The Impact of Sex Hormones on Transcranial Magnetic Stimulation Against the Oxidative Stress in the Pathogenesis of Multiple Sclerosis
Abstract
1. Introductions
2. Materials and Methods
2.1. Animals
2.2. EAE Induction
2.3. Experimental Groups and Treatments
2.4. Sample Preparation
2.5. Clinical Status
2.6. Parameters of Oxidative Stress
2.7. Statistical Analysis
3. Results
3.1. Clinical Status
3.2. Glutathione Redox System
3.3. Oxidative Stress Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMS | Transcranial magnetic stimulation |
| MS | Multiple sclerosis |
| CNS | Central nervous system |
| EAE | Experimental autoimmune encephalomyelitis |
| MOG | Myelin oligodendrocyte glycoprotein |
| PBS | Phosphate buffered saline |
| E | Estrogens |
| P | Progesterone |
| T | Testosterone |
| OVX | Ovariectomy |
| LPO | Lipid peroxidation products |
| tG | Total glutathione |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GPx | Glutathione peroxidase |
| CP | Carbonylated proteins |
| EDSS | Expanded disability status scale |
| HRT | Hormone replacement therapy |
| BBB | Blood–brain barrier |
| NLRP3 | Inflammasome-like receptor protein 3 |
References
- Hanlon, C.A.; McCalley, D.M. Sex/Gender as a Factor That Influences Transcranial Magnetic Stimulation Treatment Outcome: Three Potential Biological Explanations. Front. Psychiatry 2022, 13, 869070. [Google Scholar] [CrossRef]
- León Ruiz, M.; Sospedra, M.; Arce Arce, S.; Tejeiro-Martínez, J.; Benito-León, J. Current Evidence on the Potential Therapeutic Applications of Transcranial Magnetic Stimulation in Multiple Sclerosis: A Systematic Review of the Literature. Neurologia 2022, 37, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, K.; Chen, S.; Zhou, W.; Li, J.; Huang, Q.; Xu, T.; Gao, Z.; Wang, D.; Zhao, S.; et al. Clinical Application of Transcranial Magnetic Stimulation in Multiple Sclerosis. Front. Immunol. 2022, 13, 902658. [Google Scholar] [CrossRef]
- Alvarez-Sanchez, N.; Dunn, S.E. Potential Biological Contributers to the Sex Difference in Multiple Sclerosis Progression. Front. Immunol. 2023, 14, 1175874. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Li, R. Cellular Immunology of Relapsing Multiple Sclerosis: Interactions, Checks, and Balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Medina-Fernández, F.; Escribano, B.; Agüera, E.; Aguilar-Luque, M.; Feijoo, M.; Luque, E.; Garcia-Maceira, F.; Pascual-Leone, A.; Drucker-Colin, R.; Tunez, I. Effects of Transcranial Magnetic Stimulation on Oxidative Stress in Experimental Autoimmune Encephalomyelitis. Free Radic. Res. 2017, 51, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Medina-Fernández, F.; Escribano, B.; Luque, E.; Caballero-Villarraso, J.; Gomez-Chaparro, J.; Feijoo, M.; Garcia-Maceira, F.; Pascual-Leone, A.; Drucker-Colin, R.; Tunez, I. Comparative of Transcranial Magnetic Stimulation and Other Treatments in Experimental Autoimmune Encephalomyelitis. Brain Res. Bull. 2018, 137, 140–145. [Google Scholar] [CrossRef]
- Agüera, E.; Caballero-Villarraso, J.; Feijóo, M.; Escribano, B.M.; Bahamonde, M.C.; Conde, C.; Galván, A.; Túnez, I. Impact of Repetitive Transcranial Magnetic Stimulation on Neurocognition and Oxidative Stress in Relapsing-Remitting Multiple Sclerosis: A Case Report. Front. Neurol. 2020, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.; Muñoz-Jurado, A.; Luque, E.; Galván, A.; LaTorre, M.; Caballero-Villarraso, J.; Giraldo, A.I.; Agüera, E.; Túnez, I. Effect of the Combination of Different Therapies on Oxidative Stress in the Experimental Model of Multiple Sclerosis. Neuroscience 2023, 529, 116–128. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abu-Duhier, F.M.; Barreto, G.E.; Ashraf, G.M. Impact of Sex Differences and Gender Specificity on Behavioral Characteristics and Pathophysiology of Neurodegenerative Disorders. Neurosci. Biobehav. Rev. 2019, 102, 95–105. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef]
- Dunn, S.E.; Lee, H.; Pavri, F.R.; Zhang, M.A. Sex-Based Differences in Multiple Sclerosis (Part I): Biology of Disease Incidence. Curr. Top. Behav. Neurosci. 2015, 26, 29–56. [Google Scholar] [CrossRef]
- Gilli, F.; DiSano, K.D.; Pachner, A.R. SeXX Matters in Multiple Sclerosis. Front. Neurol. 2020, 11, 616. [Google Scholar] [CrossRef]
- Ahlgren, C.; Odén, A.; Lycke, J. High Nationwide Prevalence of Multiple Sclerosis in Sweden. Mult. Scler. 2011, 17, 901–908. [Google Scholar] [CrossRef]
- Tremlett, H.; Paty, D.; Devonshire, V. Disability Progression in Multiple Sclerosis Is Slower than Previously Reported. Neurology 2006, 66, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.C.; Dunn, S.E. Effect of Puberty on the Immune System: Relevance to Multiple Sclerosis. Front. Pediatr. 2022, 10, 1059083. [Google Scholar] [CrossRef] [PubMed]
- Midaglia, L.; Otero, S.; Baró, F.; Montalban, X.; Tintoré, M. Menopause and Multiple Sclerosis: Influence on Prognosis and Role of Disease-Modifying Drugs and Hormonal Replacement Therapy. Mult. Scler. 2022, 28, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bridge, F.; Butzkueven, H.; Van der Walt, A.; Jokubaitis, V.G. The Impact of Menopause on Multiple Sclerosis. Autoimmun. Rev. 2023, 22, 103363. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Bonsack, B.; Brinton, R.; Schumacher, M.; Kumar, N.; Lee, J.Y.; Castelli, V.; Corey, S.; Coats, A.; Sadanandan, N.; et al. Progress in Progestin-Based Therapies for Neurological Disorders. Neurosci. Biobehav. Rev. 2021, 122, 38–65. [Google Scholar] [CrossRef]
- Sparaco, M.; Bonavita, S. The Role of Sex Hormones in Women with Multiple Sclerosis: From Puberty to Assisted Reproductive Techniques. Front. Neuroendocrinol. 2021, 60, 100889. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, T.; Pawelec, P.; Ziabska, K.; Ziemka-Nalecz, M. Sexual Dimorphism in Neurodegenerative Diseases and in Brain Ischemia. Biomolecules 2022, 13, 26. [Google Scholar] [CrossRef]
- Milosevic, A.; Lavrnja, I.; Savic, D.; Milosevic, K.; Skuljec, J.; Bjelobaba, I.; Janjic, M.M. Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis. Cells 2023, 12, 1045. [Google Scholar] [CrossRef]
- Rivas-Grajales, A.M.; Barbour, T.; Camprodon, J.A.; Kritzer, M.D. The Impact of Sex Hormones on Transcranial Magnetic Stimulation Measures of Cortical Excitability: A Systematic Review and Considerations for Clinical Practice. Harv. Rev. Psychiatry 2023, 31, 114–123. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Barthelmes, J.; Tafferner, N.; Kurz, J.; de Bruin, N.; Parnham, M.J.; Geisslinger, G.; Schiffmann, S. Induction of Experimental Autoimmune Encephalomyelitis in Mice and Evaluation of the Disease-Dependent Distribution of Immune Cells in Various Tissues. J. Vis. Exp. 2016, 53933. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jurado, A.; Escribano, B.M.; Túnez, I. Animal Model of Multiple Sclerosis: Experimental Autoimmune Encephalomyelitis. Methods Cell Biol. 2024, 188, 35–60. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Zhuang, W.; Xie, L.; Liu, G.; Saimaier, K.; Han, S.; Shi, C.; Hua, Q.; Zhang, R.; et al. Induction and Diverse Assessment Indicators of Experimental Autoimmune Encephalomyelitis. J. Vis. Exp. 2022, e63866. [Google Scholar] [CrossRef]
- Drucker-Colín, R.; Verdugo-Díaz, L.; Méndez, M.; Carrillo-Ruiz, J.; Morgado-Valle, C.; Hernández-Cruz, A.; Corkidi, G. Comparison between Low Frequency Magnetic Field Stimulation and Nerve Growth Factor Treatment of Cultured Chromaffin Cells, on Neurite Growth, Noradrenaline Release, Excitable Properties, and Grafting in Nigrostriatal Lesioned Rats. Mol. Cell. Neurosci. 1994, 5, 485–498. [Google Scholar] [CrossRef]
- Medina-Fernández, F.; Luque, E.; Aguilar-Luque, M.; Agüera, E.; Feijóo, M.; García-Maceira, F.I.; Escribano, B.M.; Pascual-Leone, Á.; Drucker-Colín, R.; Túnez, I. Transcranial Magnetic Stimulation Modifies Astrocytosis, Cell Density and Lipopolysaccharide Levels in Experimental Autoimmune Encephalomyelitis. Life Sci. 2017, 169, 20–26. [Google Scholar] [CrossRef]
- Poumeau-Delille, G. Techniques Biologiques En Endocrinologie Expérimentale Chez Le Rat; Masson: Paris, France, 1953. [Google Scholar]
- Teodorov, E.; Camarini, R.; Bernardi, M.M.; Felicio, L.F. Treatment with Steroid Hormones and Morphine Alters General Activity, Sexual Behavior, and Opioid Gene Expression in Female Rats. Life Sci. 2014, 104, 47–54. [Google Scholar] [CrossRef]
- Túnez, I.; Collado, J.A.; Medina, F.J.; Peña, J.; Muñoz, M.D.C.; Jimena, I.; Franco, F.; Rueda, I.; Feijóo, M.; Muntané, J.; et al. 17 β-Estradiol May Affect Vulnerability of Striatum in a 3-Nitropropionic Acid-Induced Experimental Model of Huntington’s Disease in Ovariectomized Rats. Neurochem. Int. 2006, 48, 367–373. [Google Scholar] [CrossRef]
- Ozdamar, S.; Taskin, M.I.; Onder, G.O.; Kaymak, E.; Baran, M.; Yay, A. Progesterone Decreases the Extent of Ovarian Damage Caused by Cisplatin in an Experimental Rat Model. Adv. Clin. Exp. Med. 2019, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Túnez, I.; Feijóo, M.; Collado, J.A.; Medina, F.J.; Peña, J.; Muñoz, M.d.C.; Jimena, I.; Franco, F.; Rueda, I.; Muntané, J.; et al. Effect of Testosterone on Oxidative Stress and Cell Damage Induced by 3-Nitropropionic Acid in Striatum of Ovariectomized Rats. Life Sci. 2007, 80, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Nievas, B.G.; García-Bueno, B.; Madrigal, J.L.M.; Leza, J.C. Chronic Immobilisation Stress Ameliorates Clinical Score and Neuroinflammation in a MOG-Induced EAE in Dark Agouti Rats: Mechanisms Implicated. J. Neuroinflamm. 2010, 7, 60. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Sicotte, N.L.; Liva, S.M.; Klutch, R.; Pfeiffer, P.; Bouvier, S.; Odesa, S.; Wu, T.C.J.; Voskuhl, R.R. Treatment of Multiple Sclerosis with the Pregnancy Hormone Estriol. Ann. Neurol. 2002, 52, 421–428. [Google Scholar] [CrossRef]
- Zahaf, A.; Kassoussi, A.; Hutteau-Hamel, T.; Mellouk, A.; Marie, C.; Zoupi, L.; Tsouki, F.; Mattern, C.; Bobé, P.; Schumacher, M.; et al. Androgens Show Sex-Dependent Differences in Myelination in Immune and Non-Immune Murine Models of CNS Demyelination. Nat. Commun. 2023, 14, 1592. [Google Scholar] [CrossRef]
- Baroncini, D.; Annovazzi, P.O.; De Rossi, N.; Mallucci, G.; Torri Clerici, V.; Tonietti, S.; Mantero, V.; Ferrò, M.T.; Messina, M.J.; Barcella, V.; et al. Impact of Natural Menopause on Multiple Sclerosis: A Multicentre Study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1201–1206. [Google Scholar] [CrossRef]
- Ladeira, F.; Salavisa, M.; Caetano, A.; Barbosa, R.; Sá, F.; Correia, A.S. The Influence of Menopause in Multiple Sclerosis Course: A Longitudinal Cohort Study. Eur. Neurol. 2018, 80, 223–227. [Google Scholar] [CrossRef]
- Bove, R.; Healy, B.C.; Musallam, A.; Glanz, B.I.; De Jager, P.L.; Chitnis, T. Exploration of Changes in Disability after Menopause in a Longitudinal Multiple Sclerosis Cohort. Mult. Scler. 2016, 22, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Janjic, M.M.; Lavrnja, I.; Savic, D.; Bozic, I.D.; Tesovic, K.; Jakovljevic, M.; Pekovic, S.; Stojilkovic, S.S.; Bjelobaba, I. The Sex-Specific Patterns of Changes in Hypothalamic-Pituitary-Gonadal Axis during Experimental Autoimmune Encephalomyelitis. Brain. Behav. Immun. 2020, 89, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, P.; Wallberg, M.; Hammar, M.; Landtblom, A.M.; Brynhildsen, J. Symptoms of Multiple Sclerosis in Women in Relation to Sex Steroid Exposure. Maturitas 2006, 54, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Studd, J.W.W. A Pilot Study of the Effect upon Multiple Sclerosis of the Menopause, Hormone Replacement Therapy and the Menstrual Cycle. J. R. Soc. Med. 1992, 85, 612–613. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Lambrinoudaki, I.; Goulis, D.G. Menopause in Women with Multiple Sclerosis: A Systematic Review. Maturitas 2020, 135, 68–73. [Google Scholar] [CrossRef]
- Taylor, H.; Alhasan, S.; Saleem, M.; Poole, S.; Jiang, F.; Longbrake, E.E.; Bove, R. Influence of Menstrual Cycle and Hormonal Contraceptive Use on MS Symptom Fluctuations: A Pilot Study. Mult. Scler. Relat. Disord. 2023, 77. [Google Scholar] [CrossRef]
- Escribano, B.; Medina-Fernández, F.J.; Aguilar-Luque, M.; Agüera, E.; Feijoo, M.; Garcia-Maceira, F.I.; Lillo, R.; Vieyra-Reyes, P.; Giraldo, A.I.; Luque, E.; et al. Lipopolysaccharide Binding Protein and Oxidative Stress in a Multiple Sclerosis Model. Neurotherapeutics 2017, 14, 199–211. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Godfrey, J.A.; Wiegman, M.J. The Effect of Ovariectomy and Estrogen on Penetrating Brain Arterioles and Blood-Brain Barrier Permeability. Microcirculation 2009, 16, 685. [Google Scholar] [CrossRef]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y. Claudin-5 as a Novel Estrogen Target in Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, Inflammation and Cognition. Front. Neuroendocrinol. 2016, 40, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Collongues, N.; Patte-Mensah, C.; De Seze, J.; Mensah-Nyagan, A.G.; Derfuss, T. Testosterone and Estrogen in Multiple Sclerosis: From Pathophysiology to Therapeutics. Expert Rev. Neurother. 2018, 18, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Peng, Q.; Liu, Z.; Xie, Z.; Guo, X.; Li, Y.; Chen, C. Estrogen Plays an Important Role by Influencing the NLRP3 Inflammasome. Biomed. Pharmacother. 2023, 167, 115554. [Google Scholar] [CrossRef] [PubMed]
- Haghmorad, D.; Salehipour, Z.; Nosratabadi, R.; Rastin, M.; Kokhaei, P.; Mahmoudi, M.B.; Amini, A.A.; Mahmoudi, M. Medium-Dose Estrogen Ameliorates Experimental Autoimmune Encephalomyelitis in Ovariectomized Mice. J. Immunotoxicol. 2016, 13, 885–896. [Google Scholar] [CrossRef]
- Dalal, M.; Kim, S.; Voskuhl, R.R. Testosterone Therapy Ameliorates Experimental Autoimmune Encephalomyelitis and Induces a T Helper 2 Bias in the Autoantigen-Specific T Lymphocyte Response. J. Immunol. 1997, 159, 3–6. [Google Scholar] [CrossRef]
- Liva, S.M.; Voskuhl, R.R. Testosterone Acts Directly on CD4+ T Lymphocytes to Increase IL-10 Production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef]
- Brahmachari, S.; Pahan, K. Gender-Specific Expression of Beta1 Integrin of VLA-4 in Myelin Basic Protein-Primed T Cells: Implications for Gender Bias in Multiple Sclerosis. J. Immunol. 2010, 184, 6103–6113. [Google Scholar] [CrossRef]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone Therapy Induces an M1 to M2 Switch in Microglia Phenotype and Suppresses NLRP3 Inflammasome in a Cuprizone-Induced Demyelination Mouse Model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef]
- Bassani, T.B.; Bartolomeo, C.S.; Oliveira, R.B.; Ureshino, R.P. Progestogen-Mediated Neuroprotection in Central Nervous System Disorders. Neuroendocrinology 2023, 113, 14–35. [Google Scholar] [CrossRef]
- Labombarda, F.; González, S.L.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Effects of Progesterone on Oligodendrocyte Progenitors, Oligodendrocyte Transcription Factors, and Myelin Proteins Following Spinal Cord Injury. Glia 2009, 57, 884–897. [Google Scholar] [CrossRef] [PubMed]



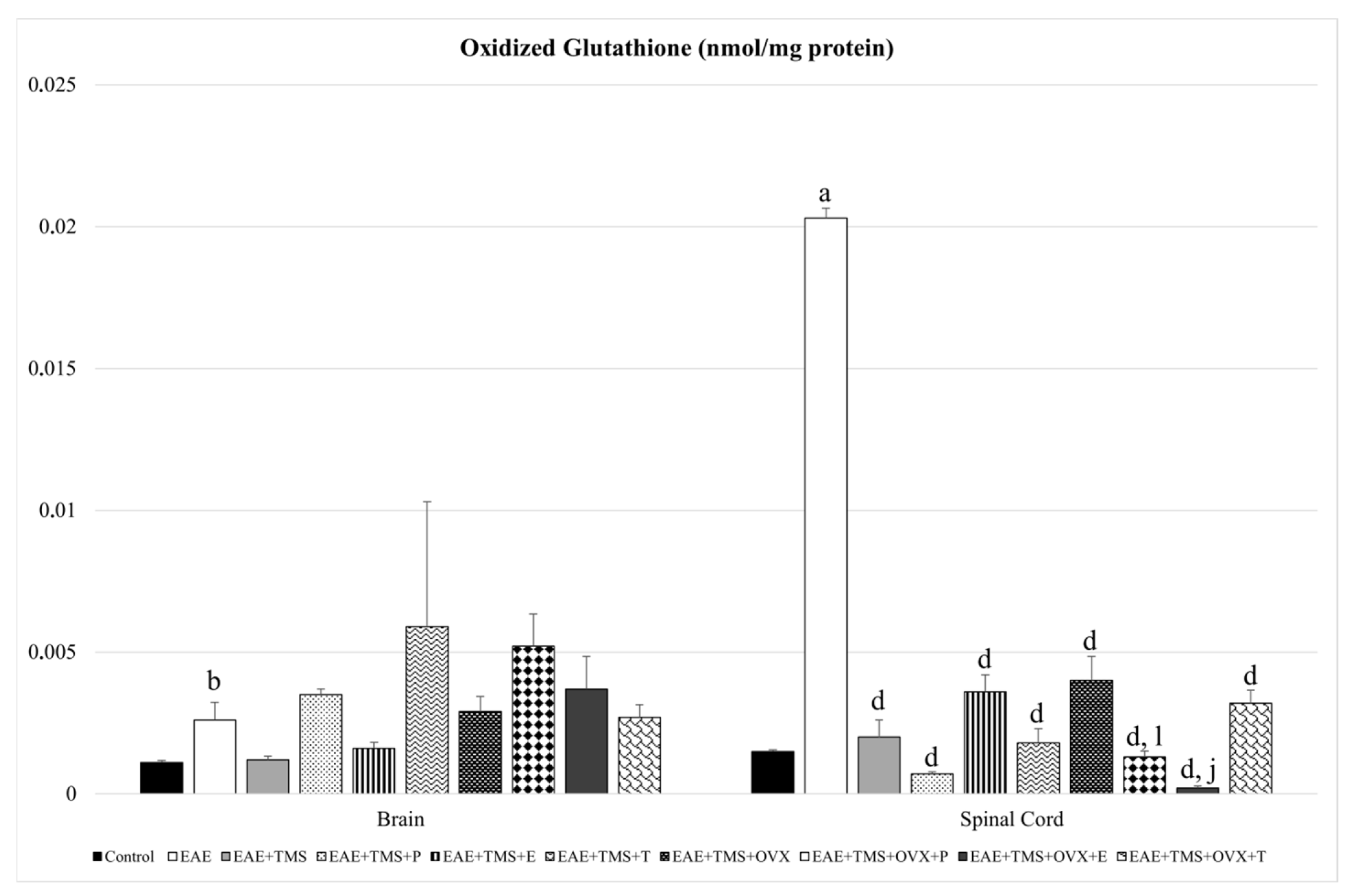
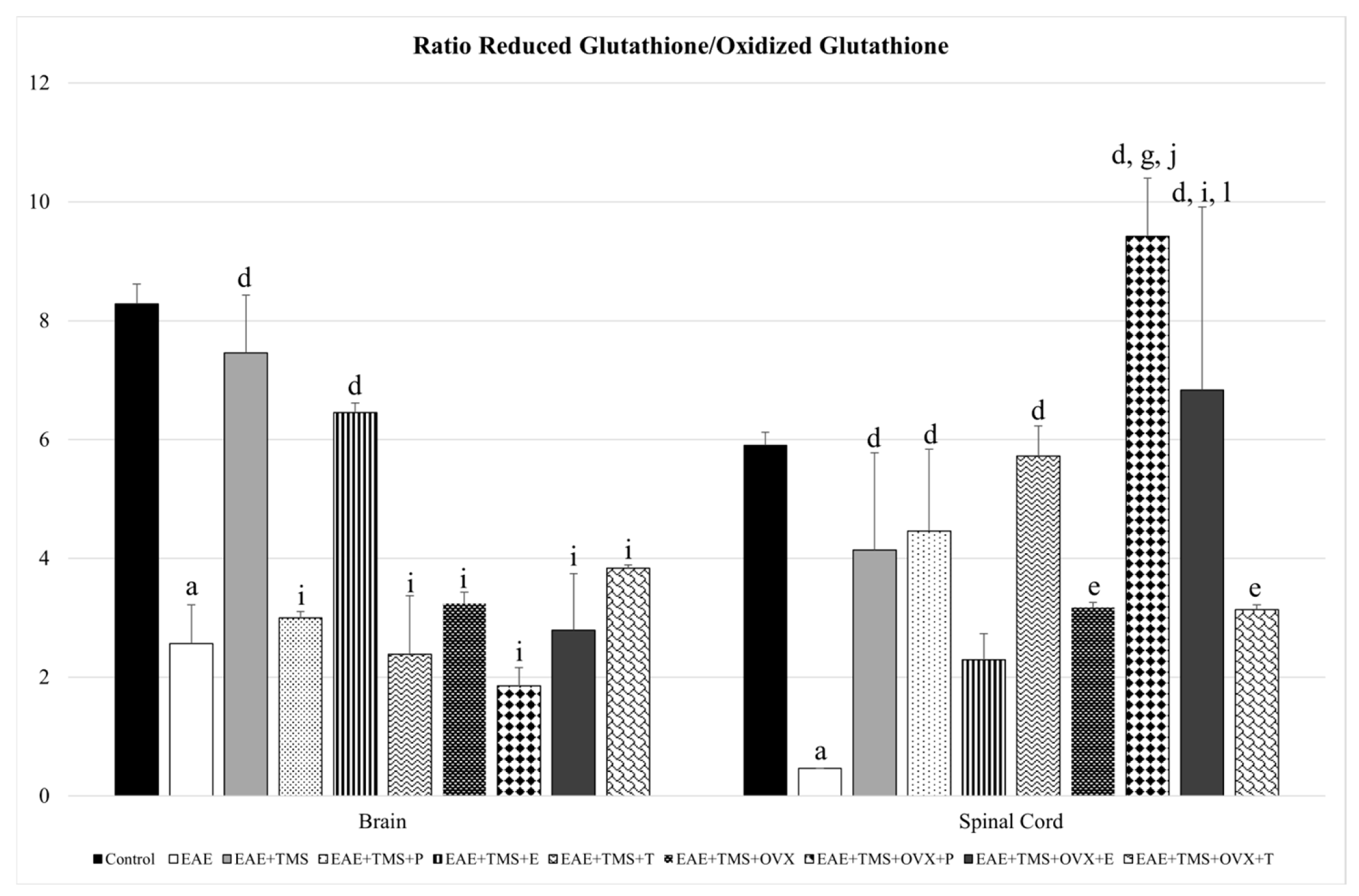

| Oxidative Stress Biomarkers | ||
|---|---|---|
| Brain | ||
| LPO (nmol/mg protein) | CP (nmol/g protein) | |
| Control | 0.8308 ± 0.0005 | 0.0100 ± 0.0002 |
| EAE | 1.8276 ± 0.0029 a | 0.0414 ± 0.0001 a |
| EAE+TMS | 0.7224 ± 0.0006 d | 0.0088 ± 0.0002 d |
| EAE+TMS+P | 1.1434 ± 0.0011 d,g | 0.0110 ± 0.0002 d |
| EAE+TMS+E | 1.1360 ± 0.0046 d,g | 0.0207 ± 0.0003 d,g |
| EAE+TMS+T | 1.2668 ± 0.0117 d,g | 0.0301 ± 0.0001 d,g |
| EAE+TMS+OVX | 0.7938 ± 0.0042 d,g | 0.0111 ± 0.0002 d |
| EAE+TMS+OVX+P | 1.0346 ± 0.0081 d,g,j | 0.0142 ± 0.0004 d,g |
| EAE+TMS+OVX+E | 1.1222 ± 0.0097 d,g,j | 0.0163 ± 0.0001 d,g,l |
| EAE+TMS+OVX+T | 1.3182 ± 0.0068 d,g,j | 0.0258 ± 0.0074 d,g,j |
| Spinal cords | ||
| Control | 0.7037 ± 0.0120 | 0.0138 ± 0.0001 |
| EAE | 2.5419 ± 0.0010 a | 0.0335 ± 0.0010 a |
| EAE+TMS | 0.5343 ± 0.0016 d | 0.0139 ± 0.0000 d |
| EAE+TMS+P | 0.8411 ± 0.0040 d,g | 0.0137 ± 0.0001 d |
| EAE+TMS+E | 1.9490 ± 0.0082 d,g | 0.0137 ± 0.0001 d |
| EAE+TMS+T | 1.4143 ± 0.0017 d,g | 0.0136 ± 0.0001 d |
| EAE+TMS+OVX | 0.6943 ± 0.0054 d,g | 0.0138 ± 0.0001 d |
| EAE+TMS+OVX+P | 0.6774 ± 0.0014 d,g | 0.0135 ± 0.0001 d |
| EAE+TMS+OVX+E | 0.8270 ± 0.0017 d,g,j | 0.0137 ± 0.0001 d |
| EAE+TMS+OVX+T | 1.5142 ± 0.00152 d,g,j | 0.0138 ± 0.0001 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escribano, B.M.; Valdevira, M.E.; Muñoz-Jurado, A.; Feijóo, M.; Agüera, E.; Caballero-Villarraso, J.; LaTorre, M.; Giraldo, A.I.; Santamaría, A.; Túnez, I. The Impact of Sex Hormones on Transcranial Magnetic Stimulation Against the Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Biomolecules 2025, 15, 1714. https://doi.org/10.3390/biom15121714
Escribano BM, Valdevira ME, Muñoz-Jurado A, Feijóo M, Agüera E, Caballero-Villarraso J, LaTorre M, Giraldo AI, Santamaría A, Túnez I. The Impact of Sex Hormones on Transcranial Magnetic Stimulation Against the Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Biomolecules. 2025; 15(12):1714. https://doi.org/10.3390/biom15121714
Chicago/Turabian StyleEscribano, Begoña M., Manuel E. Valdevira, Ana Muñoz-Jurado, Montse Feijóo, Eduardo Agüera, Javier Caballero-Villarraso, Manuel LaTorre, Ana I. Giraldo, Abel Santamaría, and Isaac Túnez. 2025. "The Impact of Sex Hormones on Transcranial Magnetic Stimulation Against the Oxidative Stress in the Pathogenesis of Multiple Sclerosis" Biomolecules 15, no. 12: 1714. https://doi.org/10.3390/biom15121714
APA StyleEscribano, B. M., Valdevira, M. E., Muñoz-Jurado, A., Feijóo, M., Agüera, E., Caballero-Villarraso, J., LaTorre, M., Giraldo, A. I., Santamaría, A., & Túnez, I. (2025). The Impact of Sex Hormones on Transcranial Magnetic Stimulation Against the Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Biomolecules, 15(12), 1714. https://doi.org/10.3390/biom15121714






