Mitochondrial Uncoupler, 2,4-Dinitrophenol, Reduces Spinal Cord Paralysis and Retinal Ganglion Cell Loss in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Induction and Scoring of EAE
2.3. DNP Treatment
2.4. Measurement of OKR
2.5. RGC Quantification
2.6. Optic Nerve Histochemistry
2.7. Protein Extraction and Western Blot
2.8. Statistical Analysis
3. Results
3.1. Effect of Daily Oral DNP on EAE Scores
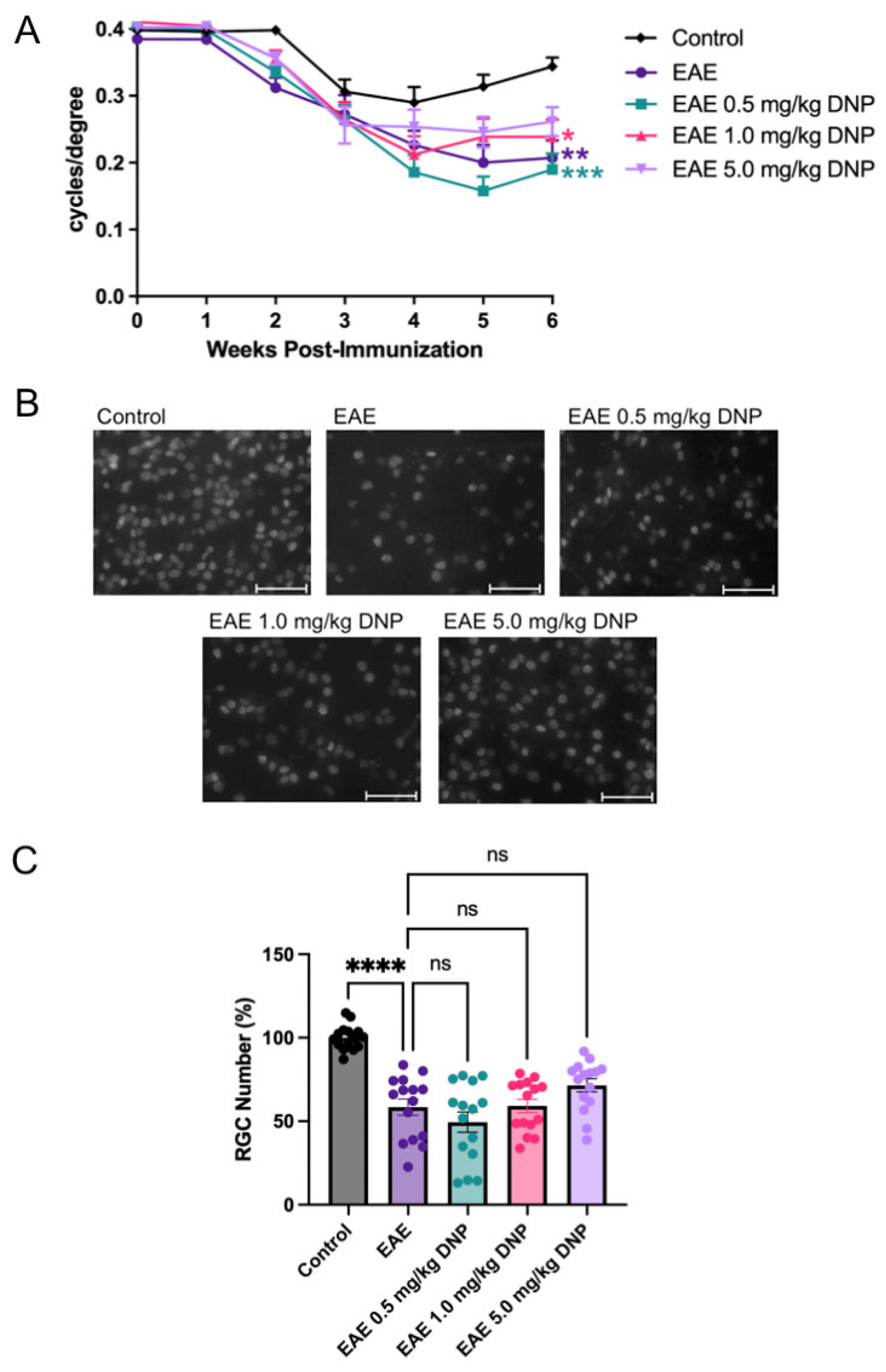
3.2. Effects of Daily Oral DNP on Visual Function
3.3. Effects of Daily Oral DNP on RGC Survival and Protein Expression
3.4. Effects of Daily Oral DNP on Optic Nerve Inflammation and Demyelination
3.5. Effect of Higher Doses of DNP on EAE Scores
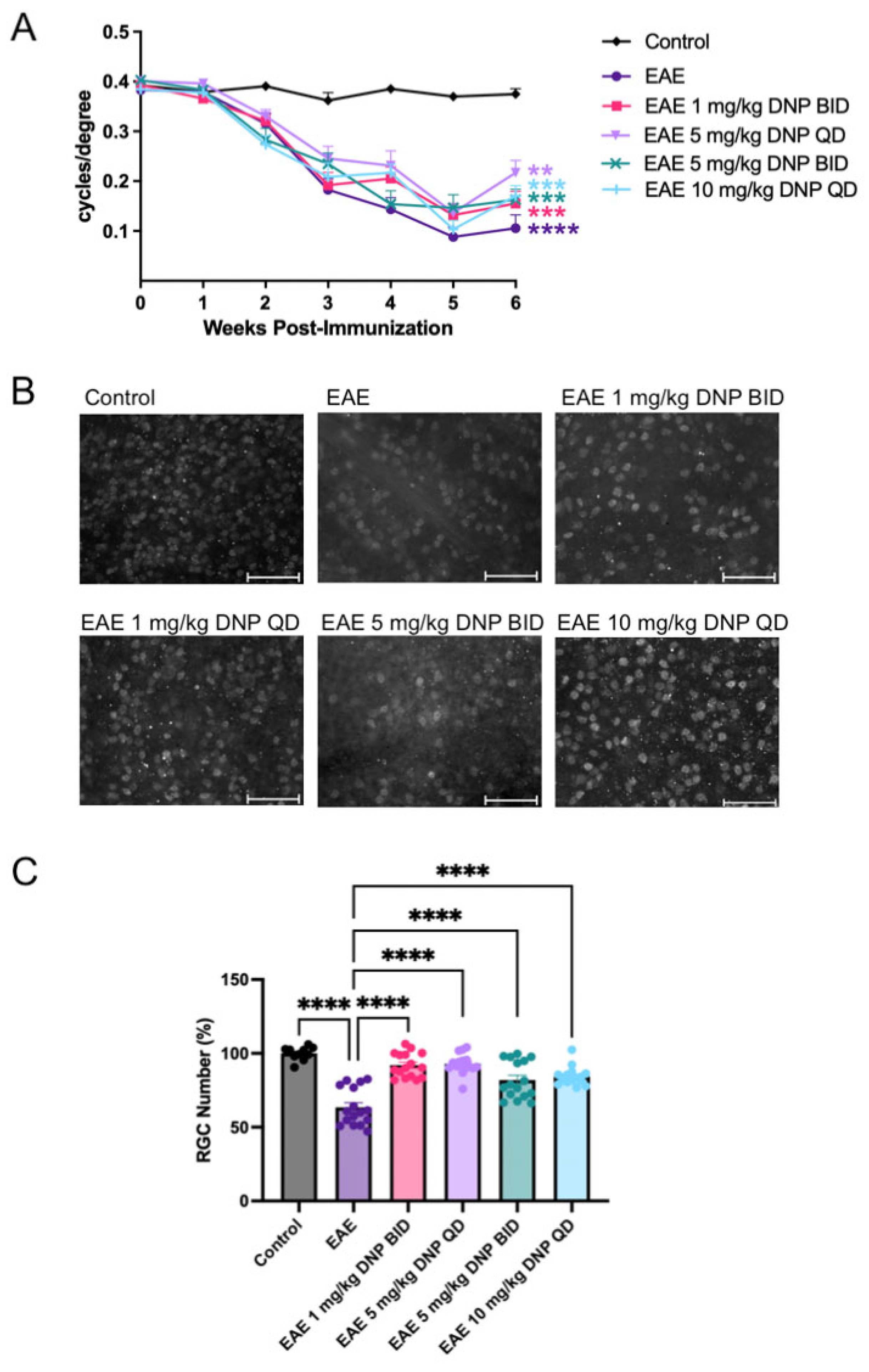
3.6. Effect of Higher Doses of DNP on Visual Function
3.7. Effect of Higher Doses of DNP on RGC Number
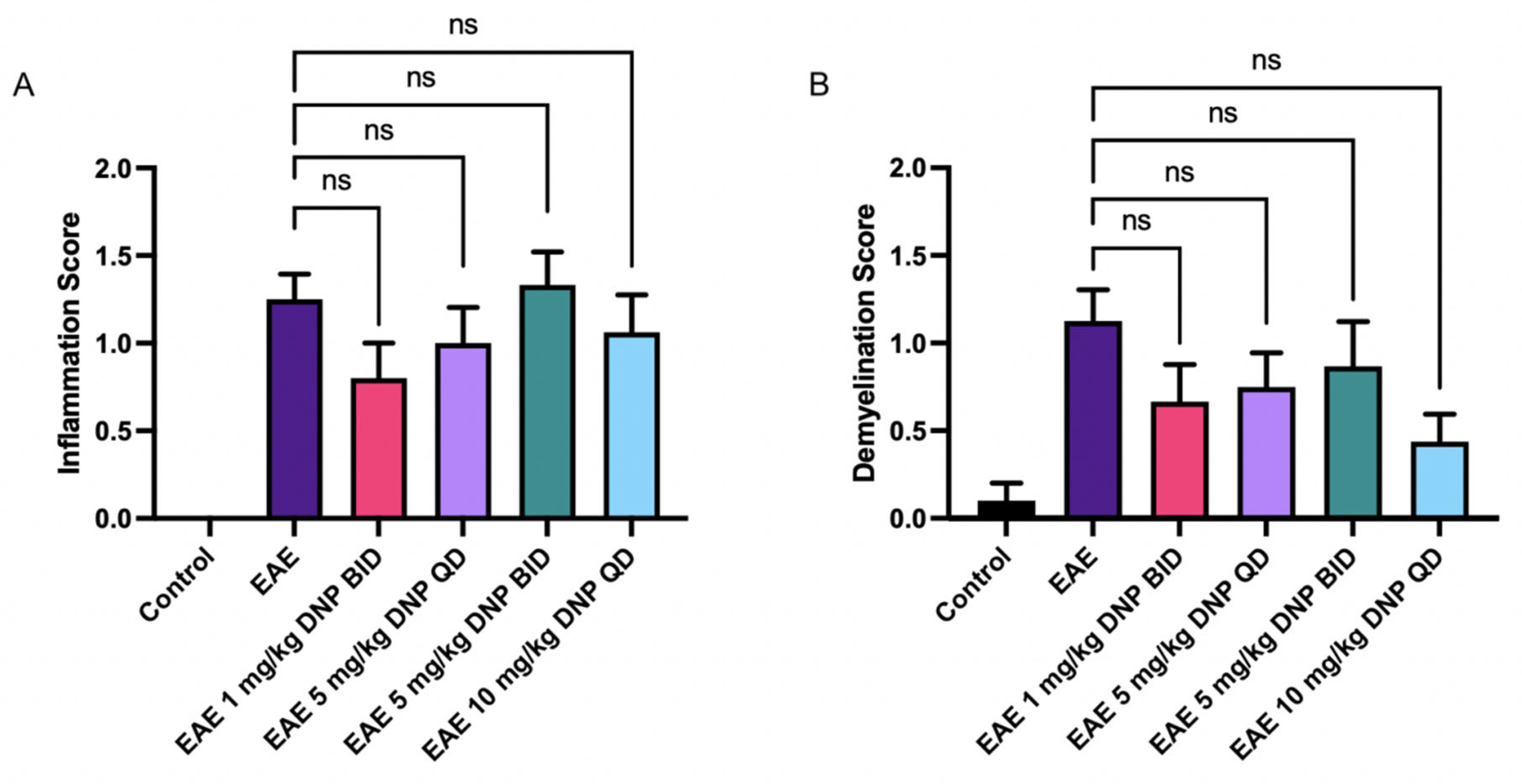
3.8. Effect of DNP in Higher Doses on Optic Nerve Inflammation and Demyelination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.L.; Costello, F.; Chen, J.J.; Petzold, A.; Biousse, V.; Newman, N.J.; Galetta, S.L. Optic neuritis and autoimmune optic neuropathies: Advances in diagnosis and treatment. Lancet Neurol. 2023, 22, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Cleary, P.A.; Anderson Jr, M.M.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R.; et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N. Engl. J. Med. 1992, 326, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Gal, R.L.; Bhatti, M.T. Visual function more than 10 years after optic neuritis: Experience of the optic neuritis treatment trial. Am. J. Ophthalmol. 2004, 137, 77–83. [Google Scholar] [PubMed]
- Costello, F.; Coupland, S.; Hodge, W.; Lorello, G.R.; Koroluk, J.; Pan, Y.I.; Freedman, M.S.; Zackon, D.H.; Kardon, R.H. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 2006, 59, 963–969. [Google Scholar] [CrossRef]
- Trip, S.; Schlottmann, P.; Jones, S.; Altmann, D.; Garway-Heath, D.; Thompson, A.; Plant, G.; Miller, D. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann. Neurol. 2005, 58, 383–391. [Google Scholar] [CrossRef]
- Mendel, I.; de Rosbo, N.K.; Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur. J. Immunol. 1995, 25, 1951–1959. [Google Scholar] [CrossRef]
- Jing, J.; Smith, M.D.; Kersbergen, C.J.; Kam, T.; Viswanathan, M.; Martin, K.; Dawson, T.M.; Dawson, V.L.; Zack, D.J.; Whartenby, K.; et al. Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2019, 7, 125. [Google Scholar]
- Quinn, T.A.; Dutt, M.; Shindler, K.S. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front. Neurol. 2011, 2, 50. [Google Scholar] [CrossRef]
- Khan, R.S.; Dine, K.; Geisler, J.G.; Shindler, K.S. Mitochondrial Uncoupler Prodrug of 2,4-Dinitrophenol, MP201, Prevents Neuronal Damage and Preserves Vision in Experimental Optic Neuritis. Oxidative Med. Cell. Long. 2017, 2017, 7180632. [Google Scholar] [CrossRef]
- Qi, X.; Lewin, A.S.; Sun, L.; Hauswirth, W.W.; Guy, J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 681–691. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Rex, T.S.; Elliott, P.; Rostami, A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.G. Targeting energy expenditure via fuel switching and beyond. Diabetologia 2011, 54, 237–244. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Divakaruni, A.S.; Jastroch, M.; Brand, M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010, 131, 463–472. [Google Scholar] [CrossRef]
- Mattiasson, G.; Shamloo, M.; Gido, G.; Mathi, K.; Tomasevic, G.; Yi, S.; Warden, C.H.; Castilho, R.F.; Melcher, T.; Gonzalez-Zulueta, M.; et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003, 9, 1062–1068. [Google Scholar] [CrossRef]
- Barnstable, C.J.; Reddy, R.; Li, H.; Horvath, T.L. Mitochondrial Uncoupling Protein 2 (UCP2) Regulates Retinal Ganglion Cell Number and Survival. J. Mol. Neurosci. 2016, 58, 461–469. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Vekaria, H.J.; Velmurugan, G.V.; Kalimon, O.J.; Prajapati, P.; Brown, E.; Geisler, J.G.; Sullivan, P.G. Mitochondrial Dysfunction After Repeated Mild Blast Traumatic Brain Injury Is Attenuated by a Mild Mitochondrial Uncoupling Prodrug. J. Neurotrauma 2023, 40, 2396–2409. [Google Scholar] [CrossRef]
- Bando, Y.; Geisler, J.G. Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis. Neurochem. Int. 2019, 131, 104561. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Johnson, J.; Fang, W.; Halpern, J.; Marosi, K.; Liu, D.; Geisler, J.G.; Mattson, M.P. A mitochondrial uncoupler prodrug protects dopaminergic neurons and improves functional outcome in a mouse model of Parkinson’s disease. Neurobiol. Aging 2020, 85, 123–130. [Google Scholar] [CrossRef]
- Caldeira da Silva, C.C.; Cerqueira, F.M.; Barbosa, L.F.; Medeiros, M.H.; Kowaltowski, A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 2008, 7, 552–560. [Google Scholar] [CrossRef]
- Korde, A.S.; Pettigrew, L.C.; Craddock, S.D.; Maragos, W.F. The mitochondrial uncoupler 2, 4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem. 2005, 94, 1676–1684. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, M.; Peng, Q.; Li, G.; Hou, Z.; Milne, G.L.; Mori, S.; Alonso, R.; Geisler, J.G.; Duan, W. 2,4 DNP improves motor function, preserves medium spiny neuronal identity, and reduces oxidative stress in a mouse model of Huntington's disease. Exp. Neurol. 2017, 293, 83–90. [Google Scholar] [CrossRef]
- Zhong, R.; Dionela, D.L.A.; Kim, N.H.; Harris, E.N.; Geisler, J.G.; Wei-LaPierre, L. Micro-Doses of DNP Preserve Motor and Muscle Function with a Period of Functional Recovery in Amyotrophic Lateral Sclerosis Mice. Ann. Neurol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Geisler, J.G. 2,4 Dinitrophenol as Medicine. Cells 2019, 8, 280. [Google Scholar] [CrossRef]
- Ross, A.G.; Chaqour, B.; McDougald, D.S.; Dine, K.E.; Duong, T.T.; Shindler, R.E.; Yue, J.; Liu, T.; Shindler, K.S. Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis. Biomolecules 2022, 12, 830. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Perkins, R.G. A study of the munitions intoxications in France. Public Health Rep. 1919, 34, 2335–2374. [Google Scholar] [CrossRef]
- Tainter, M.L.; Wood, D.A. A case of fatal dinitrophenol poisoning. J. Am. Med. Assoc. 1934, 102, 1147–1149. [Google Scholar] [CrossRef]
- Tainter, M.L.; Stockton, A.B. Cutting WC: Dinitrophenol in the treatment of obesity. J. Am. Med. Assoc. 1935, 105, 332–337. [Google Scholar] [CrossRef]
- Grandjean, P. Paracelsus Revisited: The Dose Concept in a Complex World. Basic Clin. Pharmacol. Toxicol. 2016, 119, 126–132. [Google Scholar] [CrossRef]
- Geisler, J.G.; Marosi, K.; Halpern, J.; Mattson, M.P. DNP, mitochondrial uncoupling, and neuroprotection: A little dab'll do ya. Alzheimers Dement. 2017, 13, 582–591. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, N.; Khan, R.S.; Abd Alhadi, S.; Dine, K.E.; Geisler, J.G.; Chaqour, B.; Ross, A.G.; Shindler, K.S. Mitochondrial Uncoupler, 2,4-Dinitrophenol, Reduces Spinal Cord Paralysis and Retinal Ganglion Cell Loss in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Biomolecules 2025, 15, 189. https://doi.org/10.3390/biom15020189
O’Neill N, Khan RS, Abd Alhadi S, Dine KE, Geisler JG, Chaqour B, Ross AG, Shindler KS. Mitochondrial Uncoupler, 2,4-Dinitrophenol, Reduces Spinal Cord Paralysis and Retinal Ganglion Cell Loss in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Biomolecules. 2025; 15(2):189. https://doi.org/10.3390/biom15020189
Chicago/Turabian StyleO’Neill, Nuala, Reas S. Khan, Suad Abd Alhadi, Kimberly E. Dine, John G. Geisler, Brahim Chaqour, Ahmara G. Ross, and Kenneth S. Shindler. 2025. "Mitochondrial Uncoupler, 2,4-Dinitrophenol, Reduces Spinal Cord Paralysis and Retinal Ganglion Cell Loss in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis" Biomolecules 15, no. 2: 189. https://doi.org/10.3390/biom15020189
APA StyleO’Neill, N., Khan, R. S., Abd Alhadi, S., Dine, K. E., Geisler, J. G., Chaqour, B., Ross, A. G., & Shindler, K. S. (2025). Mitochondrial Uncoupler, 2,4-Dinitrophenol, Reduces Spinal Cord Paralysis and Retinal Ganglion Cell Loss in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Biomolecules, 15(2), 189. https://doi.org/10.3390/biom15020189





