 476. https://doi.org/10.3390/biom15040476
Miljković R, Marinković E, Prodić I, Kovačević A, Protić-Rosić I, Vasić M, Lukić I, Gavrović-Jankulović M, Stojanović M. Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon. Biomolecules. 2025; 15(4):476. https://doi.org/10.3390/biom15040476
Chicago/Turabian StyleMiljković, Radmila, Emilija Marinković, Ivana Prodić, Ana Kovačević, Isidora Protić-Rosić, Marko Vasić, Ivana Lukić, Marija Gavrović-Jankulović, and Marijana Stojanović. 2025. "Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon" Biomolecules 15, no. 4: 476. https://doi.org/10.3390/biom15040476
APA StyleMiljković, R., Marinković, E., Prodić, I., Kovačević, A., Protić-Rosić, I., Vasić, M., Lukić, I., Gavrović-Jankulović, M., & Stojanović, M. (2025). Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon. Biomolecules, 15(4), 476. https://doi.org/10.3390/biom15040476






