Efficient Fabrication of Human Corneal Stromal Cell Spheroids and Promoting Cell Stemness Based on 3D-Printed Derived PDMS Microwell Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
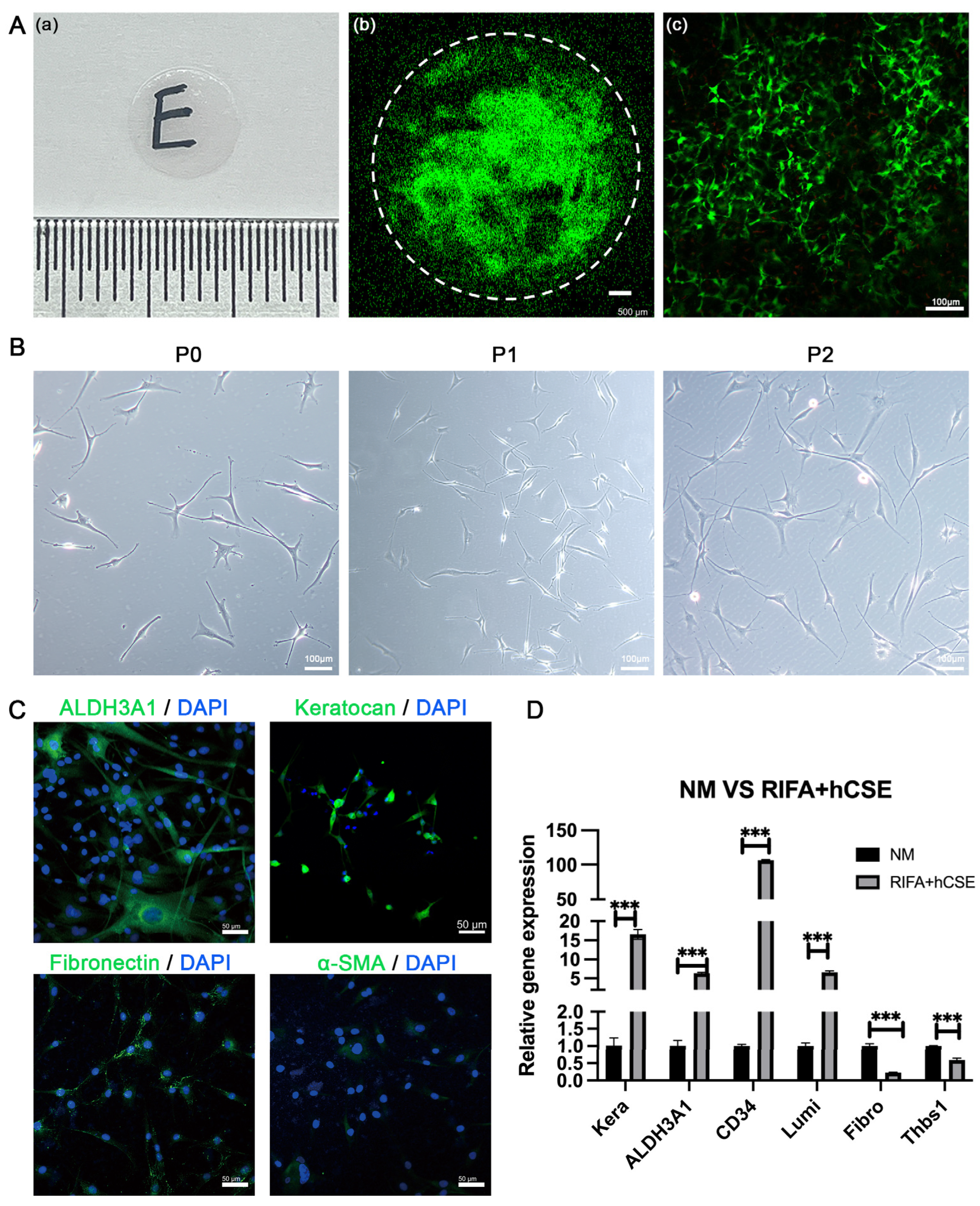
2.2. Isolation and Culture of Human Corneal Stromal Cells
2.3. Culture of Urine-Induced Pluripotent Stem Cells (iPS) and Preparation of iPS-Conditioned Medium
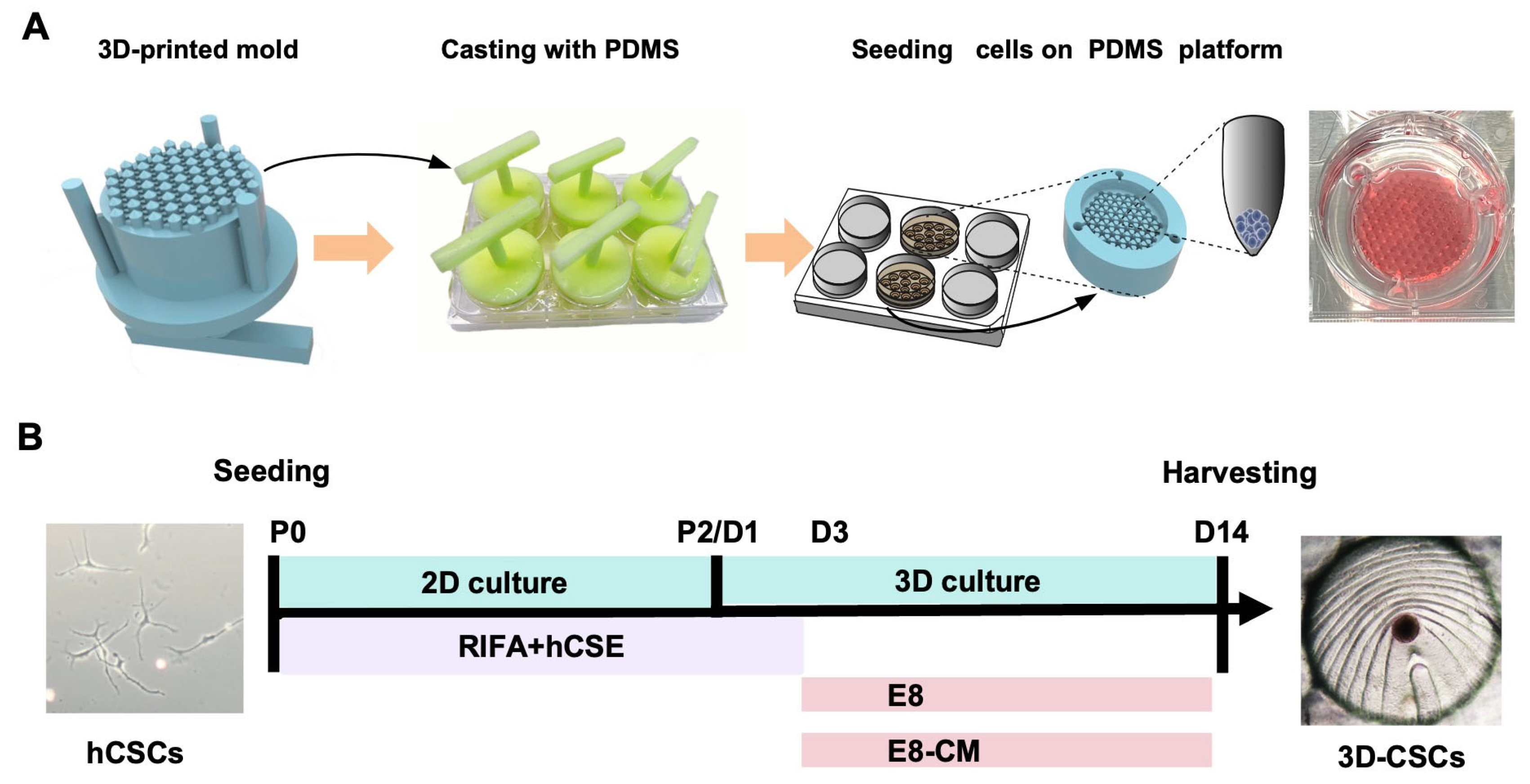
2.4. Preparation of the 3D-Printed PDMS Microwell Platform
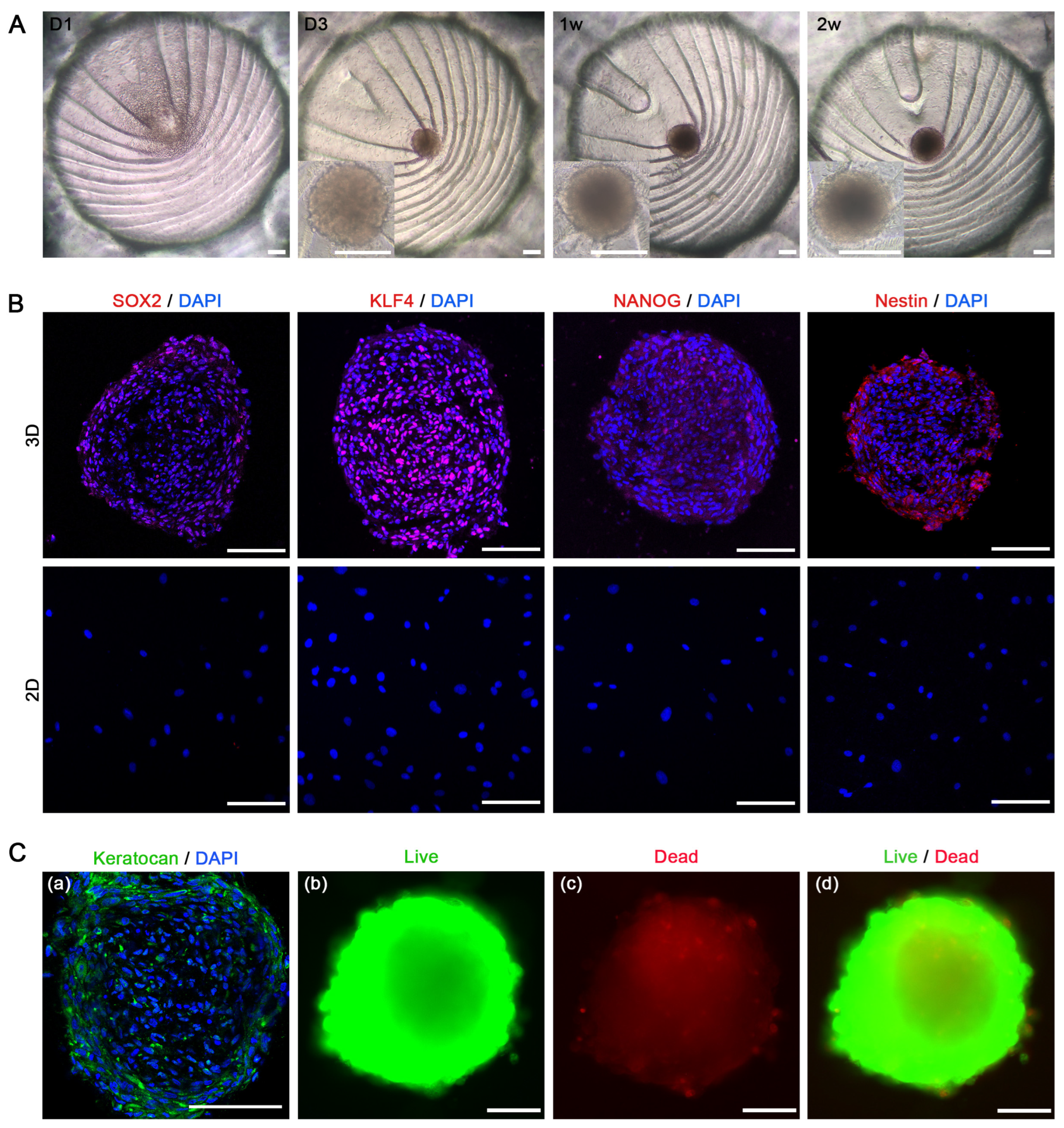
2.5. Generation and Culture of 3D-CSCs
2.6. Live/Dead Staining
2.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Immunofluorescence Staining
2.9. Western Blot Analysis
2.10. RNA-Seq Analysis
2.11. Proteomics Analysis
2.12. Statistical Analysis
3. Results
3.1. hCSCs from SMILE-Derived Lenticules
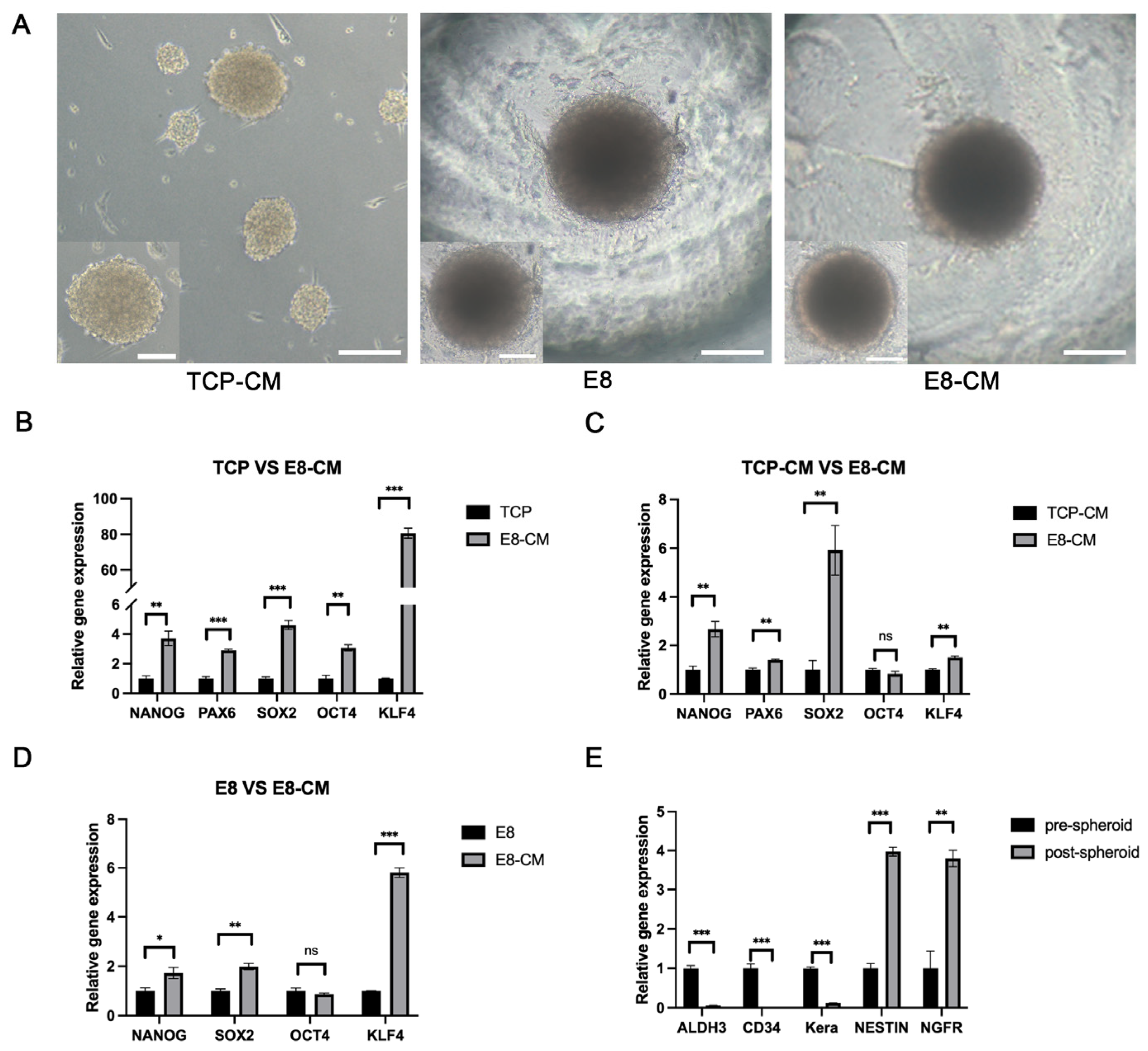
3.2. Generation and Enhancement of hCSC Stemness in 3D-CSCs
3.3. Stem Cell Characteristics in 3D-CSCs
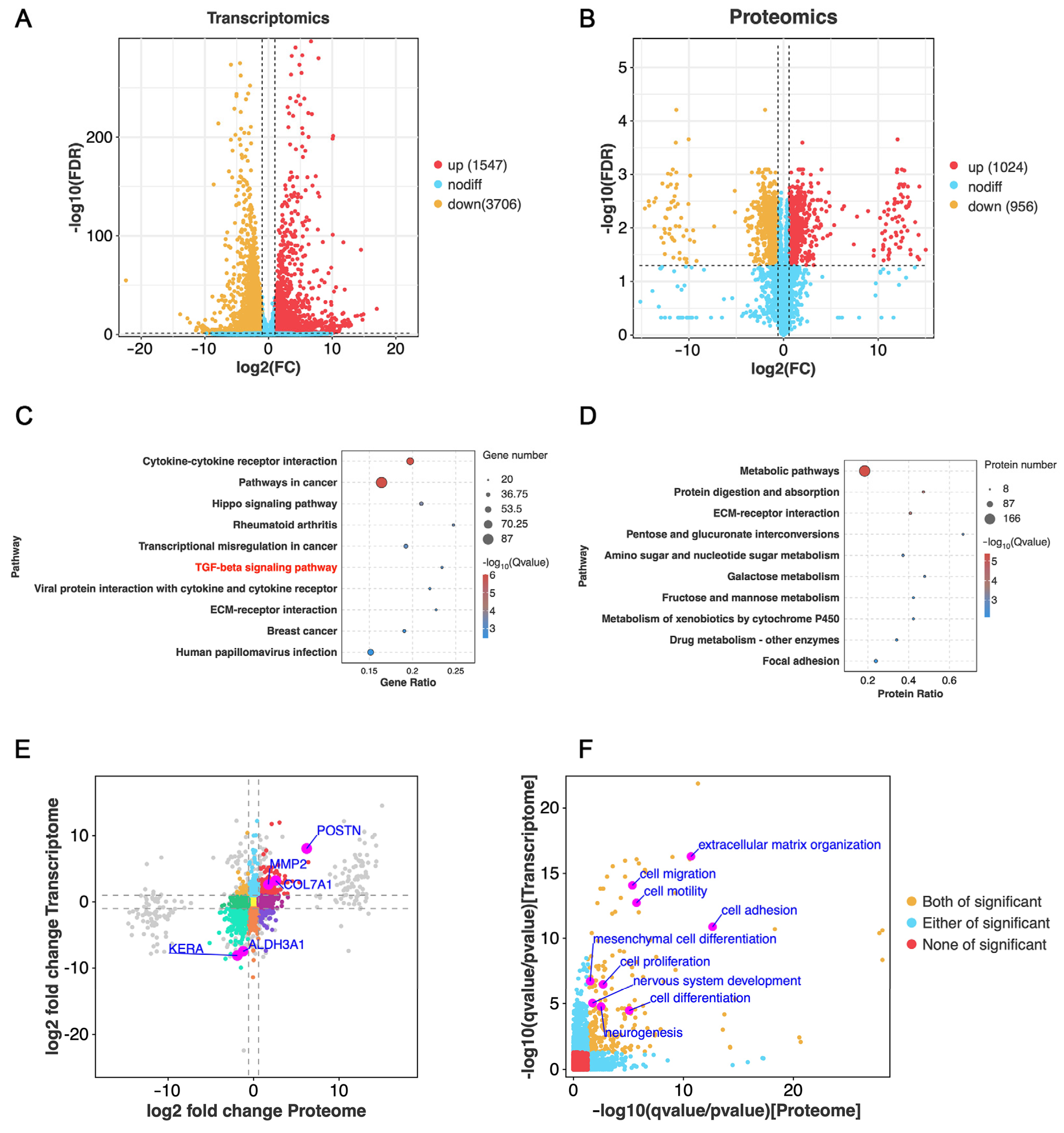
3.4. Bioinformatics Analysis of 3D-CSCs
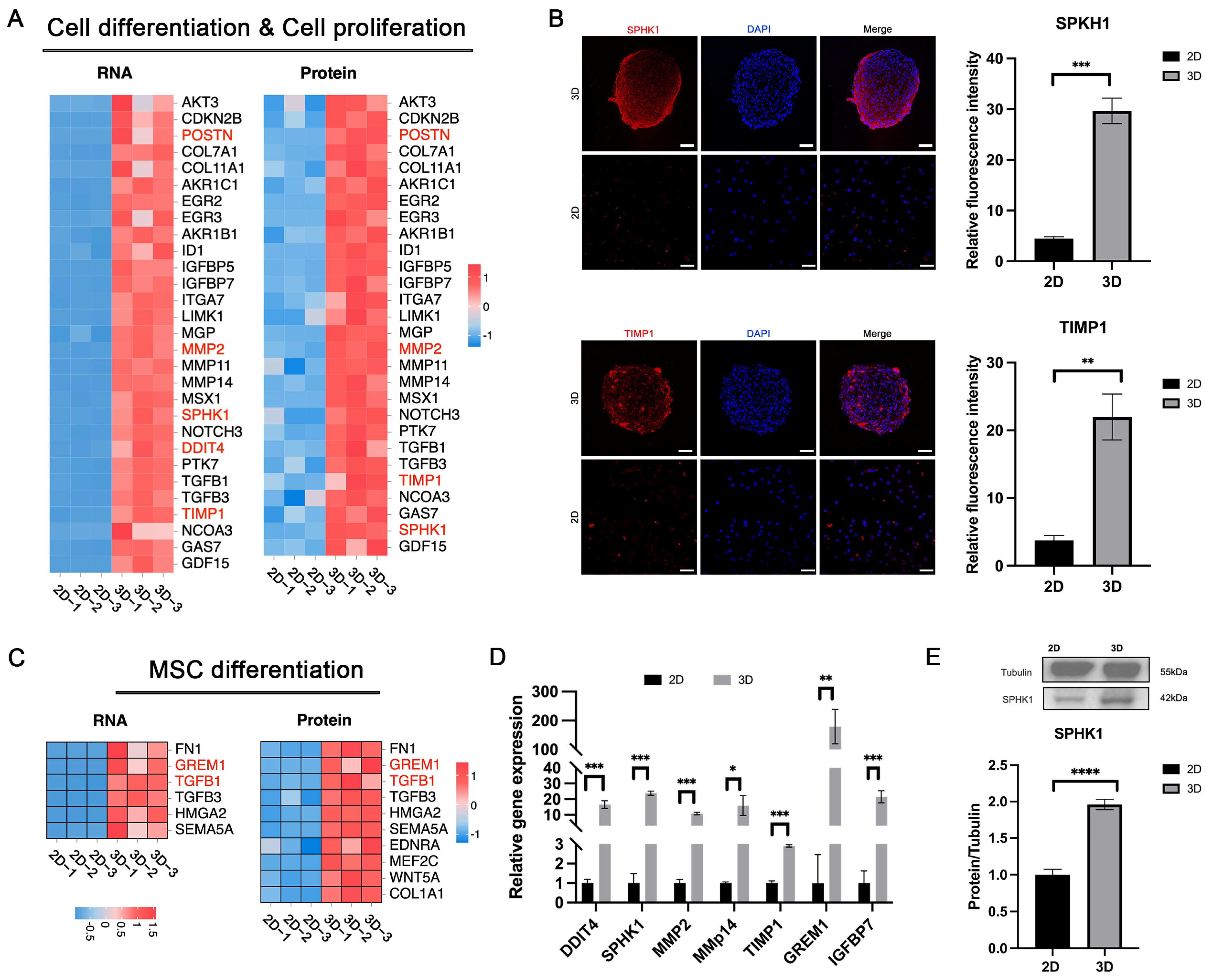
3.5. Enhanced Cell Differentiation in 3D-CSCs
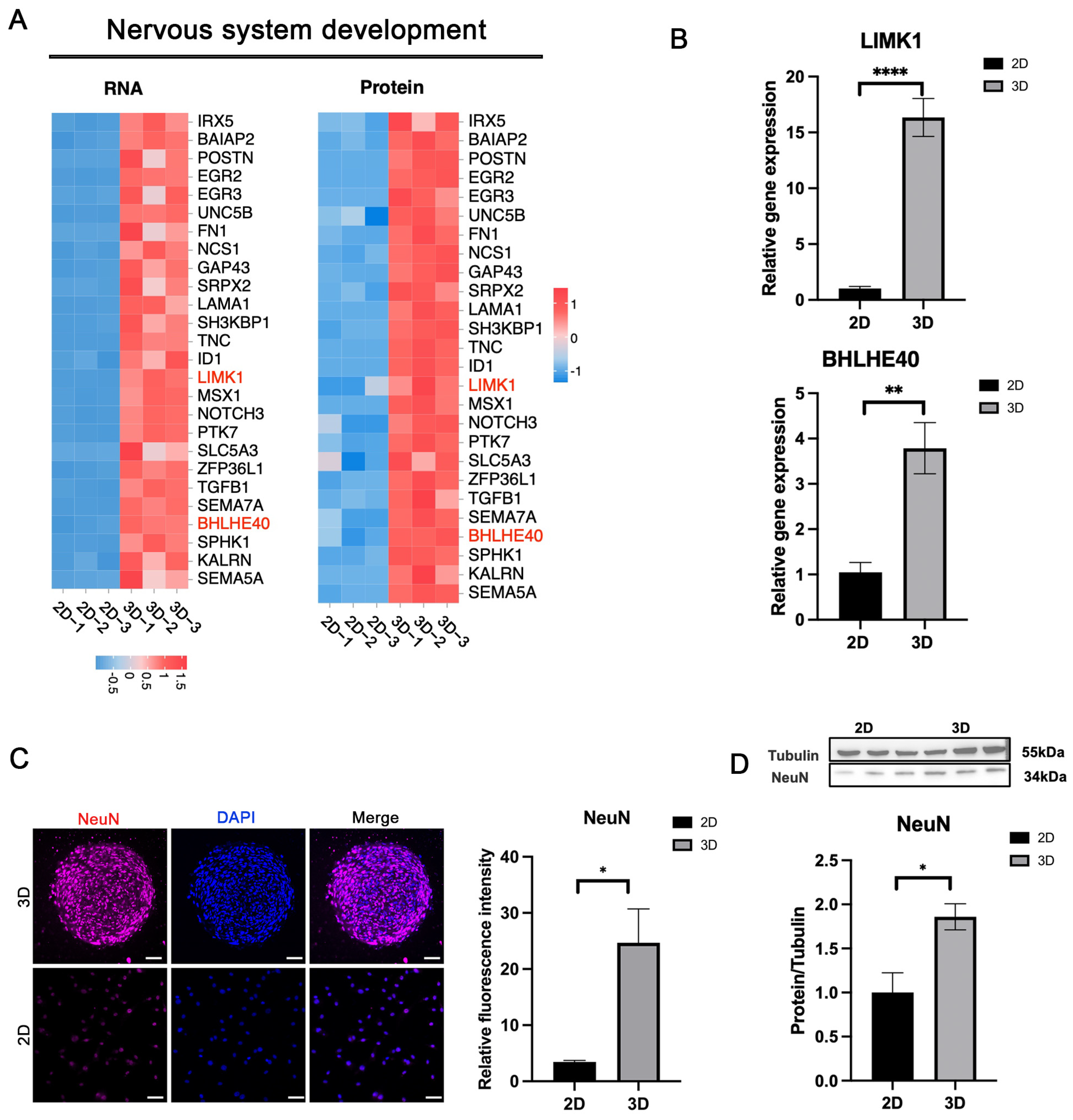
3.6. Enhanced Nervous System Development in 3D-CSCs
3.7. Enhanced Cell Migration and Adhesion in 3D-CSCs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMILE | Small Incision Lenticule Extraction |
| CM | conditioned medium |
| ECM | extracellular matrix |
| iPS | induced pluripotent stem cells |
| hCSCs | human corneal stromal cells |
| hCSE | human corneal stromal extract |
| CSSCs | corneal stromal stem cells |
Appendix A
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GAPDH | GGAAGGTGAAGGTCGGAGTC | GATCTCGCTCCTGGAAGATGG |
| LUM | GCTTCAATCAGATAGCCAGAC | CAGCCAGTTCGTTGTGAGA |
| KERA | AACCTGACCCTTCTTGACCT | ACTGCATTGTATTGGCTGGT |
| CD34 | CTACAACACCTAGTACCCTTGGA | GGTGAACACTGTGCTGATTACA |
| ALDH3A1 | TGTTCTCCAGCAACGACAAGG | AGGGCAGAGAGTGCAAGGT |
| THBS1 | TATAGCGACCCCATGTACCG | AGTCTTCCTGCCCTGAGTTG |
| FN1 | AAGACCATACCCGCCGAATG | GGCATTTGGATTGAGTCCCG |
| OCT4 | AGAGGCAACCTGGAGAAT | ATAGTCGCTGCTTGATCG |
| SOX2 | GCACAACTCGGAGATCAG | CAGCGTGTACTTATCCTTCT |
| KLF4 | TGAACTGACCAGGCACTA | TCATGTGTAAGGCGAGGT |
| NANOG | TCTCCAACATCCTGAACCT | GCGTCACACCATTGCTAT |
| PAX6 | ACATCTGGCTCCATGTTGGG | ATAACTCCGCCCATTCACCG |
| NESTIN | GCACCTCAAGATGTCCCTCAG | CTCCAGCTTGGGGTCCTGAAAG |
| NGFR | ACAAGACCTCATAGCCAGCAC | TGCAGCTGTTCCACCTCTTGA |
| Antibodies | Species | Supplier | Catalog Number |
|---|---|---|---|
| ALDH3A1 | rabbit | Abcam | Ab76976 |
| Lumican | rabbit | Abcam | Ab168348 |
| Fibronectin | rabbit | Abcam | Ab268020 |
| α-SMA | rabbit | Bioss | bs-10196R |
| Nanog | rabbit | Abcam | Ab21624 |
| Sox2 | rabbit | Abcam | Ab97959 |
| Klf4 | rabbit | Abcam | Ab215036 |
| Nestin | mouse | Santa Cruz | Sc-23927 |
| NeuN | rabbit | Abcam | Ab177487 |
| SPKH1 | rabbit | Proteintech | 10670-1-AP |
| SPP1 | rabbit | Abcam | Ab214050 |
| TIMP1 | rabbit | Abcam | Ab211926 |
Appendix B

References
- Huang, J.; Jiang, T.; Li, J.; Qie, J.; Cheng, X.; Wang, Y.; Zhou, T.; Liu, J.; Han, H.; Yao, K.; et al. Biomimetic Corneal Stroma for Scarless Corneal Wound Healing via Structural Restoration and Microenvironment Modulation. Adv. Healthc. Mater. 2023, 13, 2302889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative therapy for the Cornea. Prog. Retin. Eye Res. 2022, 87, 101011. [Google Scholar] [CrossRef] [PubMed]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Surovtseva, M.A.; Poveshchenko, O.V.; Krasner, K.Y.; Kim, I.I.; Lykov, A.P.; Bondarenko, N.A.; Shul’mina, L.A.; Trunov, A.N.; Chernykh, V.V. Morphofunctional Properties of Corneal Stromal Cells. Bull. Exp. Biol. Med. 2021, 172, 96–99. [Google Scholar] [CrossRef]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, V.K.; Singh, V.; Basu, S.; Pati, F. Recent Advancements in Molecular Therapeutics for Corneal Scar Treatment. Cells 2022, 11, 3310. [Google Scholar] [CrossRef]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Greene, C.A.; Chang, C.Y.; Fraser, C.J.; Nelidova, D.E.; Chen, J.A.; Lim, A.; Brebner, A.; McGhee, J.; Sherwin, T.; Green, C.R. Cells from the adult corneal stroma can be reprogrammed to a neuron-like cell using exogenous growth factors. Exp. Cell Res. 2014, 322, 122–132. [Google Scholar] [CrossRef]
- Bikkuzin, T.; Shi, Y.; Sun, B.; Guo, Y.; Jin, X.; Han, Z.; Pavlov, V.; Zhang, H. Human induced pluripotent stem cell line HMUi001-A derived from corneal stromal cells. Stem Cell Res. 2019, 37, 101409. [Google Scholar] [CrossRef]
- Kumar, A.; Xu, Y.; Yang, E.; Du, Y. Stemness and Regenerative Potential of Corneal Stromal Stem Cells and Their Secretome After Long-Term Storage: Implications for Ocular Regeneration. Investig. Opthalmology Vis. Sci. 2018, 59, 3728. [Google Scholar] [CrossRef] [PubMed]
- Calonge, M.; Nieto-Miguel, T.; De La Mata, A.; Galindo, S.; Herreras, J.M.; López-Paniagua, M. Goals and Challenges of Stem Cell-Based Therapy for Corneal Blindness Due to Limbal Deficiency. Pharmaceutics 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Santra, M.; Riau, A.K.; Geary, M.L.; Yang, T.; Rubin, E.; Yusoff, N.Z.B.M.; Dhaliwal, D.K.; Mehta, J.S.; Yam, G.H.-F. Combined Therapy Using Human Corneal Stromal Stem Cells and Quiescent Keratocytes to Prevent Corneal Scarring after Injury. Int. J. Mol. Sci. 2022, 23, 6980. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Hertsenberg, A.J.; Shojaati, G.; Funderburgh, M.L.; Mann, M.M.; Du, Y.; Funderburgh, J.L. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS ONE 2017, 12, e0171712. [Google Scholar] [CrossRef]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef]
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Yen, B.L.; Hsieh, C.C.; Hsu, P.J.; Chang, C.C.; Wang, L.T.; Yen, M.L. Three-Dimensional Spheroid Culture of Human Mesenchymal Stem Cells: Offering Therapeutic Advantages and In Vitro Glimpses of the In Vivo State. Stem Cells Transl. Med. 2023, 12, 235–244. [Google Scholar] [CrossRef]
- Yamada, A. Cellular memory function from 3D to 2D: Three-dimensional high density collagen microfiber cultures induce their resistance to reactive oxygen species. Mater. Today Bio 2024, 26, 101097. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lee, Y.C.; Hung, C.Y.; Yang, P.J.; Lai, P.C.; Feng, S.W. Three-dimensional spheroid culture enhances multipotent differentiation and stemness capacities of human dental pulp-derived mesenchymal stem cells by modulating MAPK and NF-kB signaling pathways. Stem Cell Rev. Rep. 2021, 17, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Wang, S.; Young, T.H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 2012, 33, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Chao, H.M.; Chern, E.; Hsu, S.H. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef]
- Fu, J.; Chuah, Y.J.; Ang, W.T.; Zheng, N.; Wang, D.-A. Optimization of a polydopamine (PD)-based coating method and polydimethylsiloxane (PDMS) substrates for improved mouse embryonic stem cell (ESC) pluripotency maintenance and cardiac differentiation. Biomater. Sci. 2017, 5, 1156–1173. [Google Scholar] [CrossRef]
- Kothapalli, C.; Mahajan, G.; Farrell, K. Substrate stiffness induced mechanotransduction regulates temporal evolution of human fetal neural progenitor cell phenotype, differentiation, and biomechanics. Biomater. Sci. 2020, 8, 5452–5464. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-catenin signaling pathways are involved in the modulation of corneal epithelial stem cell phenotype induced by substrate stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef]
- Sarvi, F.; Yue, Z.; Hourigan, K.; Thompson, M.C.; Chan, P.P.Y. Surface-functionalization of PDMS for potential micro-bioreactor and embryonic stem cell culture applications. J. Mater. Chem. B 2013, 1, 987–996. [Google Scholar] [CrossRef]
- Hung, H.S.; Yu AY, H.; Hsieh, S.C.; Kung, M.L.; Huang, H.Y.; Fu, R.H.; Yeh, C.-A.; Hsu, S.-H. Enhanced biocompatibility and differentiation capacity of mesenchymal stem cells on poly(dimethylsiloxane) by topographically patterned dopamine. ACS Appl. Mater. Interfaces 2020, 12, 44393–44406. [Google Scholar] [CrossRef]
- Razafiarison, T.; Holenstein, C.N.; Stauber, T.; Jovic, M.; Vertudes, E.; Loparic, M.; Kawecki, M.; Bernard, L.; Silvan, U.; Snedeker, J.G. Biomaterial surface energy-driven ligand assembly strongly regulates stem cell mechanosensitivity and fate on very soft substrates. Proc. Natl. Acad. Sci. USA 2018, 115, 4631–4636. [Google Scholar] [CrossRef]
- Li, S.; Ding, C.; Guo, Y.; Zhang, Y.; Wang, H.; Sun, X.; Zhang, J.; Cui, Z.; Chen, J. Mechanotransduction Regulates Reprogramming Enhancement in Adherent 3D Keratocyte Cultures. Front. Bioeng. Biotechnol. 2021, 9, 709488. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cui, Z.; Liang, Y.; Duan, C.; Chan, H.F.; Mao, S.; Gu, J.; Ding, C.; Yang, X.; Wang, Q.; et al. One-stop assembly of adherent 3D retinal organoids from hiPSCs based on 3D-printed derived PDMS microwell platform. Biofabrication 2023, 15, 035005. [Google Scholar] [CrossRef] [PubMed]
- Lian, R.L.; Guo, X.L.; Chen, J.S.; Guo, Y.L.; Zheng, J.F.; Chen, Y.W. Effects of induced pluripotent stem cells-derived conditioned medium on the proliferation and anti-apoptosis of human adipose-derived stem cells. Mol. Cell. Biochem. 2016, 413, 69–85. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.; Gao, Y.; Zheng, R.; Zhang, X.; Zhao, L.; Tan, M. Induced Pluripotent Stem Cells Inhibit Bleomycin-Induced Pulmonary Fibrosis in Mice through Suppressing TGF-β1/Smad-Mediated Epithelial to Mesenchymal Transition. Front. Pharmacol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Su VY, F.; Chiou, S.H.; Chen, W.C.; Yu, W.K.; Wu, H.H.; Chen, H.; Yang, K.Y. Induced Pluripotent Stem Cell-Derived Conditioned Medium Promotes Endogenous Leukemia Inhibitory Factor to Attenuate Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2021, 22, 5554. [Google Scholar] [CrossRef]
- Guo, X.; Gu, X.; Hareshwaree, S.; Rong, X.; Li, L.; Chu, M. Induced pluripotent stem cell-conditional medium inhibits H9C2 cardiomyocytes apoptosis via autophagy flux and Wnt/β-catenin pathway. J. Cell. Mol. Med. 2019, 23, 4358–4374. [Google Scholar] [CrossRef]
- Wann, S.; Chi, P.; Huang, W.; Cheng, C.; Chang, Y. Combination therapy of iPSC-derived conditioned medium with ceftriaxone alleviates bacteria-induced lung injury by targeting the NLRP3 inflammasome. J. Cell. Physiol. 2022, 237, 1299–1314. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Y.; Cui, Z.; Li, H.; Li, S.; Yang, X.; Yan, X.; Ding, C.; Tang, S.; Chen, J. The Construction of Retinal Pigment Epithelium Sheets with Enhanced Characteristics and Cilium Assembly Using iPS Conditioned Medium and Small Incision Lenticule Extraction Derived Lenticules. Acta Biomater. 2019, 92, 115–131. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Gu, J.; Wang, Y.; Tang, S.; Chen, J. Effect of porcine corneal stromal extract on keratocytes from SMILE-derived lenticules. J. Cell. Mol. Med. 2021, 25, 1207–1220. [Google Scholar] [CrossRef]
- Guo, X.; Lian, R.; Guo, Y.; Liu, Q.; Ji, Q.; Chen, J. bFGF and Activin A function to promote survival and proliferation of single iPS cells in conditioned half-exchange mTeSR1 medium. Hum. Cell 2015, 28, 122–132. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Yamada, T.; Hamajima, M.; Itoh, M.; Katayama, T.; Bork, P.; Goto, S.; Kanehisa, M. KEGG atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008, 36, W423–W426. [Google Scholar] [CrossRef]
- Binte MYusoff, N.Z.; Riau, A.K.; Yam, G.H.F.; binte Halim, N.S.H.; Mehta, J.S. Isolation and propagation of human corneal stromal keratocytes for tissue engineering and cell therapy. Cells 2022, 11, 178. [Google Scholar] [CrossRef]
- Alió del Barrio, J.L.; De la Mata, A.; De Miguel, M.P.; Arnalich-Montiel, F.; Nieto-Miguel, T.; El Zarif, M.; Cadenas-Martín, M.; López-Paniagua, M.; Galindo, S.; Calonge, M.; et al. Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells. Cells 2022, 11, 2549. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Li, F.; Zhang, Y.; Chen, S.Y.; Tighe, S.; Lin, S.Y.; Tseng, S.C. HC-HA/PTX3 purified from human amniotic membrane reverts human corneal fibroblasts and myofibroblasts to keratocytes by activating BMP signaling. Investig. Opthalmology Vis. Sci. 2020, 61, 62. [Google Scholar] [CrossRef]
- Kumar, P.; Pandit, A.; Zeugolis, D.I. Progress in Corneal Stromal Repair: From Tissue Grafts and Biomaterials to Modular Supramolecular Tissue-Like Assemblies. Adv. Mater. 2016, 28, 5381–5399. [Google Scholar] [CrossRef]
- Riau, A.K.; Look, Z.; Yam, G.H.F.; Boote, C.; Ma, Q.; Han, E.J.; binte M. Yusoff, N.Z.; Ong, H.S.; Goh, T.W.; binte Halim, N.S.H.; et al. Impact of keratocyte differentiation on corneal opacity resolution and visual function recovery in male rats. Nat. Commun. 2024, 15, 4959. [Google Scholar] [CrossRef]
- Li, H.; Dai, Y.; Shu, J.; Yu, R.; Guo, Y.; Chen, J. Spheroid cultures promote the stemness of corneal stromal cells. Tissue Cell 2015, 47, 39–48. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Y.; Wang, P.; Cui, Z.; Cao, J.; Liu, S.; Yu, Q.; Zeng, Q.; Zhu, D.; Xie, M.; et al. Muse cell spheroids have therapeutic effect on corneal scarring wound in mice and tree shrews. Sci. Transl. Med. 2020, 12, eaaw1120. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Goodrich, R.; Maldonado, M.; Ortiz, J.; Martinez, J.; Ico, G.; Ko, A.; Shih, H.P.; Nam, J. Nanofiber-microwell cell culture system for spatially patterned differentiation of pluripotent stem cells in 3D. Mater. Today Bio 2024, 26, 101109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, L.; Su, T.; Guo, Y.; Yu, Q.; Zhu, D.; Cui, Z.; Zhang, J.; Chen, J. Regulation of the keratocyte phenotype and cell behavior derived from human induced pluripotent stem cells by substrate stiffness. ACS Biomater. Sci. Eng. 2023, 9, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lin, F.; Ding, Y.; Zhang, Y.; Li, M.; Zhou, X.; Meng, Q.; Ma, X.; Wei, L.; Fan, H.; et al. Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res. Ther. 2021, 12, 295. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Hejazian, S.M.; Hejazian, S.S.; Mostafavi, S.M.; Hosseiniyan, S.M.; Montazersaheb, S.; Ardalan, M.; Zununi Vahed, S.; Barzegari, A. Targeting cellular senescence in kidney diseases and aging: A focus on mesenchymal stem cells and their paracrine factors. Cell Commun. Signal. 2024, 22, 609. [Google Scholar] [CrossRef]
- Yu, M.; Qin, K.; Fan, J.; Zhao, G.; Zhao, P.; Zeng, W.; Chen, C.; Wang, A.; Wang, Y.; Zhong, J.; et al. The evolving roles of wnt signaling in stem cell proliferation and differentiation, the development of human diseases, and therapeutic opportunities. Genes Dis. 2024, 11, 101026. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Zhu, Y.; Jin, R.; Wu, F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials 2021, 265, 120448. [Google Scholar] [CrossRef]
- Tan, F.; Qian, C.; Tang, K.; Abd-Allah, S.M.; Jing, N. Inhibition of transforming growth factor β (TGF-β) signaling can substitute for Oct4 protein in reprogramming and maintain pluripotency. J. Biol. Chem. 2015, 290, 4500–4511. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zhang, Y.; Fathy, A.H.; Zhou, M. The role of the wnt/β-catenin signaling pathway in the proliferation of gold nanoparticle-treated human periodontal ligament stem cells. Stem Cell Res. Ther. 2018, 9, 214. [Google Scholar] [CrossRef]
- Rothstein, M.; Azambuja, A.P.; Kanno, T.Y.; Breen, C.; Simoes-Costa, M. TGF-β signaling controls neural crest developmental plasticity via SMAD2/3. Dev. Cell 2025, S1534580725000590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, R.; Diao, S.; Du, J.; Fan, Z.; Wang, F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Chung, H.; Oh, S.J.; Kim, J.W.; Kim, S.H. Functionally enhanced cell spheroids for stem cell therapy: Role of TIMP1 in the survival and therapeutic effectiveness of stem cell spheroids. Acta Biomater. 2023, 166, 454–469. [Google Scholar] [CrossRef]
- Abbey, D. Chemical journey of somatic cells to pluripotency. Cell Regen. 2022, 11, 27. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Babai, N.; Chaudhuri, A.; Qiu, F.; Bhattacharya, S.; Dave, B.J.; Parameswaran, S.; Carson, S.D.; Thoreson, W.B.; Sharp, J.G.; et al. Non Cell-Autonomous Reprogramming of Adult Ocular Progenitors: Generation of Pluripotent Stem Cells without Exogenous Transcription Factors. Stem Cells 2009, 27, 3053–3062. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef]
- Campbell, K.H.; McWhir, J.; Ritchie, W.A.; Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996, 380, 64–66. [Google Scholar] [CrossRef]
- Wakayama, T.; Perry, A.C.; Zuccotti, M.; Johnson, K.R.; Yanagimachi, R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998, 394, 369–374. [Google Scholar] [CrossRef]
- Pesaresi, M.; Sebastian-Perez, R.; Cosma, M.P. Dedifferentiation, transdifferentiation and cell fusion: In vivo reprogramming strategies for regenerative medicine. FEBS J. 2019, 286, 1074–1093. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Deng, H. Chemical reprogramming for cell fate manipulation: Methods, applications, and perspectives. Cell Stem Cell 2023, 30, 1130–1147. [Google Scholar] [CrossRef] [PubMed]
- Maiti, G.; Monteiro de Barros, M.R.; Hu, N.; Dolgalev, I.; Roshan, M.; Foster, J.W.; Tsirigos, A.; Wahlin, K.J.; Chakravarti, S. Single cell RNA-seq of human cornea organoids identifies cell fates of a developing immature cornea. Pnas Nexus 2022, 1, pgac246. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, S.; Dhanuka, M.; Khodadadi-Jamayran, A.; Koduri, M.A.; Maiti, G.; Chakravarti, S. Matrix glycosaminoglycans and proteoglycans in human cornea organoids and similarities with fetal corneal stages. Ocul. Surf. 2025, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Swarup, A.; Phansalkar, R.; Morri, M.; Agarwal, A.; Subramaniam, V.; Li, B.; Wu, A.Y. Single-cell transcriptomic analysis of corneal organoids during development. Stem Cell Rep. 2023, 18, 2482–2497. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gu, J.; Cui, Z.; Sun, X.; Liang, Y.; Duan, C.; Li, X.; Su, Z.; Zhang, B.; Chen, J.; et al. Efficient Fabrication of Human Corneal Stromal Cell Spheroids and Promoting Cell Stemness Based on 3D-Printed Derived PDMS Microwell Platform. Biomolecules 2025, 15, 438. https://doi.org/10.3390/biom15030438
Chen Y, Gu J, Cui Z, Sun X, Liang Y, Duan C, Li X, Su Z, Zhang B, Chen J, et al. Efficient Fabrication of Human Corneal Stromal Cell Spheroids and Promoting Cell Stemness Based on 3D-Printed Derived PDMS Microwell Platform. Biomolecules. 2025; 15(3):438. https://doi.org/10.3390/biom15030438
Chicago/Turabian StyleChen, Yuexi, Jianing Gu, Zekai Cui, Xihao Sun, Yuqin Liang, Chunwen Duan, Xiaoxue Li, Zhanyu Su, Bo Zhang, Jiansu Chen, and et al. 2025. "Efficient Fabrication of Human Corneal Stromal Cell Spheroids and Promoting Cell Stemness Based on 3D-Printed Derived PDMS Microwell Platform" Biomolecules 15, no. 3: 438. https://doi.org/10.3390/biom15030438
APA StyleChen, Y., Gu, J., Cui, Z., Sun, X., Liang, Y., Duan, C., Li, X., Su, Z., Zhang, B., Chen, J., & Wang, Z. (2025). Efficient Fabrication of Human Corneal Stromal Cell Spheroids and Promoting Cell Stemness Based on 3D-Printed Derived PDMS Microwell Platform. Biomolecules, 15(3), 438. https://doi.org/10.3390/biom15030438






