Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Induction of EAE, and Clinical Scoring
2.2. Treatment with MDL72527
2.3. Electron Microscopy and Quantification of g-Ratio and Axon Count
2.4. Immunofluorescence Staining of the Optic Nerve Sections and Quantification of Fluorescence Intensity
2.5. Analysis of Visual Function Recording
2.6. Statistical Analysis
3. Results
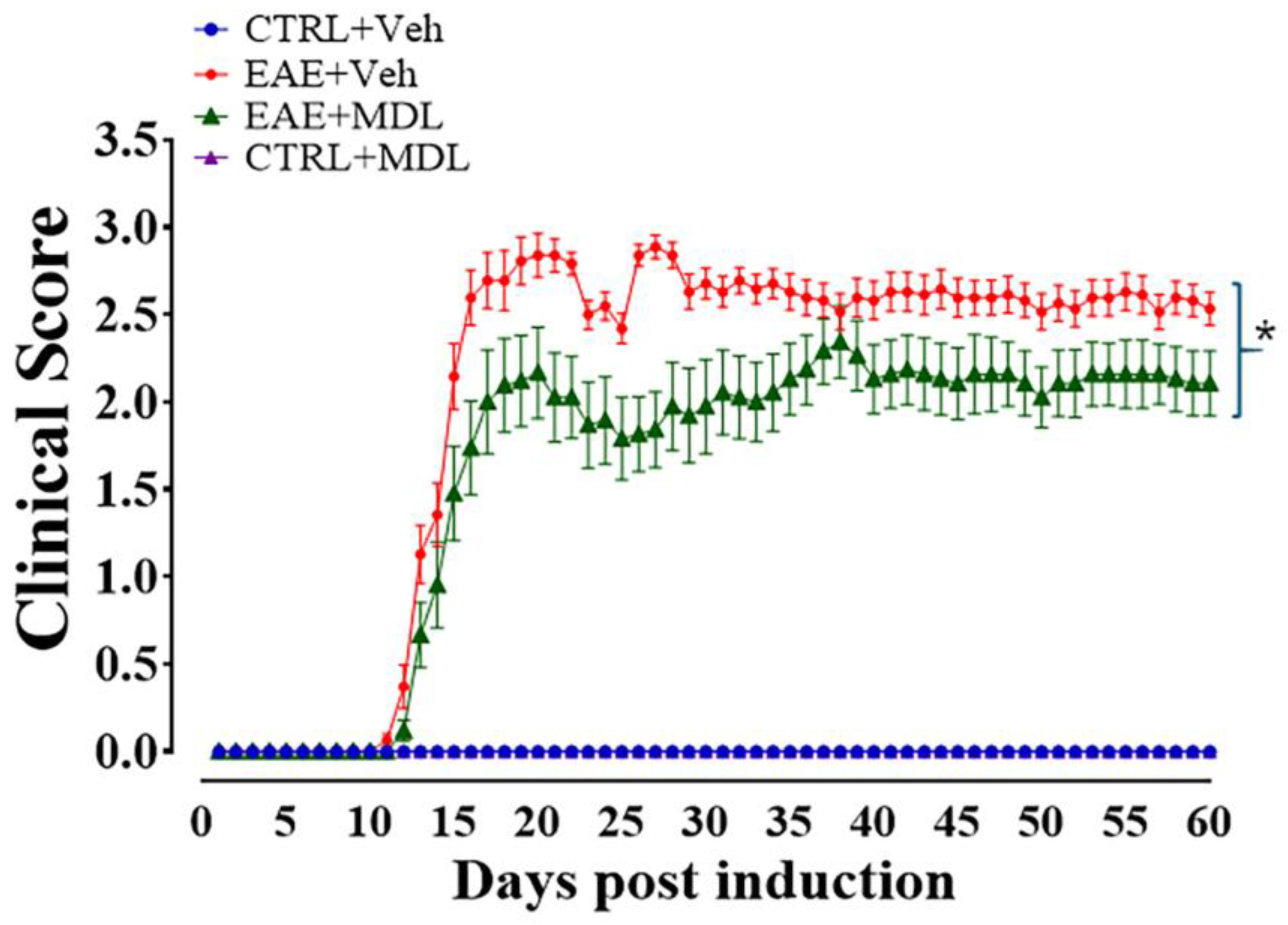
3.1. Spermine Oxidase Inhibition via MDL72527 Treatment Attenuates EAE-Induced Motor Deficits
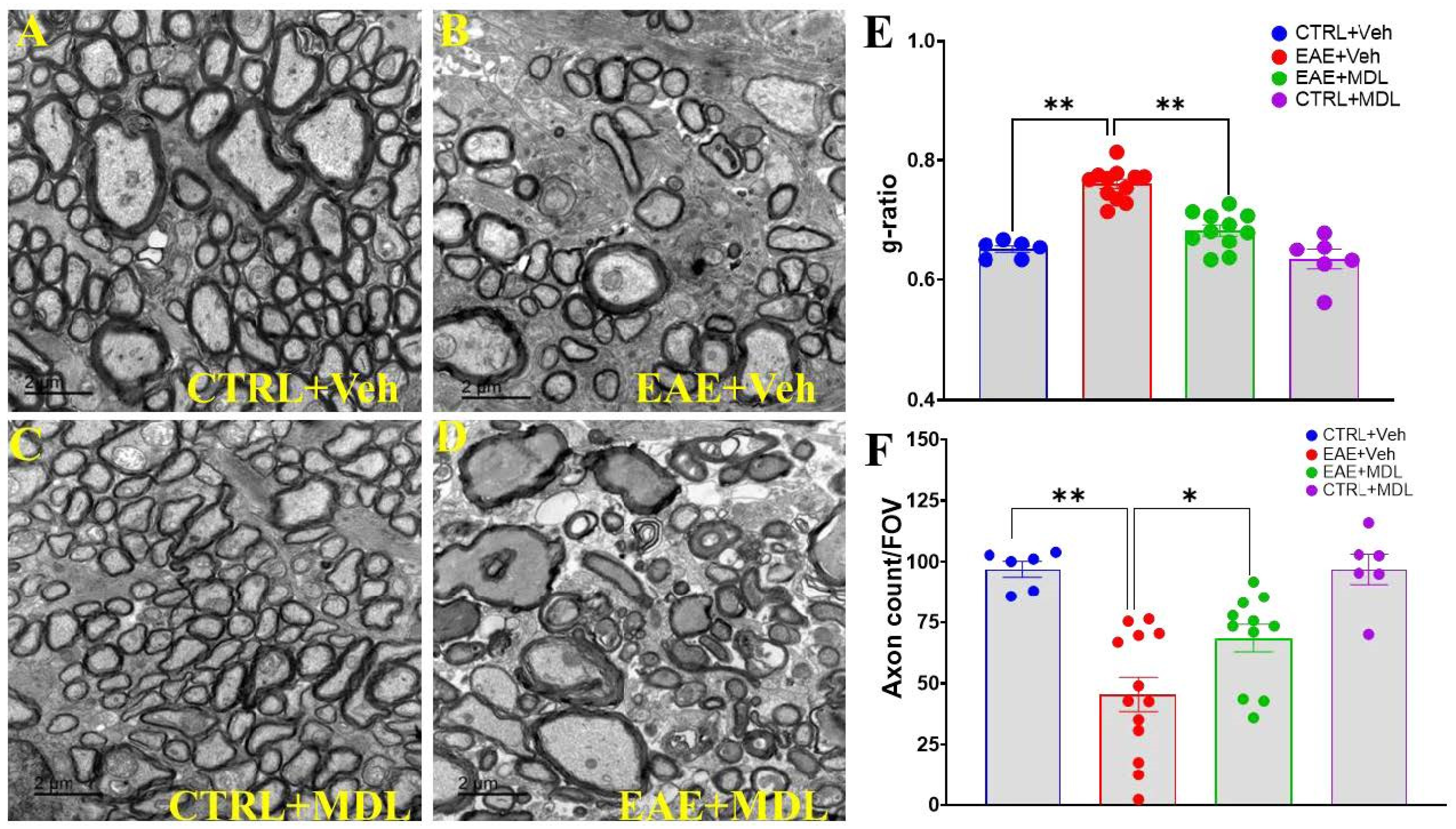
3.2. SMOX Inhibition Preserves Optic Nerve Myelin Thickness and Axonal Number in EAE Mice
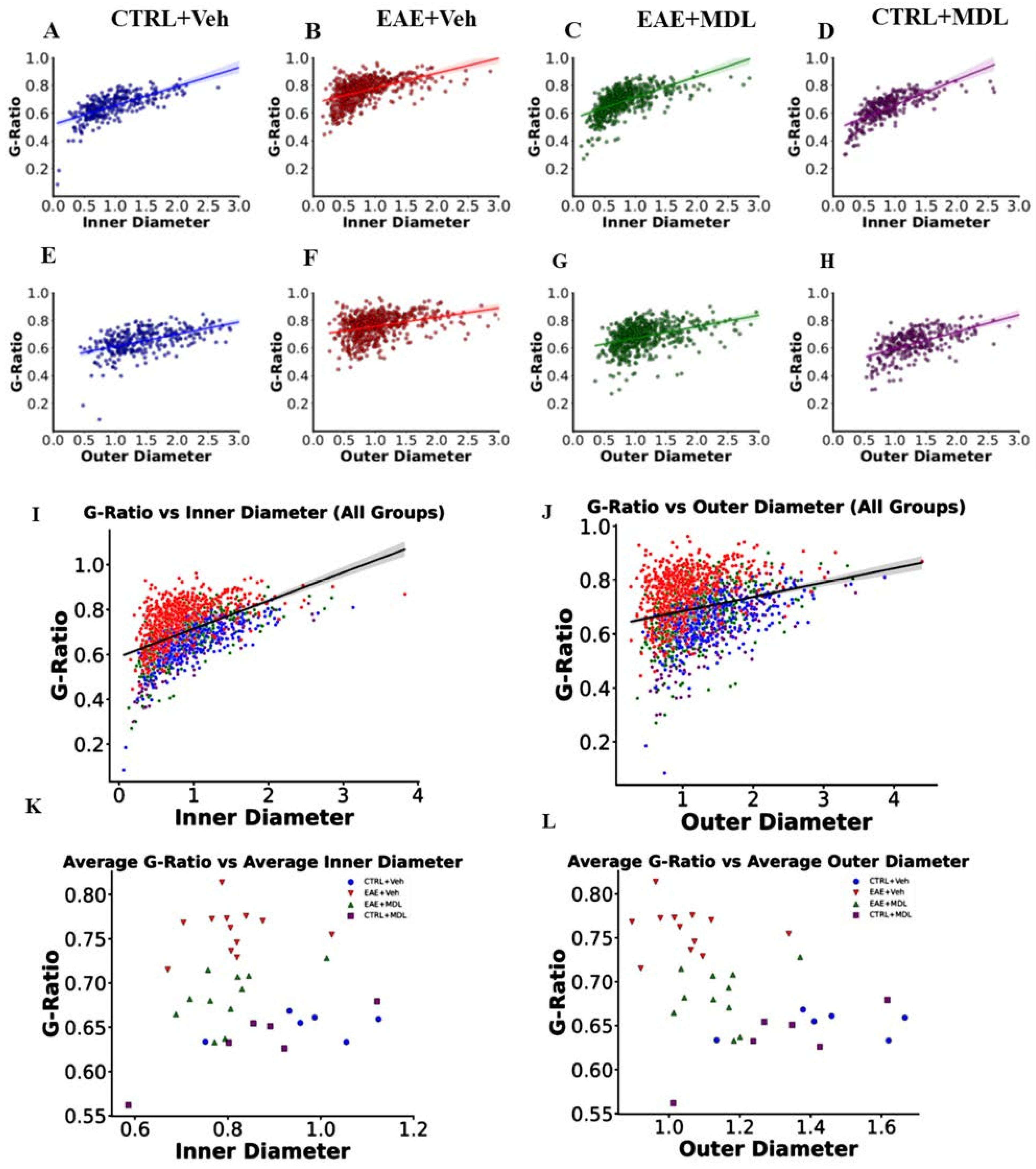
3.3. The Optic Nerve g-Ratios and Axon Diameter Relationship Is Altered in EAE
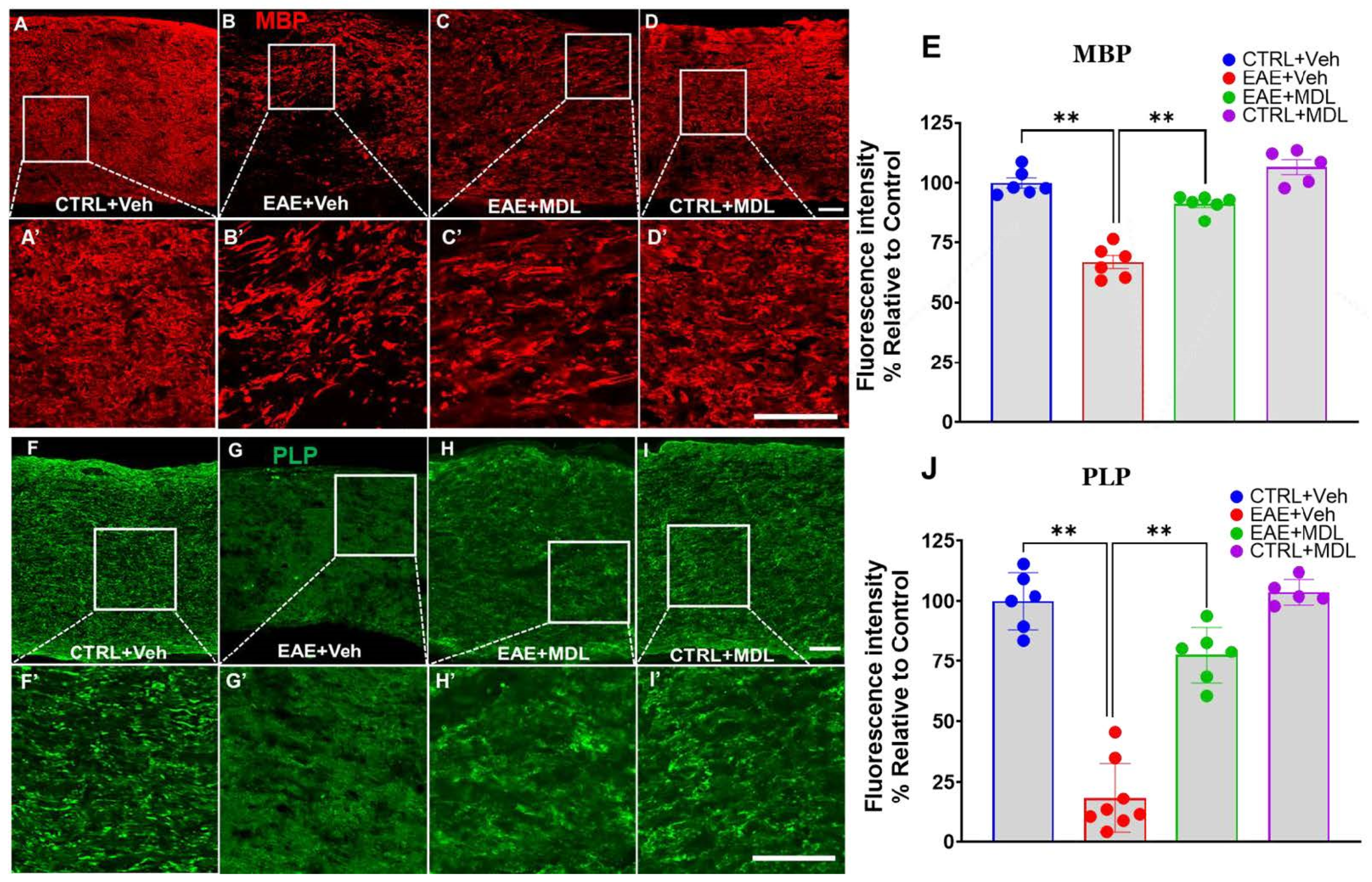
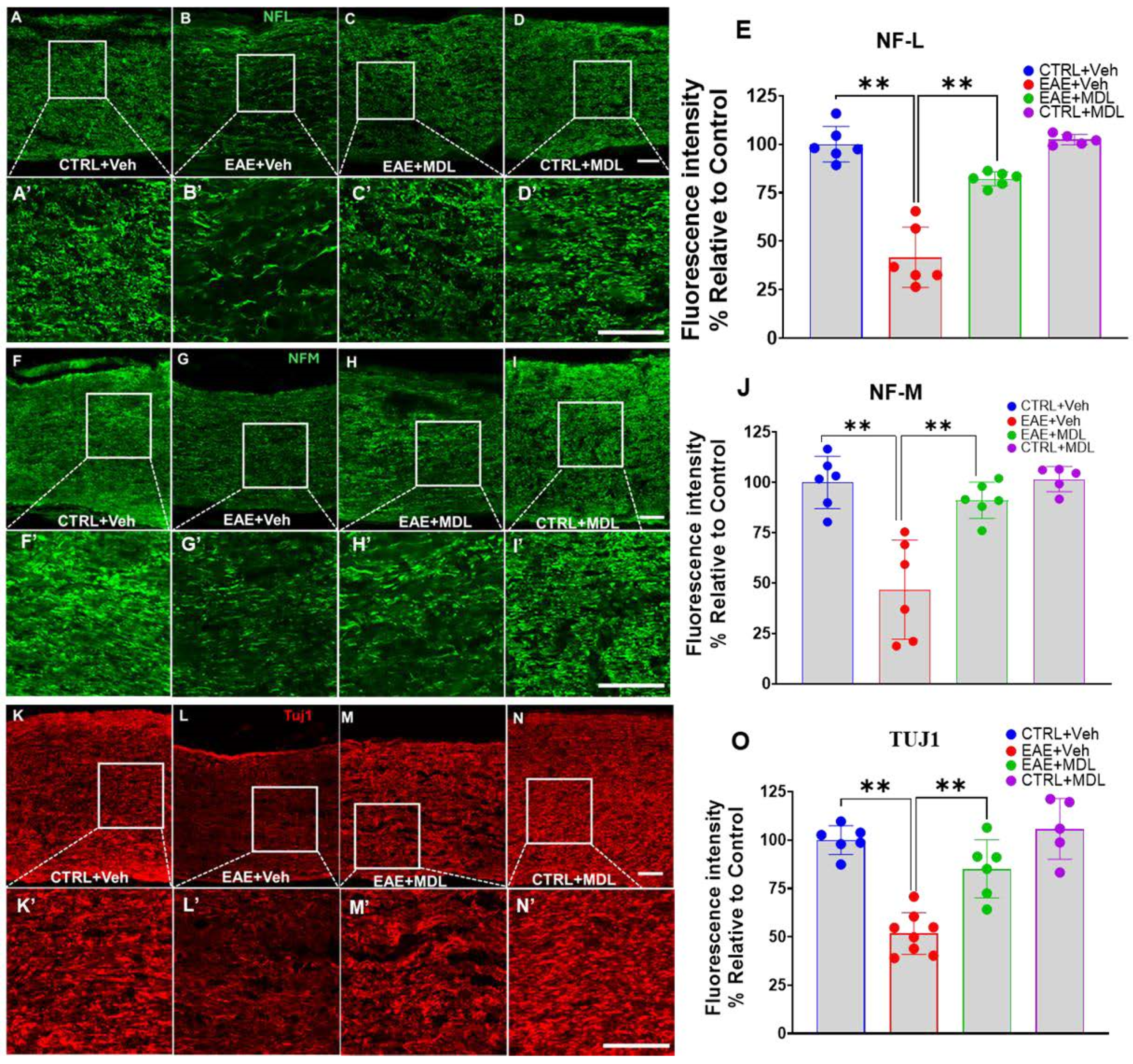
3.4. SMOX Inhibition Preserves Myelin Proteins in the EAE Optic Nerve
3.5. EAE-Induced Axonal Damage Is Reduced by SMOX Inhibition
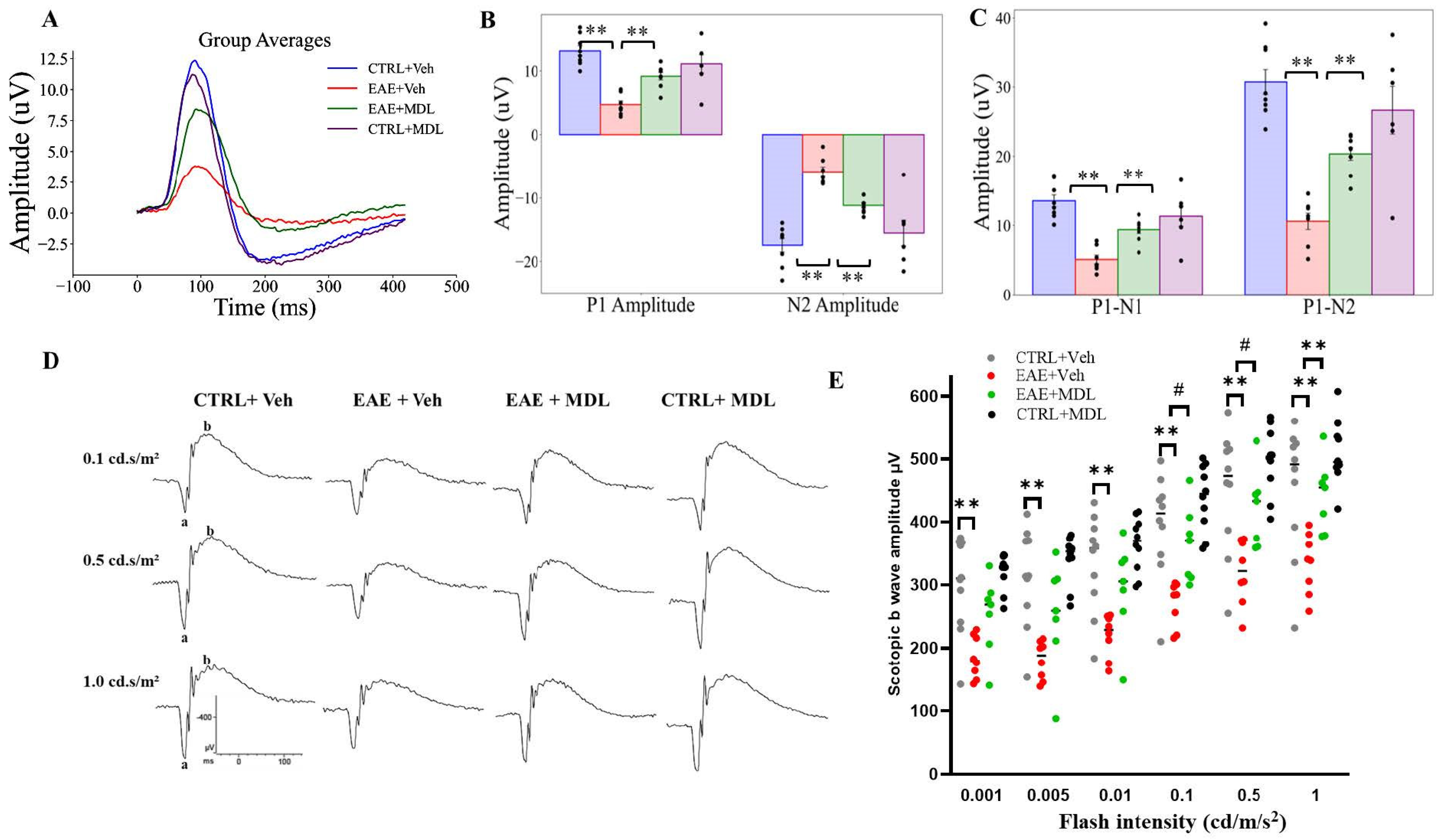
3.6. MDL72527 Treatment Rescues RGC Dysfunction in EAE Mice
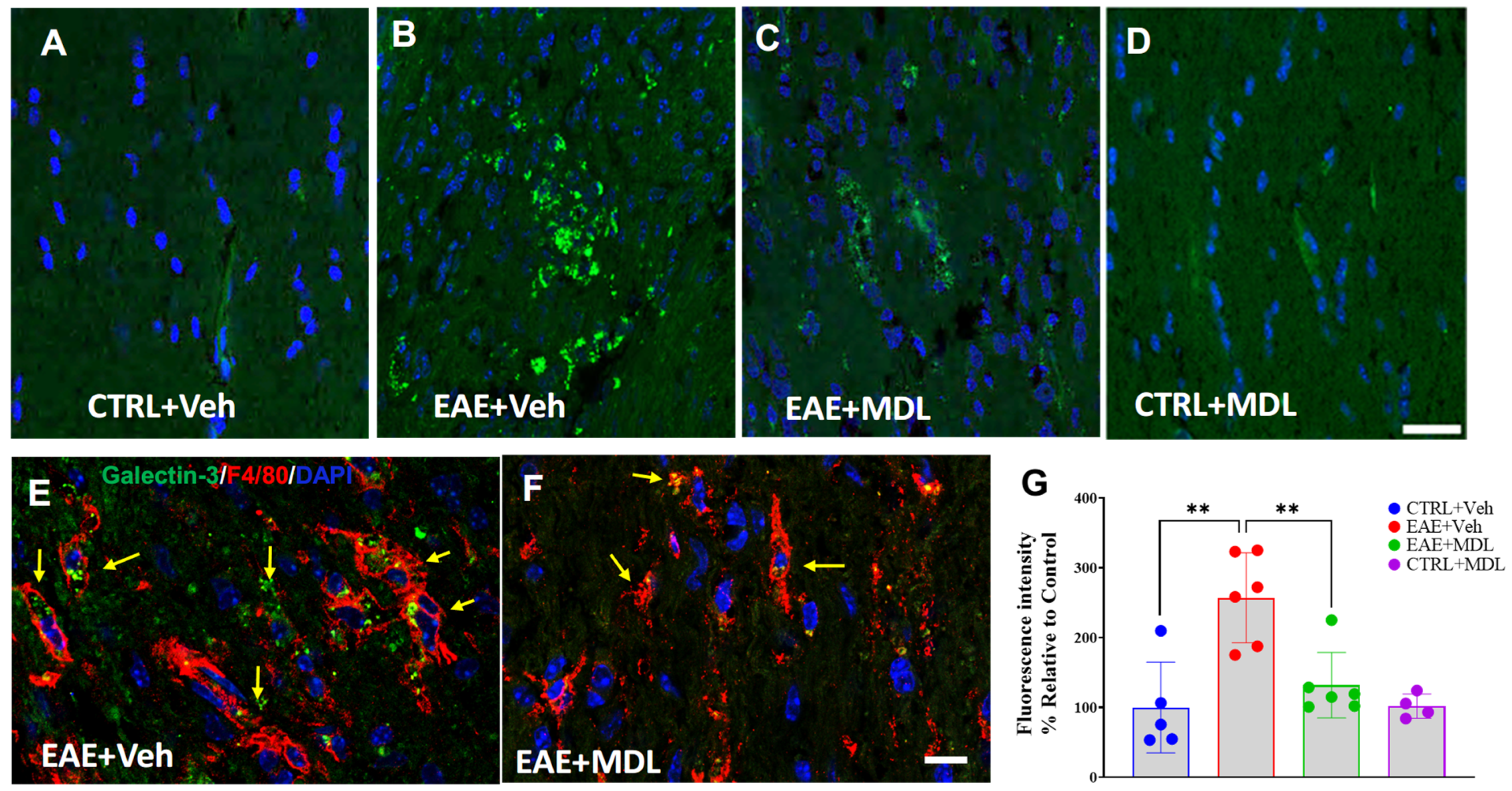
3.7. SMOX Inhibition Suppresses EAE-Induced Upregulation of Galectin 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, Z.; Li, Y.; Guan, Z.; Guo, P.; Zheng, K.; Du, Y.; Yin, S.; Chen, B.; Wang, H.; Jiang, J.; et al. Global, regional, and national burden of multiple sclerosis from 1990 to 2019: Findings of global burden of disease study 2019. Front. Public Health 2023, 11, 1073278. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sorensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Mike, A.; Glanz, B.I.; Hildenbrand, P.; Meier, D.; Bolden, K.; Liguori, M.; Dell’Oglio, E.; Healy, B.C.; Bakshi, R.; Guttmann, C.R. Identification and clinical impact of multiple sclerosis cortical lesions as assessed by routine 3T MR imaging. AJNR Am. J. Neuroradiol. 2011, 32, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M. Multiple sclerosis: Diagnosis, differential diagnosis, and clinical presentation. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 122, pp. 269–290. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Patti, F. Cognitive impairment in multiple sclerosis. Mult. Scler. 2009, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenet. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Barnett, M.H.; Yiannikas, C.; Parratt, J.; Matthews, J.; Graham, S.L.; Klistorner, A. Chronic demyelination exacerbates neuroaxonal loss in patients with MS with unilateral optic neuritis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e700. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Blanco, L.; Marzin, M.; Leistra, A.; van der Valk, P.; Nutma, E.; Amor, S. Immunopathology of the optic nerve in multiple sclerosis. Clin. Exp. Immunol. 2022, 209, 236–246. [Google Scholar] [CrossRef]
- Costello, F.; Coupland, S.; Hodge, W.; Lorello, G.R.; Koroluk, J.; Pan, Y.I.; Freedman, M.S.; Zackon, D.H.; Kardon, R.H. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 2006, 59, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kale, N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain 2016, 8, 195–202. [Google Scholar] [CrossRef]
- Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch. Neurol. 2008, 65, 727–732. [Google Scholar] [CrossRef]
- Wilhelm, H.; Schabet, M. The Diagnosis and Treatment of Optic Neuritis. Dtsch. Arztebl. Int. 2015, 112, 616–625, quiz 626. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.; Costello, F.; Chen, J.J.; Petzold, A.; Biousse, V.; Newman, N.J.; Galetta, S.L. Optic neuritis and autoimmune optic neuropathies: Advances in diagnosis and treatment. Lancet Neurol. 2023, 22, 89–100. [Google Scholar] [CrossRef]
- Kraker, J.A.; Chen, J.J. An update on optic neuritis. J. Neurol. 2023, 270, 5113–5126. [Google Scholar] [CrossRef] [PubMed]
- Benard-Seguin, E.; Costello, F. Optic neuritis: Current challenges in diagnosis and management. Curr. Opin. Neurol. 2023, 36, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L. Optic Neuritis. Continuum 2019, 25, 1236–1264. [Google Scholar] [CrossRef] [PubMed]
- Miura, G.; Wang, M.H.; Ivers, K.M.; Frishman, L.J. Retinal pathway origins of the pattern ERG of the mouse. Exp. Eye Res. 2009, 89, 49–62. [Google Scholar] [CrossRef]
- Stromnes, I.M.; Goverman, J.M. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006, 1, 1810–1819. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Martin, R.; Bergman, C.; Dalal, M.; Ruddle, N.H.; McFarland, H.F. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity 1993, 15, 137–143. [Google Scholar] [CrossRef]
- Gold, R.; Hartung, H.P.; Toyka, K.V. Animal models for autoimmune demyelinating disorders of the nervous system. Mol. Med. Today 2000, 6, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D.J.; McGown, A.; Jain, S.A.; De Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal models of multiple sclerosis: From rodents to zebrafish. Mult. Scler. 2019, 25, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, 2364. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Wilson, J.; Kolathur, K.K.; Mudgal, J.; Lewis, S.A.; Arora, D.; Nampoothiri, M. Spermidine as an epigenetic regulator of autophagy in neurodegenerative disorders. Eur. J. Pharmacol. 2024, 979, 176823. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Klotz, A.; Guven, A.; Kapadnis, U.; Ravipaty, S.; Tolstikov, V.; Vemulapalli, V.; Rodrigues, L.O.; Li, H.; Kellogg, M.D.; et al. Identification and validation of N-acetylputrescine in combination with non-canonical clinical features as a Parkinson’s disease biomarker panel. Sci. Rep. 2024, 14, 10036. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s Disease: Balancing Between Neurotoxicity and Neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H.; Zhang, J.; Yu, H.; Lin, Z.; Cai, Y. Spermidine Exhibits Protective Effects Against Traumatic Brain Injury. Cell. Mol. Neurobiol. 2020, 40, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Huttinger, F.; Morrison, R.; Murray-Stewart, T.; Casero, R.A.; Strauss, K.I. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 2010, 27, 515–525. [Google Scholar] [CrossRef]
- Yang, P.; Shi, M.; Jia, Y.; Zhong, C.; Peng, H.; Sun, L.; Guo, D.; Chen, J.; Wang, A.; Xu, T.; et al. Plasma Polyamines and Short-Term Adverse Outcomes Among Patients with Ischemic Stroke: A Prospective Cohort Study. J. Am. Heart Assoc. 2024, 13, e035837. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, T.W.; Lin, Y.Y.; Ho, W.C.; Tsai, H.C.; Chen, S.P.; Lin, A.M.; Liu, T.Y.; Wang, H.T. Acrolein is involved in ischemic stroke-induced neurotoxicity through spermidine/spermine-N1-acetyltransferase activation. Exp. Neurol. 2020, 323, 113066. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Komotar, R.J.; McCullough-Hicks, M.E.; Otten, M.L.; Starke, R.M.; Kellner, C.P.; Garrett, M.C.; Merkow, M.B.; Rynkowski, M.; Dash, K.A.; et al. The role of polyamine metabolism in neuronal injury following cerebral ischemia. Can. J. Neurol. Sci. 2009, 36, 14–19. [Google Scholar] [CrossRef]
- Wu, R.; Chen, X.; Kang, S.; Wang, T.; Gnanaprakasam, J.R.; Yao, Y.; Liu, L.; Fan, G.; Burns, M.R.; Wang, R. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci. Adv. 2020, 6, eabc4275. [Google Scholar] [CrossRef]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kaminski, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.E20. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine oxidase: Ten years after. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Saul, A.B.; Pichavaram, P.; Xu, Z.; Rudraraju, M.; Somanath, P.R.; Smith, S.B.; Caldwell, R.B.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Reduces Neurodegeneration and Improves Retinal Function in Diabetic Mice. J. Clin. Med. 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Brooks, M.; Barone, S.; Rahmati, N.; Murray Stewart, T.; Dunworth, M.; Destefano-Shields, C.; Dasgupta, N.; Davidson, S.; Lindquist, D.M.; et al. Ablation of polyamine catabolic enzymes provokes Purkinje cell damage, neuroinflammation, and severe ataxia. J. Neuroinflamm. 2020, 17, 301. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, M.; Wang, X.; Tian, Z.; Wang, J.; Fan, D.; Zeng, J.; Zhang, K.; Dai, X. Targeting Smox Is Neuroprotective and Ameliorates Brain Inflammation in Cerebral Ischemia/Reperfusion Rats. Toxicol. Sci. 2019, 168, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Sun, W.; Zheng, L.; Brookes, S.; Tully, M.; Shi, R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience 2011, 173, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Tang, J.; Zheng, L.; Acosta, G.; Tian, R.; Hayward, L.; Race, N.; Mattson, D.; Shi, R. Systemic Acrolein Elevations in Mice with Experimental Autoimmune Encephalomyelitis and Patients with Multiple Sclerosis. Front. Neurol. 2018, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Alfarhan, M.; Baker, L.; Shenoy, N.; Liao, Y.; Henry-Ojo, H.O.; Somanath, P.R.; Narayanan, S.P. Treatment with MDL72527 Ameliorated Clinical Symptoms, Retinal Ganglion Cell Loss, Optic Nerve Inflammation, and Improved Visual Acuity in an Experimental Model of Multiple Sclerosis. Cells 2022, 11, 4100. [Google Scholar] [CrossRef]
- Alfarhan, M.; Liu, F.; Shan, S.; Pichavaram, P.; Somanath, P.R.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Suppresses Excitotoxicity Induced Neuroinflammation in Mouse Retina. Int. J. Mol. Sci. 2022, 23, 2133. [Google Scholar] [CrossRef]
- Dogan, A.; Rao, A.M.; Hatcher, J.; Rao, V.L.; Baskaya, M.K.; Dempsey, R.J. Effects of MDL72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J. Neurochem. 1999, 72, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; DeFeo-Mattox, K.; Woster, P.M.; Gilmour, S.K. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids 2014, 46, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Alhonen, L.; Parkkinen, J.J.; Keinanen, T.; Sinervirta, R.; Herzig, K.H.; Janne, J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc. Natl. Acad. Sci. USA 2000, 97, 8290–8295. [Google Scholar] [CrossRef]
- Diaz, E.; Adhikary, S.; Tepper, A.; Riley, D.; Ortiz-Meoz, R.; Krosky, D.; Buyck, C.; Lamenca, C.M.; Llaveria, J.; Fang, L.; et al. Structure of human spermine oxidase in complex with a highly selective allosteric inhibitor. Commun. Biol. 2022, 5, 787. [Google Scholar] [CrossRef] [PubMed]
- Palani, C.D.; Fouda, A.Y.; Liu, F.; Xu, Z.; Mohamed, E.; Giri, S.; Smith, S.B.; Caldwell, R.B.; Narayanan, S.P. Deletion of Arginase 2 Ameliorates Retinal Neurodegeneration in a Mouse Model of Multiple Sclerosis. Mol. Neurobiol. 2019, 56, 8589–8602. [Google Scholar] [CrossRef] [PubMed]
- Candadai, A.A.; Liu, F.; Fouda, A.Y.; Alfarhan, M.; Palani, C.D.; Xu, Z.; Caldwell, R.B.; Narayanan, S.P. Deletion of arginase 2 attenuates neuroinflammation in an experimental model of optic neuritis. PLoS ONE 2021, 16, e0247901. [Google Scholar] [CrossRef] [PubMed]
- Mysona, B.A.; Segar, S.; Hernandez, C.; Kim, C.; Zhao, J.; Mysona, D.; Bollinger, K.E. QuPath Automated Analysis of Optic Nerve Degeneration in Brown Norway Rats. Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Bohorquez, J.; Toft-Nielsen, J.; Ozdamar, O.; Porciatti, V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Feuer, W.J.; Monsalve, P.; Triolo, G.; Vazquez, L.; McSoley, J.; Ventura, L.M. Head-down Posture in Glaucoma Suspects Induces Changes in IOP, Systemic Pressure, and PERG That Predict Future Loss of Optic Nerve Tissue. J. Glaucoma 2017, 26, 459–465. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, H.; Barwick, S.; Liu, Y.; Smith, S.B. Optimal timing for activation of sigma 1 receptor in the Pde6b(rd10)/J (rd10) mouse model of retinitis pigmentosa. Exp. Eye Res. 2021, 202, 108397. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, J.; Saul, A.; Smith, S.B. Comparison of Neuroprotective Effects of Monomethylfumarate to the Sigma 1 Receptor Ligand (+)-Pentazocine in a Murine Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5. [Google Scholar] [CrossRef]
- Stassart, R.M.; Mobius, W.; Nave, K.A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Marenna, S.; Huang, S.C.; Castoldi, V.; d’Isa, R.; Costa, G.D.; Comi, G.; Leocani, L. Functional evolution of visual involvement in experimental autoimmune encephalomyelitis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320963474. [Google Scholar] [CrossRef]
- Cairns, J.; Vavasour, I.M.; Traboulsee, A.; Carruthers, R.; Kolind, S.H.; Li, D.K.B.; Moore, G.R.W.; Laule, C. Diffusely abnormal white matter in multiple sclerosis. J. Neuroimaging 2022, 32, 5–16. [Google Scholar] [CrossRef]
- Schaffner, E.; Bosch-Queralt, M.; Edgar, J.M.; Lehning, M.; Strauss, J.; Fleischer, N.; Kungl, T.; Wieghofer, P.; Berghoff, S.A.; Reinert, T.; et al. Myelin insulation as a risk factor for axonal degeneration in autoimmune demyelinating disease. Nat. Neurosci. 2023, 26, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Groh, J.; Abdelwahab, T.; Kattimani, Y.; Horner, M.; Loserth, S.; Gudi, V.; Adalbert, R.; Imdahl, F.; Saliba, A.E.; Coleman, M.; et al. Microglia-mediated demyelination protects against CD8(+) T cell-driven axon degeneration in mice carrying PLP defects. Nat. Commun. 2023, 14, 6911. [Google Scholar] [CrossRef]
- Zhu, Y.; Pappas, A.C.; Wang, R.; Seifert, P.; Sun, D.; Jakobs, T.C. Ultrastructural Morphology of the Optic Nerve Head in Aged and Glaucomatous Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3984–3996. [Google Scholar] [CrossRef] [PubMed]
- Rashid Khan, M.; Fayaz Ahmad, S.; Nadeem, A.; Imam, F.; Al-Harbi, N.O.; Shahnawaz Khan, M.; Alsahli, M.; Alhosaini, K. Cathepsin-B inhibitor CA-074 attenuates retinopathy and optic neuritis in experimental autoimmune encephalomyelitis induced in SJL/J mice. Saudi Pharm. J. 2023, 31, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Alrashdi, B.; Dawod, B.; Tacke, S.; Kuerten, S.; Cote, P.D.; Marshall, J.S. Mice Heterozygous for the Sodium Channel Scn8a (Nav1.6) Have Reduced Inflammatory Responses During EAE and Following LPS Challenge. Front. Immunol. 2021, 12, 533423. [Google Scholar] [CrossRef] [PubMed]
- Jahn, O.; Siems, S.B.; Kusch, K.; Hesse, D.; Jung, R.B.; Liepold, T.; Uecker, M.; Sun, T.; Werner, H.B. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front. Cell. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Garbay, B.; Heape, A.M.; Sargueil, F.; Cassagne, C. Myelin synthesis in the peripheral nervous system. Prog. Neurobiol. 2000, 61, 267–304. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.C., 3rd; O’Muircheartaigh, J.; Dirks, H.; Travers, B.G.; Adluru, N.; Alexander, A.L.; Deoni, S.C.L. Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. Neuroimage 2016, 132, 225–237. [Google Scholar] [CrossRef]
- Duval, T.; Le Vy, S.; Stikov, N.; Campbell, J.; Mezer, A.; Witzel, T.; Keil, B.; Smith, V.; Wald, L.L.; Klawiter, E.; et al. g-Ratio weighted imaging of the human spinal cord in vivo. Neuroimage 2017, 145, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Rushton, W.A. A theory of the effects of fibre size in medullated nerve. J. Physiol. 1951, 115, 101–122. [Google Scholar] [CrossRef]
- West, K.L.; Kelm, N.D.; Carson, R.P.; Alexander, D.C.; Gochberg, D.F.; Does, M.D. Experimental studies of g-ratio MRI in ex vivo mouse brain. Neuroimage 2018, 167, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Stikov, N.; Campbell, J.S.; Stroh, T.; Lavelee, M.; Frey, S.; Novek, J.; Nuara, S.; Ho, M.K.; Bedell, B.J.; Dougherty, R.F.; et al. Quantitative analysis of the myelin g-ratio from electron microscopy images of the macaque corpus callosum. Data Brief. 2015, 4, 368–373. [Google Scholar] [CrossRef]
- Kaneko, S.; Wang, J.; Kaneko, M.; Yiu, G.; Hurrell, J.M.; Chitnis, T.; Khoury, S.J.; He, Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 2006, 26, 9794–9804. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Zhu, K.; Carlstrom, K.E. Axonal mitochondria adjust in size depending on g-ratio of surrounding myelin during homeostasis and advanced remyelination. J. Neurosci. Res. 2021, 99, 793–805. [Google Scholar] [CrossRef]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mork, S.; Bo, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lovas, G.; Szilagyi, N.; Majtenyi, K.; Palkovits, M.; Komoly, S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 2000, 123 Pt 2, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Smith, M.D.; Kersbergen, C.J.; Kam, T.I.; Viswanathan, M.; Martin, K.; Dawson, T.M.; Dawson, V.L.; Zack, D.J.; Whartenby, K.; et al. Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2019, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Fraher, J.; Dockery, P. A strong myelin thickness-axon size correlation emerges in developing nerves despite independent growth of both parameters. J. Anat. 1998, 193 Pt 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Cercignani, M.; Giulietti, G.; Dowell, N.G.; Gabel, M.; Broad, R.; Leigh, P.N.; Harrison, N.A.; Bozzali, M. Characterizing axonal myelination within the healthy population: A tract-by-tract mapping of effects of age and gender on the fiber g-ratio. Neurobiol. Aging 2017, 49, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Stikov, N.; Campbell, J.S.; Stroh, T.; Lavelee, M.; Frey, S.; Novek, J.; Nuara, S.; Ho, M.K.; Bedell, B.J.; Dougherty, R.F.; et al. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 2015, 118, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kleerekooper, I.; Del Porto, L.; Dell’Arti, L.; Guajardo, J.; Leo, S.; Robson, A.G.; Trip, S.A.; Petzold, A.; Plant, G.T.; Holder, G.E. Pattern ERGs suggest a possible retinal contribution to the visual acuity loss in acute optic neuritis. Doc. Ophthalmol. 2022, 145, 185–195. [Google Scholar] [CrossRef]
- Elwood, B.W.; Godwin, C.R.; Anders, J.J.; Kardon, R.H.; Gramlich, O.W. Correlation of Visual System Biomarkers with Motor Deficits in Experimental Autoimmune Encephalomyelitis-Optic Neuritis. Transl. Vis. Sci. Technol. 2024, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, O.W.; Brown, A.J.; Godwin, C.R.; Chimenti, M.S.; Boland, L.K.; Ankrum, J.A.; Kardon, R.H. Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, A.T.; Reinehr, S.; Kuhn, S.; Pedreiturria, X.; Petrikowski, L.; Faissner, S.; Ayzenberg, I.; Stute, G.; Gold, R.; Dick, H.B.; et al. Laquinimod protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2018, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Mdzomba, J.B.; Rodriguez, L.; Morin, F.; Vallieres, L.; Pernet, V. B cell-dependent EAE induces visual deficits in the mouse with similarities to human autoimmune demyelinating diseases. J. Neuroinflamm. 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Cordano, C.; Werneburg, S.; Abdelhak, A.; Bennett, D.J.; Beaudry-Richard, A.; Duncan, G.J.; Oertel, F.C.; Boscardin, W.J.; Yiu, H.H.; Jabassini, N.; et al. Synaptic injury in the inner plexiform layer of the retina is associated with progression in multiple sclerosis. Cell Rep. Med. 2024, 5, 101490. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Omi, N. Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front. Neuroanat. 2017, 11, 92. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Muller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Hare, W.A. Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vis. Res. 2000, 40, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.P.; Barnett, M.H.; Parratt, J.D.; Prineas, J.W. Multiple sclerosis: Distribution of inflammatory cells in newly forming lesions. Ann. Neurol. 2009, 66, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; Garcia-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennstrom, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Lozinski, B.M.; Ta, K.; Dong, Y. Emerging role of galectin 3 in neuroinflammation and neurodegeneration. Neural Regen. Res. 2024, 19, 2004–2009. [Google Scholar] [CrossRef]
- Xue, S.; Lozinski, B.M.; Ghorbani, S.; Ta, K.; D’Mello, C.; Yong, V.W.; Dong, Y. Elevated Galectin-3 Is Associated with Aging, Multiple Sclerosis, and Oxidized Phosphatidylcholine-Induced Neurodegeneration. J. Neurosci. 2023, 43, 4725–4737. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.E17. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.E9. [Google Scholar] [CrossRef] [PubMed]
- Barake, F.; Soza, A.; Gonzalez, A. Galectins in the brain: Advances in neuroinflammation, neuroprotection and therapeutic opportunities. Curr. Opin. Neurol. 2020, 33, 381–390. [Google Scholar] [CrossRef]
- Paganelli, A.; Diomede, F.; Marconi, G.D.; Pizzicannella, J.; Rajan, T.S.; Trubiani, O.; Paganelli, R. Inhibition of LPS-Induced Inflammatory Response of Oral Mesenchymal Stem Cells in the Presence of Galectin-3. Biomedicines 2023, 11, 1519. [Google Scholar] [CrossRef]
- Gobert, A.P.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Finley, J.L.; Smith, T.M.; McNamara, K.M.; Singh, K.; Sierra, J.C.; et al. Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology 2022, 162, 813–827.e8. [Google Scholar] [CrossRef]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Al-Greene, N.T.; Singh, K.; Coburn, L.A.; Sierra, J.C.; Verriere, T.G.; Luis, P.B.; Schneider, C.; Asim, M.; Allaman, M.M.; et al. Distinct Immunomodulatory Effects of Spermine Oxidase in Colitis Induced by Epithelial Injury or Infection. Front. Immunol. 2018, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Z.; Lin, P.; Zhang, H.; Wang, Y.; Zhang, T.; Wang, H.; Li, H.; Lin, L.; Zhao, Y.; et al. Knockdown of Smox protects the integrity of the blood-brain barrier through antioxidant effect and Nrf2 pathway activation in stroke. Int. Immunopharmacol. 2024, 126, 111183. [Google Scholar] [CrossRef]
- Pietropaoli, S.; Leonetti, A.; Cervetto, C.; Venturini, A.; Mastrantonio, R.; Baroli, G.; Persichini, T.; Colasanti, M.; Maura, G.; Marcoli, M.; et al. Glutamate Excitotoxicity Linked to Spermine Oxidase Overexpression. Mol. Neurobiol. 2018, 55, 7259–7270. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Mou, Z.; Jing, Y.; Jiang, R.; Sun, T. CircSmox knockdown alleviates PC12 cell apoptosis and inflammation in spinal cord injury by miR-340-5p/Smurf1 axis. Immun. Inflamm. Dis. 2023, 11, e824. [Google Scholar] [CrossRef] [PubMed]
- Fairley, L.H.; Lejri, I.; Grimm, A.; Eckert, A. Spermidine Rescues Bioenergetic and Mitophagy Deficits Induced by Disease-Associated Tau Protein. Int. J. Mol. Sci. 2023, 24, 5297. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Garg, G.; Singh, A.K.; Verma, A.K.; Bissoyi, A.; Rizvi, S.I. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging. Biogerontology 2021, 22, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Kochlamazashvili, G.; Eisenberg, T.; Racz, B.; Michael, E.; Toppe, D.; Stumpf, A.; Wirth, A.; Zeug, A.; Muller, F.E.; et al. Spermidine protects from age-related synaptic alterations at hippocampal mossy fiber-CA3 synapses. Sci. Rep. 2019, 9, 19616. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Shirooie, S.; Noori, T.; Sheibani, M.; Tavangar, S.M.; Hemmati, S.; Sadeghi, M.A.; Akbarniakhaky, H.; Mohammadi, Z.; Foroutani, L.; et al. Spermidine reduced neuropathic pain in chronic constriction injury-induced peripheral neuropathy in rats. Fundam. Clin. Pharmacol. 2023, 37, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis. 2015, 6, e1720. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanigawa, T.; Nadatani, Y.; Otani, K.; Machida, H.; Okazaki, H.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; et al. Mitochondrial disorders in NSAIDs-induced small bowel injury. J. Clin. Biochem. Nutr. 2011, 48, 117–121. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Chaturvedi, R.; de Sablet, T.; Asim, M.; Piazuelo, M.B.; Barry, D.P.; Verriere, T.G.; Sierra, J.C.; Hardbower, D.M.; Delgado, A.G.; Schneider, B.G.; et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015, 34, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Tempera, G.; Dalla Vedova, L.; Condello, M.; Arancia, G. MDL 72527 and spermine oxidation products induce a lysosomotropic effect and mitochondrial alterations in tumour cells. Biochem. Soc. Trans. 2007, 35, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Allanach, J.R.; Farrell, J.W., 3rd; Mesidor, M.; Karimi-Abdolrezaee, S. Current status of neuroprotective and neuroregenerative strategies in multiple sclerosis: A systematic review. Mult. Scler. 2022, 28, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.D.; Banerji, T.; Tagge, I.J.; Kirkemo, L.L.; Chaudhary, P.; Calkins, E.; Galipeau, D.; Shokat, M.D.; DeBell, M.J.; Van Leuven, S.; et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight 2019, 4, e126329. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Su, T.; Verkman, A.S. Clobetasol promotes remyelination in a mouse model of neuromyelitis optica. Acta Neuropathol. Commun. 2016, 4, 42. [Google Scholar] [CrossRef]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A.; International Conference on Cell-Based Therapies for Multiple Sclerosis. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Bardia, A.; Tiwari, S.K.; Paspala, S.A.; Khan, A.A. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J. Adv. Res. 2014, 5, 277–294. [Google Scholar] [CrossRef]
- Gresle, M.M.; Liu, Y.; Kilpatrick, T.J.; Kemper, D.; Wu, Q.Z.; Hu, B.; Fu, Q.L.; So, K.F.; Sheng, G.; Huang, G.; et al. Blocking LINGO-1 in vivo reduces degeneration and enhances regeneration of the optic nerve. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316641704. [Google Scholar] [CrossRef]
| Primary Antibody | Species | Dilution | Source/Catalog No |
|---|---|---|---|
| Myelin basic protein (MBP) | Rat | 1:200 | EMD-Millipore/MAB386 (Burlington, MA, USA) |
| Proteolipid protein (PLP) | Chicken | 1:200 | Novus Biological/Nb100-1608 (Centennial, CO, USA) |
| Neurofilament L (NF-L) | Rabbit | 1:100 | EMD-Millipore/AB9568 (Burlington, MA, USA) |
| Neurofilament M (NF-M) | Chicken | 1:100 | EMD-Millipore/AB5735 (Burlington, MA, USA) |
| anti-βIII-tubulin (TUJ1) | Mouse | 1:200 | Biolegend/801202 (San diego, CA, USA) |
| F4/80 | Rat | 1:200 | Abcam/ab6640 (Waltham, MA, USA) |
| Galectin3 (Gal3) | Rabbit | 1:150 | Lifespan Biosciences/C357474 (Newark, CA, USA) |
| Secondary Antibody | Dilution | Catalog No | |
| Donkey anti-rabbit | 1:500 | Invitrogen (A21206) (Carlsbad, CA, USA) | |
| Donkey anti-rat | 1:500 | Invitrogen (A21208) (Carlsbad, CA, USA) | |
| Donkey anti-mouse | 1:500 | Invitrogen (A31570) (Carlsbad, CA, USA) | |
| Donkey anti-chicken | 1:500 | Invitrogen (A11039) (Carlsbad, CA, USA) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry-Ojo, H.O.; Liu, F.; Narayanan, S.P. Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis. Biomolecules 2025, 15, 158. https://doi.org/10.3390/biom15020158
Henry-Ojo HO, Liu F, Narayanan SP. Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis. Biomolecules. 2025; 15(2):158. https://doi.org/10.3390/biom15020158
Chicago/Turabian StyleHenry-Ojo, Harry O., Fang Liu, and S. Priya Narayanan. 2025. "Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis" Biomolecules 15, no. 2: 158. https://doi.org/10.3390/biom15020158
APA StyleHenry-Ojo, H. O., Liu, F., & Narayanan, S. P. (2025). Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis. Biomolecules, 15(2), 158. https://doi.org/10.3390/biom15020158






