Molecular Profiling and FTIR Characterization of Wheat Germ Oil, Supported by the Screening of Its Anti-Inflammatory and Cytotoxic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Germ Oil (WGO)
2.2. Determination of Fatty Acid Profile in WGO
2.3. Analysis of Squalene Concentration in WGO
2.4. Antioxidant Activity and Total Phenolic Content in WGO
2.5. Fourier Transform Infrared Spectroscopy
2.6. Cytotoxic Activity of WGO
2.7. Anti-Inflammatory Activity of WGO
2.8. Nitric Oxide Release
2.9. TNF-Alpha and IL-6 Analysis
2.10. Fluorometric Measurements
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Profile and Squalene Content
3.2. Antioxidant Activity and Total Phenolic Compunds
3.3. Fourier Transform Infrared Spectroscopy
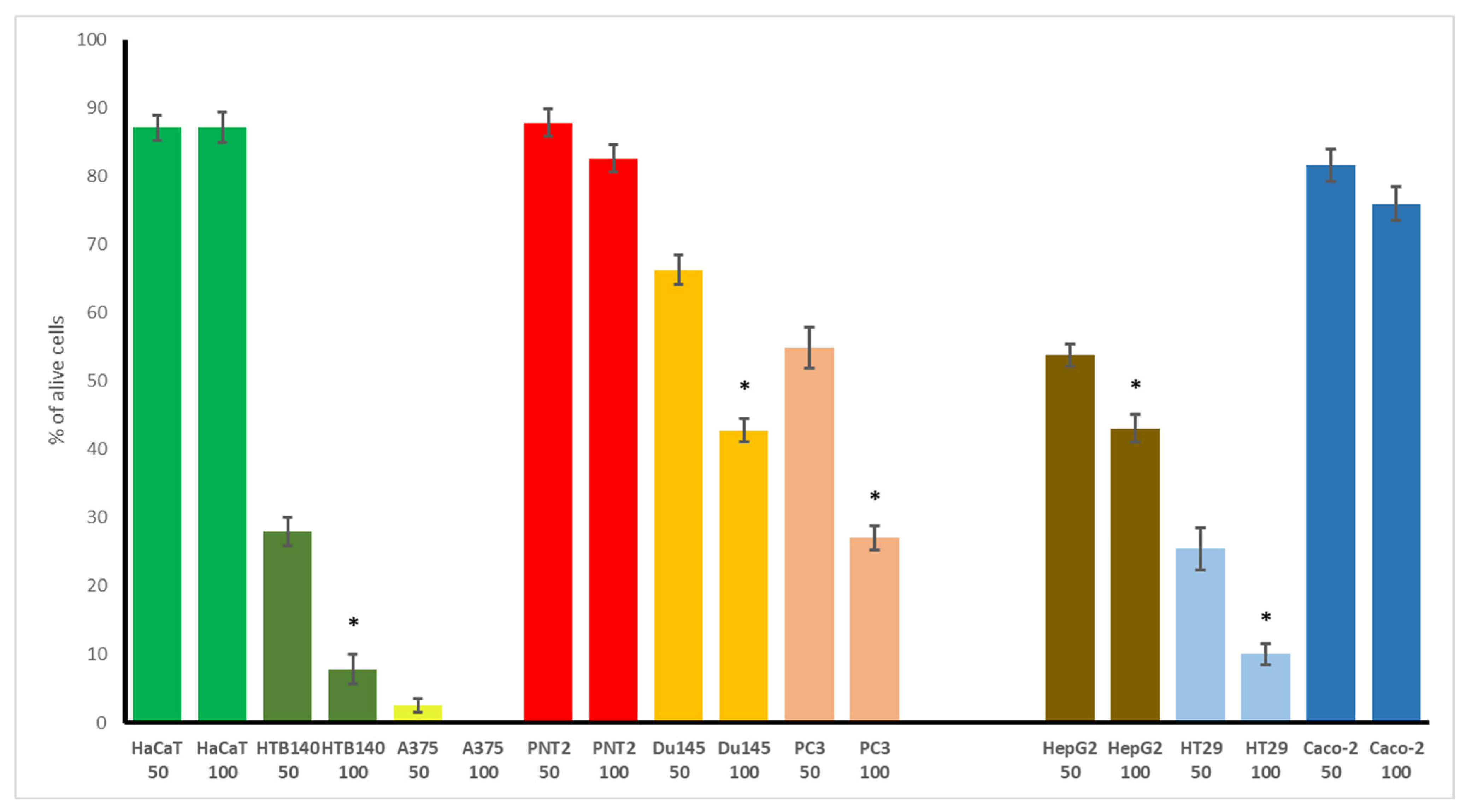
3.4. Cytotoxic Potential of WGO
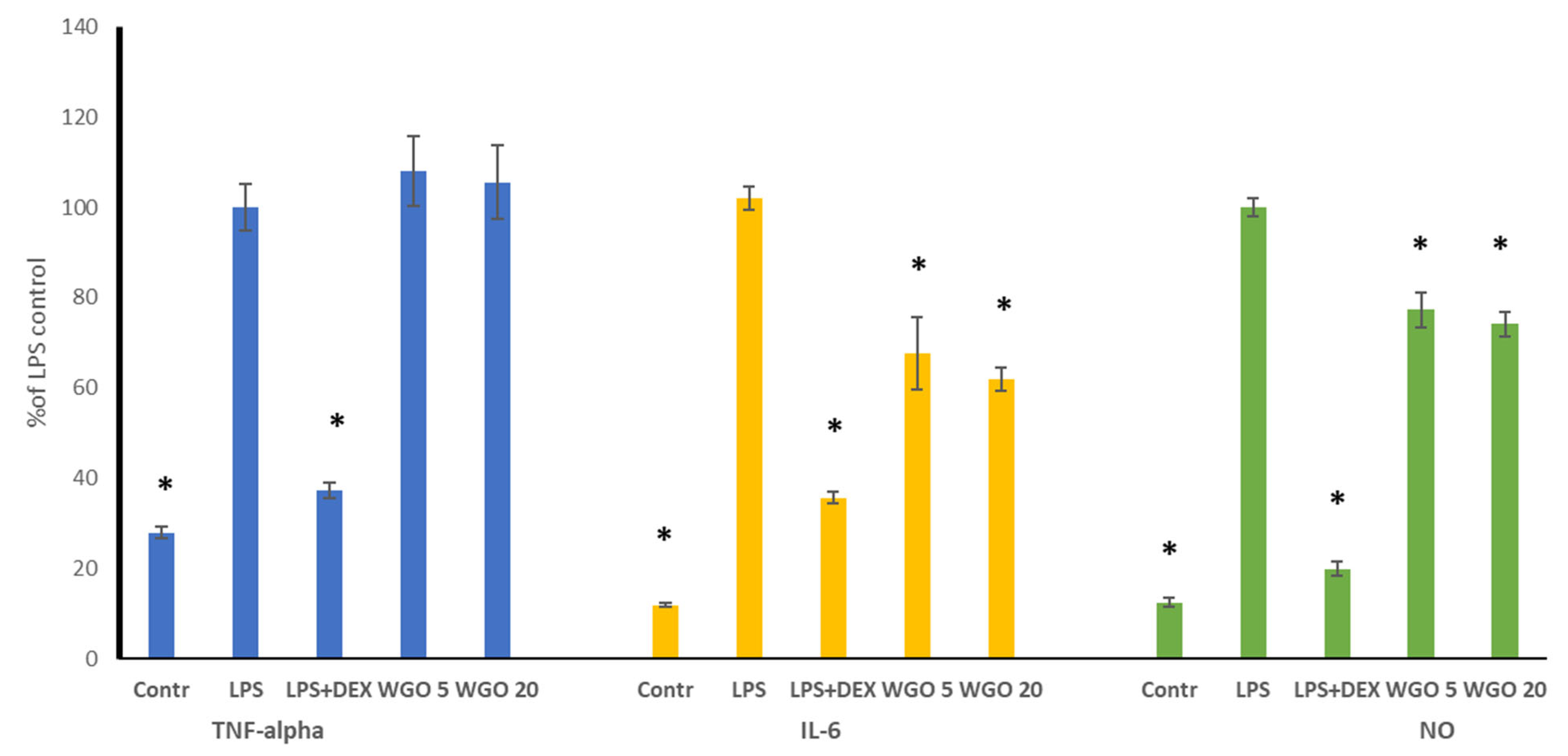
3.5. Anti-Inflammatory Activity of WGO
3.6. Human Serum Albumin Binding Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, G.S.; Krishna, A.G. Studies on the nutraceuticals composition of wheat-derived oils: Wheat bran oil and wheat germ oil. J. Food Sci. Technol. 2015, 52, 1145–1151. [Google Scholar] [CrossRef]
- Yuldasheva, N.K.; Ul’chenko, N.T.; Glushenkova, A.I. Wheat germ oil. Chem. Nat. Compd. 2010, 46, 97–98. [Google Scholar]
- Hassanein, M.M.M.; Abedel-Razek, A.G. Chromatographic quantitation of some bioactive minor components in oils of wheat germ and grape seeds produced as by-products. J. Oleo Sci. 2009, 58, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Jiang, S.T.; Pan, L.J.; Pang, M. Characterization of wheat germ oil in terms of volatile compounds, lipid composition, thermal behavior, and structure. Int. J. Food Prop. 2013, 16, 1740–1749. [Google Scholar] [CrossRef]
- Barnes, P.J. Lipid composition of wheat germ and wheat germ oil. Fette Seifen Anstrichm. 1982, 84, 256–269. [Google Scholar]
- Ghafoor, K.; Özcan, M.; Al-Juhaimi, F.; Babiker, E.; Sarker, Z.; Ahmed, I.; Ahmed, M. Nutritional composition, extraction, and utilization of wheat germ oil: A review. Eur. J. Lipid Sci. Technol. 2017, 119, 1600160. [Google Scholar]
- Harrabi, S.; Ferchichi, A.; Fellah, H.; Feki, M.; Hosseinian, F. Chemical composition and in vitro anti-inflammatory activity of wheat germ oil depending on the extraction procedure. J. Oleo Sci. 2021, 70, 1051–1058. [Google Scholar]
- Deiana, M.; Corongiu, F.P.; Dessi, M.A.; Scano, P.; Casu, M.; Lai, A. NMR determination of site-specific deuterium distribution (SNIF-NMR) in squalene from different sources. Magn. Reson. Chem. 2001, 39, 29–32. [Google Scholar]
- Zargar, S.; Wani, T.A.; Rizwan Ahamad, S. An insight into wheat germ oil nutrition, identification of its bioactive constituents, and computer-aided multidimensional data analysis of its potential anti-inflammatory effect via molecular connections. Life 2023, 13, 526. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. S1), 71–74. [Google Scholar]
- Arshad, M.U.; Zakir, S.; Anjum, F.M.; Zahoor, T.; Nawaz, H. Nutritive value of cookies containing wheat germ oil. Pak. J. Life Soc. Sci. 2008, 6, 127–134. [Google Scholar]
- Siraj, N. Wheat germ oil: A comprehensive review. Food Sci. Technol. 2022, 42, e113721. [Google Scholar] [CrossRef]
- Shen, W.; Koirala, N.; Mukherjee, D.; Lee, K.; Zhao, M.; Li, J. Tween 20 stabilized conventional heavy crude oil-in-water emulsions formed by mechanical homogenization. Front. Environ. Sci. 2022, 10, 873730. [Google Scholar] [CrossRef]
- Shen, P.; Tang, H. Effects of salts on oxidative stability of lipids in Tween-20 stabilized oil-in-water emulsions. Food Chem. 2015, 188, 439–445. [Google Scholar]
- Paśko, P.; Galanty, A.; Ramos-Zambrano, E.; Ayala, A.L.M.; Delgado, E.; Argasińska, J.G.; Gorinstein, S. Pseudocereal oils, authenticated by Fourier transform infrared spectroscopy, and their chemopreventive properties. Plant Foods Hum. Nutr. 2024, 79, 151–158. [Google Scholar] [CrossRef]
- Popa, O.; Băbeanu, N.E.; Popa, I.; Niță, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. Biomed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed]
- Cowan, E.A.; Tran, H.; Watson, C.H.; Blount, B.C.; Valentín-Blasini, L. The quantitation of squalene and squalane in bronchoalveolar lavage fluid using gas chromatography mass spectrometry. Front. Chem. 2022, 10, 874373. [Google Scholar] [CrossRef]
- Santivañez-Veliz, M.; Moreno-Viguri, E.; Pérez-Silanes, S.; Varela, J.; Cerecetto, H.; González, M.; Lizarraga, E. Development, validation and application of a GC–MS method for the simultaneous detection and quantification of neutral lipid species in Trypanosoma cruzi. J. Chromatogr. B 2017, 1061, 225–232. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits–A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40, 100888. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alvarez, V.H.; Alonso, E.; Cocero, M.J.; Saldaña, M.D. Pressurized aqueous ethanol extraction of β-glucans and phenolic compounds from waxy barley. Food Res. Int. 2015, 75, 252–259. [Google Scholar] [CrossRef]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Park, Y.S.; Kim, Y.M.; Paśko, P.; Gorinstein, S. Unraveling the antioxidant, binding, and health-protecting properties of phenolic compounds of beers with main human serum proteins: In vitro and in silico approaches. Molecules 2020, 25, 4962. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, M.; Zhang, G.; He, H.; Yang, T. Antioxidant activities and phenolic compositions of wheat germ as affected by the roasting process. J. Am. Oil Chem. Soc. 2015, 92, 1303–1312. [Google Scholar] [CrossRef]
- Małecka, M. Antioxidant properties of the unsaponifiable matter isolated from tomato seeds, oat grains, and wheat germ oil. Food Chem. 2002, 79, 327–330. [Google Scholar]
- Drzewiecki, J.; Martinez-Ayala, A.L.; Lozano-Grande, M.A.; Leontowicz, H.; Leontowicz, M.; Jastrzebski, Z.; Gorinstein, S. In vitro screening of bioactive compounds in some gluten-free plants. Appl. Biochem. Biotechnol. 2018, 186, 847–860. [Google Scholar]
- Tarhan, İ. A new and rapid analysis method for the most important herbal squalene source: Comparison of UV–visible, fluorescence, and FTIR techniques for the quantification of squalene in amaranth seed oil. Microchem. J. 2021, 168, 106446. [Google Scholar]
- Deng, D.H.; Xu, L.; Ye, Z.H.; Cui, H.F.; Cai, C.B.; Yu, X.P. FTIR spectroscopy and chemometric class modeling techniques for authentication of Chinese sesame oil. J. Am. Oil Chem. Soc. 2012, 89, 1003–1009. [Google Scholar]
- Arslan, F.N. ATR–FTIR spectroscopy combined with chemometrics for rapid classification of extra virgin olive oils and edible oils from different cultivars available on the Turkish markets. Eskişehir Tech. Univ. J. Sci. Technol. A Appl. Sci. Eng. 2018, 19, 926–947. [Google Scholar]
- Ergül, M.; Bekele, A.; Terzi, H.; Altun, A. Evaluation of in vitro anticancer activity of wheat germ oil in MCF-7 breast cancer cell lines. Cumhur. Med. J. 2019, 41, 500–505. [Google Scholar]
- Şeleci, D.A.; Gümüş, Z.P.; Yavuz, M.; Şeleci, M.; Bongartz, R.; Stahl, F.; Scheper, T. A case study on in vitro investigations of the potent biological activities of wheat germ and black cumin seed oil. Turk. J. Chem. 2015, 39, 801–812. [Google Scholar]
- Gömeç, M.; İpek, G.; Öztürk, A.; İnan, D.Ş. Effect of wheat germ oil on wound healing: An in vitro study in fibroblast cells. Turk. J. Sci. Health 2022, 3, 230–235. [Google Scholar] [CrossRef]
- Liu, V.N.; Van Blarigan, E.L.; Zhang, L.; Graff, R.E.; Loeb, S.; Langlais, C.S.; Cowan, J.E.; Carroll, P.R.; Chan, J.M.; Kenfield, S.A. Plant-based diets and disease progression in men with prostate cancer. JAMA Netw. Open 2024, 7, e249053. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, V.; Klotz, L.H. Diet and prostate cancer: Mechanisms of action and implications for chemoprevention. Nat. Rev. Urol. 2010, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.U.; Kang, B.K.; Kim, K.B.W.R.; Kim, M.J.; Bark, S.W.; Pak, W.M.; Kim, B.R.; Ahn, N.K.; Park, J.H.; Bae, N.Y.; et al. Anti-inflammatory effects of wheat germ oil on RAW264.7 macrophages. Korean Soc. Food Sci. Nutr. 2014, 10, 287–288. [Google Scholar]
- Paśko, P.; Tyszka-Czochara, M.; Namieśnik, J.; Jastrzębski, Z.; Leontowicz, H.; Drzewiecki, J.; Gorinstein, S. Cytotoxic, antioxidant, and binding properties of polyphenols from selected gluten-free pseudocereals and their by-products: In vitro model. J. Cereal Sci. 2019, 87, 325–333. [Google Scholar]
- Dai, T.; Yan, X.; Li, Q.; Li, T.; Liu, C.; McClements, D.; Chen, J. Characterization of binding interaction between rice glutelin and gallic acid: Multi-spectroscopic analyses and computational docking simulation. Food Res. Int. 2017, 102, 274–281. [Google Scholar] [CrossRef]
- Keerati-U-Rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil-water interface studied by fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2012, 93, 41–48. [Google Scholar] [CrossRef]
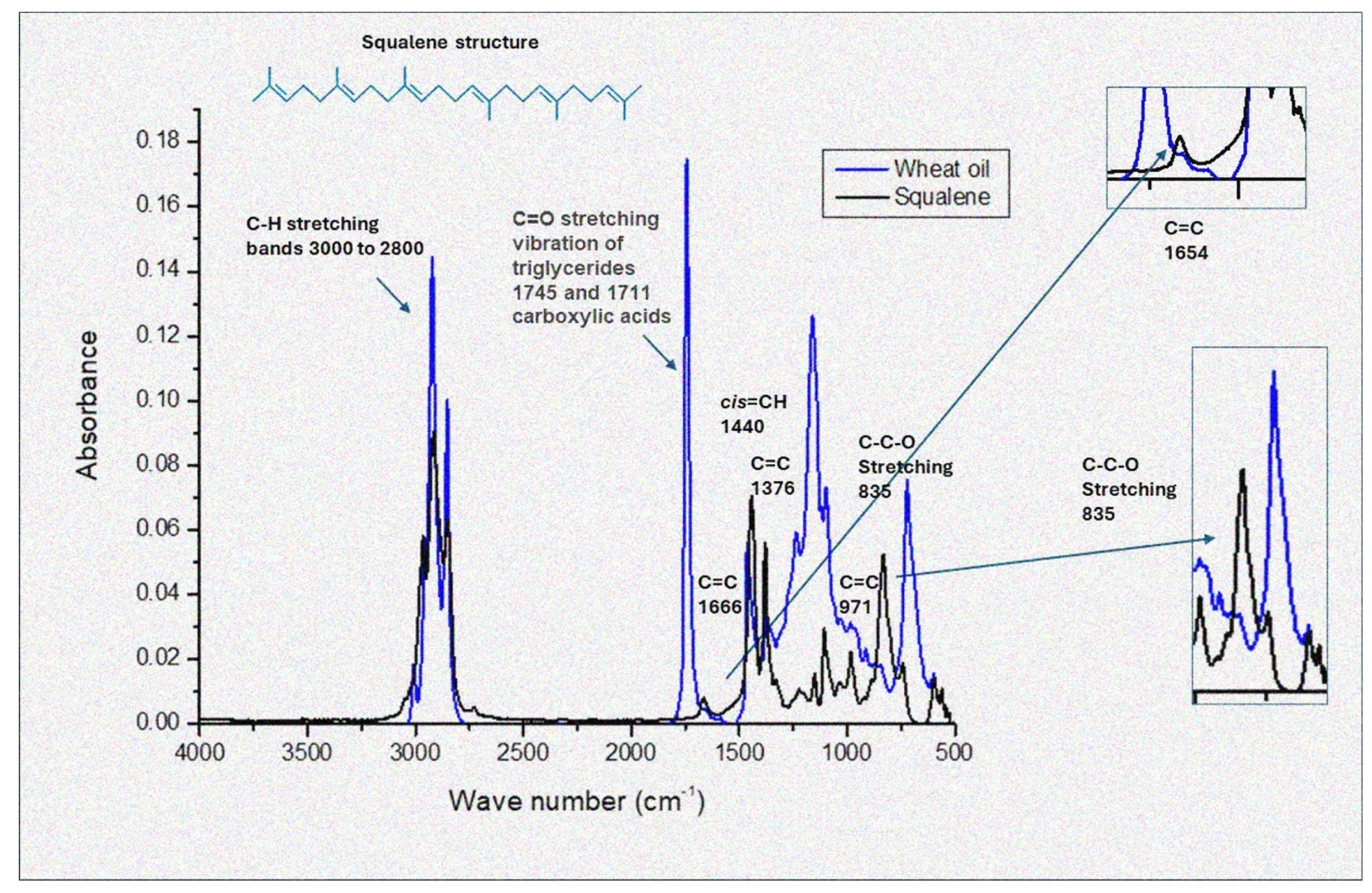

| Fatty Acids Profile [%] | |
| Palmitic acid C16:0 | 19.4 ± 2.1 |
| Stearic acid C18:0 | 4.6 ± 0.7 |
| Oleic acid C18:1 n-9 | 26.8 ± 3.7 |
| Linoleic acid C18:2 n-6 | 45.3 ± 5.1 |
| α-linolenic acid C18:3 n-3 | 3.9 ± 0.6 |
| Squalene [g/100 g] | |
| 2.52 ± 0.32 | |
| Antioxidant activity | CUPRAC [μM TE/mL] |
| WGO | 0.38 ± 0.07 |
| Lipophilic fraction of WGO | 0.13 ± 0.05 * |
| Hydrophilic fraction of WGO | 0.24 ± 0.04 * |
| Total polyphenols | TPC [µg GAE/mL] |
| WGO | 7.40 ± 0.80 |
| Lipophilic fraction of WGO | 2.70 ± 0.40 * |
| Hydrophilic fraction of WGO | 4.69 ± 0.70 * |
| Type of Cells | WGO | Doxorubicin |
|---|---|---|
| Skin panel | ||
| HaCaT | >Cmax | 3.20 |
| HTB140 | 37.2 | 4.45 |
| A375 | 18.0 | 0.37 |
| Prostate panel | ||
| PNT2 | >Cmax | 0.99 |
| Du145 | 82.5 | 2.29 |
| PC3 | 52.3 | >40 |
| LNCaP | 15.4 | 3.48 |
| Gastrointestinal panel | ||
| HT29 | 25.2 | 1.04 |
| HepG2 | 62.4 | 1.11 |
| Caco-2 | >Cmax | 2.79 |
| Sample | Peak a | Peak b | ||
|---|---|---|---|---|
| λex/λem (nm/nm) Int F0 | λex/λem (nm/nm) Int F0 | |||
| HSA + water | 228/347 | 746.30 | 280/350 | 854.34 |
| HSA + Tween + NaCl | 227/347 | 444.43 | 280/355 | 822.00 |
| HSA + WGO | 227/355 | 372.34 | 280/358 | 799.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśko, P.; Galanty, A.; Ramos-Zambrano, E.; Ayala, A.L.M.; Gralak, M.; Gdula-Argasińska, J.; Pavlov, D.; Deutsch, J.; Gorinstein, S. Molecular Profiling and FTIR Characterization of Wheat Germ Oil, Supported by the Screening of Its Anti-Inflammatory and Cytotoxic Properties. Biomolecules 2025, 15, 464. https://doi.org/10.3390/biom15040464
Paśko P, Galanty A, Ramos-Zambrano E, Ayala ALM, Gralak M, Gdula-Argasińska J, Pavlov D, Deutsch J, Gorinstein S. Molecular Profiling and FTIR Characterization of Wheat Germ Oil, Supported by the Screening of Its Anti-Inflammatory and Cytotoxic Properties. Biomolecules. 2025; 15(4):464. https://doi.org/10.3390/biom15040464
Chicago/Turabian StylePaśko, Paweł, Agnieszka Galanty, Emilia Ramos-Zambrano, Alma Leticia Martinez Ayala, Mikołaj Gralak, Joanna Gdula-Argasińska, Danail Pavlov, Joseph Deutsch, and Shela Gorinstein. 2025. "Molecular Profiling and FTIR Characterization of Wheat Germ Oil, Supported by the Screening of Its Anti-Inflammatory and Cytotoxic Properties" Biomolecules 15, no. 4: 464. https://doi.org/10.3390/biom15040464
APA StylePaśko, P., Galanty, A., Ramos-Zambrano, E., Ayala, A. L. M., Gralak, M., Gdula-Argasińska, J., Pavlov, D., Deutsch, J., & Gorinstein, S. (2025). Molecular Profiling and FTIR Characterization of Wheat Germ Oil, Supported by the Screening of Its Anti-Inflammatory and Cytotoxic Properties. Biomolecules, 15(4), 464. https://doi.org/10.3390/biom15040464









