Chikungunya Particle and RNA Induce Mechanical and Heat Hypersensitivities in a TRPV1-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. CHIKV Sample
2.2. Mice
2.3. Mechanical Hypernociceptive Responses
2.4. Knee Joint Histology
2.5. TRPV1 Immunohistochemistry
2.6. Statistical Analyses
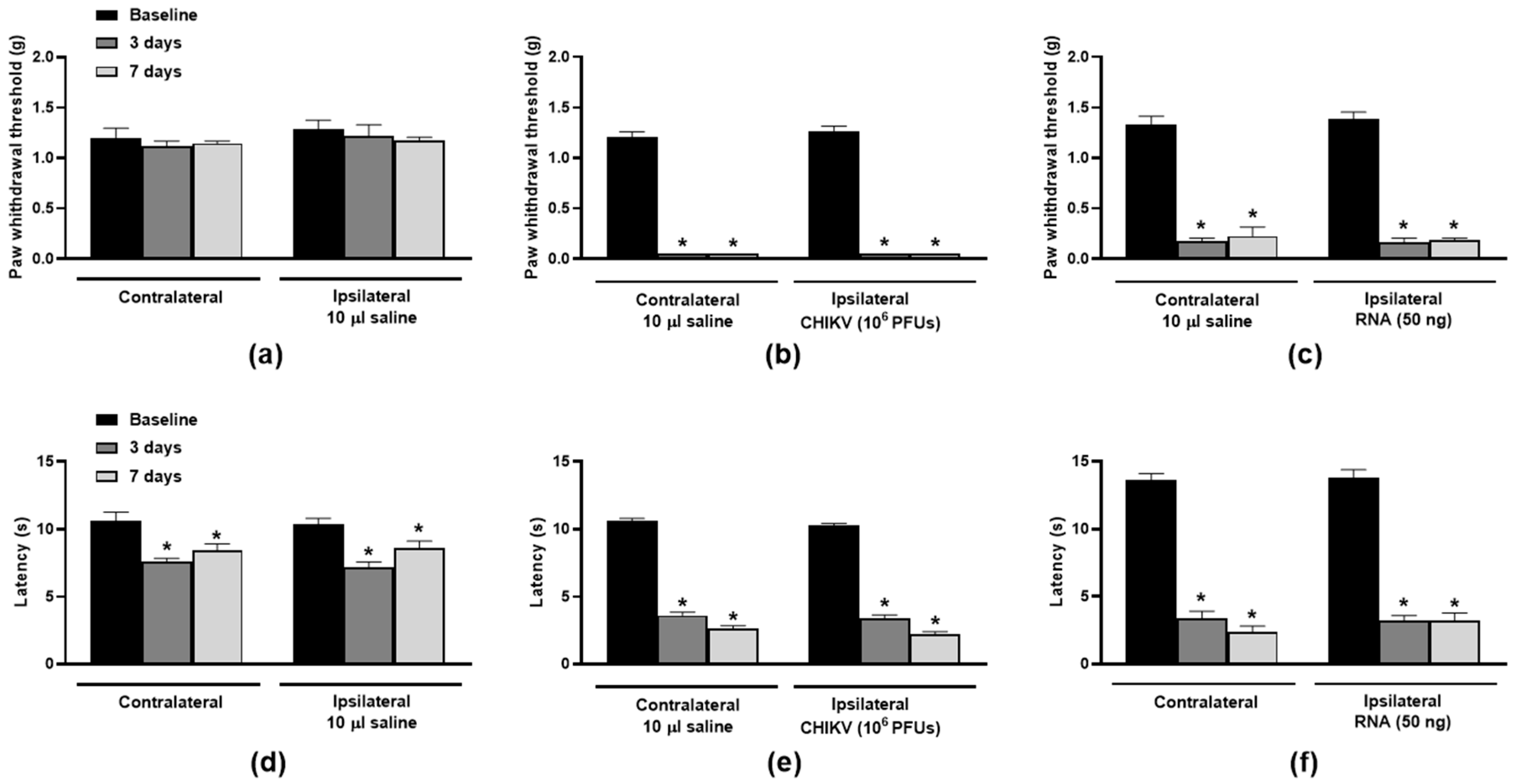
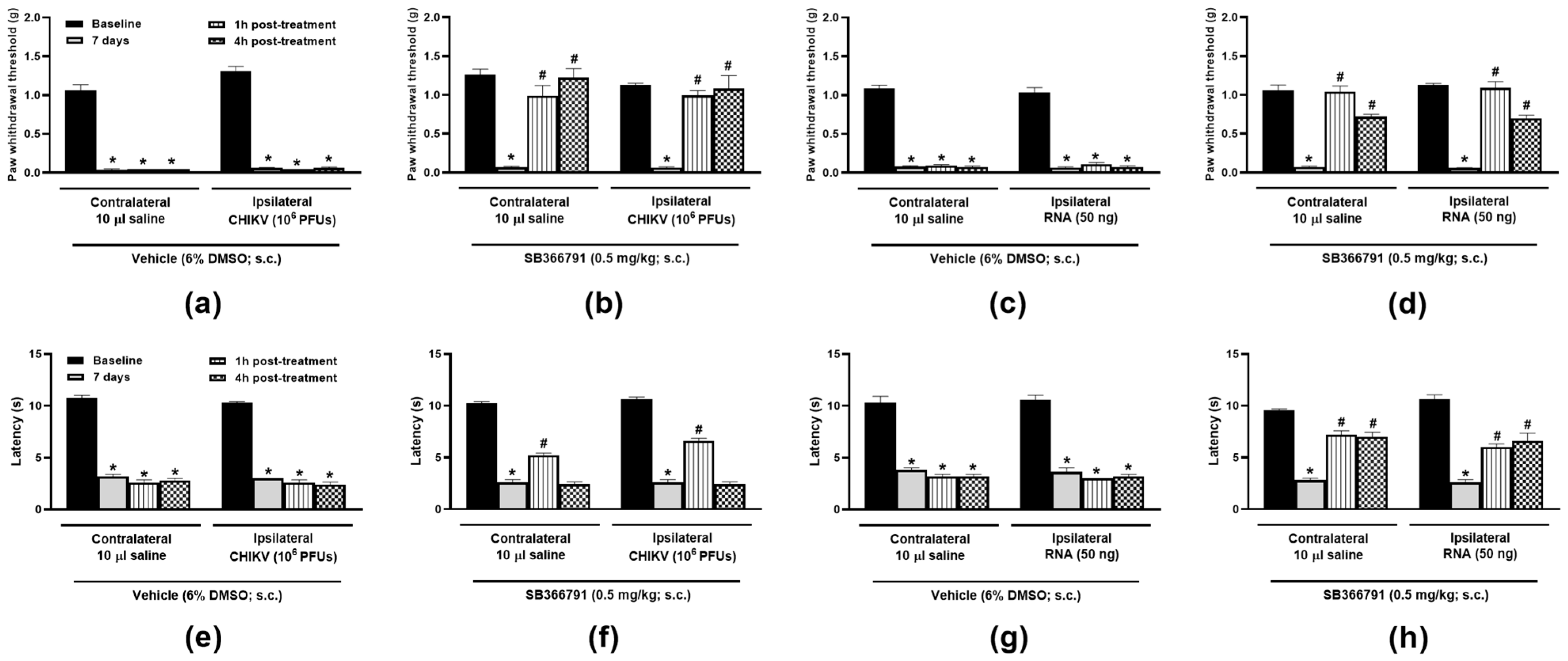
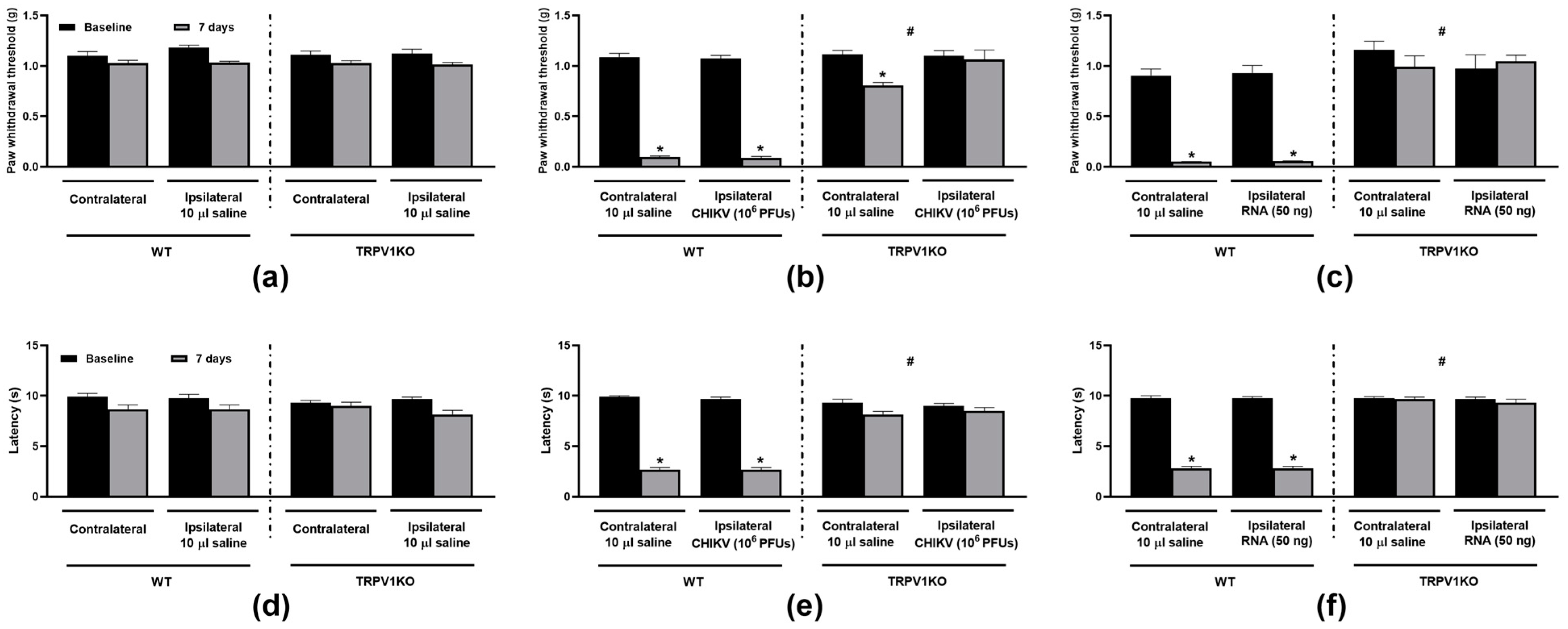
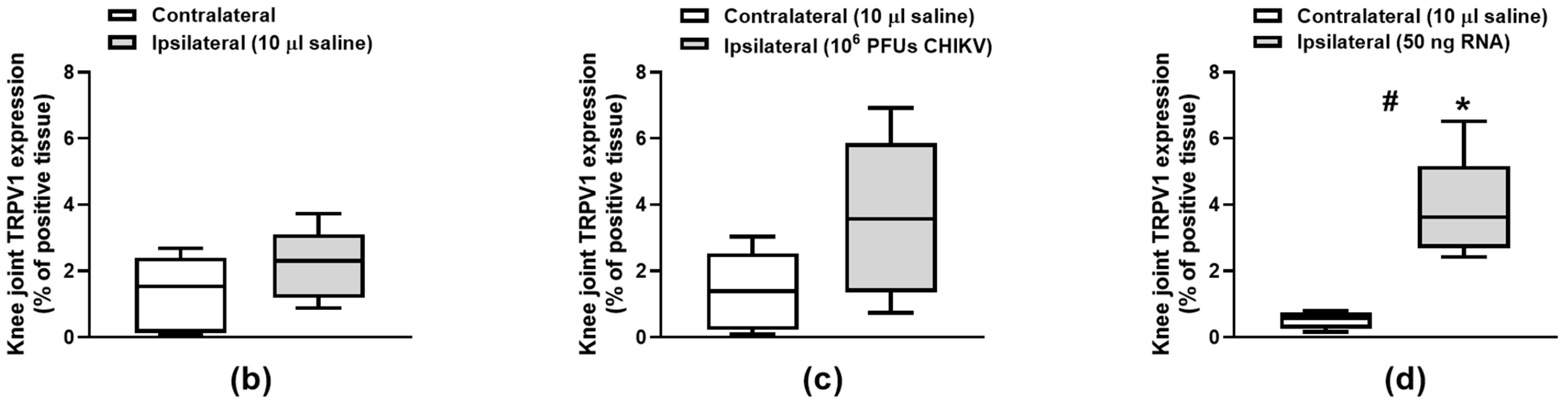
3. Results
3.1. TRPV1 Antagonism and Ablation Attenuate CHIKV- and Viral RNA-Induced Nociception
3.2. TRPV1 Protein Expression in the Knee Joint Cartilage Is Induced by CHIKV
3.3. CHIKV-Induced Histology Changes Are Not Affected by Loss of TRPV1 Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ECDC (European Centre for Disease Prevention and Control). Chikungunya Monthly Update. ECDC. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly (accessed on 27 November 2024).
- Manzoor, K.N.; Javed, F.; Ejaz, M.; Ali, M.; Mujaddadi, N.; Khan, A.A.; Khattak, A.A.; Zaib, A.; Ahmad, I.; Saeed, W.K.; et al. The global emergence of Chikungunya infection: An integrated view. Rev. Med. Virol. 2022, 32, e2287. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.S.; Utt, A.; Tan, Y.B.; Zheng, J.; Wang, S.; Chen, M.W.; Griffin, P.R.; Merits, A.; Luo, D. Structural insights into RNA recognition by the Chikungunya virus nsP2 helicase. Proc. Natl. Acad. Sci. USA 2019, 116, 9558–9567. [Google Scholar] [CrossRef]
- Couturier, E.; Guillemin, F.; Mura, M.; Leon, L.; Virion, J.M.; Letort, M.J.; De Valk, H.; Simon, F.; Vaillant, V. Impaired quality of life after chikungunya virus infection: A 2-year follow-up study. Rheumatology 2012, 51, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Staikowsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Edington, F.; Varjao, D.; Melo, P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Jt. Bone Spine 2018, 85, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Jain, S. Chikungunya: A rheumatologist’s perspective. Int. J. Rheum. Dis. 2018, 21, 584–601. [Google Scholar] [CrossRef]
- Runowska, M.; Majewski, D.; Niklas, K.; Puszczewicz, M. Chikungunya virus: A rheumatologist’s perspective. Clin. Exp. Rheumatol. 2018, 36, 494–501. [Google Scholar] [PubMed]
- Chirathaworn, C.; Chansaenroj, J.; Poovorawan, Y. Cytokines and Chemokines in Chikungunya Virus Infection: Protection or Induction of Pathology. Pathogens 2020, 9, 415. [Google Scholar] [CrossRef]
- Brito, M.; Marchi, M.S.; Perin, M.Y.; Cosso, I.D.S.; Bumlai, R.U.M.; Silva Junior, W.V.D.; Prado, A.Y.M.; Cruz, T.C.D.; Avila, E.T.P.; Damazo, A.S.; et al. Inflammation, fibrosis and E1 glycoprotein persistence in joint tissue of patients with post-Chikungunya chronic articular disease. Rev. Soc. Bras. Med. Trop. 2023, 56, e02782023. [Google Scholar] [CrossRef]
- Karimi, S.A.; Zahra, F.T.; Martin, L.J. IUPHAR review: Navigating the role of preclinical models in pain research. Pharmacol. Res. 2024, 200, 107073. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2024, 22, 6–14. [Google Scholar] [CrossRef]
- Mugo, A.; Chou, R.; Chin, F.; Liu, B.; Jiang, Q.X.; Qin, F. A suicidal mechanism for the exquisite temperature sensitivity of TRPV1. Proc. Natl. Acad. Sci. USA 2023, 120, e2300305120. [Google Scholar] [CrossRef] [PubMed]
- Segato-Vendrameto, C.Z.; Zanluca, C.; Zucoloto, A.Z.; Zaninelli, T.H.; Bertozzi, M.M.; Saraiva-Santos, T.; Ferraz, C.R.; Staurengo-Ferrari, L.; Badaro-Garcia, S.; Manchope, M.F.; et al. Chikungunya Virus and Its Envelope Protein E2 Induce Hyperalgesia in Mice: Inhibition by Anti-E2 Monoclonal Antibodies and by Targeting TRPV1. Cells 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Sanjai Kumar, P.; Nayak, T.K.; Mahish, C.; Sahoo, S.S.; Radhakrishnan, A.; De, S.; Datey, A.; Sahu, R.P.; Goswami, C.; Chattopadhyay, S.; et al. Inhibition of transient receptor potential vanilloid 1 (TRPV1) channel regulates chikungunya virus infection in macrophages. Arch. Virol. 2021, 166, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kochukov, M.Y.; McNearney, T.A.; Fu, Y.; Westlund, K.N. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am. J. Physiol. Cell Physiol. 2006, 291, C424–C432. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Fu, Y.; Liu, Y.; Liu, C.; Xu, B.; Zhang, Q.; Jiang, P. The role of TRPV1 in RA pathogenesis: Worthy of attention. Front. Immunol. 2023, 14, 1232013. [Google Scholar] [CrossRef]
- Liao, Z.; Umar, M.; Huang, X.; Qin, L.; Xiao, G.; Chen, Y.; Tong, L.; Chen, D. Transient receptor potential vanilloid 1: A potential therapeutic target for the treatment of osteoarthritis and rheumatoid arthritis. Cell Prolif. 2024, 57, e13569. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Silva, C.R.; Ferreira, J.; Oliveira, S.M. Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications. Brain Sci. 2023, 13, 941. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, Y.; Li, B.; Xu, L.; Kamau, P.M.; Zheng, J.; Yang, F.; Yang, S.; Lai, R. Molecular basis for heat desensitization of TRPV1 ion channels. Nat. Commun. 2019, 10, 2134. [Google Scholar] [CrossRef]
- Rossato, M.F.; Rigo, F.K.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Cunha, T.M.; Gomez, M.V.; Ferreira, J.; Trevisan, G. Participation of transient receptor potential vanilloid 1 in paclitaxel-induced acute visceral and peripheral nociception in rodents. Eur. J. Pharmacol. 2018, 828, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Russell, F.A.; Spina, D.; McDougall, J.J.; Graepel, R.; Gentry, C.; Staniland, A.A.; Mountford, D.M.; Keeble, J.E.; Malcangio, M.; et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011, 63, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.F.; Araújo, L.M.C.; Carmo, V.J.G.; Cardoso, E.J.A.; Raposo, M.C.F.; Melo, R.S. Prevalence, affected joints and intensity of the arthralgias in individuals in the chronic phase of Chikungunya fever. BrJP 2021, 4, 108–112. [Google Scholar] [CrossRef]
- Watson, H.; Tritsch, S.R.; Encinales, L.; Cadena, A.; Cure, C.; Ramirez, A.P.; Mendoza, A.R.; Chang, A.Y. Stiffness, pain, and joint counts in chronic chikungunya disease: Relevance to disability and quality of life. Clin. Rheumatol. 2020, 39, 1679–1686. [Google Scholar] [CrossRef]
- Cavalcante, A.F.L.; Okano, A.H.; Micussi, M.T.; Souza, C.G.; Passos, J.O.S.; Morya, E.; Freitas, R.P.A. Artralgia crônica por Chikungunya reduz funcionalidade, qualidade de vida e performance ocupacional: Estudo descritivo transversal. BrJP 2022, 5, 233–238. [Google Scholar] [CrossRef]
- Russell, F.A.; Fernandes, E.S.; Courade, J.P.; Keeble, J.E.; Brain, S.D. Tumour necrosis factor alpha mediates transient receptor potential vanilloid 1-dependent bilateral thermal hyperalgesia with distinct peripheral roles of interleukin-1beta, protein kinase C and cyclooxygenase-2 signalling. Pain 2009, 142, 264–274. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 25404048. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 2014, 291, 11–15. [Google Scholar] [CrossRef]
- Bailey, M.; Christoforidou, Z.; Lewis, M.C. The evolutionary basis for differences between the immune systems of man, mouse, pig and ruminants. Vet. Immunol. Immunopathol. 2013, 152, 13–19. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Voets, T.; Iadarola, M.J.; Szallasi, A. Targeting TRP channels for pain relief: A review of current evidence from bench to bedside. Curr. Opin. Pharmacol. 2024, 75, 102447. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, L.C.M.; dos Santos Maia, A.C.; de Sousa, N.C.F.; Pavi, C.P.; Savi, B.P.; Nagashima, S.; Damasceno, S.; Schneider, A.H.; Mascarin, L.Z.; Rodrigues, J.F.S.; et al. Chikungunya Particle and RNA Induce Mechanical and Heat Hypersensitivities in a TRPV1-Dependent Manner. Biomolecules 2025, 15, 171. https://doi.org/10.3390/biom15020171
da Silva LCM, dos Santos Maia AC, de Sousa NCF, Pavi CP, Savi BP, Nagashima S, Damasceno S, Schneider AH, Mascarin LZ, Rodrigues JFS, et al. Chikungunya Particle and RNA Induce Mechanical and Heat Hypersensitivities in a TRPV1-Dependent Manner. Biomolecules. 2025; 15(2):171. https://doi.org/10.3390/biom15020171
Chicago/Turabian Styleda Silva, Liziane C. M., Andressa C. dos Santos Maia, Nágila C. F. de Sousa, Catielen P. Pavi, Beatriz P. Savi, Seigo Nagashima, Samara Damasceno, Ayda H. Schneider, Lucas Z. Mascarin, João F. S. Rodrigues, and et al. 2025. "Chikungunya Particle and RNA Induce Mechanical and Heat Hypersensitivities in a TRPV1-Dependent Manner" Biomolecules 15, no. 2: 171. https://doi.org/10.3390/biom15020171
APA Styleda Silva, L. C. M., dos Santos Maia, A. C., de Sousa, N. C. F., Pavi, C. P., Savi, B. P., Nagashima, S., Damasceno, S., Schneider, A. H., Mascarin, L. Z., Rodrigues, J. F. S., Monteiro, C. R. A. V., Silva, I. T., Fongaro, G., Monteiro-Neto, V., Bomfim, M. R. Q., Cunha, T. M., de Sousa Valente, J., Calixto, J. B., de Noronha, L., ... Fernandes, E. S. (2025). Chikungunya Particle and RNA Induce Mechanical and Heat Hypersensitivities in a TRPV1-Dependent Manner. Biomolecules, 15(2), 171. https://doi.org/10.3390/biom15020171









