Sodium-Dependent Conformational Change in Flagellar Stator Protein MotS from Bacillus subtilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Mutagenesis
2.2. Protein Expression and Purification
2.3. Analytical Size-Exclusion Column Chromatography
2.4. Crystallization
2.5. Data Collection and Structural Determination
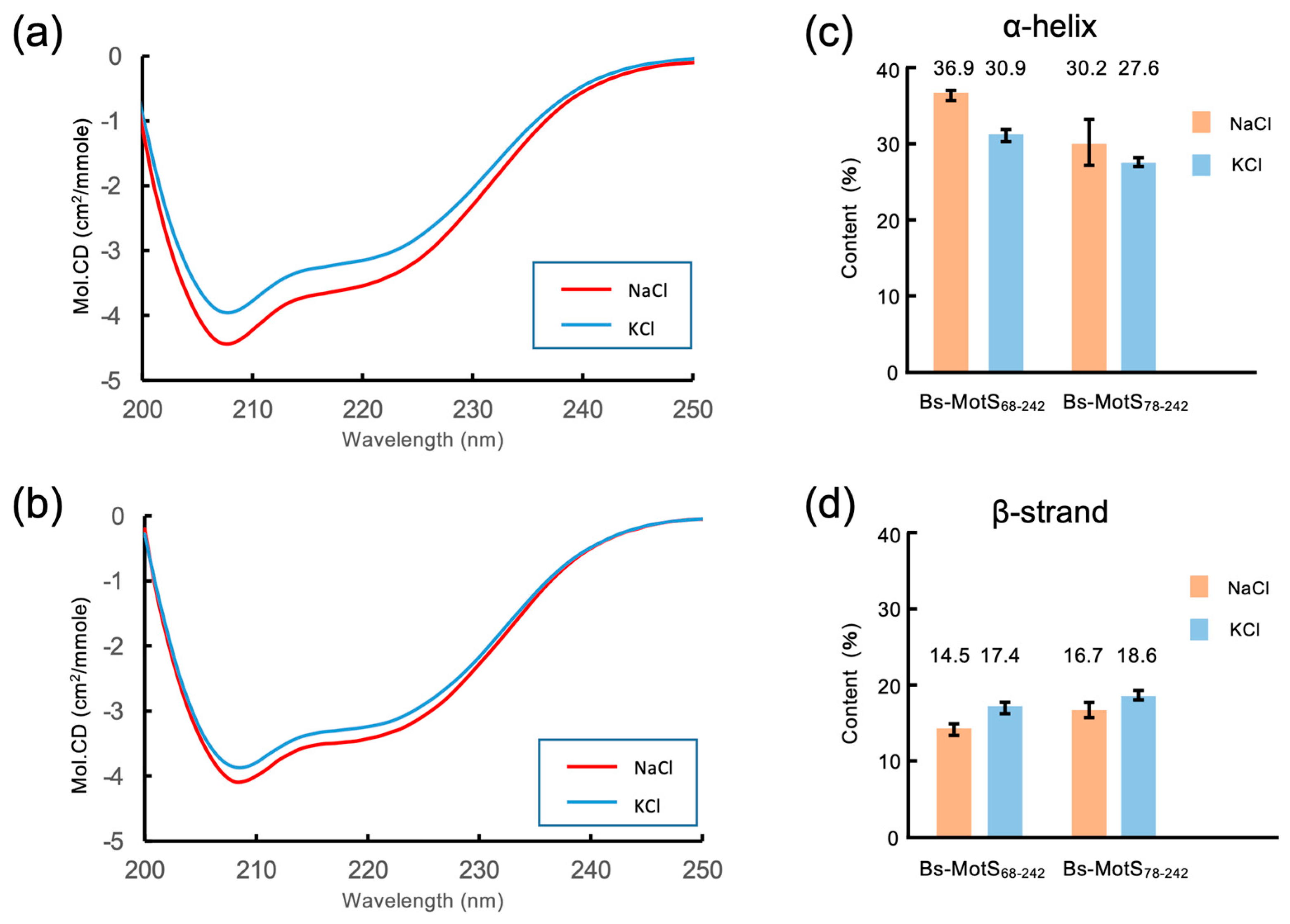
2.6. Far-UV CD Spectroscopy
3. Results
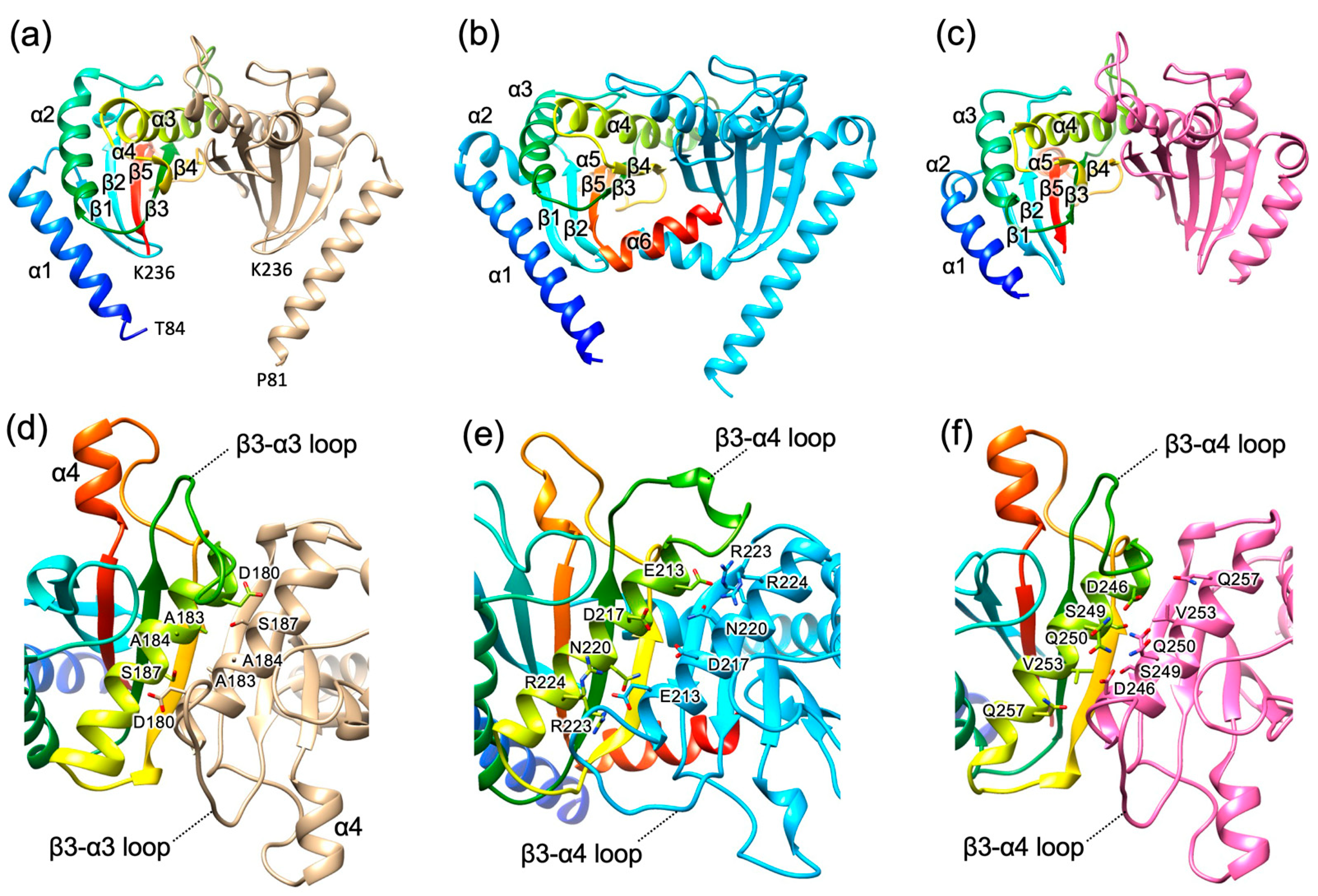
3.1. Structure of Bs-MotS68–242
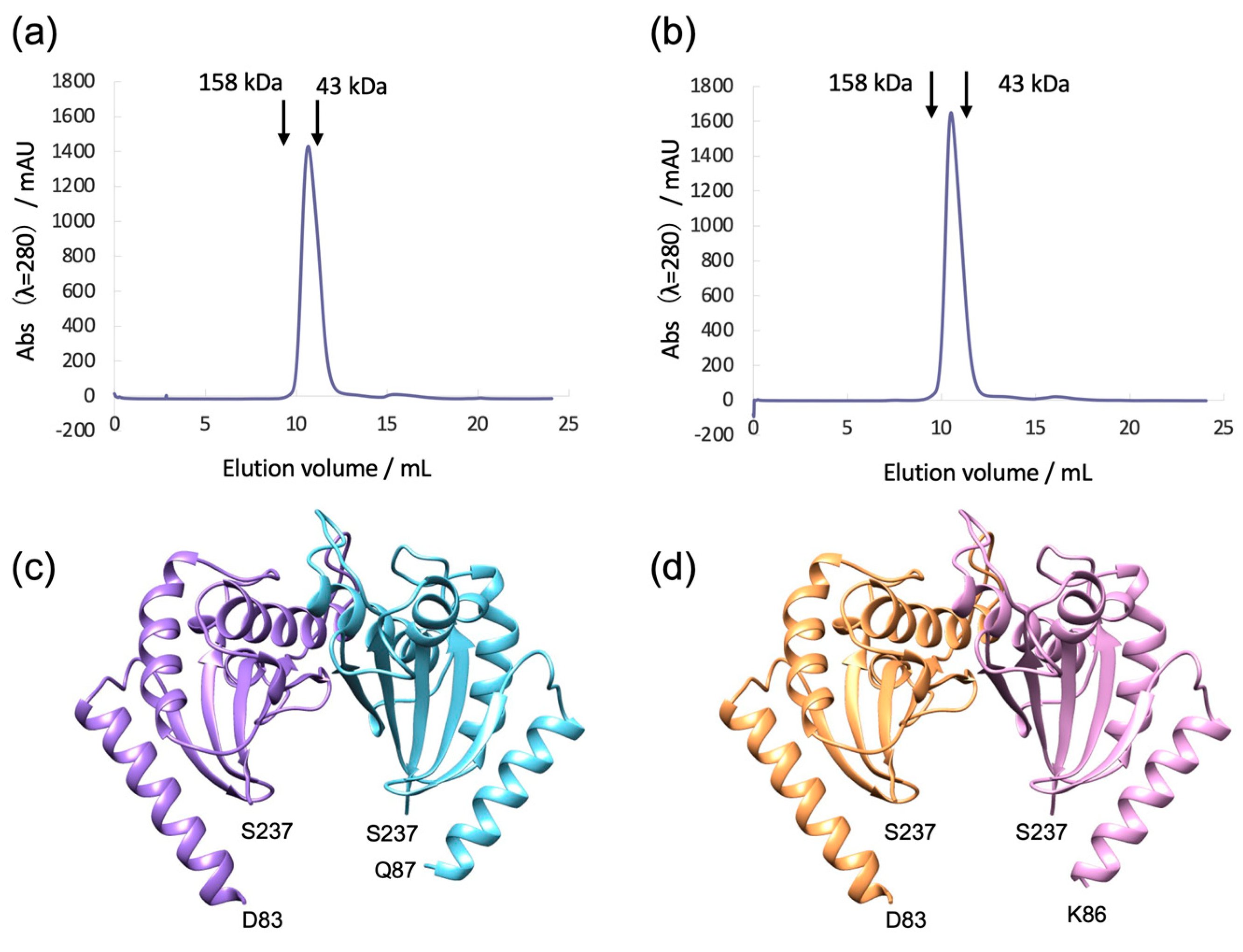
3.2. Bs-MotSc Forms a Stable Dimer Regardless of Sodium Concentration
3.3. Structure of Bs-MotS68–242 at High and Low Na+ Concentrations
3.4. Na+-Dependent Coil–Helix Transition in the N-Terminal Disordered Region
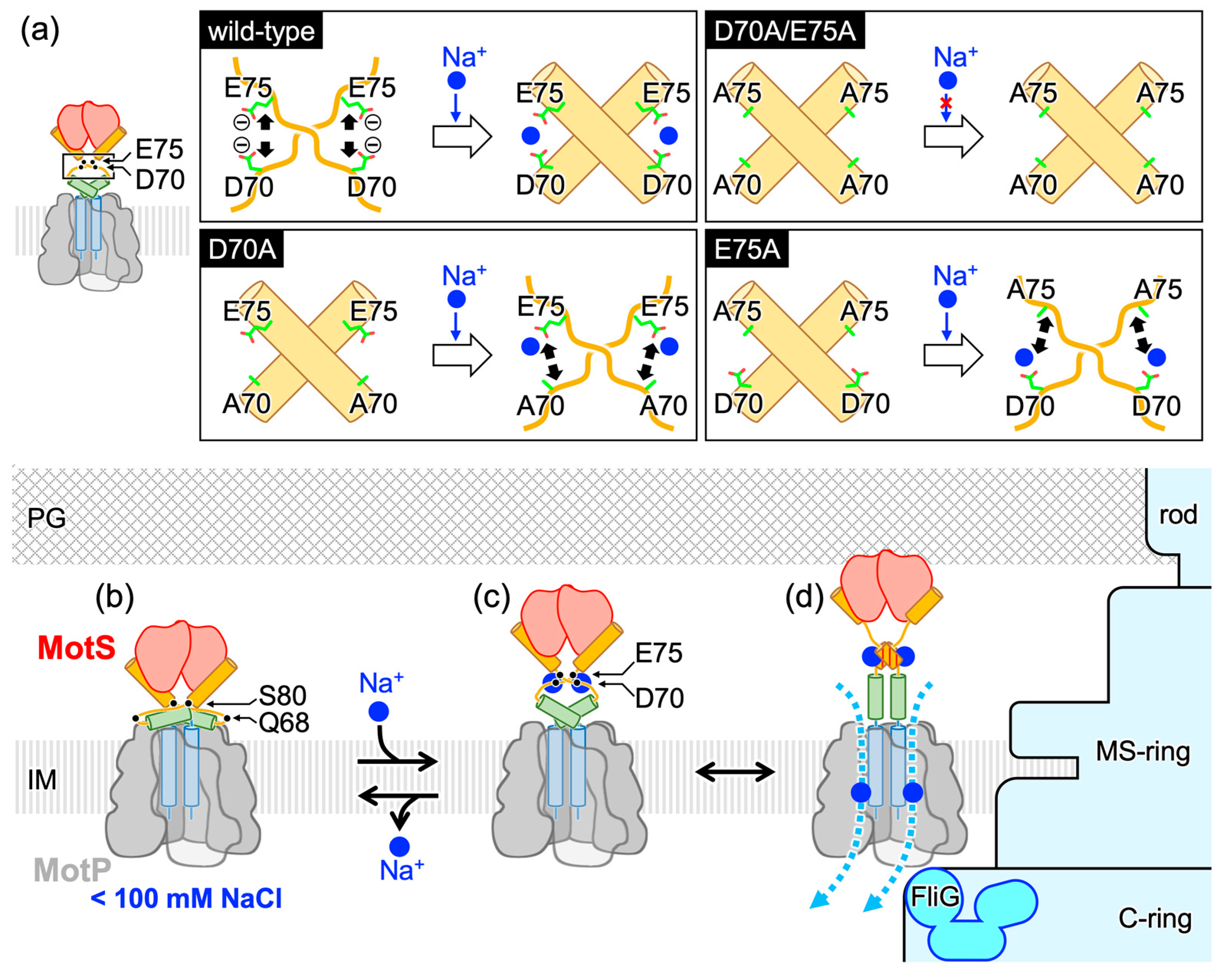
3.5. Possible Na+-Binding Sites in Bs-MotSc
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, H. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Imada, K. Bacterial flagellar axial structure and its construction. Biophys. Rev. 2018, 10, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Kojima, S. The Periplasmic Domain of the Ion-Conducting Stator of Bacterial FlagellaRegulates Force Generation. Front. Microbiol. 2022, 13, 869187. [Google Scholar] [CrossRef]
- Nandel, V.; Scadden, J.; Baker, M.A.B. Ion-Powered Rotary Motors: Where Did They Come from and Where They Are Going? Int. J. Mol. Sci. 2023, 24, 10601. [Google Scholar] [CrossRef]
- Blair, D.F. Flagellar movement driven by proton translocation. FEBS Lett. 2003, 545, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; Kojima, S.; Kato, H.; Nishioka, N.; Kawagishi, I.; Homma, M. Putative channel components for the fast-rotating sodium-driven flagellar motor of a marine bacterium. J. Bacteriol. 1997, 179, 5104–5110. [Google Scholar] [CrossRef]
- Ito, M.; Hicks, D.; Henkin, T.; Guffanti, A.; Powers, B.; Zvi, L.; Uematsu, K.; Krulwich, T. MotPS is the stator-force generator for motility of alkaliphilic Bacillus, and its homologue is a second functional Mot in Bacillus subtilis. Mol. Microbiol. 2004, 53, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Deme, J.C.; Johnson, S.; Vickery, O.; Aron, A.; Monkhouse, H.; Griffiths, T.; James, R.H.; Berks, B.C.; Coulton, J.W.; Stansfeld, P.J.; et al. Structures of the stator complex that drives rotation of the bacterial flagellum. Nat. Microbiol. 2020, 5, 1553–1564. [Google Scholar] [CrossRef]
- Santiveri, M.; Roa-Eguiara, A.; Kühne, C.; Wadhwa, N.; Hu, H.; Berg, H.C.; Erhardt, M.; Taylor, N.M.I. Structure and Function of Stator Units of the Bacterial Flagellar Motor. Cell 2020, 183, 244–257.e216. [Google Scholar] [CrossRef]
- Hu, H.; Popp, P.F.; Santiveri, M.; Roa-Eguiara, A.; Yan, Y.; Martin, F.J.O.; Liu, Z.; Wadhwa, N.; Wang, Y.; Erhardt, M.; et al. Ion selectivity and rotor coupling of the Vibrio flagellar sodium-driven stator unit. Nat. Commun. 2023, 14, 4411. [Google Scholar] [CrossRef]
- Nishikino, T.; Takekawa, N.; Kishikawa, J.-i.; Hirose, M.; Kojima, S.; Homma, M.; Kato, T.; Imada, K. Structural insight into sodium ion pathway in the bacterial flagellar stator from marine Vibrio. Proc. Natl. Acad. Sci. USA 2025, 122, e2415713122. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, K.; Carroll, B.L.; Zhao, X.; Charon, N.W.; Norris, S.J.; Motaleb, M.A.; Li, C.; Liu, J. Molecular mechanism for rotational switching of the bacterial flagellar motor. Nat. Struct. Mol. Biol. 2020, 27, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fazzio, R.T.; Blair, D.F. Membrane topology of the MotA protein of Escherichia coli. J. Mol. Biol. 1995, 251, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, N.; Kojima, S.; Homma, M. Contribution of many charged residues at the stator-rotor interface of the Na+-driven flagellar motor to torque generation in Vibrio Alginolyticus. J. Bacteriol. 2014, 196, 1377–1385. [Google Scholar] [CrossRef]
- Zhou, J.; Lloyd, S.A.; Blair, D.F. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 1998, 95, 6436–6441. [Google Scholar] [CrossRef] [PubMed]
- Yakushi, T.; Yang, J.; Fukuoka, H.; Homma, M.; Blair, D.F. Roles of charged residues of rotor and stator in flagellar rotation: Comparative study using H+-driven and Na+-driven motors in Escherichia coli. J. Bacteriol. 2006, 188, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.A.; Blair, D.F. Charged residues of the rotor protein FliG essential for torque generation in the flagellar motor of Escherichia coli. J. Mol. Biol. 1997, 266, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sharp, L.L.; Tang, H.L.; Lloyd, S.A.; Billings, S.; Braun, T.F.; Blair, D.F. Function of protonatable residues in the flagellar motor of Escherichia coli: A critical role for Asp 32 of MotB. J. Bacteriol. 1998, 180, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Sudo, Y.; Kitade, Y.; Furutani, Y.; Kojima, M.; Kojima, S.; Homma, M.; Kandori, H. Interaction between Na+ ion and carboxylates of the PomA-PomB stator unit studied by ATR-FTIR spectroscopy. Biochemistry 2009, 48, 11699–11705. [Google Scholar] [CrossRef]
- Kojima, S.; Imada, K.; Sakuma, M.; Sudo, Y.; Kojima, C.; Minamino, T.; Homma, M.; Namba, K. Stator assembly and activation mechanism of the flagellar motor by the periplasmic region of MotB. Mol. Microbiol. 2009, 73, 710–718. [Google Scholar] [CrossRef]
- Kojima, S.; Takao, M.; Almira, G.; Kawahara, I.; Sakuma, M.; Homma, M.; Kojima, C.; Imada, K. The helix rearrangement in the periplasmic domain of the flagellar stator B subunit activates peptidoglycan binding and ion influx. Structure 2018, 26, 590–598.e5. [Google Scholar] [CrossRef]
- Zhu, S.; Nishikino, T.; Takekawa, N.; Terashima, H.; Kojima, S.; Imada, K.; Homma, M.; Liu, J. In situ structure of the Vibrio polar flagellum reveals a distinct outer membrane complex and its specific interaction with the stator. J. Bacteriol. 2020, 202, e00592-19. [Google Scholar] [CrossRef]
- De Mot, R.; Vanderleyden, J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 1994, 12, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.F.; Berg, H.C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell 1990, 60, 439–449. [Google Scholar] [CrossRef]
- Stolz, B.; Berg, H.C. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J. Bacteriol. 1991, 173, 7033–7037. [Google Scholar] [CrossRef]
- Hosking, E.; Vogt, C.; Bakker, E.; Manson, M. The Escherichia coli MotAB proton channel unplugged. J. Mol. Biol. 2006, 364, 921–937. [Google Scholar] [CrossRef]
- Li, N.; Kojima, S.; Homma, M. Characterization of the Periplasmic Region of PomB, a Na+-Driven Flagellar Stator Protein in Vibrio Alginolyticus. J. Bacteriol. 2011, 193, 3773–3784. [Google Scholar] [CrossRef]
- Morimoto, Y.V.; Che, Y.S.; Minamino, T.; Namba, K. Proton-conductivity assay of plugged and unplugged MotA/B proton channel by cytoplasmic pHluorin expressed in Salmonella. FEBS Lett. 2010, 584, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Terashima, H.; Koiwa, H.; Kojima, S. Putative Spanner Function of the. J. Bacteriol. 2021, 203, e0015921. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Wada, T.; Kojima, S.; Ishijima, A.; Homma, M. Sodium-dependent dynamic assembly of membrane complexes in sodium- driven flagellar motors. Mol. Microbiol. 2009, 71, 825–835. [Google Scholar] [CrossRef]
- Leake, M.C.; Chandler, J.H.; Wadhams, G.H.; Bai, F.; Berry, R.M.; Armitage, J.P. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature 2006, 443, 355–358. [Google Scholar] [CrossRef]
- Paulick, A.; Delalez, N.J.; Brenzinger, S.; Steel, B.C.; Berry, R.M.; Armitage, J.P.; Thormann, K.M. Dual stator dynamics in the Shewanella onei- densis MR-1 flagellar motor. Mol. Microbiol. 2015, 96, 993–1001. [Google Scholar] [CrossRef]
- Zhu, S.; Takao, M.; Li, N.; Sakuma, M.; Nishino, Y.; Homma, M.; Kojima, S.; Imada, K. Conformational change in the periplamic region of the flagellar stator coupled with the assembly around the rotor. Proc. Natl. Acad. Sci. USA 2014, 111, 13523–13528. [Google Scholar] [CrossRef]
- Ito, M.; Terahara, N.; Fujinami, S.; Krulwich, T.A. Properties of motility in Bacillus subtilis powered by the H+-coupled MotAB flagellar stator, Na+-coupled MotPS or hybrid stators MotAS or MotPB. J. Mol. Biol. 2005, 352, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N.; Noguchi, Y.; Nakamura, S.; Kami-ike, N.; Ito, M.; Namba, K.; Minamino, T. Load- and polysaccharide-dependent activation of the Na+-type MotPS stator in the Bacillus subtilis flagellar motor. Sci. Rep. 2017, 7, 46081. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N.; Kodera, N.; Uchihashi, T.; Ando, T.; Namba, K.; Minamino, T. Na+-induced structural transition of MotPS for stator assembly of the Bacillusflagellar motor. Sci. Adv. 2017, 3, eaao4119. [Google Scholar] [CrossRef]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 2011, 67 Pt 4, 271–281. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67 Pt 4, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Liebschner, D.; Afonine, P.; Baker, M.; Bunkoczi, G.; Chen, V.; Croll, T.; Hintze, B.; Hung, L.; Jain, S.; McCoy, A.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D-Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D-Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins 2012, 80, 374–381. [Google Scholar] [CrossRef]
- O'Neill, J.; Xie, M.; Hijnen, M.; Roujeinikova, A. Role of the MotB linker in the assembly and activation of the bacterial flagellar motor. Acta Crystallogr. D Biol. Crystallogr. 2011, 67 Pt 12, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G.; Jesseet, J.; Bloomt, F.R.; Hanahan, D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 1990, 87, 4645–4649. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takekawa, N.; Yamaguchi, A.; Nishiuchi, K.; Uehori, M.; Kinoshita, M.; Minamino, T.; Imada, K. Sodium-Dependent Conformational Change in Flagellar Stator Protein MotS from Bacillus subtilis. Biomolecules 2025, 15, 302. https://doi.org/10.3390/biom15020302
Takekawa N, Yamaguchi A, Nishiuchi K, Uehori M, Kinoshita M, Minamino T, Imada K. Sodium-Dependent Conformational Change in Flagellar Stator Protein MotS from Bacillus subtilis. Biomolecules. 2025; 15(2):302. https://doi.org/10.3390/biom15020302
Chicago/Turabian StyleTakekawa, Norihiro, Ayaka Yamaguchi, Koki Nishiuchi, Maria Uehori, Miki Kinoshita, Tohru Minamino, and Katsumi Imada. 2025. "Sodium-Dependent Conformational Change in Flagellar Stator Protein MotS from Bacillus subtilis" Biomolecules 15, no. 2: 302. https://doi.org/10.3390/biom15020302
APA StyleTakekawa, N., Yamaguchi, A., Nishiuchi, K., Uehori, M., Kinoshita, M., Minamino, T., & Imada, K. (2025). Sodium-Dependent Conformational Change in Flagellar Stator Protein MotS from Bacillus subtilis. Biomolecules, 15(2), 302. https://doi.org/10.3390/biom15020302






