Application of Thyroid Hormones in Women’s Hair for the Non-Invasive Prediction of Graves’ Disease
Abstract
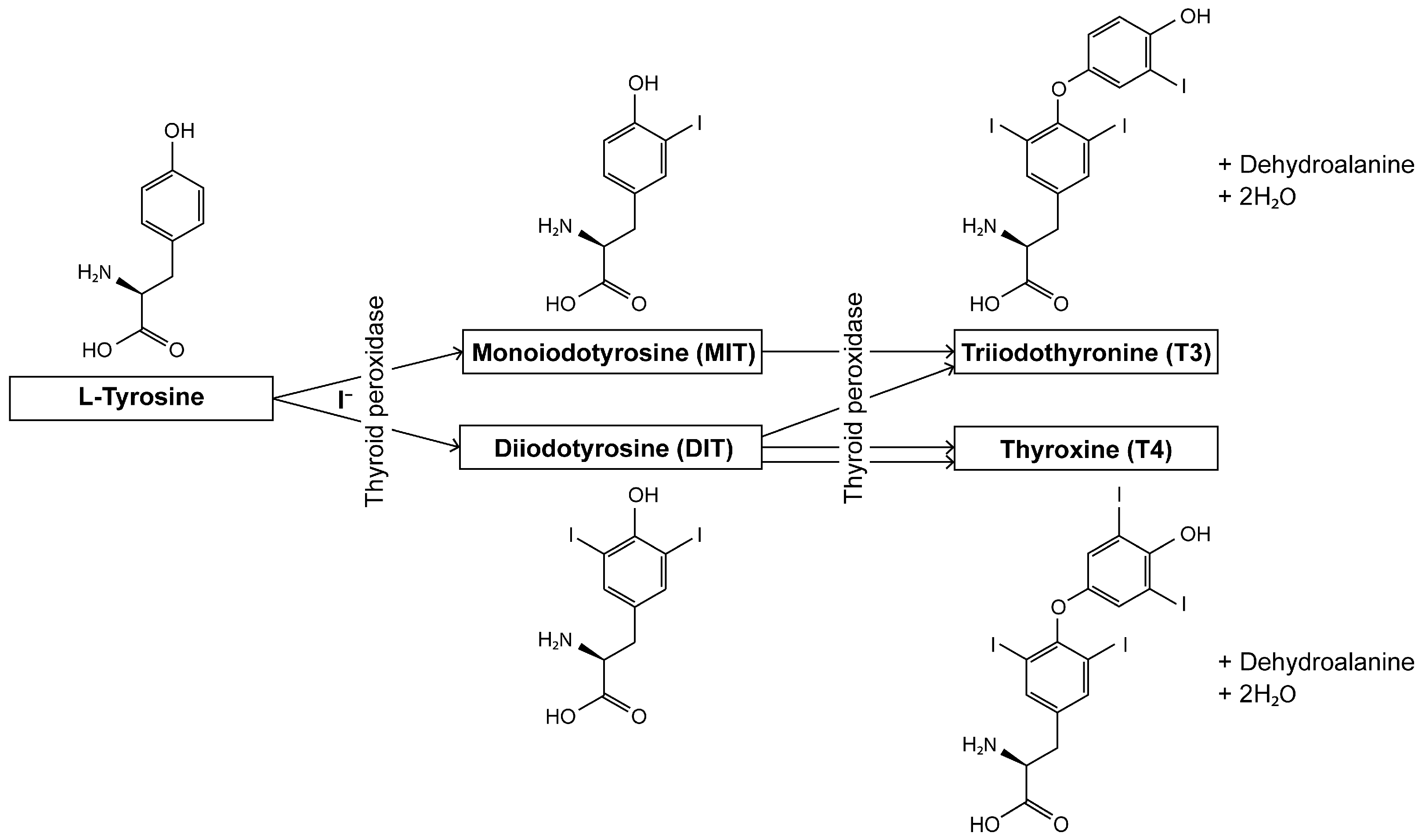
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Reagents and Standards
2.3. Hair Sample Preparation
2.4. Blood Tests
2.5. LC-MS Analysis
2.6. Data Analysis
3. Results
3.1. Establishment of Highly Sensitive System for Analyzing Thyroid Hormones in Hair
3.2. Evaluation of Accuracy of GD Determination Using Thyroid Hormones in Hair
3.3. Verification of Correlation Between Blood and Hair Tests for GD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CE | Collision energy |
| CI | Confidence interval |
| DIT | Diiodotyrosine |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GD | Graves’ disease |
| HCT | Healthy control |
| HD | Hashimoto’s disease |
| HTg | Human thyroglobulin |
| LOD | Limit of detection |
| MIT | Monoiodotyrosine |
| MRM | Multiple reaction monitoring |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| ROC | Receiver operating characteristic |
| RPA | Relative peak areas |
| RSD | Relative standard deviation |
| RT | Retention time |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TgAb | Thyroglobulin antibodies |
| TPO TPOAb | Thyroid peroxidase Thyroid peroxidase antibody |
| TPP | Thyrotoxic periodic paralysis |
| TRAb | TSH receptor antibody |
| TSAb | Thyroid-stimulating antibody |
| TSH | Thyroid-stimulating hormone |
| TSI | Thyroid-stimulating immunoglobulin |
References
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Teixeira, P.F.D.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Zawalna, N.; Gut, P.; Ruchała, M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol. Rep. 2022, 74, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Rydzewska, M.; Jaromin, M.; Pasierowska, I.E.; Stożek, K.; Bossowski, A. Role of the T and B lymphocytes in pathogenesis of autoimmune thyroid diseases. Thyroid. Res. 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef]
- Suzuki, N.; Inoue, K.; Yoshimura, R.; Kinoshita, A.; Suzuki, A.; Fukushita, M.; Matsumoto, M.; Yoshihara, A.; Watanabe, N.; Noh, J.Y.; et al. The mediation role of thyrotropin receptor antibody in the relationship between age and severity of hyperthyroidism in Graves’ disease. Thyroid 2022, 32, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Nappi, R.E.; Regalbuto, C.; De Silvestri, A.; Incardona, A.; Amariti, R.; Bassanese, F.; Clemente, A.M.; Vinci, F.; Albertini, R.; et al. Gender differences at the onset of autoimmune thyroid diseases in children and adolescents. Front. Endocrinol. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, K.; Franklyn, J.A. Thyroid hormone in health and disease. J. Endocrinol. 2005, 187, 1–15. [Google Scholar] [CrossRef]
- Mintziori, G.; Veneti, S.; Poppe, K.; Goulis, D.G.; Armeni, E.; Erel, C.T.; Fistonić, I.; Hillard, T.; Hirschberg, A.L.; Meczekalski, B.; et al. EMAS position statement: Thyroid disease and menopause. Maturitas 2024, 185, 107991. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Kalaria, T.; Gherman-Ciolac, C.; Raghavan, R.; Buch, H.N.; Kar, N. Impact of hyperthyroidism and its treatment on the outcome of mental health, occupational functioning, and quality of life: A naturalistic, prospective study. Indian J. Psychiatry 2023, 65, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Suwalska, A.; Lacka, K.; Lojko, D.; Rybakowski, J.K. Quality of life, depressive symptoms and anxiety in hyperthyroid patients. Rocz. Akad. Med. Bialymst 2005, 50, 61–63. [Google Scholar] [PubMed]
- Kollerits, E.; Zsila, Á.; Matuszka, B. Quality of life, social support, and adherence in female patients with thyroid disorders. BMC Womens Health 2023, 23, 567. [Google Scholar] [CrossRef]
- Shimizu, T.; Koide, S.; Noh, J.Y.; Sugino, K.; Ito, K.; Nakazawa, H. Hyperthyroidism and the management of atrial fibrillation. Thyroid. 2002, 12, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Amin, P.; Lazarus, J.H. Hyperthyroidism and pregnancy. BMJ 2008, 336, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Kuo, S.W.; Pei, D.; Hung, Y.J.; Chyi-Fan, S.; Wu, L.I.; He, C.T.; Yang, T.C.; Lian, W.C.; Lee, C.H. Thyrotoxic periodic paralysis: An overview. Ann. Saudi Med. 2004, 24, 418–422. [Google Scholar] [CrossRef]
- Okinaka, S.; Shizume, K.; Iino, S.; Watanabe, A.; Irie, M.; Noguchi, A.; Kuma, S.; Kuma, K.; Ito, T. The association of periodic paralysis and hyperthyroidism in Japan. J. Clin. Endocrinol. Metab. 1957, 17, 1454–1459. [Google Scholar] [CrossRef]
- Grova, N.; Wang, X.; Hardy, E.M.; Palazzi, P.; Chata, C.; Appenzeller, B.M.R. Ultra performance liquid chromatography—tandem mass spectrometer method applied to the analysis of both thyroid and steroid hormones in human hair. J. Chromatogr. A 2020, 1612, 460648. [Google Scholar] [CrossRef]
- Gao, W.; Penz, M.; Wekenborg, M.; Walther, A.; Kirschbaum, C. Determination of thyroid hormones in human hair with online SPE LC-MS/MS: Analytical protocol and application in study of burnout. Psychoneuroendocrinology 2019, 106, 129–137. [Google Scholar] [CrossRef]
- Kronstrand, R.; Nyström, I.; Strandberg, J.; Druid, H. Screening for drugs of abuse in hair with ion spray LC–MS–MS. Forensic Sci. Int. 2004, 145, 183–190. [Google Scholar] [CrossRef]
- Nouioui, M.A.; Araoud, M.; Milliand, M.L.; Bessueille-Barbier, F.; Amira, D.; Ayouni-Derouiche, L.; Hedhili, A. Evaluation of the status and the relationship between essential and toxic elements in the hair of occupationally exposed workers. Environ. Monit. Assess. 2018, 190, 731. [Google Scholar] [CrossRef] [PubMed]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holčapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for good practice in MS-based lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef] [PubMed]
- Lipidomics Standards Initiative Consortium. Lipidomics needs more standardization. Nat. Metab. 2019, 1, 745–747. [Google Scholar] [CrossRef]
- Stout, P.R.; Dehn, D.; Ruth, J.A. Deposition and retention of radiolabeled serum constituents in hair after systemic administration. Drug Metab. Dispos. 1998, 26, 900–906. [Google Scholar] [PubMed]
- Alvin Mathew, A.; Papaly, R.; Maliakal, A.; Chandra, L.; Antony, M.A. Elevated Graves’ Disease-Specific Thyroid-Stimulating Immunoglobulin and Thyroid Stimulating Hormone Receptor Antibody in a Patient with Subacute Thyroiditis. Cureus 2021, 13, e19448. [Google Scholar] [PubMed]
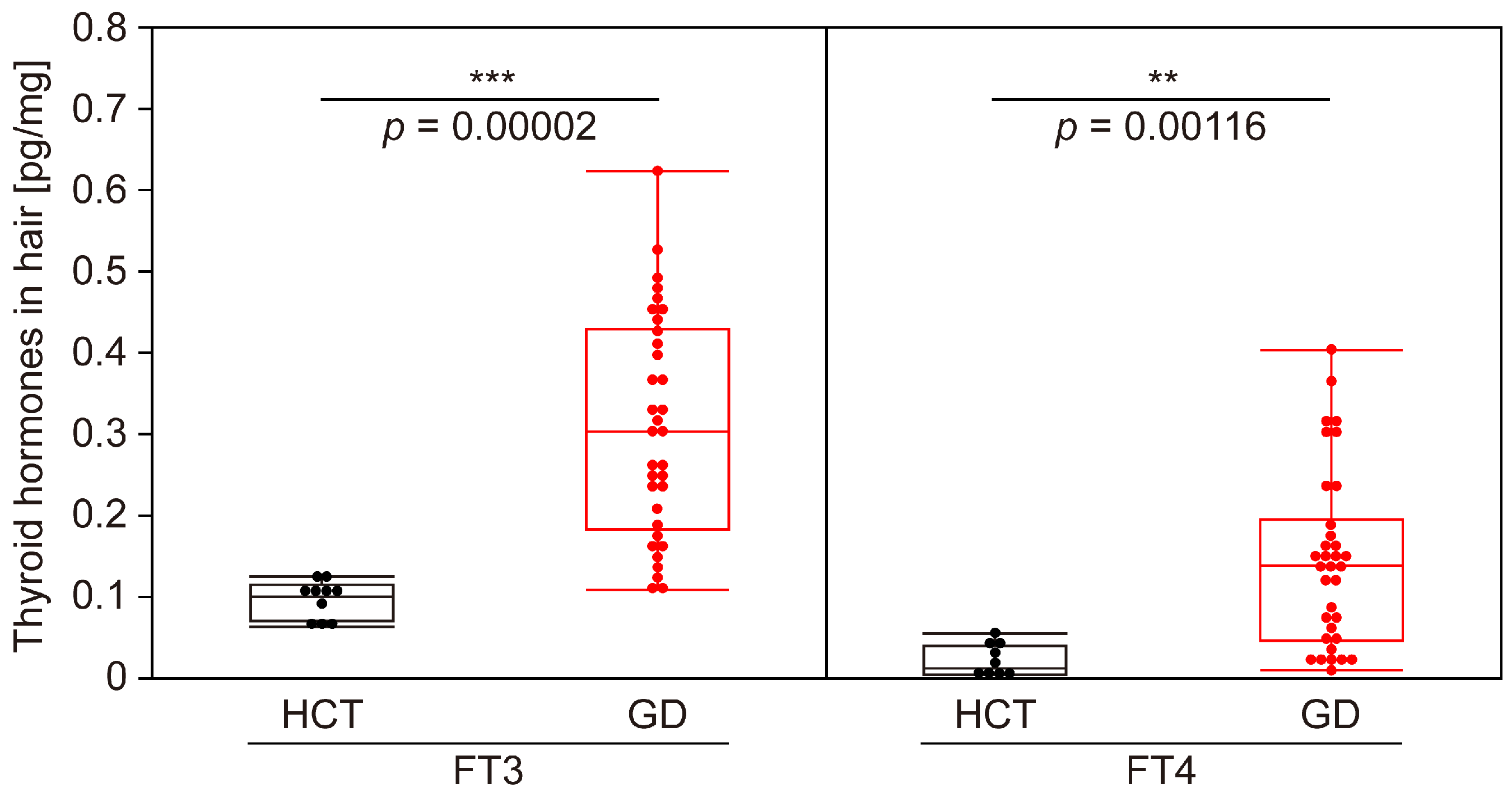



| HCT | GD | |
|---|---|---|
| Number | 10 | 34 |
| Age (mean ± SD, median, min–max) [years] | 36.8 ± 7.03, 39, 26–50 | 41.7 ± 10.24, 43, 20–59 |
| FT3 (median, min–max) [pg/mL] | 2.8, 2.2–3.3 | 13.8, 3.6–32.5 ≤ 3 |
| FT3 abnormal rate | 0 (normal:abnormal = 10:0) | 0.941 (normal:abnormal = 2:32) |
| FT4 (median, min–max) [ng/dL] | 1.25, 0.89–1.60 | 4.24, 1.69–7.77 ≤ 3 |
| FT4 abnormal rate | 0 (normal:abnormal = 10:0) | 1 (normal:abnormal = 0:34) |
| TSH (median, min–max) [μIU/mL] | 1.58, 0.72–3.35 | ≤30.01, ≤30.01–0.02 |
| TgAb (mean ± SD, median, min–max) [IU/mL] | 12.8 ± 1.40, 12.5, 10.7–15.2 | |
| HTg (mean ± SD, median, min–max) [ng/mL] | 9.89 ± 4.52, 8.88, 3.46–17.90 |
| Compound Name | Molecular Formula | Monoisotopic Mass | Precursor ion (Adduct) | Transition of Confirmation | CE (V) | Transition of Quantification | CE (V) |
|---|---|---|---|---|---|---|---|
| Triiodothyronine (T3) | C15H12I3NO4 | 650.7901(656.8102) *1 | [M + H]+ | 651.80 > 651.80 | −9.0 | 651.80 > 605.80 | −23.0 |
| (657.90 > 657.90) *1 | −9.0 | (657.90 > 611.90) *1 | −23.0 | ||||
| Thyroxine (T4) | C15H11I4NO4 | 776.6867(782.7068) *2 | [M + H]+ | 777.70 > 777.70 | −14.0 | 777.70 > 731.60 | −25.0 |
| (783.80 > 783.80) *2 | −14.0 | (783.80 > 737.80) *2 | −25.0 |
| RT RSD (%, n = 5) | RPA RSD (%, n = 5) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Day 1 | Day 2 | Day 3 | Inter Day | Day 1 | Day 2 | Day 3 | Inter Day | LOD (pM) | Linearity Range (pm) | Linearity (R2) | Recovery (%) |
| T3 | 0.07 | 0.04 | 0.05 | 0.05 | 1.53 | 6.49 | 5.78 | 3.09 | 1.0 | 10–1000 (Low) *1 | 0.9962 | 91.6 |
| 1000–10,000 (High) *2 | 0.9997 | |||||||||||
| T4 | 0.03 | 0.05 | 0.04 | 0.03 | 3.02 | 3.50 | 2.81 | 3.22 | 10.0 | 25–1000 (Low) *1 | 0.9971 | 92.3 |
| 1000–10,000 (High) *2 | 0.9982 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igarashi, K.; Takita, C.; Matsumoto, M.; Kitagawa, W.; Ota, A.; Miyazaki, N.; Ito, K.; Ikeda, K. Application of Thyroid Hormones in Women’s Hair for the Non-Invasive Prediction of Graves’ Disease. Biomolecules 2025, 15, 353. https://doi.org/10.3390/biom15030353
Igarashi K, Takita C, Matsumoto M, Kitagawa W, Ota A, Miyazaki N, Ito K, Ikeda K. Application of Thyroid Hormones in Women’s Hair for the Non-Invasive Prediction of Graves’ Disease. Biomolecules. 2025; 15(3):353. https://doi.org/10.3390/biom15030353
Chicago/Turabian StyleIgarashi, Kouhei, Chie Takita, Masako Matsumoto, Wataru Kitagawa, Atsuko Ota, Naoko Miyazaki, Koichi Ito, and Kazutaka Ikeda. 2025. "Application of Thyroid Hormones in Women’s Hair for the Non-Invasive Prediction of Graves’ Disease" Biomolecules 15, no. 3: 353. https://doi.org/10.3390/biom15030353
APA StyleIgarashi, K., Takita, C., Matsumoto, M., Kitagawa, W., Ota, A., Miyazaki, N., Ito, K., & Ikeda, K. (2025). Application of Thyroid Hormones in Women’s Hair for the Non-Invasive Prediction of Graves’ Disease. Biomolecules, 15(3), 353. https://doi.org/10.3390/biom15030353







