Investigating the Eye as a Biomarker of Gulf War Illness: Sphingolipid and Eicosanoid Composition in Tears and Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Clinical Examination
2.4. Sample Collection
2.4.1. Tear Collection
2.4.2. Plasma Collection
2.5. Tear Biomarker Analysis
2.5.1. Tear Sphingolipid Extraction and Analysis
2.5.2. Tear Eicosanoid Extraction and Analysis
2.6. Plasma Biomarker Analysis
2.6.1. Plasma Sphingolipid Extraction and Analysis
2.6.2. Plasma Eicosanoid Extraction and Analysis
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Ocular Symptoms and Signs in GWI Cases and Controls
3.3. Tear Biomarkers
3.4. Plasma Biomarkers
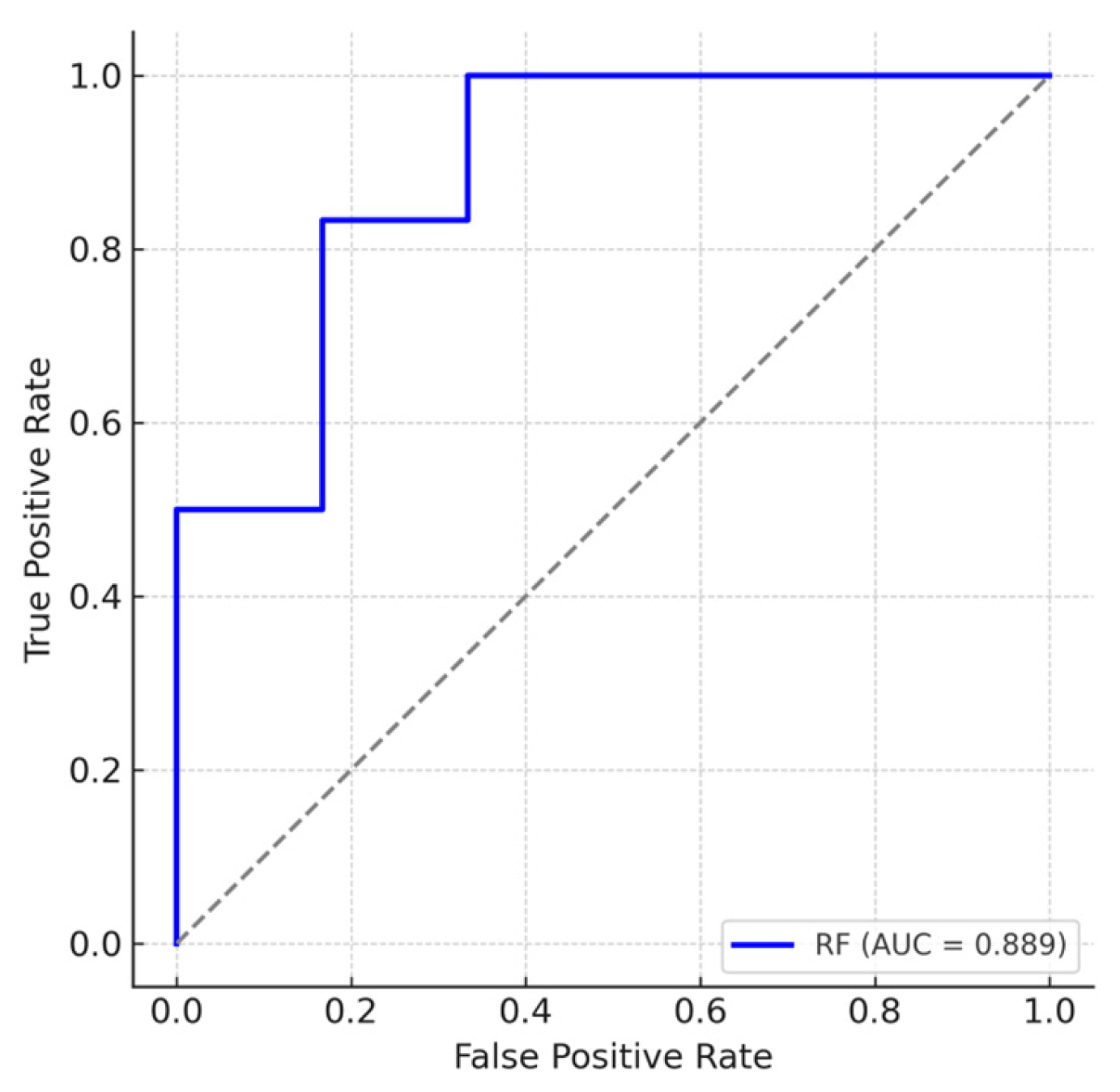
3.5. Multivariate Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (±)11,12-DHET | 11,12-Dihydroxyeicosatrienoic Acid (racemic mixture) |
| 12-HETE | 12-Hydroxyeicosatetraenoic Acid |
| (±)14(15)-EET | 14(15)-Epoxyeicosatrienoic Acid (racemic mixture) |
| (±)14,15-DHET | 14,15-Dihydroxyeicosatrienoic Acid (racemic mixture) |
| 15-HETE | 15-Hydroxyeicosatetraenoic Acid |
| 20-HETE | 20-Hydroxyeicosatetraenoic Acid |
| 5-HETE | 5-Hydroxyeicosatetraenoic Acid |
| 5-OxoETE | 5-Oxo-Eicosatetraenoic Acid |
| (±)8(9)-EET | 8(9)-Epoxyeicosatrienoic Acid (racemic mixture) |
| (±)8,9-DHET | 8,9-Dihydroxyeicosatrienoic Acid (racemic mixture) |
| AA | Arachidonic Acid |
| ASA | Aspirin |
| AUC | Area Under the Receiver Operating Characteristic (ROC) Curve |
| Bcl6 | B-Cell Lymphoma 6 |
| BH | Benjamini–Hochberg (multiple comparison adjustment) |
| C1P | Ceramide-1-Phosphate |
| CAD | Collisionally Activated Dissociation |
| CDC | Centers for Disease Control and Prevention |
| Cer | Ceramides (chain length indicated by Cxx) |
| Cer% of Total | Ceramide Percent of Total Sphingolipids |
| CI | Confidence Interval |
| CISS | Convergence Insufficiency Symptoms Survey |
| CRP | C-Reactive Protein |
| Csf2 | Colony-Stimulating Factor 2 |
| CYP 450 | Cytochrome P450 |
| CYP–EET | Cytochrome P450–Epoxyeicosatrienoic Acid Pathway |
| d18:1–C16:0 | Ceramide with sphingoid base d18:1 and fatty acid 16:0 |
| DED | Dry Eye Disease |
| DEET | N,N-Diethyl-Meta-Toluamide |
| DEQ-5 | 5-Item Dry Eye Questionnaire |
| DH | Dihydro-form of the lipid species (saturated sphingoid base) |
| DHA | Docosahexaenoic Acid |
| DHETs | Dihydroxyeicosatrienoic Acids |
| DHGLA | Dihomo-γ-Linolenic Acid |
| EDTA | Ethylenediaminetetraacetic Acid |
| eLPE | Ether Lysophosphatidylethanolamine |
| EPA | Eicosapentaenoic Acid |
| ESI-MS/MS | Electrospray Ionization Tandem Mass Spectrometry |
| EtOH:dH2O | Ethanol–Distilled Water mixture |
| F2α | Prostaglandin F2 Alpha |
| FDR | False Discovery Rate |
| GW | Gulf War |
| GWI | Gulf War Illness |
| GWIRCs | Gulf War Illness–Related Chemicals |
| Hex-Cer | Hexosylceramide |
| HIPAA | Health Insurance Portability and Accountability Act |
| IL | Interleukin |
| IS | Internal Standard |
| LB4 | Leukotriene B4 |
| LC | Liquid Chromatography |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| MeOH:CHCl3 | Methanol:chloroform |
| MFIS | Modified Fatigue Impact Scale |
| MGD | Meibomian Gland Dysfunction |
| MHC | Monohexosyl Ceramide (chain length indicated by Cxx) |
| MHC % of Total | Monohexosyl Ceramide Percent of Total Sphingolipids |
| MMP | Matrix Metalloproteinase |
| MRM | Multiple Reaction Monitoring |
| ND | Non-Detectable |
| NFκB1 | Nuclear Factor Kappa B Subunit 1 |
| NPSI-E | Neuropathic Pain Symptom Inventory modified for the Eye |
| NRS | Numeric Rating Scale |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| OD | Right Eye (Oculus Dexter) |
| OR | Odds Ratio |
| OS | Left Eye (Oculus Sinister) |
| OSDI | Ocular Surface Disease Index |
| PB | Pyridostigmine Bromide |
| PC | Phosphatidylcholine |
| PCL-M | PTSD Checklist—Military Version |
| PER | Permethrin (used as shorthand for the rodent model exposure) |
| PET | Positron Emission Tomography |
| PGA2 | Prostaglandin A2 |
| PGD2 | Prostaglandin D2 |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2 Alpha |
| PHQ-9 | Patient Health Questionnaire-9 |
| PL | Phospholipid |
| PSQI | Pittsburgh Sleep Quality Index |
| PTSD | Post-Traumatic Stress Disorder |
| PUFA | Polyunsaturated Fatty Acid |
| Q1 | Quadrupole 1 |
| Q2 | Quadrupole 2 |
| Q3 | Quadrupole 3 |
| Resolvin D1 | Specialized Pro-resolving Mediator Resolvin D1 |
| ROC | Receiver Operating Characteristic |
| S/N | Signal-to-Noise Ratio |
| S1P | Sphingosine-1-Phosphate |
| Sa | Sphinganine |
| Sa1P | Sphinganine-1-Phosphate |
| SCUD | Surface-to-Surface Missile (common term for Iraqi missiles in Gulf War) |
| SD | Standard Deviation |
| sEH | Soluble Epoxide Hydrolase |
| SF-12 | 12-Item Short Form Survey |
| SM | Sphingomyelins (chain length indicated by Cxx) |
| SM% of Total | Sphingomyelins Percent of Total Sphingolipids |
| So | Sphingosine |
| SPE | Solid-Phase Extraction |
| Sph | Sphingosine |
| SPL | Sphingolipids |
| SPL Total | Total Sphingolipids |
| SPSS | IBM Statistical Package for the Social Sciences |
| SS | Symptom Severity Scale |
| T2D | Type 2 Diabetes |
| TBUT | Tear Break-Up Time |
| TNF-α | Tumor Necrosis Factor alpha |
| Total Cer | Total Ceramides |
| Total MHC | Total Monohexosyl Ceramide |
| Total SM | Total Sphingomyelins |
| TSPO | 18 kDa Translocator Protein |
| TXB2 | Thromboxane B2 |
| UPLC | Ultra-Performance Liquid Chromatography |
| VA | Veterans Affairs |
| WPI | Widespread Pain Index |
References
- Institute of Medicine (US) Committee on Gulf War and Health. Gulf War and Health: Volume 8: Update of Health Effects of Serving in the Gulf War; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- White, R.F.; Steele, L.; O’Callaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C.; et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef]
- Steele, L. Prevalence and Patterns of Gulf War Illness in Kansas Veterans: Association of Symptoms with Characteristics of Person, Place, and Time of Military Service. Am. J. Epidemiol. 2000, 152, 992–1002. [Google Scholar] [CrossRef]
- Baksh, B.S.; Zayan, K.L.; Goldhardt, R.; Felix, E.R.; Klimas, N.; Galor, A. Ocular manifestations and biomarkers of Gulf War Illness in US veterans. Sci. Rep. 2021, 11, 6548. [Google Scholar] [CrossRef]
- Sanchez, V.; Baksh, B.S.; Cabrera, K.; Choudhury, A.; Jensen, K.; Klimas, N.; Galor, A. Dry Eye Symptoms and Signs in US Veterans with Gulf War Illness. Am. J. Ophthalmol. 2022, 237, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Madhu, L.N.; Attaluri, S.; Kodali, M.; Shuai, B.; Upadhya, R.; Gitai, D.; Shetty, A.K. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav. Immun. 2019, 81, 430–443. [Google Scholar] [CrossRef]
- Kerr, K.J. Gulf War illness: An overview of events, most prevalent health outcomes, exposures, and clues as to pathogenesis. Rev. Environ. Health 2015, 30, 273–286. [Google Scholar] [CrossRef]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex factors in the etiology of Gulf War illness: Wartime exposures and risk factors in veteran subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef]
- Alshelh, Z.; Albrecht, D.S.; Bergan, C.; Akeju, O.; Clauw, D.J.; Conboy, L.; Edwards, R.R.; Kim, M.; Lee, Y.C.; Protsenko, E.; et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav. Immun. 2020, 87, 498–507. [Google Scholar] [CrossRef]
- Shetty, G.A.; Hattiangady, B.; Upadhya, D.; Bates, A.; Attaluri, S.; Shuai, B.; Kodali, M.; Shetty, A.K. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front. Mol. Neurosci. 2017, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Ottenweller, J.E.; Dickens, B.F.; Mahmoud, F.F.; Levine, P.H. Activity of Paraoxonase/Arylesterase and Butyrylcholinesterase in Peripheral Blood of Gulf War Era Veterans with Neurologic Symptom Complexes or Post-Traumatic Stress Disorder. J. Occup. Environ. Med. 2017, 59, 1000–1006, Correction in J. Occup. Environ. Med. 2018, 60, e74–e75.. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; DeMeirleir, K.L.; Rawat, S.; Berk, G.S.; Gaynor-Berk, R.S.; Mijatovic, T.; Blatt, N.; Rizvanov, A.A.; Young, S.G.; Lombardi, V.C. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine 2015, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Fletcher, M.A.; Gallagher, M.; Barnes, Z.; Vernon, S.D.; Klimas, N.G. Exploring the diagnostic potential of immune biomarker coexpression in Gulf War Illness. Methods Mol. Biol. 2012, 934, 145–164. [Google Scholar]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Wang, L.; Monk, J.M.; Bright, A.T.; Koslik, H.J.; Ritchie, J.B.; Golomb, B.A. Metabolic features of Gulf War illness. PLoS ONE 2019, 14, e0219531. [Google Scholar] [CrossRef]
- Emmerich, T.; Zakirova, Z.; Klimas, N.; Sullivan, K.; Shetty, A.K.; Evans, J.E.; Ait-Ghezala, G.; Laco, G.S.; Hattiangady, B.; Shetty, G.A.; et al. Phospholipid profiling of plasma from GW veterans and rodent models to identify potential biomarkers of Gulf War Illness. PLoS ONE 2017, 12, e0176634. [Google Scholar] [CrossRef]
- Dickey, B.; Madhu, L.N.; Shetty, A.K. Gulf War Illness: Mechanisms Underlying Brain Dysfunction and Promising Therapeutic Strategies. Pharmacol. Ther. 2021, 220, 107716. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, V.; Tan, J.; Nguyen, J.; Lee, J.; Allegood, J.; Galor, A.; Mandal, N. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul. Surf. 2019, 17, 318–326. [Google Scholar] [CrossRef]
- Gary, A.A.; Prislovsky, A.; Tovar, A.; Locatelli, E.; Felix, E.R.; Stephenson, D.; Chalfant, C.E.; Lai, J.; Kim, C.; Mandal, N.; et al. Lipids from ocular meibum and tears may serve as biomarkers for depression and post-traumatic stress disorder. Clin. Exp. Ophthalmol. 2024, 52, 516–527. [Google Scholar] [CrossRef]
- Stimpson, N.J.; Thomas, H.V.; Weightman, A.L.; Dunstan, F.; Lewis, G. Psychiatric disorder in veterans of the Persian Gulf War of 1991: Systematic review. Br. J. Psychiatry 2003, 182, 391–403. [Google Scholar] [CrossRef]
- Sidebottom, A.C.; Harrison, P.A.; Godecker, A.; Kim, H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch. Womens Ment. Health 2012, 15, 367–374. [Google Scholar] [CrossRef]
- Wilkins, K.C.; Lang, A.J.; Norman, S.B. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety 2011, 28, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Insana, S.P.; Hall, M.; Buysse, D.J.; Germain, A. Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J. Trauma. Stress 2013, 26, 192–200. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Motl, R.W. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J. Neurol. Sci. 2013, 331, 102–107. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.; Feuer, W.; Galor, A.; Bouhassira, D.; Levitt, R.C.; Sarantopoulos, C.D.; Felix, E.R. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019, 160, 1541–1550. [Google Scholar] [CrossRef]
- Rouse, M.; Borsting, E.; Mitchell, G.L.; Cotter, S.A.; Kulp, M.; Scheiman, M.; Barnhardt, C.; Bade, A.; Yamada, T. Validity of the convergence insufficiency symptom survey: A confirmatory study. Optom. Vis. Sci. 2009, 86, 357–363, Correction in Optom. Vis. Sci. 2009, 86, 786.. [Google Scholar] [CrossRef]
- Crane, A.M.; Feuer, W.; Felix, E.R.; Levitt, R.C.; McClellan, A.L.; Sarantopoulos, K.D.; Galor, A. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: Incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br. J. Ophthalmol. 2017, 101, 1238–1243. [Google Scholar] [CrossRef]
- Pult 5-Grade Scale for Meibomian Gland Atrophy. Dr. Heiko Pult, Optometry & Vision Research, Germany. Available online: www.heiko-pult.de (accessed on 1 June 2025).
- Shaner, R.L.; Allegood, J.C.; Park, H.; Wang, E.; Kelly, S.; Haynes, C.A.; Sullards, M.C.; Merrill, A.H., Jr. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 2009, 50, 1692–1707. [Google Scholar] [CrossRef]
- Qi, H.; Priyadarsini, S.; Nicholas, S.E.; Sarker-Nag, A.; Allegood, J.; Chalfant, C.E.; Mandal, N.A.; Karamichos, D. Analysis of sphingolipids in human corneal fibroblasts from normal and keratoconus patients. J. Lipid Res. 2017, 58, 636–648. [Google Scholar] [CrossRef]
- Stephenson, D.J.; MacKnight, H.P.; Hoeferlin, L.A.; Washington, S.L.; Sawyers, C.; Archer, K.J.; Strauss, J.F., 3rd; Walsh, S.W.; Chalfant, C.E. Bioactive lipid mediators in plasma are predictors of preeclampsia irrespective of aspirin therapy. J. Lipid Res. 2023, 64, 100377. [Google Scholar] [CrossRef]
- Maus, K.D.; Stephenson, D.J.; Macknight, H.P.; Vu, N.T.; Hoeferlin, L.A.; Kim, M.; Diegelmann, R.F.; Xie, X.; Chalfant, C.E. Skewing cPLAα activity toward oxoeicosanoid production promotes neutrophil N2 polarization, wound healing, and the response to sepsis. Sci. Signal 2023, 16, eadd6527. [Google Scholar] [CrossRef] [PubMed]
- Al-Husseini, A.; Wijesinghe, D.S.; Farkas, L.; Kraskauskas, D.; Drake, J.I.; Van Tassel, B.; Abbate, A.; Chalfant, C.E.; Voelkel, N.F. Increased eicosanoid levels in the Sugen/chronic hypoxia model of severe pulmonary hypertension. PLoS ONE 2015, 10, e0120157. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. Omega-3 Tear Film Lipids Correlate with Clinical Measures of Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Dutta, D.; Lu, S.; Bellen, H.J. Sphingolipids in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1137893. [Google Scholar] [CrossRef]
- Gorica, E.; Calderone, V. Arachidonic Acid Derivatives and Neuroinflammation. CNS Neurol. Disord. Drug Targets 2022, 21, 118–129. [Google Scholar] [CrossRef]
- Dagogo-Jack, S.; Asuzu, P.; Wan, J.; Grambergs, R.; Stentz, F.; Mandal, N. Plasma Ceramides and Other Sphingolipids in Relation to Incident Prediabetes in a Longitudinal Biracial Cohort. J. Clin. Endocrinol. Metab. 2024, 109, 2530–2540. [Google Scholar] [CrossRef]
- Mielke, M.M.; Bandaru, V.V.R.; Haughey, N.J.; Xia, J.; Fried, L.P.; Yasar, S.; Albert, M.; Varma, V.; Harris, G.; Schneider, E.B.; et al. Serum ceramides increase the risk of Alzheimer disease. Neurology 2012, 79, 633–641. [Google Scholar] [CrossRef]
- Golomb, B.A.; Koslik, H.J.; Christians, U.; Ritchie, J.; Wilson, P.; Elkins, N.; Klawitter, J.; Klawitter, J.; Smith, D.; Repine, J.E. Depressed prostaglandins and leukotrienes in veterans with Gulf War illness. J. Environ. Sci. Health B 2019, 54, 623–639. [Google Scholar] [CrossRef]
- Ek, M.; Engblom, D.; Saha, S.; Blomqvist, A.; Jakobsson, P.J.; Ericsson-Dahlstrand, A. Inflammatory response: Pathway across the blood-brain barrier. Nature 2001, 410, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.P.; Srivastava, P.K.; Dev, R.; Dastidar, S.G.; Ray, A. Prostaglandin E synthase inhibition as a therapeutic target. Expert Opin. Ther. Targets 2009, 13, 849–865. [Google Scholar] [CrossRef]
- Shim, J.; Park, C.; Lee, H.S.; Park, M.S.; Lim, H.T.; Chauhan, S.; Dana, R.; Lee, H.; Lee, H.K. Change in Prostaglandin Expression Levels and Synthesizing Activities in Dry Eye Disease. Ophthalmology 2012, 119, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009, 50, S52–S56. [Google Scholar] [CrossRef]
- Gulbins, E.; Walter, S.; Becker, K.A.; Halmer, R.; Liu, Y.; Reichel, M.; Edwards, M.J.; Müller, C.P.; Fassbender, K.; Kornhuber, J. A central role for the acid sphingomyelinase/ceramide system in neurogenesis and major depression. J. Neurochem. 2015, 134, 183–192. [Google Scholar] [CrossRef]
- Schumacher, F.; Edwards, M.J.; Mühle, C.; Carpinteiro, A.; Wilson, G.C.; Wilker, B.; Soddemann, M.; Keitsch, S.; Scherbaum, N.; Müller, B.W.; et al. Ceramide levels in blood plasma correlate with major depressive disorder severity and its neutralization abrogates depressive behavior in mice. J. Biol. Chem. 2022, 298, 102185. [Google Scholar] [CrossRef]
- Mühle, C.; Reichel, M.; Gulbins, E.; Kornhuber, J. Sphingolipids in psychiatric disorders and pain syndromes. In Handbook of Experimental Pharmacology; Springer: Vienna, Austria, 2013; Volume 216, pp. 431–456. [Google Scholar]
- Konjevod, M.; Sáiz, J.; Nikolac Perkovic, M.; Nedic Erjavec, G.; Tudor, L.; Uzun, S.; Kozumplik, O.; Barbas, C.; Zarkovic, N.; Pivac, N.; et al. Plasma lipidomics in subjects with combat posttraumatic stress disorder. Free Radic. Biol. Med. 2022, 189, 169–177. [Google Scholar] [CrossRef]
- Carreras, I.; Jung, Y.; Lopez-Benitez, J.; Tognoni, C.M.; Dedeoglu, A. Fingolimod mitigates memory loss in a mouse model of Gulf War Illness amid decreasing the activation of microglia, protein kinase R, and NFkappaB. Neurotoxicology 2023, 96, 197–206. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | GWI (n = 19) | Controls (n = 21) | Raw p-Value | Adjusted p-Value (FDR) |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean ± SD (years) | 56.7 ± 5.2 | 55.7 ± 5.4 | 0.57 | 0.72 |
| Sex, male % (n) | 89.5% (17) | 85.7% (18) | 1.00 | 1.00 |
| Race, White % (n) | 63.2% (12) | 42.9% (9) | 0.15 | 0.28 |
| Race, Black % (n) | 31.6% (6) | 57.1% (12) | ||
| Race, Asian % (n) | 5.3% (1) | 0% (0) | ||
| Ethnicity, Hispanic % (n) | 36.8% (7) | 38.1% (8) | 0.94 | 0.96 |
| Comorbidities % (n) | ||||
| Diabetes mellitus | 21.1% (4) | 19.0% (4) | 1.00 | 0.93 |
| PTSD | 47.4% (9) | 14.3% (3) | 0.02 | 0.19 |
| Depression | 36.8% (7) | 23.8% (5) | 0.37 | 0.57 |
| Sleep apnea | 68.4% (13) | 23.8% (5) | 0.005 | 0.07 |
| Hypertension | 31.6% (6) | 61.9% (13) | 0.055 | 0.26 |
| Hyperlipidemia | 42.1% (8) | 52.4% (11) | 0.52 | 0.70 |
| Medications % (n) | ||||
| Ocular medications | 47.4% (9) | 33.3% (7) | 0.37 | 0.57 |
| NSAIDs | 63.2% (12) | 33.3% (7) | 0.06 | 0.26 |
| ASA | 21.1% (4) | 14.3% (3) | 0.57 | 0.74 |
| Fish oil | 36.8% (7) | 19.0% (4) | 0.21 | 0.44 |
| Statins | 36.8% (7) | 42.9% (9) | 0.70 | 0.83 |
| Antidepressants | 36.8% (7) | 0% (0) | 0.002 | 0.04 |
| Antianxiety | 26.3% (5) | 14.3% (3) | 0.34 | 0.56 |
| Systemic Symptoms, mean (SD) | ||||
| Depression (PHQ-9) | 12.7 (5.1) | 7.9 (7.4) | 0.01 | 0.25 |
| PTSD (PCL-M) | 47.9 (19.1) | 37.7 (16.5) | 0.13 | 0.36 |
| Insomnia (PSQI) | 13.2 (4.5) | 10.6 (5.5) | 0.11 | 0.32 |
| Fatigue (MFIS) | 54.5 (14.5) | 28.4 (22.7) | <0.001 | 0.007 |
| Widespread Pain Index | 9.1 (5.2) | 4.7 (4.0) | 0.01 | 0.17 |
| Symptom Severity Scale | 8.2 (2.0) | 4.1 (2.8) | <0.001 | 0.007 |
| SF-12 physical score | 32.5 (9.7) | 40.2 (13.1) | 0.12 | 0.35 |
| SF-12 mental score | 39.4 (16.3) | 47.3 (11.1) | 0.17 | 0.39 |
| Measure | GWI Mean (SD) (n = 19) | Control Mean (SD) (n = 21) | Raw p-Value | Adjusted p-Value (FDR) |
|---|---|---|---|---|
| Ocular Symptoms (Questionnaires) | ||||
| OSDI | 40.4 (25.0) | 24.5 (22.5) | 0.047 | 0.23 |
| DEQ-5 | 9.6 (4.1) | 7.4 (5.1) | 0.14 | 0.36 |
| NPSI-Eye total score | 24.3 (21.3) | 8.9 (13.3) | 0.02 | 0.13 |
| CISS | 29.3 (11.5) | 11.8 (11.7) | <0.001 | 0.003 |
| Ocular Pain Ratings | ||||
| Right eye (OD), now | 2.1 (2.7) | 0.6 (0.9) | 0.19 | 0.19 |
| Left eye (OS), now | 2.5 (3.3) | 1.0 (1.4) | 0.28 | 0.26 |
| Right eye (OD), average 1 week | 2.1 (2.3) | 1.0 (1.3) | 0.15 | 0.27 |
| Left eye (OS), average 1 week | 2.6 (2.8) | 1.1 (1.3) | 0.12 | 0.23 |
| Right eye (OD), worst 1 week | 2.9 (3.1) | 1.1 (1.5) | 0.10 | 0.19 |
| Left eye (OS), worst 1 week | 3.2 (3.3) | 1.2 (1.5) | 0.09 | 0.19 |
| Pre-anesthetic OD | 2.2 (2.5) | 1.1 (2.4) | 0.11 | 0.36 |
| Pre-anesthetic OS | 2.0 (2.5) | 1.0 (2.2) | 0.13 | 0.38 |
| Post-anesthetic OD | 1.0 (2.3) | 0.3 (0.9) | 0.67 | 0.42 |
| Post-anesthetic OS | 1.1 (2.2) | 0.3 (0.9) | 0.50 | 0.39 |
| Clinical Signs | ||||
| InflammaDry (MMP) OD | 1.4 (1.0) | 0.8 (0.9) | 0.07 | 0.27 |
| InflammaDry (MMP) OS | 0.8 (0.8) | 0.8 (0.8) | 0.90 | 0.92 |
| TBUT OD (seconds) | 9.3 (3.9) | 9.6 (5.1) | 0.86 | 0.92 |
| TBUT OS (seconds) | 9.4 (4.7) | 9.5 (5.0) | 0.95 | 0.97 |
| Corneal staining OD | 1.2 (2.0) | 1.8 (2.6) | 0.47 | 0.61 |
| Corneal staining OS | 1.5 (2.1) | 1.8 (3.3.) | 0.69 | 0.85 |
| Schirmer OD (mm/5 min) | 16.1 (8.3) | 17.0 (10.9) | 0.78 | 0.78 |
| Schirmer OS (mm/5 min) | 17.8 (7.2) | 20.1 (12.1) | 0.73 | 0.73 |
| Eyelid vascularity OD | 0.5 (0.7) | 0.2 (0.4) | 0.41 | 0.42 |
| Eyelid vascularity OS | 0.5 (0.7) | 0.2 (0.4) | 0.41 | 0.42 |
| Meibum quality OD | 1.1 (1.0) | 0.8 (0.4) | 0.34 | 0.36 |
| Meibum quality OS | 1.2 (1.0) | 1.0 (0.9) | 0.46 | 0.68 |
| MG gland dropout OD | 1.9 (1.1) | 1.3 (0.9) | 0.08 | 0.28 |
| MG gland dropout OS | 2.2 (1.3) | 1.5 (0.8) | 0.13 | 0.27 |
| Biomarkers | GWI Mean (SD) (n = 19) | Control Mean (SD) (n = 21) | p-Value | Adjusted p-Value (FDR) |
|---|---|---|---|---|
| Tear Sphingolipids (pmol) | ||||
| Cer C14:0 | 0.33 (0.30) | 0.16 (0.10) | 0.046 | 0.23 |
| Cer C24:0 | 3.70 (3.75) | 1.75 (1.49) | 0.04 | 0.26 |
| MHC C18:0 | 0.10 (0.08) | 0.05 (0.03) | 0.03 | 0.19 |
| MHC C22:0 | 0.24 (0.20) | 0.12 (0.07) | 0.02 | 0.17 |
| MHC C24:0 | 0.42 (0.39) | 0.20 (0.11) | 0.02 | 0.55 |
| Tear Sphingolipids (mol %) | ||||
| Cer C16:0 DH | 1.31 (0.52) | 1.72 (0.56) | 0.01 | 0.23 |
| Cer C18:1 | 2.05 (1.03) | 2.89 (1.38) | 0.04 | 0.25 |
| Cer C18:0 | 4.42 (1.14) | 5.45 (1.15) | 0.01 | 0.17 |
| Cer C20:0 | 4.02 (1.14) | 4.88 (0.82) | 0.01 | 0.17 |
| Cer C26:0 | 5.20 (3.33) | 7.72 (4.02) | 0.03 | 0.22 |
| Tear Eicosanoids (pmol/mL) | ||||
| PGE2 | 0.01 (0.01) | 0.004 (0.003) | 0.04 | 0.19 |
| 15-HETE | 0.12 (0.15) | 0.04 (0.04) | 0.02 | 0.19 |
| (±)14(15)-EET | 0.04 (0.03) | 0.01 (0.01) | 0.002 | 0.04 |
| 5-OxoETE | 0.38 (0.35) | 0.20 (0.21) | 0.02 | 0.25 |
| (±)8(9)-EET | 0.03 (0.03) | 0.01 (0.01) | 0.008 | 0.09 |
| AA | 29.83 (22.64) | 15.70 (15.93) | 0.02 | 0.29 |
| Biomarkers | GWI Mean (SD) (n = 19) | Control Mean (SD) (n = 21) | p-Value | Adjusted p-Value (FDR) |
|---|---|---|---|---|
| Plasma Sphingolipids (pmol) | ||||
| Cer C18:0 | 8.82 (3.12) | 11.84 (4.39) | 0.02 | 0.18 |
| Cer C20:0 | 20.83 (6.58) | 26.16 (9.49) | 0.048 | 0.25 |
| SM C16:0 DH | 537.32 (68.77) | 622.58 (127.11) | 0.01 | 0.17 |
| SM C20:0 | 1334.88 (229.70) | 1152.93 (157.26) | 0.005 | 0.09 |
| SM C22:0 | 2175.69 (452.10) | 1911.47 (262.31) | 0.03 | 0.19 |
| Plasma Sphingolipids (mol %) | ||||
| Cer C22:0 | 18.47 (2.33) | 16.78 (2.30) | 0.01 | 0.19 |
| MHC C16:0 | 15.14 (2.28) | 17.89 (2.78) | 0.002 | 0.04 |
| MHC C24:0 | 39.72 (5.50) | 36.28 (3.56) | 0.02 | 0.36 |
| SM C14:0 | 8.09 (0.75) | 10.10 (2.76) | 0.005 | 0.07 |
| SM C16:0 | 4.24 (0.70) | 4.67 (0.61) | 0.048 | 0.25 |
| SM C16:0 DH | 4.75 (0.31) | 5.44 (0.47) | <0.001 | 0.001 |
| SM C20:0 | 11.74 (1.00) | 10.19 (0.95) | <0.001 | 0.002 |
| SM C22:0 | 19.05 (1.98) | 16.87 (1.26) | <0.001 | 0.007 |
| Plasma Eicosanoids (pmol/mL) | ||||
| (±)11,12-DHET | 1.18 (0.30) | 1.38 (0.30) | 0.04 | 0.23 |
| 5-OxoETE | 31.28 (13.30) | 46.38 (29.92) | 0.05 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paule Jimenez, L.B.; Prislovsky, A.; Parchejo, L.A.; Cabrera, K.; Nafziger, A.J.; Stephenson, D.J.; Chalfant, C.E.; Aenlle, K.; Klimas, N.; Tang, F.; et al. Investigating the Eye as a Biomarker of Gulf War Illness: Sphingolipid and Eicosanoid Composition in Tears and Plasma. Biomolecules 2025, 15, 1716. https://doi.org/10.3390/biom15121716
Paule Jimenez LB, Prislovsky A, Parchejo LA, Cabrera K, Nafziger AJ, Stephenson DJ, Chalfant CE, Aenlle K, Klimas N, Tang F, et al. Investigating the Eye as a Biomarker of Gulf War Illness: Sphingolipid and Eicosanoid Composition in Tears and Plasma. Biomolecules. 2025; 15(12):1716. https://doi.org/10.3390/biom15121716
Chicago/Turabian StylePaule Jimenez, Laura Beatriz, Amanda Prislovsky, Loralei Ann Parchejo, Kim Cabrera, Andrew J. Nafziger, Daniel J. Stephenson, Charles E. Chalfant, Kristina Aenlle, Nancy Klimas, Fei Tang, and et al. 2025. "Investigating the Eye as a Biomarker of Gulf War Illness: Sphingolipid and Eicosanoid Composition in Tears and Plasma" Biomolecules 15, no. 12: 1716. https://doi.org/10.3390/biom15121716
APA StylePaule Jimenez, L. B., Prislovsky, A., Parchejo, L. A., Cabrera, K., Nafziger, A. J., Stephenson, D. J., Chalfant, C. E., Aenlle, K., Klimas, N., Tang, F., Mandal, N., & Galor, A. (2025). Investigating the Eye as a Biomarker of Gulf War Illness: Sphingolipid and Eicosanoid Composition in Tears and Plasma. Biomolecules, 15(12), 1716. https://doi.org/10.3390/biom15121716







