Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies
Abstract
1. Introduction
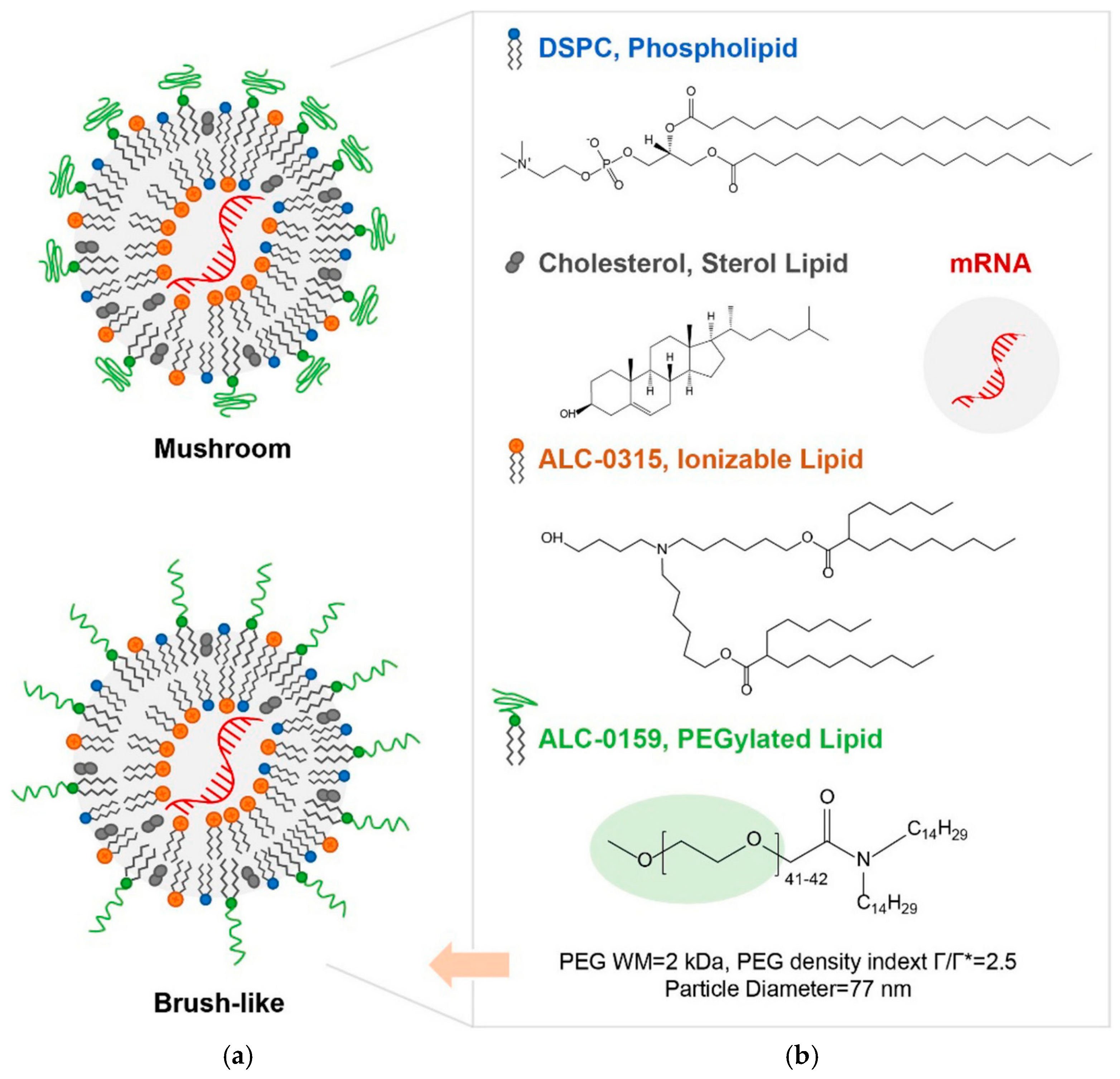
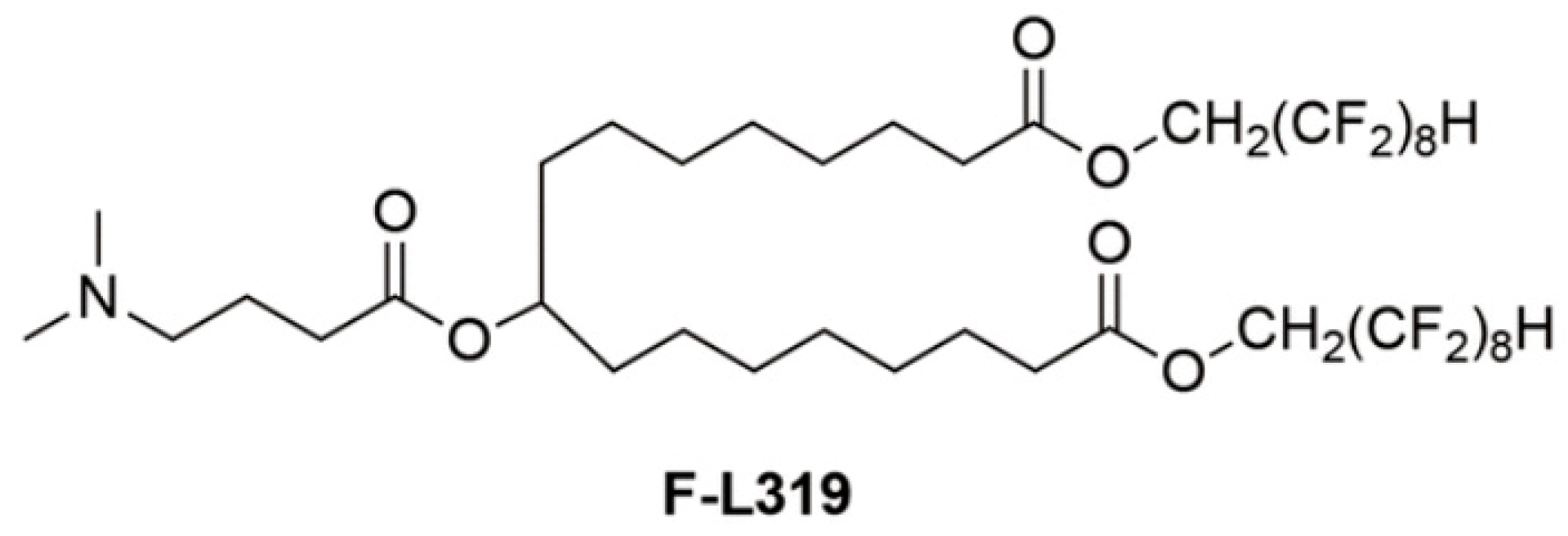
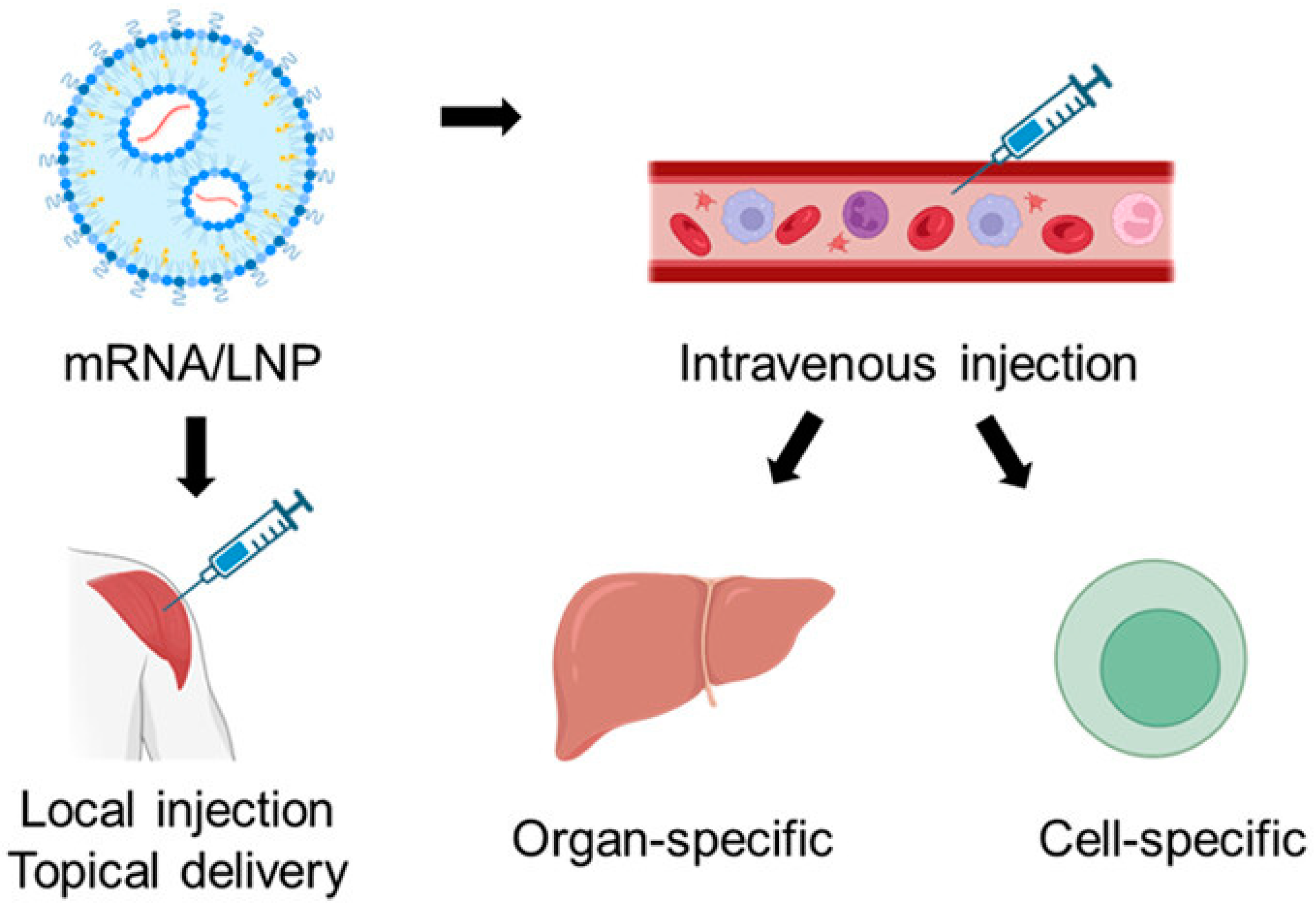
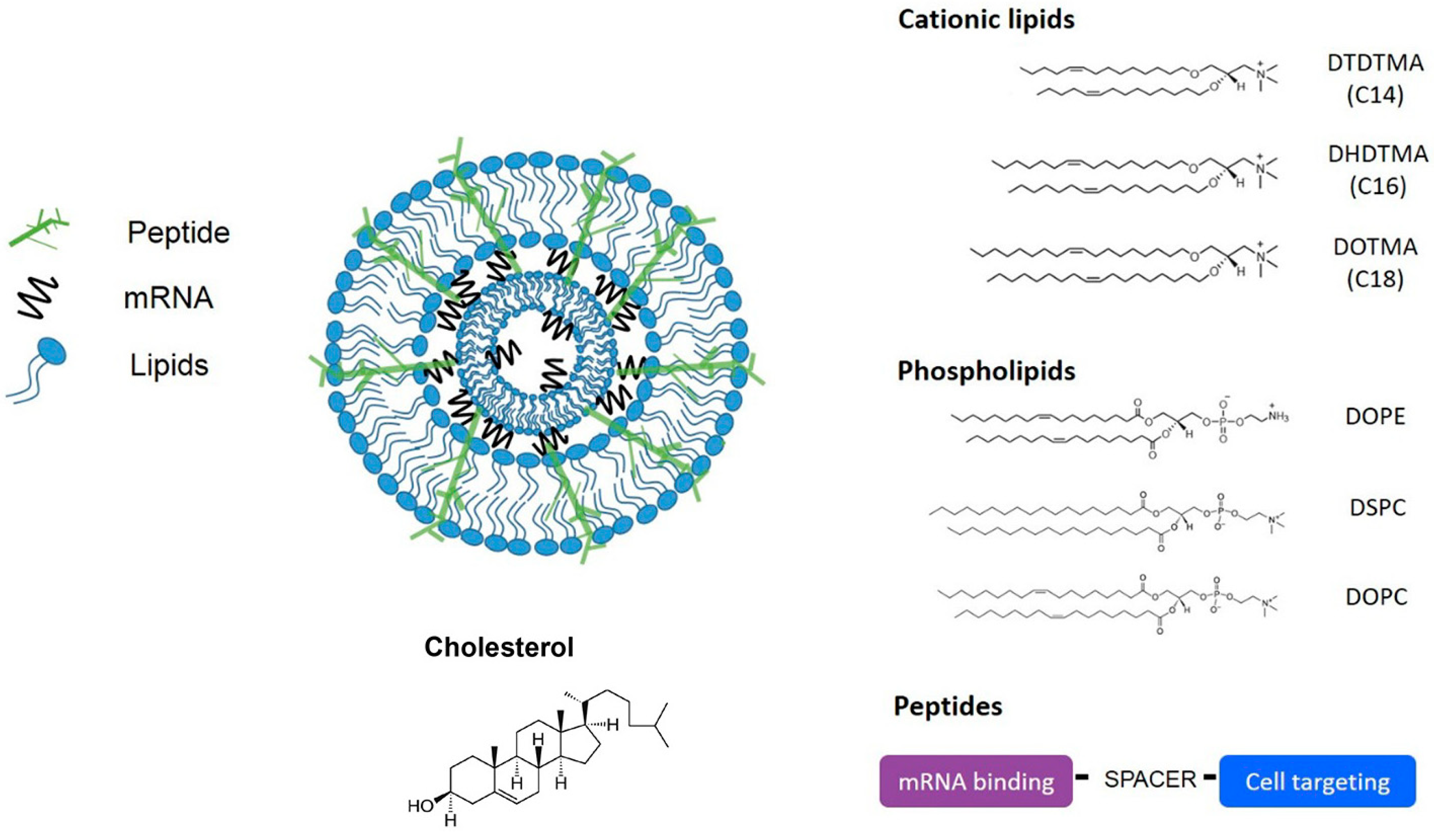
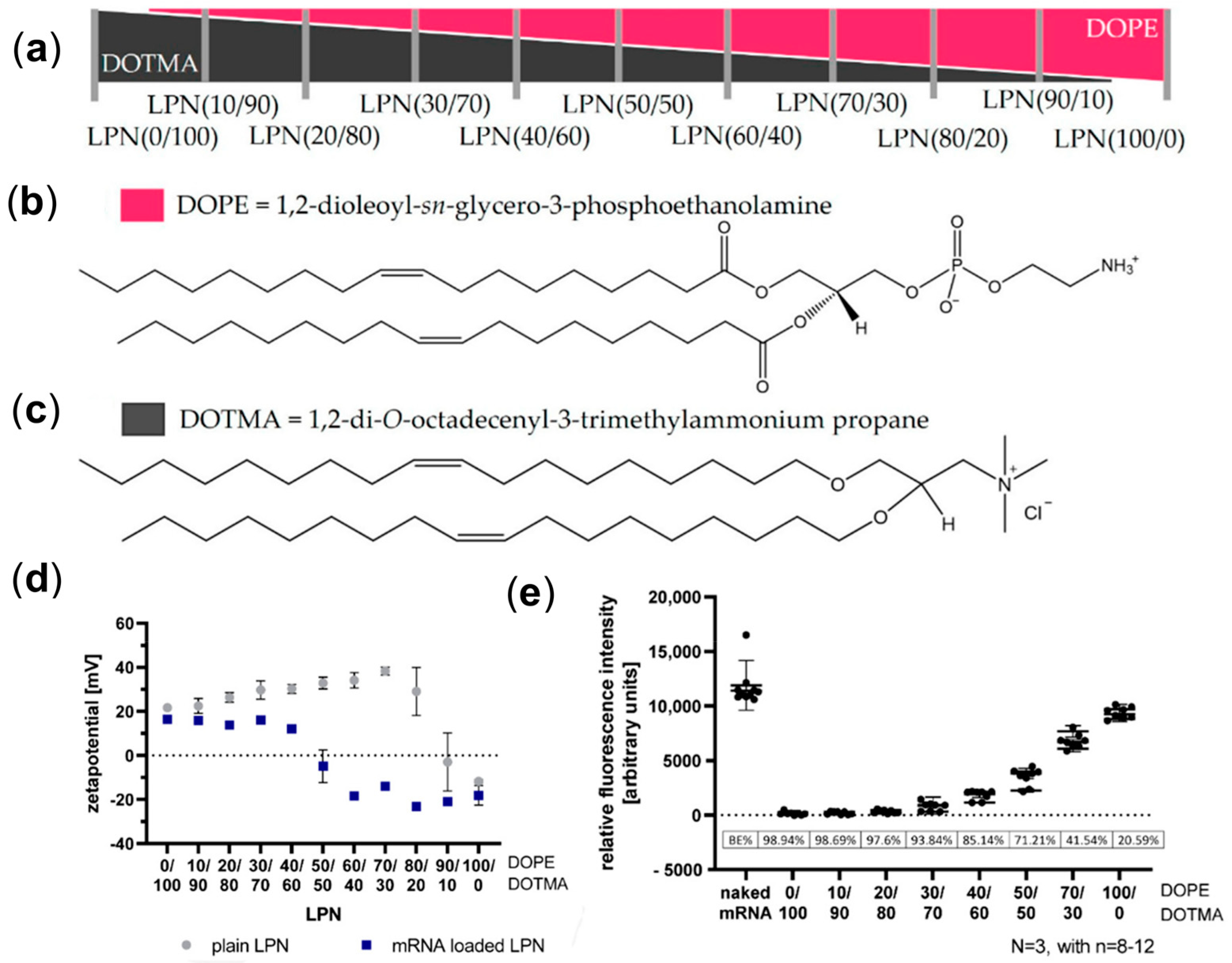
2. Lipid Nanoparticles (LNPs)
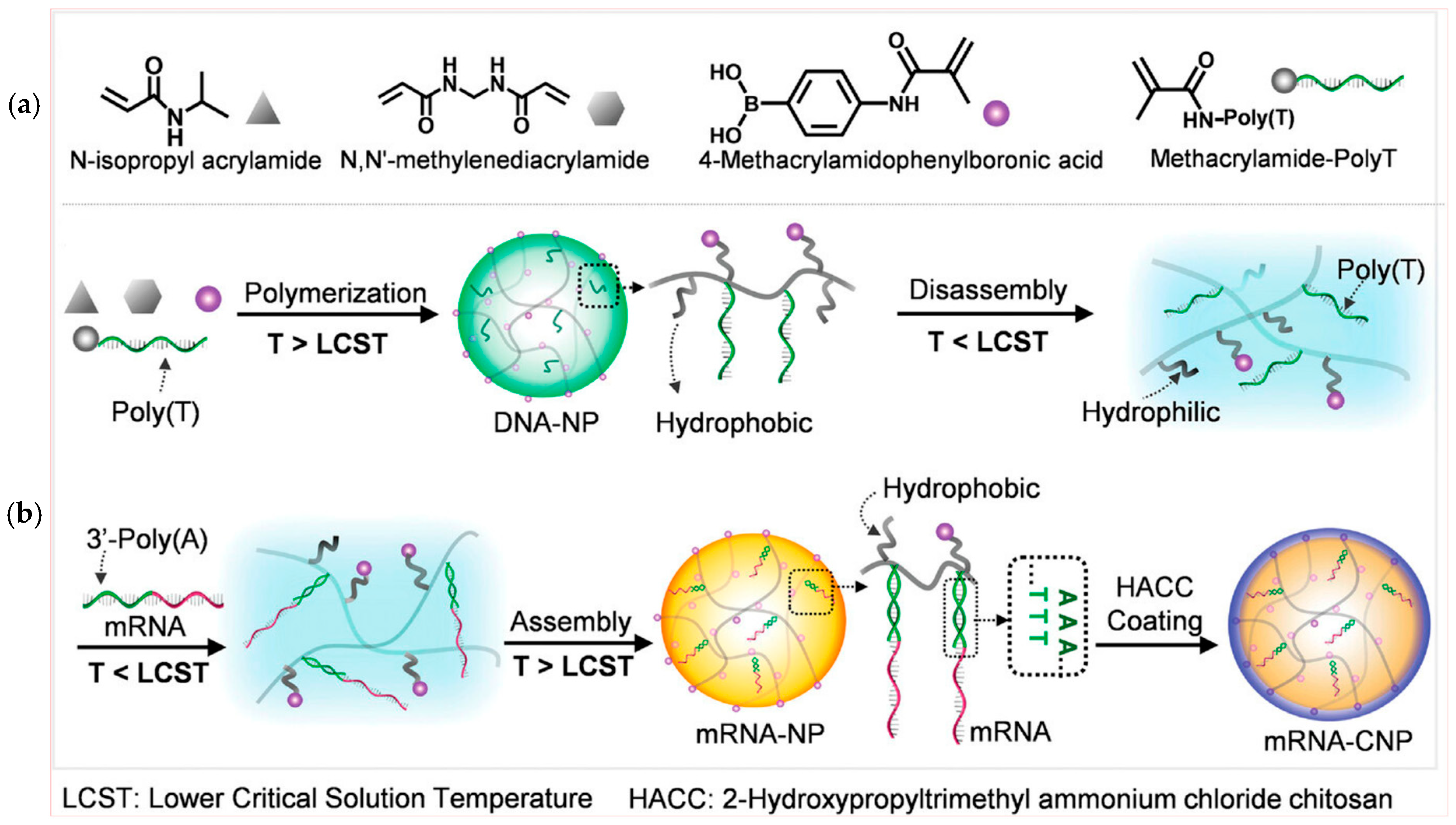
3. Polymeric Nanoparticles
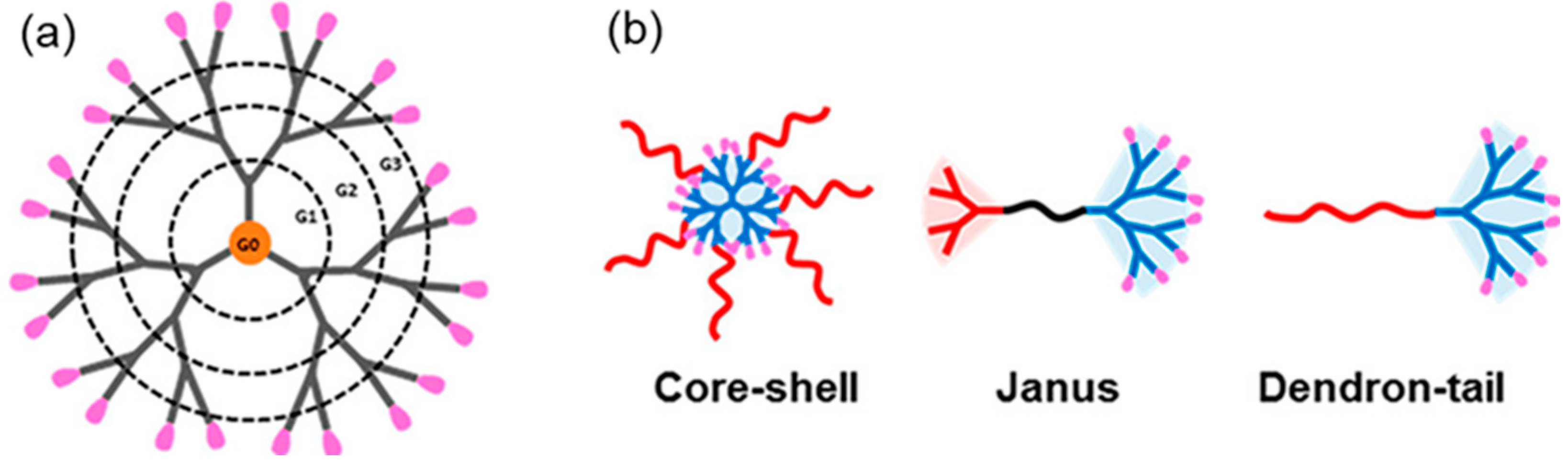
4. Dendrimer Nanoparticles
5. Polysaccharide-Based Nanoparticles
6. Peptide-Derived Nanoparticles
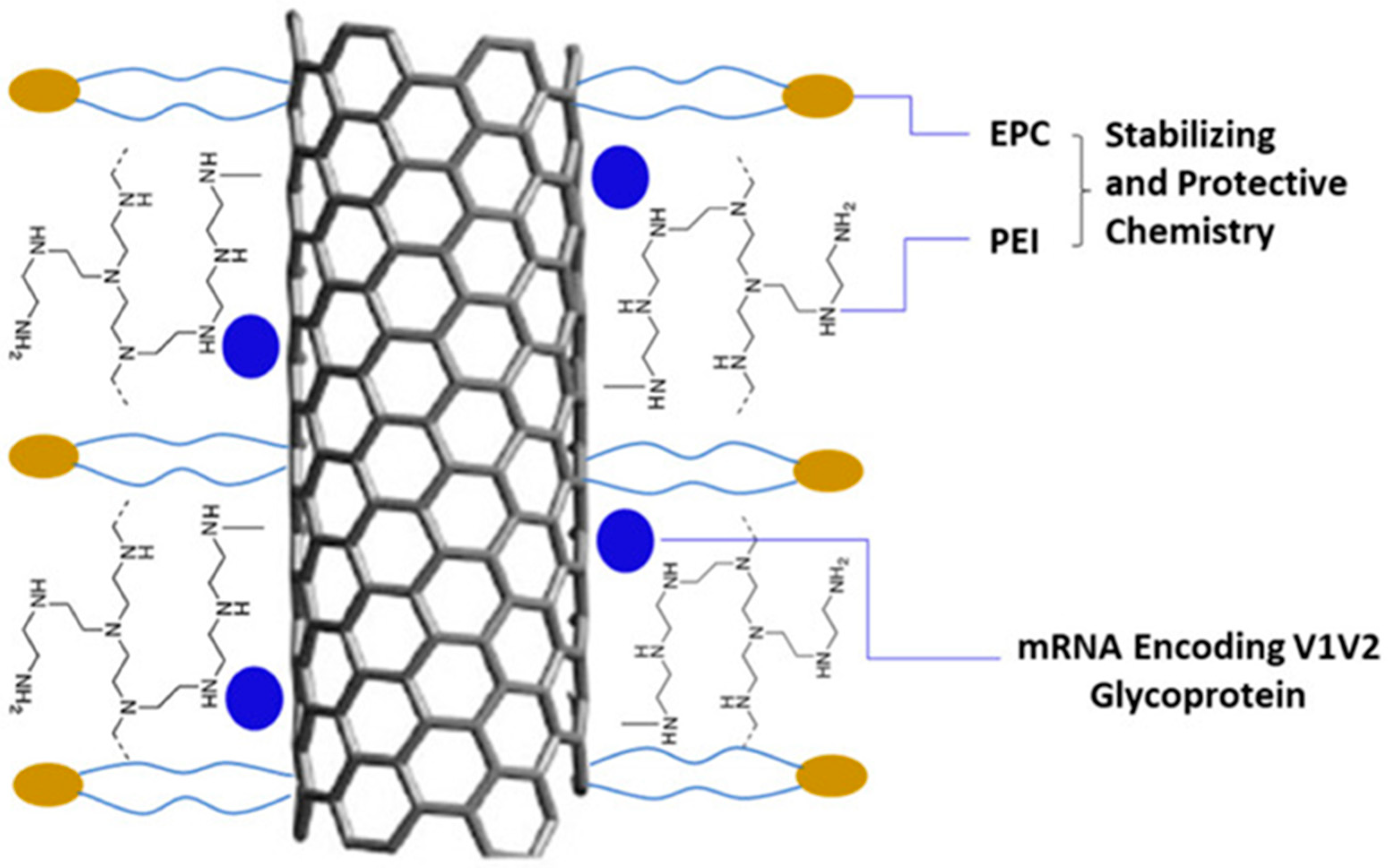
7. Carbon-Based Nanoparticles
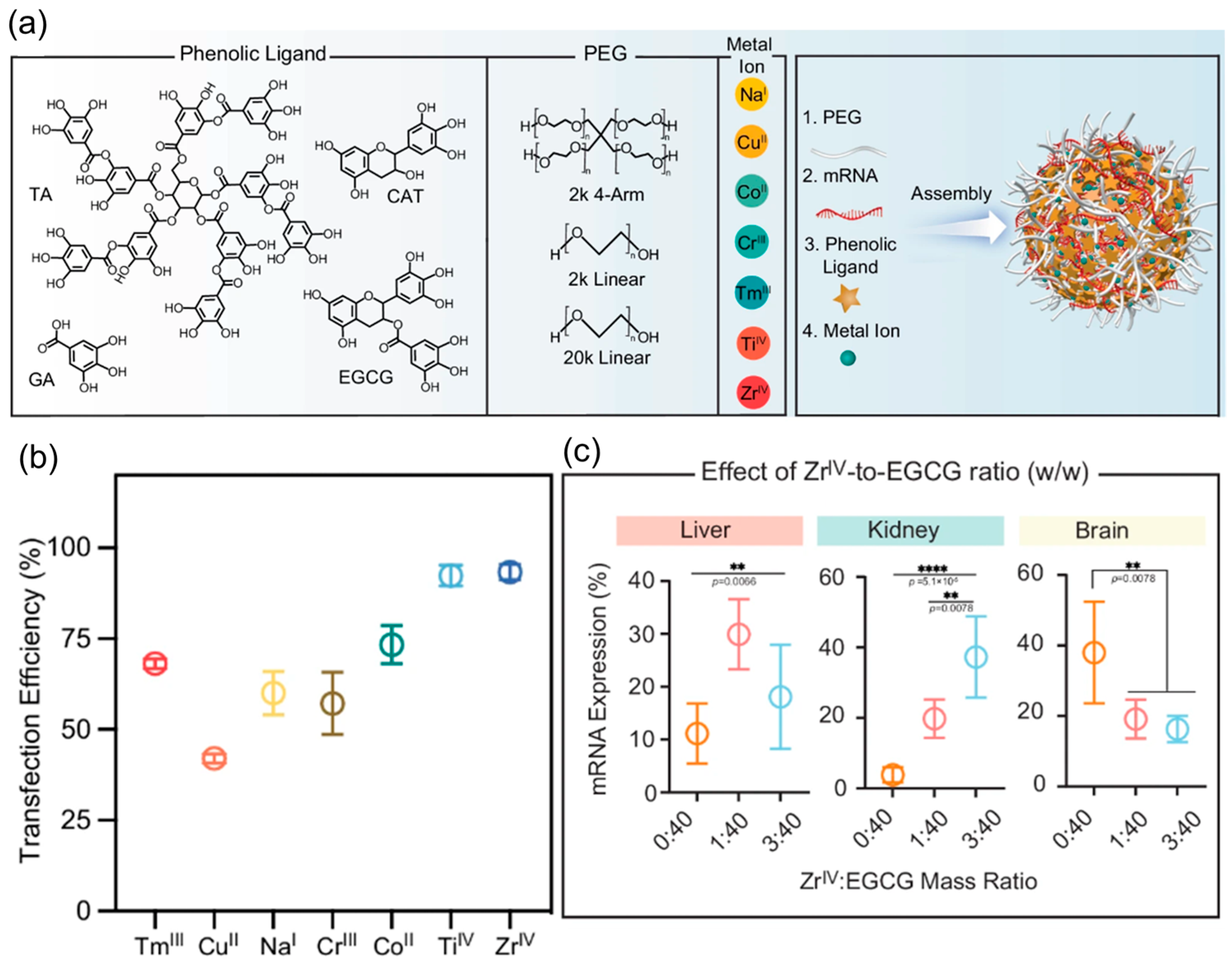
8. Metal-Based Nanoparticles (MNPs)
9. DNA Nanostructures
10. Hybrid Nanoparticles
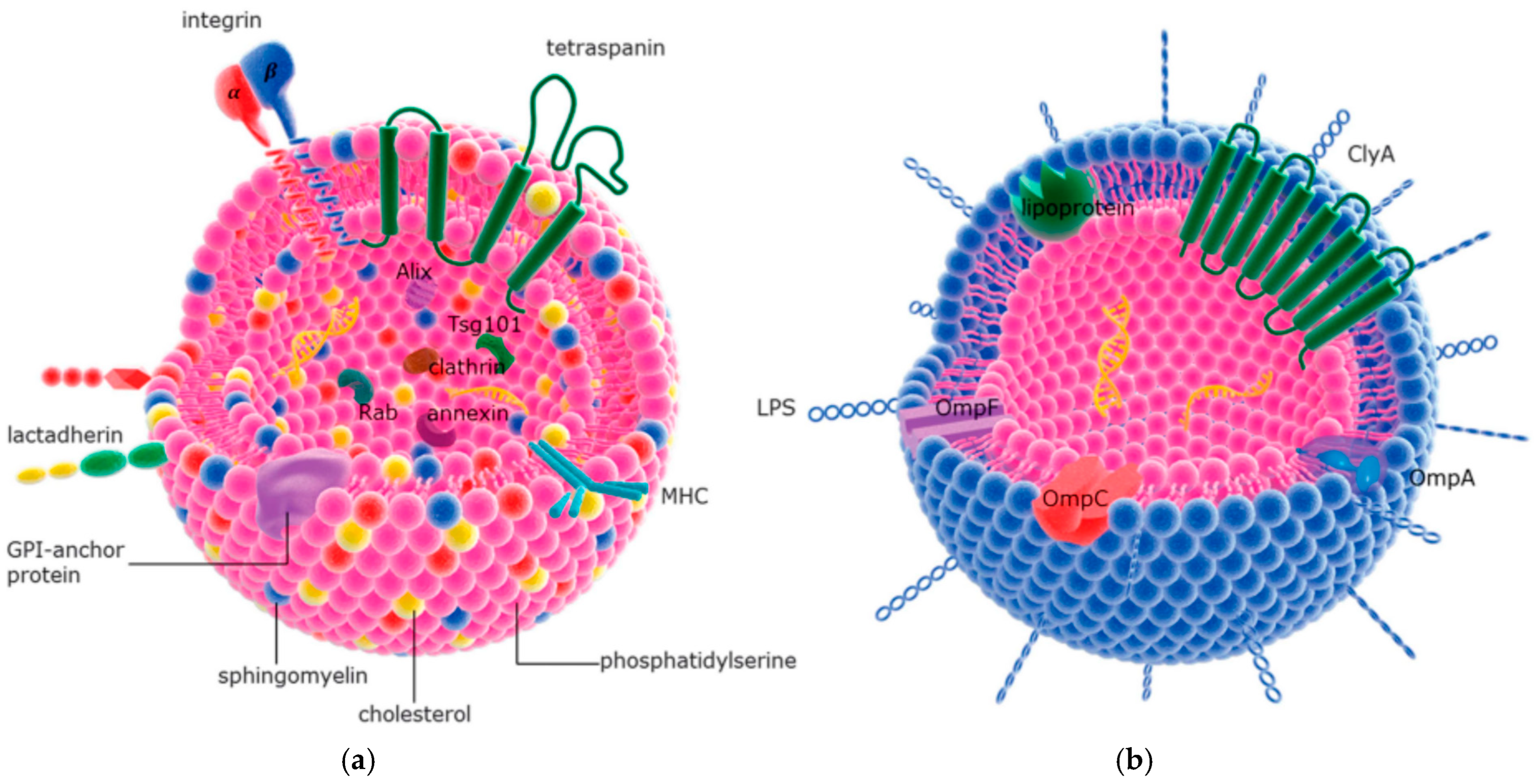
11. Liposomes and Extracellular Vesicles
12. Challenges and Opportunities
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brenner, S.; Jacob, F.; Meselson, M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 1961, 190, 576–581. [Google Scholar] [CrossRef]
- Krieg, P.A.; Melton, D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984, 12, 7057–7070. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research Advances on the Stability of mRNA Vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Thoresen, D.; Wang, W.; Galls, D.; Guo, R.; Xu, L.; Pyle, A.M. The molecular mechanism of RIG-I activation and signaling. Immunol. Rev. 2021, 304, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Cao, Q.; Fang, H.; Tian, H. mRNA vaccines contribute to innate and adaptive immunity to enhance immune response in vivo. Biomaterials 2024, 310, 122628. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Takano, T.; Takahashi, Y. Immune responses related to the immunogenicity and reactogenicity of COVID-19 mRNA vaccines. Int. Immunol. 2023, 35, 213–220. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Sergeeva, O.V.; Koteliansky, V.E.; Zatsepin, T.S. mRNA-Based Therapeutics—Advances and Perspectives. Biochem. (Mosc) 2016, 81, 709–722. [Google Scholar] [CrossRef]
- Lin, Y.X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics 2020, 10, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Teresa, A.; Alfonso, T.-C.; Laura, F.-R.; Andrea, R.; Andrei Mihai, S.; Luna, P.; Victor, J.N.; Juan, M.-O.; Diego de, M. Comprehensive Optimization of a Freeze-Drying Process Achieving Enhanced Long-Term Stability and In Vivo Performance of Lyophilized mRNA-LNPs. Int. J. Mol. Sci. 2024, 25, 10603. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kon, E.; Han, X.; Zhang, X.; Kong, N.; Mitchell, M.J.; Peer, D.; Tao, W. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 2022, 17, 1027–1037. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Granot, Y.; Peer, D. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics-An innate immune system standpoint. Semin. Immunol. 2017, 34, 68–77. [Google Scholar] [CrossRef]
- Patel, A.K.; Kaczmarek, J.C.; Bose, S.; Kauffman, K.J.; Mir, F.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv. Mater. 2019, 31, e1805116. [Google Scholar] [CrossRef]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef]
- Kong, N.; Zhang, R.; Wu, G.; Sui, X.; Wang, J.; Kim, N.Y.; Blake, S.; De, D.; Xie, T.; Cao, Y.; et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2112696119. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Hajiaghapour Asr, M.; Dayani, F.; Saedi Segherloo, F.; Kamedi, A.; Neill, A.O.; MacLoughlin, R.; Doroudian, M. Lipid Nanoparticles as Promising Carriers for mRNA Vaccines for Viral Lung Infections. Pharmaceutics 2023, 15, 1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Sarmadi, M.; Ying, B.; Jaklenec, A.; Langer, R. Recent advances in nano- and micro-scale carrier systems for controlled delivery of vaccines. Biomaterials 2023, 303, 122345. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Wang, M.M.; Wappelhorst, C.N.; Jensen, E.L.; Chi, Y.-C.T.; Rouse, J.C.; Zou, Q. Elucidation of lipid nanoparticle surface structure in mRNA vaccines. Sci. Rep. 2023, 13, 16744. [Google Scholar] [CrossRef]
- Huo, H.; Cheng, X.; Xu, J.; Lin, J.; Chen, N.; Lu, X. A fluorinated ionizable lipid improves the mRNA delivery efficiency of lipid nanoparticles. J. Mater. Chem. B 2023, 11, 4171–4180. [Google Scholar] [CrossRef]
- Han, X.; Alameh, M.G.; Butowska, K.; Knox, J.J.; Lundgreen, K.; Ghattas, M.; Gong, N.; Xue, L.; Xu, Y.; Lavertu, M.; et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 2023, 18, 1105–1114. [Google Scholar] [CrossRef]
- Hunter, M.R.; Cui, L.; Porebski, B.T.; Pereira, S.; Sonzini, S.; Odunze, U.; Iyer, P.; Engkvist, O.; Lloyd, R.L.; Peel, S.; et al. Understanding Intracellular Biology to Improve mRNA Delivery by Lipid Nanoparticles. Small Methods 2023, 7, e2201695. [Google Scholar] [CrossRef]
- Xu, X.; Xia, T. Recent Advances in Site-Specific Lipid Nanoparticles for mRNA Delivery. ACS Nanosci. Au 2023, 3, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tang, Z.; Huang, X.; Chen, W.; Zhou, J.; Liu, H.; Liu, C.; Kong, N.; Tao, W. Emerging mRNA technologies: Delivery strategies and biomedical applications. Chem. Soc. Rev. 2022, 51, 3828–3845. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.R.; Tzeng, S.Y.; Rui, Y.; Neshat, S.Y.; Conge, M.J.; Luly, K.M.; Wang, E.; Firestone, J.L.; McAuliffe, J.; Maruggi, G.; et al. Biodegradable Polyester Nanoparticle Vaccines Deliver Self-Amplifying mRNA in Mice at Low Doses. Adv Ther (Weinh) 2023, 6, 2200219. [Google Scholar] [CrossRef]
- Suberi, A.; Grun, M.K.; Mao, T.; Israelow, B.; Reschke, M.; Grundler, J.; Akhtar, L.; Lee, T.; Shin, K.; Piotrowski-Daspit, A.S.; et al. Inhalable polymer nanoparticles for versatile mRNA delivery and mucosal vaccination. bioRxiv 2022. [Google Scholar] [CrossRef]
- Suberi, A.; Grun, M.K.; Mao, T.; Israelow, B.; Reschke, M.; Grundler, J.; Akhtar, L.; Lee, T.; Shin, K.; Piotrowski-Daspit, A.S.; et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci. Transl. Med. 2023, 15, eabq0603. [Google Scholar] [CrossRef]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(beta-amino ester)s as gene delivery vehicles: Challenges and opportunities. Expert. Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Wilson, D.R.; Tzeng, S.Y.; Yamagata, H.M.; Sudhakar, D.; Conge, M.; Berlinicke, C.A.; Zack, D.J.; Tuesca, A.; Green, J.J. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci. Adv. 2022, 8, eabk2855. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef]
- Kavanagh, E.W.; Tzeng, S.Y.; Sharma, N.; Cutting, G.R.; Green, J.J. Ligand-free biodegradable poly(beta-amino ester) nanoparticles for targeted systemic delivery of mRNA to the lungs. Biomaterials 2025, 313, 122753. [Google Scholar] [CrossRef]
- Sonawane, S.; Pingale, P.; Amrutkar, S. PLGA: A Wow Smart Biodegradable Polymer in Drug Delivery System. Indian. J. Pharm. Educ. Res. 2023, 57, s189–s197. [Google Scholar] [CrossRef]
- Sharifnia, Z.; Bandehpour, M.; Hamishehkar, H.; Mosaffa, N.; Kazemi, B.; Zarghami, N. In-vitro Transcribed mRNA Delivery Using PLGA/PEI Nanoparticles into Human Monocyte-derived Dendritic Cells. Iran. J. Pharm. Res. 2019, 18, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Guangsheng, D.; Xun, S. Recent Advances in the Noninvasive Delivery of mRNA. Acc. Chem. Res. 2021, 10, 493. [Google Scholar] [CrossRef]
- Neary, M.T.; Mulder, L.M.; Kowalski, P.S.; MacLoughlin, R.; Crean, A.M.; Ryan, K.B. Nebulised delivery of RNA formulations to the lungs: From aerosol to cytosol. J. Control Release 2024, 366, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Melike, O.; Abhijeet, L.; Saahil, B.; Zhenning, S.; Signe Tandrup, S.; Henrik, F.; Rades, T.; Federica, S.; Aneesh, T.; Camilla, F. Lipid nanoparticles for local delivery of mRNA to the respiratory tract: Effect of PEG-lipid content and administration route. Eur. J. Pharm. Biopharm. 2024, 198, 114266. [Google Scholar] [CrossRef]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-Based Nanoparticles for Nucleic Acid Delivery: Technological Aspects, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korovkina, O.; Gubina, N.; Ekimova, V.; Ishutinova, A.; Korzhikova-Vlakh, E.; Tennikova, T.; Korzhikov-Vlakh, V. Random Copolymers of Lysine and Isoleucine for Efficient mRNA Delivery. Int. J. Mol. Sci. 2022, 23, 5363. [Google Scholar] [CrossRef]
- Meyer, R.A.; Hussmann, G.P.; Peterson, N.C.; Santos, J.L.; Tuesca, A.D. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Ratautaite, V.; Ramanaviciene, A.; Ramanavicius, A. Detection of the SARS-CoV-2 nucleoprotein by electrochemical biosensor based on molecularly imprinted polypyrrole formed on self-assembled monolayer. Biosens. Bioelectron. 2025, 272, 117092. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Lim, C.C.; Chia, L.Y.; Kumar, P.V. Dendrimer-based nanocomposites for the production of RNA delivery systems. OpenNano 2023, 13, 100173. [Google Scholar] [CrossRef]
- Kisakova, L.A.; Apartsin, E.K.; Nizolenko, L.F.; Karpenko, L.I. Dendrimer-Mediated Delivery of DNA and RNA Vaccines. Pharmaceutics 2023, 15, 1106. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, D.; Liu, X.; Peng, L. Amphiphilic Dendrimer Vectors for RNA Delivery: State-of-the-Art and Future Perspective. Acc. Mater. Res. 2022, 3, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Kumbhar, S.A.; Gorain, B.; Choudhury, H.; Kesharwani, P. Chapter 9—Safety and toxicity issues of dendrimers. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 143–162. [Google Scholar]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef]
- Andreea, M.C.; Alexandru, M.; Alina Simona, Ş.; Anne-Marie, C.; Carmen, C.; Rahela, C.; Alina Gabriela, D. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment—An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef]
- Li, X.; Naeem, A.; Xiao, S.; Hu, L.; Zhang, J.; Zheng, Q. Safety Challenges and Application Strategies for the Use of Dendrimers in Medicine. Pharmaceutics 2022, 14, 1292. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Joubert, F.; Munson, M.J.; Sabirsh, A.; England, R.M.; Hemmerling, M.; Alexander, C.; Ashford, M.B. Precise and systematic end group chemistry modifications on PAMAM and poly(l-lysine) dendrimers to improve cytosolic delivery of mRNA. J. Control. Release 2023, 356, 580–594. [Google Scholar] [CrossRef]
- Li, X.; Qi, J.; Wang, J.; Hu, W.; Zhou, W.; Wang, Y.; Li, T. Nanoparticle technology for mRNA: Delivery strategy, clinical application and developmental landscape. Theranostics 2024, 14, 738–760. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, X.; Mao, C.; Jiang, Y. The Distinct Properties of Polysaccharide Nanoparticles Tune Immune Responses against mRNA Antigen via Stimulator of Interferon Genes-Mediated Autophagy and Inflammasome. ACS Nano 2023, 17, 21782–21798. [Google Scholar] [CrossRef]
- Dacoba, T.G.; Omange, R.W.; Li, H.; Crecente-Campo, J.; Luo, M.; Alonso, M.J. Polysaccharide Nanoparticles Can Efficiently Modulate the Immune Response against an HIV Peptide Antigen. ACS Nano 2019, 13, 4947–4959. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502. [Google Scholar] [CrossRef]
- Jamila, D.; Javier, F.-L.; Hugo, M.S.; Julia, L.; Sergi, R.-C.; Emilia, B.; José Luis, C.-M.; Carlos, L. Study and Preparation of Multifunctional Poly(L-Lysine)@Hyaluronic Acid Nanopolyplexes for the Effective Delivery of Tumor Suppressive MiR-34a into Triple-Negative Breast Cancer Cells. Materials 2020, 13, 5309. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, J.H.; Yoon, H.Y. Method for nucleic acid delivery using hyaluronic acid. U.S. Patent 9,744,241, 2014. [Google Scholar]
- Su Sundee, M.; Chavee, L.; Sirikool, T.; Supakarn, C.; Jittima Amie, L. Hyaluronic Acid Nanogels: A Promising Platform for Therapeutic and Theranostic Applications. Pharmaceutics 2023, 15, 2671. [Google Scholar] [CrossRef]
- Ansari, M.T.; Murteza, S.; Ahsan, M.N.; Hasnain, M.S.; Nayak, A.K. Chapter 15—Chitosan as a responsive biopolymer in drug delivery. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 389–410. [Google Scholar]
- Garcia, B.B.; Stefania, D.; Omar, M.; Enrico, M.; Sang, W.H. Efficacy of Chitosan-N-Arginine Chitosomes in mRNA Delivery and Cell Viability Enhancement. ACS Appl. Bio Mater. 2024, 7, 8261–8271. [Google Scholar] [CrossRef] [PubMed]
- van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- van der Lubben, I.M.; Kersten, G.; Fretz, M.M.; Beuvery, C.; Coos Verhoef, J.; Junginger, H.E. Chitosan microparticles for mucosal vaccination against diphtheria: Oral and nasal efficacy studies in mice. Vaccine 2003, 21, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine 2014, 10, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Sahithya, M.; Induja, M.; Ashok, B.; Rushil, K.; Srinidhi, G.; Sathiya, K.; Abinaya, S.; Raghav, G.; Selvamurugan, N. Chitosan nanocarriers for non-coding RNA therapeutics: A review. Int. J. Biol. Macromol. 2024, 263, 130361. [Google Scholar] [CrossRef]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Özbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control Release 2014, 189, 141–149. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef]
- Porosk, L.; Hark, H.H.; Arukuusk, P.; Haljasorg, U.; Peterson, P.; Kurrikoff, K. The Development of Cell-Penetrating Peptides for Efficient and Selective In Vivo Expression of mRNA in Spleen Tissue. Pharmaceutics 2023, 15, 952. [Google Scholar] [CrossRef]
- Udhayakumar, V.K.; De Beuckelaer, A.; McCaffrey, J.; McCrudden, C.M.; Kirschman, J.L.; Vanover, D.; Van Hoecke, L.; Roose, K.; Deswarte, K.; De Geest, B.G.; et al. Arginine-Rich Peptide-Based mRNA Nanocomplexes Efficiently Instigate Cytotoxic T Cell Immunity Dependent on the Amphipathic Organization of the Peptide. Adv. Heal. Mater. 2017, 6, 1412. [Google Scholar] [CrossRef]
- Herrera-Barrera, M.; Ryals, R.C.; Gautam, M.; Jozic, A.; Landry, M.; Korzun, T.; Gupta, M.; Acosta, C.; Stoddard, J.; Reynaga, R.; et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023, 9, eadd4623. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Kim, E.H.; Jang, H.; Jang, Y.; Chi, S.G.; Yang, Y.; Kim, S.H. The Potential of Cell-Penetrating Peptides for mRNA Delivery to Cancer Cells. Pharmaceutics 2022, 14, 1271. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Grant-Serroukh, D.; Hunter, M.R.; Maeshima, R.; Tagalakis, A.D.; Aldossary, A.M.; Allahham, N.; Williams, G.R.; Edbrooke, M.; Desai, A.; Hart, S.L. Lipid-peptide nanocomplexes for mRNA delivery in vitro and in vivo. J. Control. Release 2022, 348, 786–797. [Google Scholar] [CrossRef]
- Xinxi, C.; Aftab, U.; Rui, Q.; Junming, C.; Lin, W.; Song, S. Membrane-coated protein nanoparticles for mRNA delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105427. [Google Scholar] [CrossRef]
- Hiroshi, K.; Yutaro, Y.; Loreto, B.F.; Hitomi, E.; Keiji, I.; Katsuro, T. Efficient mRNA Delivery with Lyophilized Human Serum Albumin-Based Nanobubbles. Nanomaterials 2023, 13, 1283. [Google Scholar] [CrossRef]
- Hassanin, I.A.; Elzoghby, A.O. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Rama, P.; Álvaro, S. Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions. Cancers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Jiarui, Z.; Yianghao, L.; Li Qian, B.; Gan, G.; Yuxuan, L.; Heyun, S. Ph Responsive Poly(Amino Acid) Nanoparticles As Potent Carrier Adjuvants For Enhancing Cellular Immunity. Macromol. Biosci. 2023, 23, e2200520. [Google Scholar] [CrossRef]
- Yingying, X.; Yi-xin, Z.; Chengyan, W.; Xiaoman, H.; Feng, Z. PEGylated pH-responsive peptide-mRNA nano self-assemblies enhance the pulmonary delivery efficiency and safety of aerosolized mRNA. Drug Deliv. 2023, 30, 2219870. [Google Scholar] [CrossRef]
- Jiaojiao, Z.; Zixuan, W.; Jiwei, M.; Xuelin, Z.; Rongxin, S.; Yuefei, W.; Wei, Q. Self-Assembly of Peptide-Lipid Nanoparticles for the Efficient Delivery of Nucleic Acids. Langmuir 2023, 39, 7484–7494. [Google Scholar] [CrossRef]
- Xu, Y.; Ferguson, T.; Masuda, K.; Siddiqui, M.A.; Smith, K.P.; Vest, O.; Brooks, B.; Zhou, Z.; Obliosca, J.; Kong, X.P.; et al. Short Carbon Nanotube-Based Delivery of mRNA for HIV-1 Vaccines. Biomolecules 2023, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, X.; Hu, Y.; Tian, X.L.; Yu, X.Q.; Zhang, J. Spleen-Targeted mRNA Delivery by Amphiphilic Carbon Dots for Tumor Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 19937–19950. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics 2021, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Yuang, G.; Jingqu, C.; Zhaoran, W.; Chang, L.; Tianzheng, W.; Chan-Jin, K.; Helena, Ď.; Soraia, F.; Darryl, N.J.; Robert De, R.; et al. mRNA delivery enabled by metal–organic nanoparticles. Nat. Commun. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Zhang, D.; Huang, X.; Dong, Y.; Gao, W.; Ye, Y.; Ren, K.; Zhao, W.; Qiao, H.; et al. Black Phosphorus Nanosheets Assist Nanoerythrosomes for Efficient mRNA Vaccine Delivery and Immune Activation. Adv. Heal. Mater. 2023, 12, e2300935. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, L.; Hao, Z.; Liu, D. DNA-based nanostructures for RNA delivery. Med. Rev. 2024, 4, 207–224. [Google Scholar] [CrossRef]
- Lee, H.; Lytton-Jean, A.K.; Chen, Y.; Love, K.T.; Park, A.I.; Karagiannis, E.D.; Sehgal, A.; Querbes, W.; Zurenko, C.S.; Jayaraman, M.; et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012, 7, 389–393. [Google Scholar] [CrossRef]
- Bujold, K.E.; Hsu, J.C.C.; Sleiman, H.F. Optimized DNA “Nanosuitcases” for Encapsulation and Conditional Release of siRNA. J. Am. Chem. Soc. 2016, 138, 14030–14038. [Google Scholar] [CrossRef]
- Fu, X.; Chen, T.; Song, Y.; Feng, C.; Chen, H.; Zhang, Q.; Chen, G.; Zhu, X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small 2021, 17, e2101224. [Google Scholar] [CrossRef]
- Parsons, M.F.; Allan, M.F.; Li, S.; Shepherd, T.R.; Ratanalert, S.; Zhang, K.; Pullen, K.M.; Chiu, W.; Rouskin, S.; Bathe, M. 3D RNA-scaffolded wireframe origami. Nat. Commun. 2023, 14, 382. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.; Yang, J.; Ren, J.; Huang, M.; Wang, S.; Yang, D. A Thermal and Enzymatic Dual-Stimuli Responsive DNA-Based Nanomachine for Controlled mRNA Delivery. Adv. Sci. 2023, 10, e2204905. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Huang, M.; Yang, J.; Li, P.; Chang, L.; Tang, Q.; Chen, X.; Wang, S.; Yao, C.; Liu, P.; et al. A Smart DNA-Based Nanosystem Containing Ribosome-Regulating siRNA for Enhanced mRNA Transfection. Adv. Mater. 2023, 35, e2300823. [Google Scholar] [CrossRef]
- Hu, M.; Feng, C.; Yuan, Q.; Liu, C.; Ge, B.; Sun, F.; Zhu, X. Lantern-shaped flexible RNA origami for Smad4 mRNA delivery and growth suppression of colorectal cancer. Nat. Commun. 2023, 14, 1307. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Kliesch, L.; Delandre, S.; Gabelmann, A.; Koch, M.; Schulze, K.; Guzmán, C.A.; Loretz, B.; Lehr, C.M. Lipid-Polymer Hybrid Nanoparticles for mRNA Delivery to Dendritic Cells: Impact of Lipid Composition on Performance in Different Media. Pharmaceutics 2022, 14, 2675. [Google Scholar] [CrossRef]
- Li, B.; Luo, X.; Deng, B.; Wang, J.; McComb, D.W.; Shi, Y.; Gaensler, K.M.; Tan, X.; Dunn, A.L.; Kerlin, B.A.; et al. An Orthogonal Array Optimization of Lipid-like Nanoparticles for mRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, C.; Li, B.; Zhang, X.; Luo, X.; Zeng, C.; Li, W.; Gao, M.; Dong, Y. Lipid Polymer Hybrid Nanomaterials for mRNA Delivery. Cell Mol. Bioeng. 2018, 11, 397–406. [Google Scholar] [CrossRef]
- Yadava, S.K.; Reddy, B.P.K.; Prausnitz, M.R.; Cicerone, M.T. Hybrid Lipid Nanocapsules: A Robust Platform for mRNA Delivery. ACS Appl. Mater. Interfaces 2024, 16, 15981–15992. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Duskunovic, N.; Im, S.H.; Lee, J.; Chung, H.J. Effective mRNA Delivery by Condensation with Cationic Nanogels Incorporated into Liposomes. Mol. Pharm. 2023, 20, 3088–3099. [Google Scholar] [CrossRef]
- Vysochinskaya, V.; Shishlyannikov, S.; Zabrodskaya, Y.; Shmendel, E.; Klotchenko, S.; Dobrovolskaya, O.; Gavrilova, N.; Makarova, D.; Plotnikova, M.; Elpaeva, E.; et al. Influence of Lipid Composition of Cationic Liposomes 2X3-DOPE on mRNA Delivery into Eukaryotic Cells. Pharmaceutics 2022, 15, 8. [Google Scholar] [CrossRef]
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G.; et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Sagawa, A.; Inoue, N.; Torii, S.; Tomita, K.; Hattori, Y. Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method. Pharmaceutics 2023, 15, 1141. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, P.; Li, Z.; Zhang, R.; Wei, M.; Yang, Y.; Yuan, L.; Han, Y.; Yang, G. Improved extracellular vesicle-based mRNA delivery for familial hypercholesterolemia treatment. Theranostics 2023, 13, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; De Rosa, F.G.; Camussi, G. Oral Delivery of mRNA Vaccine by Plant-Derived Extracellular Vesicle Carriers. Cells 2023, 12, 1826. [Google Scholar] [CrossRef]
- Jiang, L.; Vader, P.; Schiffelers, R.M. Extracellular vesicles for nucleic acid delivery: Progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017, 24, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L.; et al. Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. 2022, 34, e2109984. [Google Scholar] [CrossRef]
- Verma, A.; Awasthi, A. Innovative Strategies to Enhance mRNA Vaccine Delivery and Effectiveness: Mechanisms and Future Outlook. Curr. Pharm. Des. 2024, 30, 1049–1059. [Google Scholar] [CrossRef]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in mRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Pei, Y.; Li, Z.; Luo, D. Progress and challenges of mRNA vaccines. Interdiscip. Med. 2022, 1, 8. [Google Scholar] [CrossRef]
- Geddie, M.L.; Kirpotin, D.B.; Kohli, N.; Kornaga, T.; Boll, B.; Razlog, M.; Drummond, D.C.; Lugovskoy, A.A. Development of disulfide-stabilized Fabs for targeting of antibody-directed nanotherapeutics. MAbs 2022, 14, 2083466. [Google Scholar] [CrossRef]
- Huang, J.; Yuen, D.; Mintern, J.D.; Johnston, A.P.R. Opportunities for innovation: Building on the success of lipid nanoparticle vaccines. Curr. Opin. Colloid. Interface Sci. 2021, 55, 101468. [Google Scholar] [CrossRef]
- Subramanya, S.; Armant, M.; Salkowitz, J.R.; Nyakeriga, A.M.; Haridas, V.; Hasan, M.; Bansal, A.; Goepfert, P.A.; Wynn, K.K.; Ladell, K.; et al. Enhanced induction of HIV-specific cytotoxic T lymphocytes by dendritic cell-targeted delivery of SOCS-1 siRNA. Mol. Ther. 2010, 18, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Fu, X.; Qi, Y.; Zhao, Y.; Zhang, S. Development and applications of mRNA treatment based on lipid nanoparticles. Biotechnol. Adv. 2023, 65, 108130. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.Q.; Ho, W.; Bai, X.; Jaijyan, D.K.; Li, F.; Kumar, R.; Kolloli, A.; Subbian, S.; Zhu, H.; et al. Lipid-Polymer Hybrid “Particle-in-Particle” Nanostructure Gene Delivery Platform Explored for Lyophilizable DNA and mRNA COVID-19 Vaccines. Adv. Funct. Mater. 2022, 32, 2204462. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Ahsan, W.; Aggarwal, G.; Kohli, K. Nanocarriers-Assisted Needle-Free Vaccine Delivery Through Oral and Intranasal Transmucosal Routes: A Novel Therapeutic Conduit. Front. Pharmacol. 2021, 12, 757761. [Google Scholar] [CrossRef]
- Abramson, A.; Kirtane, A.R.; Shi, Y.; Zhong, G.; Collins, J.E.; Tamang, S.; Ishida, K.; Hayward, A.; Wainer, J.; Rajesh, N.U.; et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 2022, 5, 975–987. [Google Scholar] [CrossRef]
- Altay Benetti, A.; Tan, E.Y.Z.; Chang, Z.W.; Bae, K.H.; Thwin, M.T.; Muthuramalingam, R.P.K.; Liao, K.-C.; Wan, Y.; Ng, L.F.P.; Renia, L.; et al. Design and Characterization of a New Formulation for the Delivery of COVID-19-mRNA Vaccine to the Nasal Mucosa. Vaccines 2024, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Puigmal, N.; Ramos, V.; Artzi, N.; Borrós, S. Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics 2023, 15, 1262. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, F.; Wels, M.; Nguyen, P.; Roels, D.; Paulus, Y.M.; Braeckmans, K.; De Smedt, S. Photoporation of the ocular surface for enhanced mRNA delivery. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3595. [Google Scholar]
- Zhao, R.; Guo, J.; Wu, M. It is time to thoroughly evaluate the risks of mRNA drug and vaccine toxicity. MedComm–Biomater. Appl. 2024, 3, 78. [Google Scholar] [CrossRef]
- Bitounis, D.; Jacquinet, E.; Rogers, M.A.; Amiji, M.M. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat. Rev. Drug Discov. 2024, 23, 281–300. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Jiaxuan, L.; Yuning, Z.; Yong-Guang, Y.; Tianmeng, S. Advancing mRNA Therapeutics: The Role and Future of Nanoparticle Delivery Systems. Mol. Pharm. 2024, 21, 3743–3763. [Google Scholar] [CrossRef]
- Boettler, T.; Csernalabics, B.; Salié, H.; Luxenburger, H.; Wischer, L.; Salimi Alizei, E.; Zoldan, K.; Krimmel, L.; Bronsert, P.; Schwabenland, M.; et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 2022, 77, 653–659. [Google Scholar] [CrossRef]
- Bril, F.; Al Diffalha, S.; Dean, M.; Fettig, D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol 2021, 75, 222–224. [Google Scholar] [CrossRef]
- Chavda, V.P.; Gogoi, N.R.; Shah, Y.; Shah, D.; Mazumder, B. Chapter 21—New approaches to vaccines for autoimmunity. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Chavda, V.P., Vora, L.K., Apostolopoulos, V., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 391–414. [Google Scholar]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022, 25, 205–214. [Google Scholar] [CrossRef]
- Mingyuan, L.; Lin, J.; Yanbo, X.; Wenlin, M.; Zhihong, Y.; Fufeng, L.; Jie, D.; Ali, Z.; Xue, S.; Wen, S.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. npj Vaccines 2023, 8, 1–13. [Google Scholar] [CrossRef]
- Alexander, L.; Joris, L.; Emily De, L.; Zifu, Z.; Mark, G.; Yong, C.; Thomas De, B.; Bruno, G.D.G. Successful batch and continuous lyophilization of mRNA LNP formulations depend on cryoprotectants and ionizable lipids. Biomater. Sci. 2023, 11, 4327–4334. [Google Scholar] [CrossRef]
- Liangxia, A.; Yafei, L.; Li, Z.; Wenrong, Y.; Hao, Z.; Zhaoyu, H.; Jinyu, H.; Weijie, W.; Junmiao, W.; Pan, X.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2022, 9, 4327–4334. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Z.; Wang, L.; Wu, L.; Zhang, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. Mbio 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Kis, Z. Stability Modelling of mRNA Vaccine Quality Based on Temperature Monitoring throughout the Distribution Chain. Pharmaceutics 2022, 14, 430. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The nano delivery systems and applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef]
- Estapé Senti, M.; García Del Valle, L.; Schiffelers, R.M. mRNA delivery systems for cancer immunotherapy: Lipid nanoparticles and beyond. Adv. Drug Deliv. Rev. 2024, 206, 115190. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Adlsadabad, S.; Hanrahan, J.W.; Kakkar, A. mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1739. [Google Scholar] [CrossRef] [PubMed]
- Itziar, G.-A.; Julen, R.-C.; Marina, B.-M.; Alicia, R.-G.; Ana del, P.-R.; María Ángeles, S. mRNA delivery technologies: Toward clinical translation. Int. Rev. Cell Mol. Biol. 2022, 372, 207–293. [Google Scholar]
- Héloïse, R.; Fabienne, D.; Véronique, P.; Robert, L.; Daniel, G.A. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert. Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; An, T.; Hong, K.-J. Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies. Biomolecules 2025, 15, 359. https://doi.org/10.3390/biom15030359
Fatima M, An T, Hong K-J. Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies. Biomolecules. 2025; 15(3):359. https://doi.org/10.3390/biom15030359
Chicago/Turabian StyleFatima, Munazza, Timothy An, and Kee-Jong Hong. 2025. "Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies" Biomolecules 15, no. 3: 359. https://doi.org/10.3390/biom15030359
APA StyleFatima, M., An, T., & Hong, K.-J. (2025). Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies. Biomolecules, 15(3), 359. https://doi.org/10.3390/biom15030359






