Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer’s Disease Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Lines
2.2. Rotarod Test
2.3. Wire Hang Test
2.4. Antibodies
2.5. Preparation of Brain Homogenates for Biochemical Analysis
2.6. Analysis of Sarkosyl-Insoluble PHF-Tau Fraction
2.7. WB
2.8. Histological Staining and Immunocytochemistry
2.9. Quantitative Analyses of Histological Staining
2.10. Statistical Analyses
3. Results
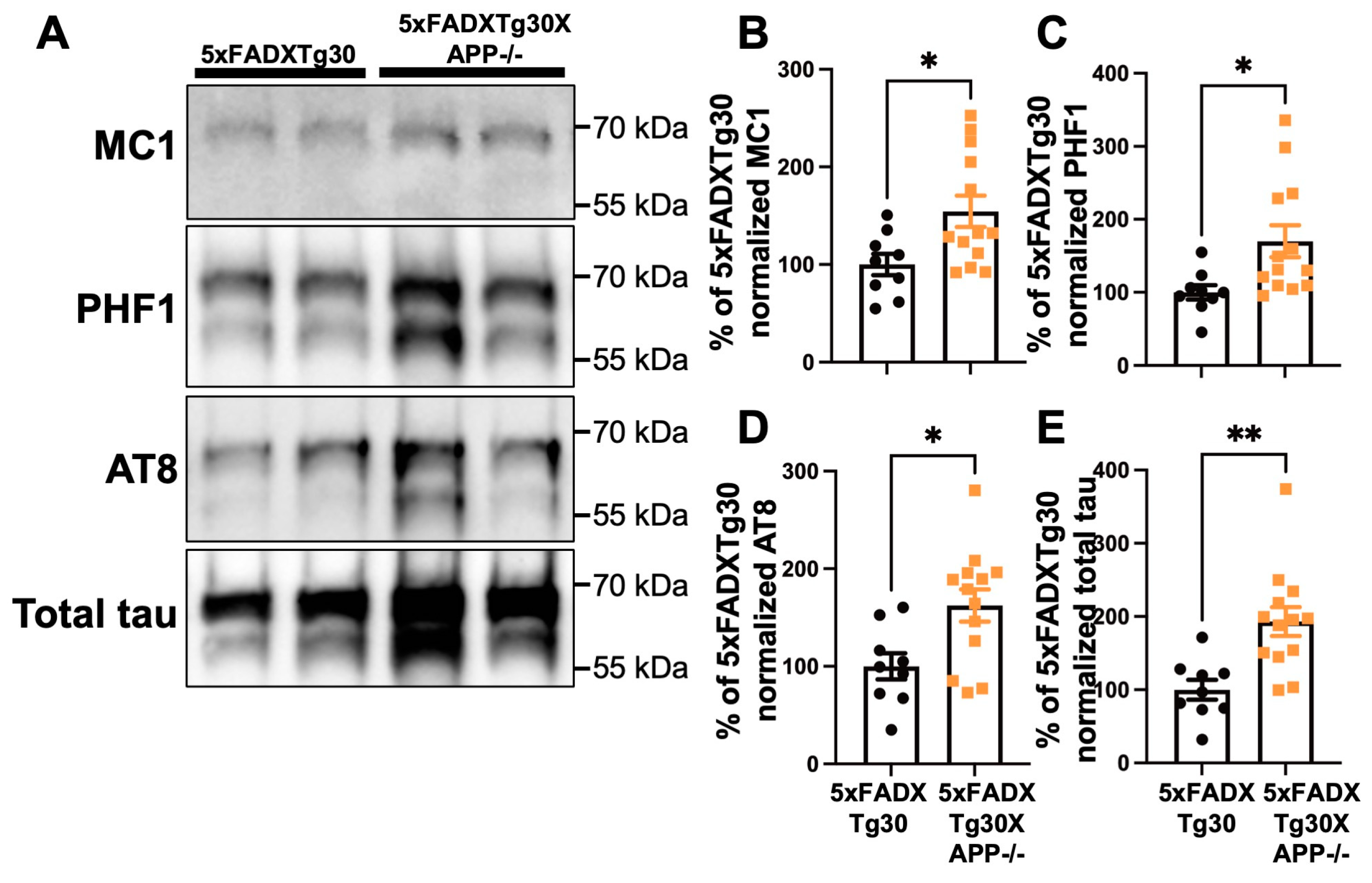
3.1. Expression of APP and Tau Proteins in 5xFADXTg30 and 5xFADXTg30XAPP-/- Mouse Brains
3.2. Murine APP Deletion in 5xFADXTg30 Mouse Model Aggravates Muscle Weakness and Motor Phenotype
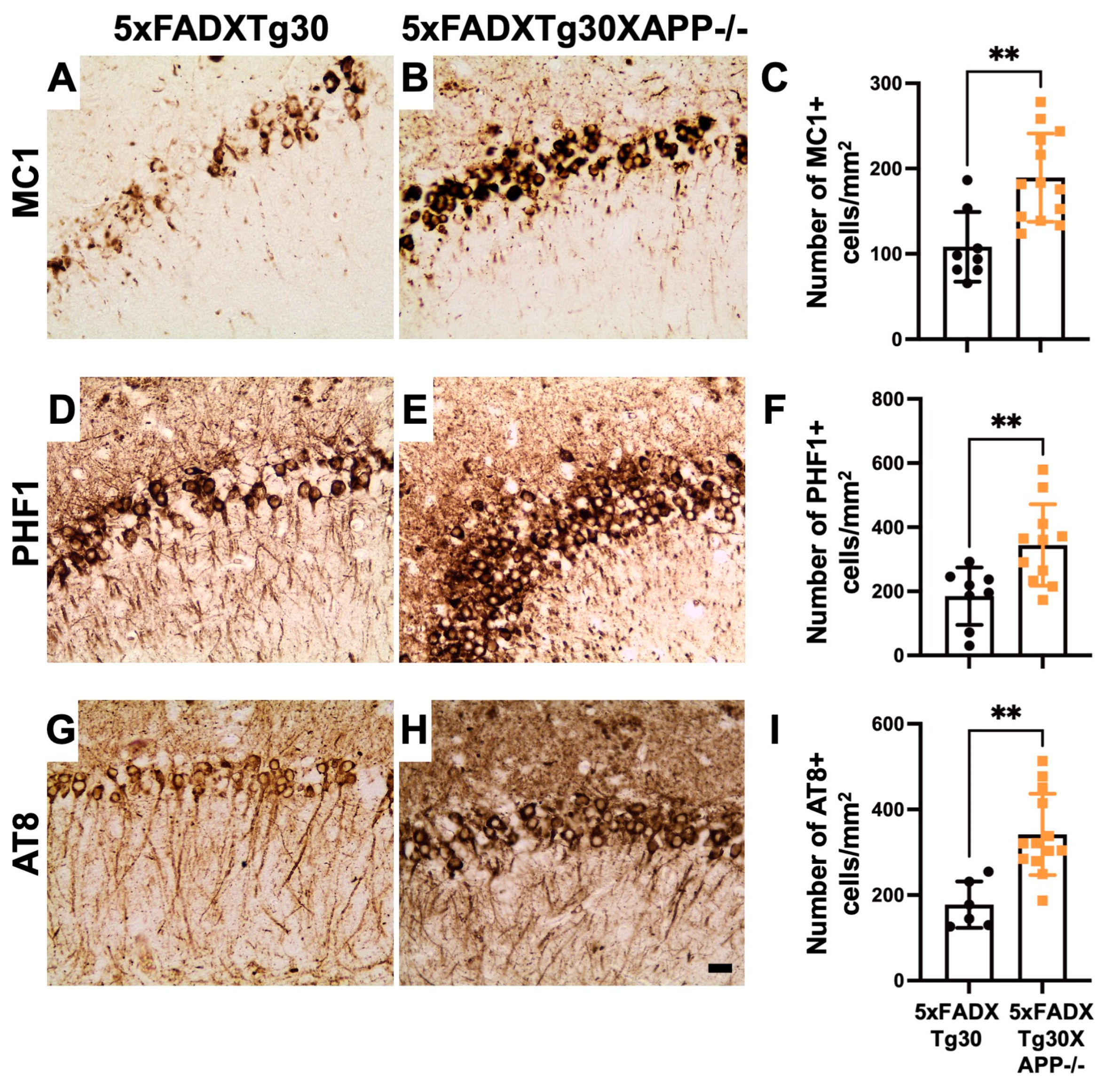
3.3. Tau Pathology Is Aggravated in 5xFADXTg30XAPP-/- Mouse Brains Compared to 5xFADXTg30
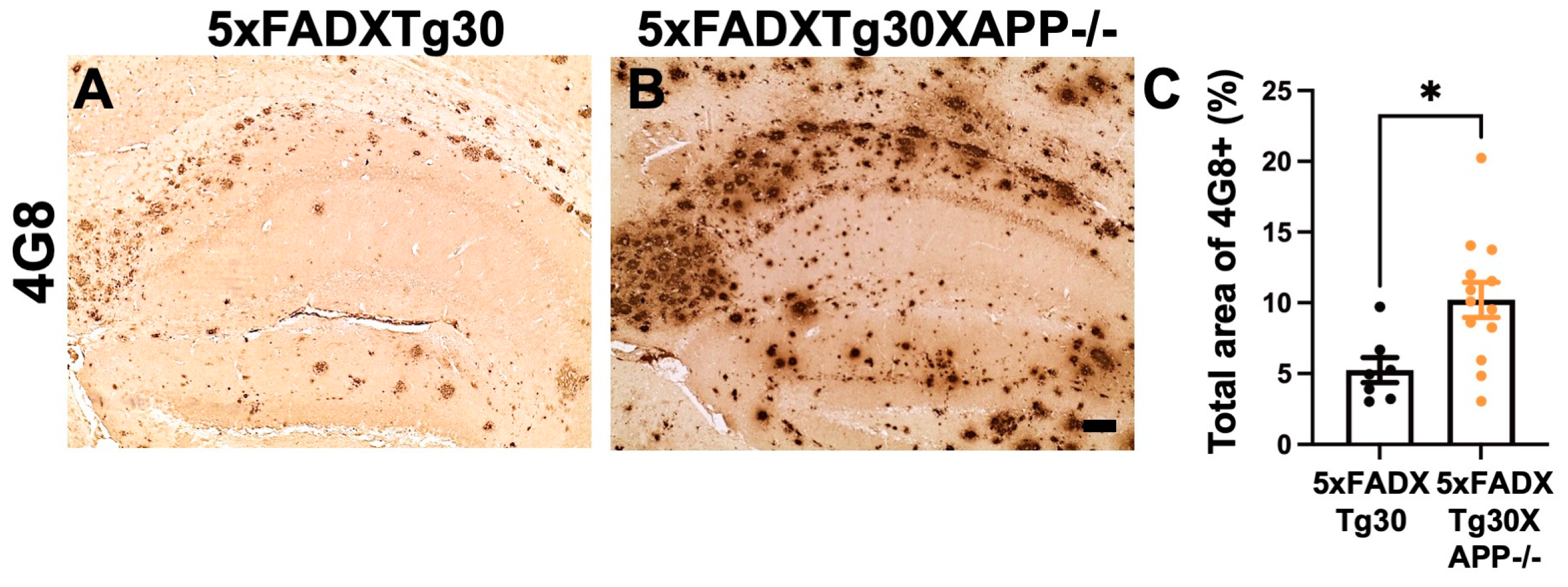
3.4. Amyloid Pathology Is Aggravated in the Hippocampus of 5xFADXTg30APP-/- Mice Compared to 5xFADXTg30 Mice
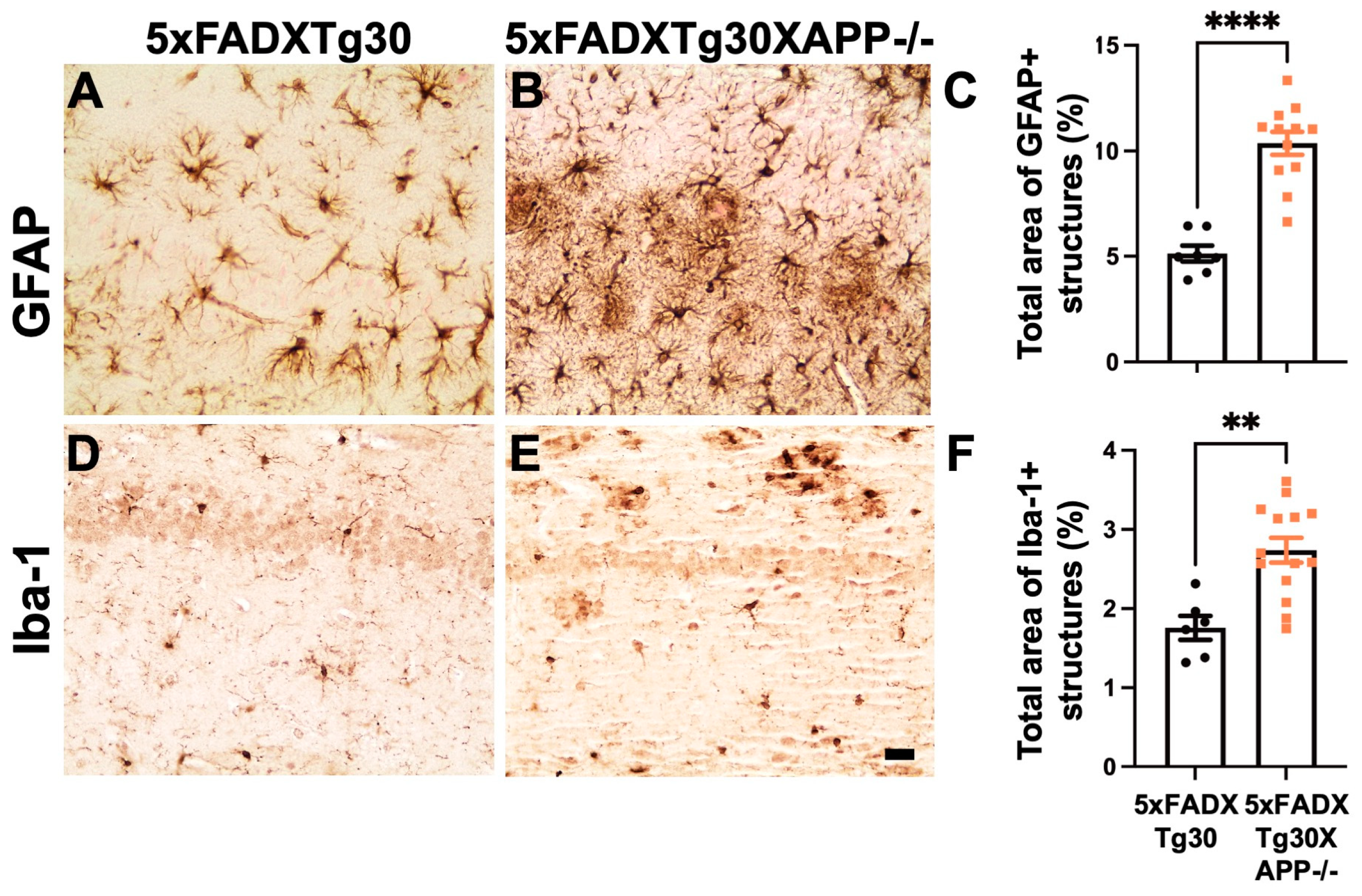
3.5. Gliosis Is Aggravated in 5xFADXTg30XAPP-/- Mice Compared to 5xFADXTg30 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated-virus |
| Aβ | amyloid β |
| AD | Alzheimer’s disease |
| AICD | amyloid precursor protein intracellular domain |
| APP | amyloid precursor protein |
| CTF | C-terminal fragment |
| FAD | familial AD |
| FTLD | frontotemporal lobar degeneration |
| GFAP | Glial Fibrillary Acidic Protein |
| LTP | Long-term potential |
| NFTs | neurofibrillary tangles |
| PBS | phosphate buffer saline |
| PHF-tau | paired-helical filament |
| PS1 | presenilin-1 |
| sAPPα | soluble N-terminal APPα |
| sAPPβ | soluble N-terminal APPβ |
| WB | Western blot |
References
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef] [PubMed]
- Gakhar-Koppole, N.; Hundeshagen, P.; Mandl, C.; Weyer, S.W.; Allinquant, B.; Muller, U.; Ciccolini, F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 2008, 28, 871–882. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Ryan, N.S.; Nicholas, J.M.; Weston, P.S.J.; Liang, Y.; Lashley, T.; Guerreiro, R.; Adamson, G.; Kenny, J.; Beck, J.; Chavez-Gutierrez, L.; et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: A case series. Lancet Neurol. 2016, 15, 1326–1335. [Google Scholar] [CrossRef]
- Brion, J.P.; Couck, A.M.; Passareiro, E.; Flament-Durand, J. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J. Submicrosc. Cytol. 1985, 17, 89–96. [Google Scholar] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Buee, L.; Bussiere, T.; Buee-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Jeremic, D.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: A systematic review. Ageing. Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, M.; Trumbauer, M.E.; Sirinathsinghji, D.J.; Hopkins, R.; Smith, D.W.; Heavens, R.P.; Dawson, G.R.; Boyce, S.; Conner, M.W.; et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 1995, 81, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Stanga, S.; Zanou, N.; Audouard, E.; Tasiaux, B.; Contino, S.; Vandermeulen, G.; Rene, F.; Loeffler, J.P.; Clotman, F.; Gailly, P.; et al. APP-dependent glial cell line-derived neurotrophic factor gene expression drives neuromuscular junction formation. FASEB J. 2016, 30, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Vanden Dries, V.; Stygelbout, V.; Pierrot, N.; Yilmaz, Z.; Suain, V.; De Decker, R.; Buee, L.; Octave, J.N.; Brion, J.P.; Leroy, K. Amyloid precursor protein reduction enhances the formation of neurofibrillary tangles in a mutant tau transgenic mouse model. Neurobiol. Aging 2017, 55, 202–212. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Schindowski, K.; Bretteville, A.; Leroy, K.; Begard, S.; Brion, J.P.; Hamdane, M.; Buee, L. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol. 2006, 169, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Bretteville, A.; Schindowski, K.; Gilissen, E.; Authelet, M.; De Decker, R.; Yilmaz, Z.; Buee, L.; Brion, J.P. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am. J. Pathol. 2007, 171, 976–992. [Google Scholar] [CrossRef]
- Frederick, C.; Ando, K.; Leroy, K.; Heraud, C.; Suain, V.; Buee, L.; Brion, J.P. Rapamycin ester analog CCI-779/Temsirolimus alleviates tau pathology and improves motor deficit in mutant tau transgenic mice. J. Alzheimers. Dis. 2015, 44, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Ando, K.; Laporte, V.; Dedecker, R.; Suain, V.; Authelet, M.; Heraud, C.; Pierrot, N.; Yilmaz, Z.; Octave, J.N.; et al. Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice. Am. J. Pathol. 2012, 181, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Philippe, B.; Brion, J.P.; Macq, A.F.; Octave, J.N. A new monoclonal antibody against the anionic domain of the amyloid precursor protein of Alzheimer’s disease. Neuroreport 1993, 5, 289–292. [Google Scholar] [CrossRef][Green Version]
- Ando, K.; Leroy, K.; Heraud, C.; Yilmaz, Z.; Authelet, M.; Suain, V.; De Decker, R.; Brion, J.P. Accelerated human mutant tau aggregation by knocking out murine tau in a transgenic mouse model. Am. J. Pathol. 2011, 178, 803–816. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Feiner, L.; Lang, E.; Szendrei, G.I.; Goedert, M.; Lee, V.M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J. Neurosci. Res. 1994, 39, 669–673. [Google Scholar] [CrossRef]
- Jicha, G.A.; Bowser, R.; Kazam, I.G.; Davies, P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 1997, 48, 128–132. [Google Scholar] [CrossRef]
- Ando, K.; Brion, J.P.; Stygelbout, V.; Suain, V.; Authelet, M.; Dedecker, R.; Chanut, A.; Lacor, P.; Lavaur, J.; Sazdovitch, V.; et al. Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer’s brains. Acta Neuropathol. 2013, 125, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Kucukali, F.; Doeraene, E.; Nagaraj, S.; Antonelli, E.M.; Thazin Htut, M.; Yilmaz, Z.; Kosa, A.C.; Lopez-Guitierrez, L.; Quintanilla-Sanchez, C.; et al. Alteration of gene expression and protein solubility of the PI 5-phosphatase SHIP2 are correlated with Alzheimer’s disease pathology progression. Acta Neuropathol. 2024, 147, 94. [Google Scholar] [CrossRef]
- Greenberg, S.G.; Davies, P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA 1990, 87, 5827–5831. [Google Scholar] [CrossRef] [PubMed]
- Brion, J.P.; Hanger, D.P.; Bruce, M.T.; Couck, A.M.; Flament-Durand, J.; Anderton, B.H. Tau in Alzheimer neurofibrillary tangles. N- and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem. J. 1991, 273 Pt 1, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Kabova, A.; Stygelbout, V.; Leroy, K.; Heraud, C.; Frederick, C.; Suain, V.; Yilmaz, Z.; Authelet, M.; Dedecker, R.; et al. Vaccination with Sarkosyl Insoluble PHF-Tau Decrease Neurofibrillary Tangles Formation in Aged Tau Transgenic Mouse Model: A Pilot Study. J. Alzheimer’s Dis. 2014, 40, S135–S145. [Google Scholar] [CrossRef]
- Ando, K.; Ndjim, M.; Turbant, S.; Fontaine, G.; Pregoni, G.; Dauphinot, L.; Yilmaz, Z.; Suain, V.; Mansour, S.; Authelet, M.; et al. The lipid phosphatase Synaptojanin 1 undergoes a significant alteration in expression and solubility and is associated with brain lesions in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 79. [Google Scholar] [CrossRef]
- Vergara, C.; Houben, S.; Suain, V.; Yilmaz, Z.; De Decker, R.; Vanden Dries, V.; Boom, A.; Mansour, S.; Leroy, K.; Ando, K.; et al. Amyloid-beta pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol. 2019, 137, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Heraud, C.; Goufak, D.; Ando, K.; Leroy, K.; Suain, V.; Yilmaz, Z.; De Decker, R.; Authelet, M.; Laporte, V.; Octave, J.N.; et al. Increased misfolding and truncation of tau in APP/PS1/tau transgenic mice compared to mutant tau mice. Neurobiol. Dis. 2013, 62C, 100–112. [Google Scholar] [CrossRef]
- Mahler, J.; Morales-Corraliza, J.; Stolz, J.; Skodras, A.; Radde, R.; Duma, C.C.; Eisele, Y.S.; Mazzella, M.J.; Wong, H.; Klunk, W.E.; et al. Endogenous murine Abeta increases amyloid deposition in APP23 but not in APPPS1 transgenic mice. Neurobiol. Aging 2015, 36, 2241–2247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marazuela, P.; Paez-Montserrat, B.; Bonaterra-Pastra, A.; Sole, M.; Hernandez-Guillamon, M. Impact of Cerebral Amyloid Angiopathy in Two Transgenic Mouse Models of Cerebral beta-Amyloidosis: A Neuropathological Study. Int. J. Mol. Sci. 2022, 23, 4972. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.R.; Seabrook, G.R.; Zheng, H.; Smith, D.W.; Graham, S.; O’Dowd, G.; Bowery, B.J.; Boyce, S.; Trumbauer, M.E.; Chen, H.Y.; et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 1999, 90, 1–13. [Google Scholar] [CrossRef]
- Cai, W.; Li, L.; Sang, S.; Pan, X.; Zhong, C. Physiological Roles of beta-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology. Neurosci. Bull. 2023, 39, 1289–1308. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.R.; O’Connor, K.; Tate, W.P.; Abraham, W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003, 70, 1–32. [Google Scholar] [CrossRef]
- Almkvist, O.; Basun, H.; Wagner, S.L.; Rowe, B.A.; Wahlund, L.O.; Lannfelt, L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch. Neurol. 1997, 54, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Colciaghi, F.; Borroni, B.; Pastorino, L.; Marcello, E.; Zimmermann, M.; Cattabeni, F.; Padovani, A.; Di Luca, M. [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol. Med. 2002, 8, 67–74. [Google Scholar] [CrossRef]
- Fellgiebel, A.; Kojro, E.; Muller, M.J.; Scheurich, A.; Schmidt, L.G.; Fahrenholz, F. CSF APPs alpha and phosphorylated tau protein levels in mild cognitive impairment and dementia of Alzheimer’s type. J. Geriatr. Psychiatry. Neurol. 2009, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lannfelt, L.; Basun, H.; Wahlund, L.O.; Rowe, B.A.; Wagner, S.L. Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nat. Med. 1995, 1, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Tyler, S.J.; Dawbarn, D.; Wilcock, G.K.; Allen, S.J. alpha- and beta-secretase: Profound changes in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 299, 373–376. [Google Scholar] [CrossRef]
- Fol, R.; Braudeau, J.; Ludewig, S.; Abel, T.; Weyer, S.W.; Roederer, J.P.; Brod, F.; Audrain, M.; Bemelmans, A.P.; Buchholz, C.J.; et al. Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016, 131, 247–266. [Google Scholar] [CrossRef]
- Ring, S.; Weyer, S.W.; Kilian, S.B.; Waldron, E.; Pietrzik, C.U.; Filippov, M.A.; Herms, J.; Buchholz, C.; Eckman, C.B.; Korte, M.; et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007, 27, 7817–7826. [Google Scholar] [CrossRef]
- Bold, C.S.; Baltissen, D.; Ludewig, S.; Back, M.K.; Just, J.; Kilian, L.; Erdinger, S.; Banicevic, M.; Rehra, L.; Almouhanna, F.; et al. APPsalpha Rescues Tau-Induced Synaptic Pathology. J. Neurosci. 2022, 42, 5782–5802. [Google Scholar] [CrossRef]
- Hartl, D.; Klatt, S.; Roch, M.; Konthur, Z.; Klose, J.; Willnow, T.E.; Rohe, M. Soluble alpha-APP (sAPPalpha) regulates CDK5 expression and activity in neurons. PLoS ONE 2013, 8, e65920. [Google Scholar] [CrossRef]
- Han, P.; Dou, F.; Li, F.; Zhang, X.; Zhang, Y.W.; Zheng, H.; Lipton, S.A.; Xu, H.; Liao, F.F. Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: A novel excitoprotective mechanism involving modulation of tau phosphorylation. J. Neurosci. 2005, 25, 11542–11552. [Google Scholar] [CrossRef]
- Baltissen, D.; Bold, C.S.; Rehra, L.; Banicevic, M.; Fricke, J.; Just, J.; Ludewig, S.; Buchholz, C.J.; Korte, M.; Muller, U.C. APPsalpha rescues CDK5 and GSK3beta dysregulation and restores normal spine density in Tau transgenic mice. Front. Cell. Neurosci. 2023, 17, 1106176. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Nakaya, T.; Pedrini, S.; Takeda, S.; Iijima-Ando, K.; Iijima, K.; Mathews, P.M.; Itohara, S.; Gandy, S.; Suzuki, T. Physiological mouse brain Abeta levels are not related to the phosphorylation state of threonine-668 of Alzheimer’s APP. PLoS ONE 2006, 1, e51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef]
- Barman, A.; Schurer, S.; Prabhakar, R. Computational modeling of substrate specificity and catalysis of the beta-secretase (BACE1) enzyme. Biochemistry 2011, 50, 4337–4349. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Kosa, A.-C.; Mehadji, Y.; Lasri, H.; Lopez-Gutierrez, L.; Quintanilla-Sánchez, C.; Aydin, E.; Doeraene, E.; Wathelet-Depauw, A.; Nagaraj, S.; et al. Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer’s Disease Model. Biomolecules 2025, 15, 159. https://doi.org/10.3390/biom15020159
Ando K, Kosa A-C, Mehadji Y, Lasri H, Lopez-Gutierrez L, Quintanilla-Sánchez C, Aydin E, Doeraene E, Wathelet-Depauw A, Nagaraj S, et al. Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer’s Disease Model. Biomolecules. 2025; 15(2):159. https://doi.org/10.3390/biom15020159
Chicago/Turabian StyleAndo, Kunie, Andreea-Claudia Kosa, Yasmina Mehadji, Hinde Lasri, Lidia Lopez-Gutierrez, Carolina Quintanilla-Sánchez, Emmanuel Aydin, Emilie Doeraene, Alain Wathelet-Depauw, Siranjeevi Nagaraj, and et al. 2025. "Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer’s Disease Model" Biomolecules 15, no. 2: 159. https://doi.org/10.3390/biom15020159
APA StyleAndo, K., Kosa, A.-C., Mehadji, Y., Lasri, H., Lopez-Gutierrez, L., Quintanilla-Sánchez, C., Aydin, E., Doeraene, E., Wathelet-Depauw, A., Nagaraj, S., Brion, J.-P., & Leroy, K. (2025). Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer’s Disease Model. Biomolecules, 15(2), 159. https://doi.org/10.3390/biom15020159







