Serum PTH ≥ 40 pg/mL as a Marker of Bone Fragility and Vitamin D Deficiency in Periodontitis Patients: Biochemical, Densitometric and Genetic Evidence
Abstract
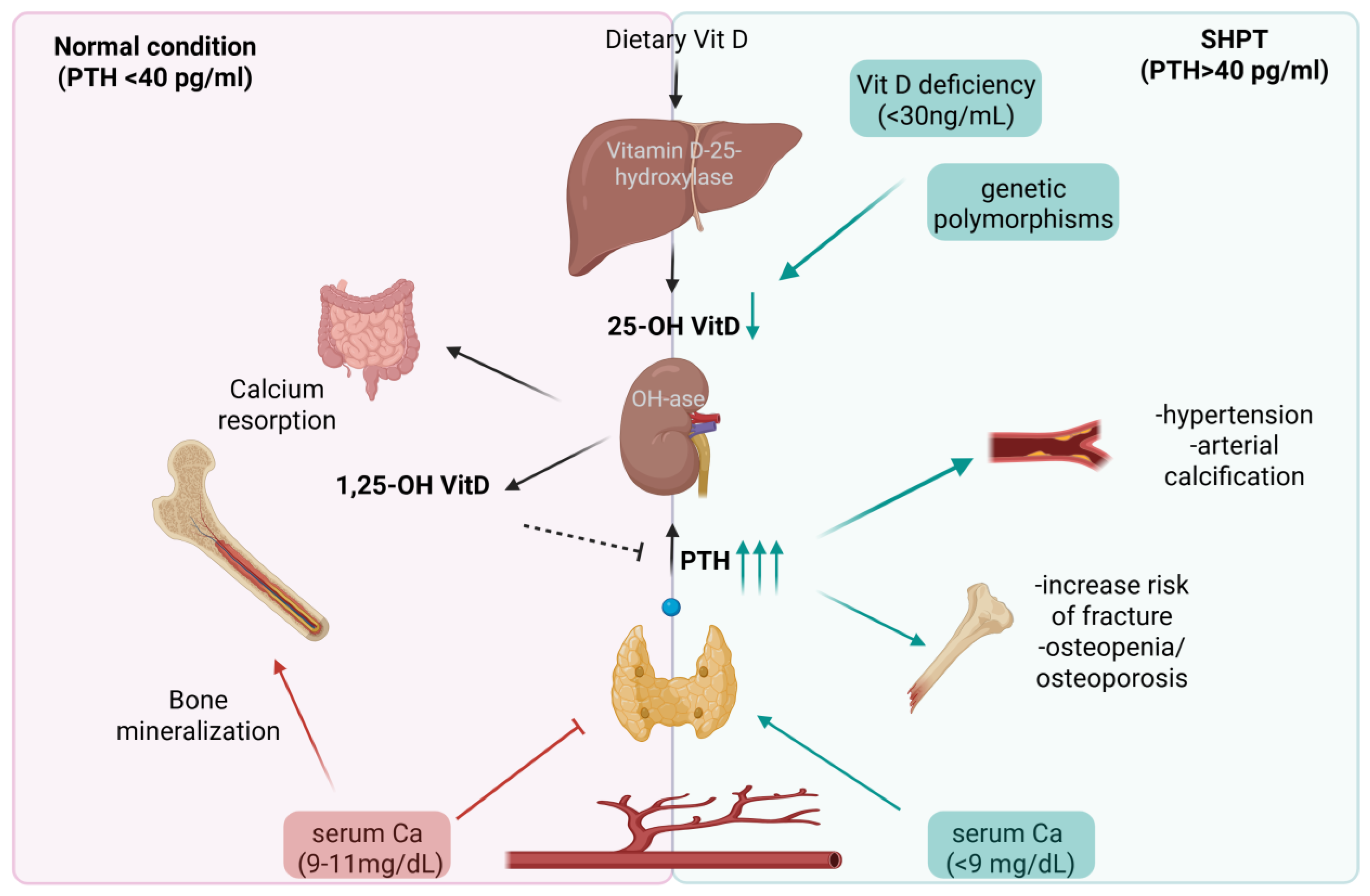
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Tests
2.3. Bone Densitometry
2.4. DNA Genotyping
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
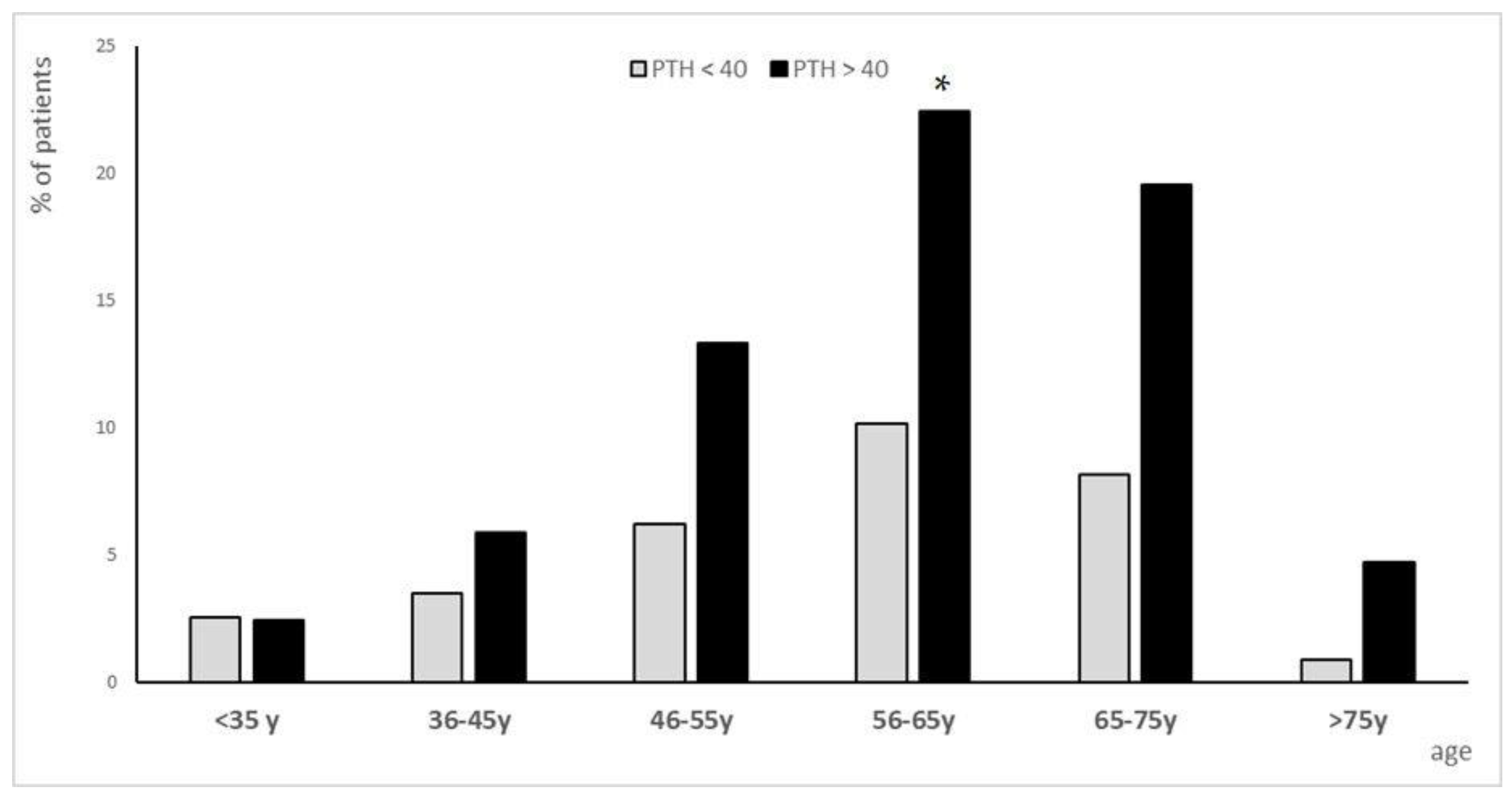
3.1.1. Population Analysis for PTH Subgroups
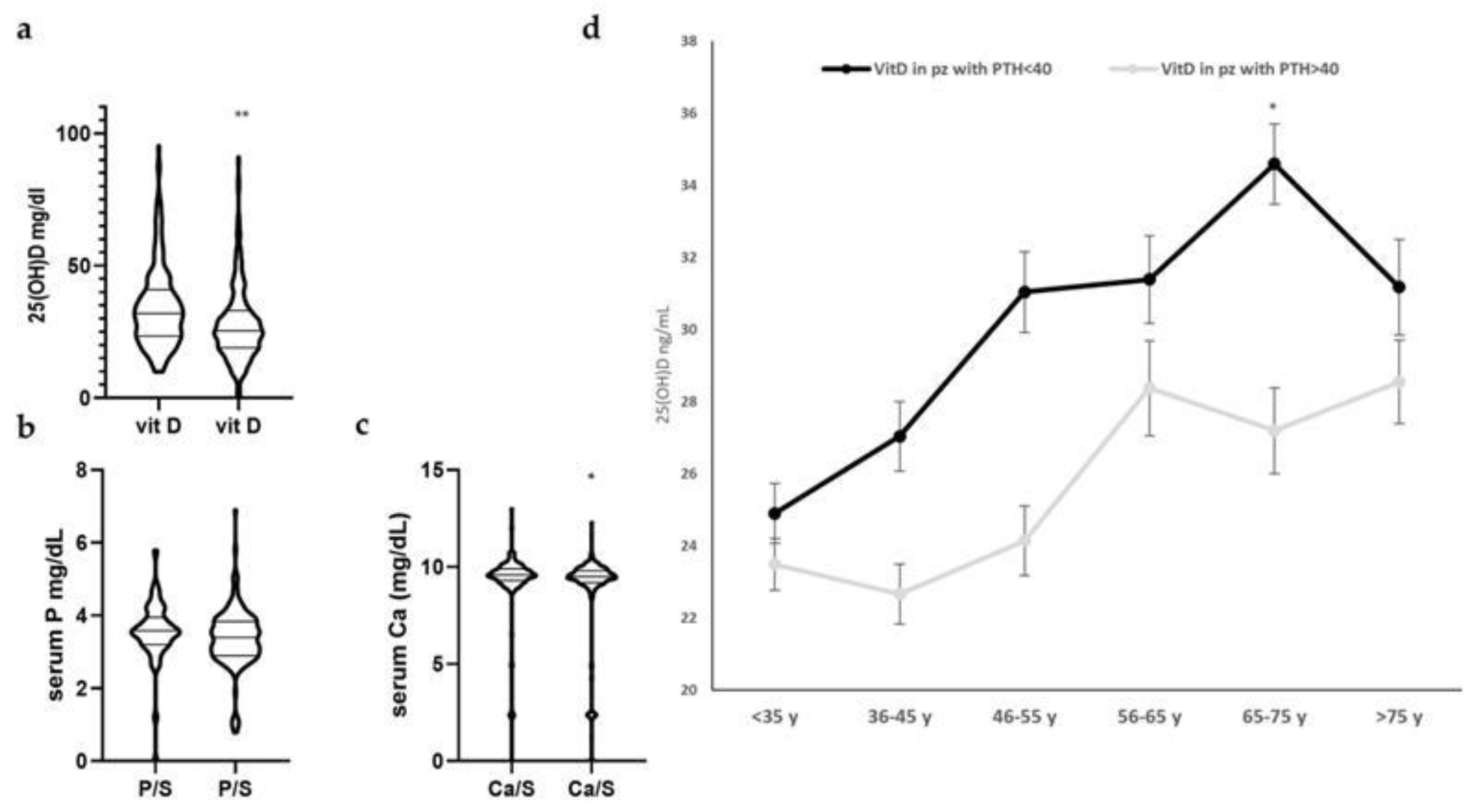
3.1.2. Characterization of Subgroups
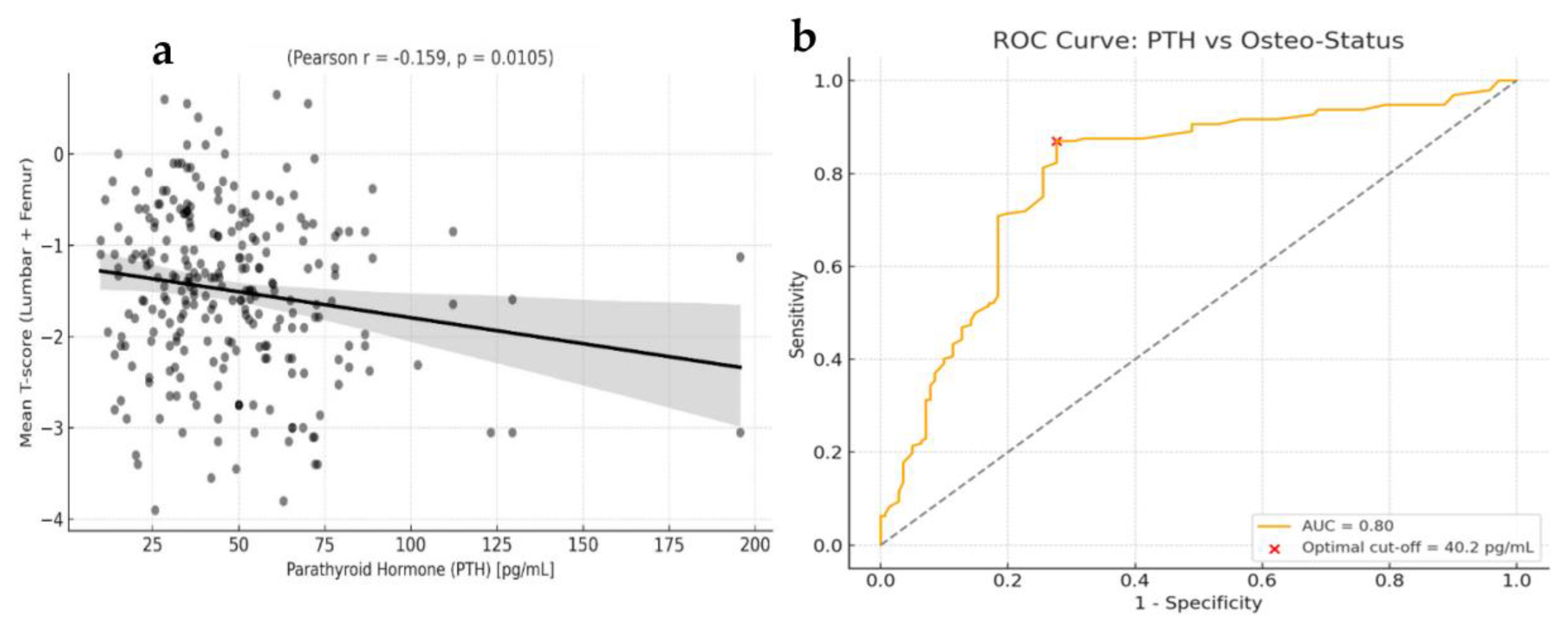
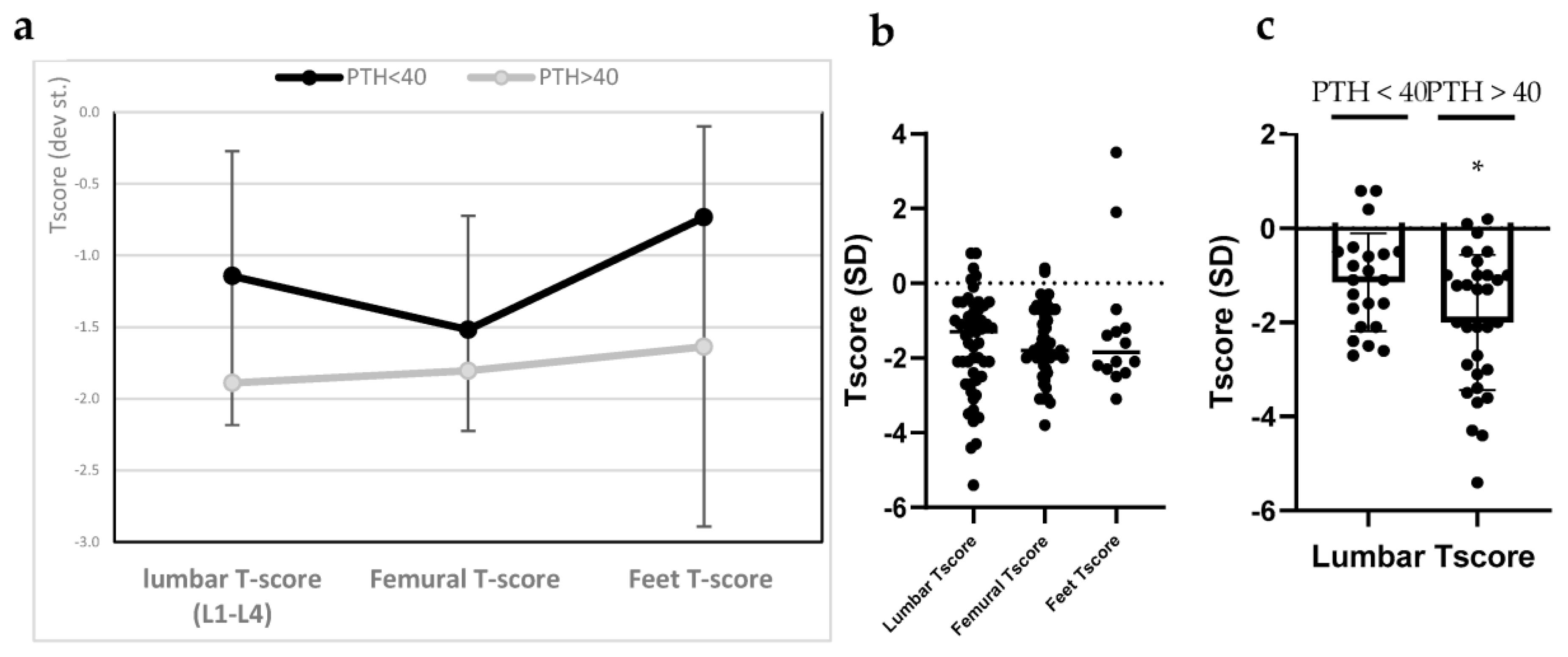
3.2. Bone Mineral Density (BMD)
3.3. Interaction Between Genetic Polymorphisms and PTH Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, E.M.; Pollak, M.; Seidman, C.E.; Seidman, J.G.; Chou, Y.H.; Riccardi, D.; Hebert, S.C. Calcium-ion-sensing cell-surface receptors. N. Engl. J. Med. 1995, 333, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Wolf, M. Calcium and Phosphate Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 8f3, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Just, A.; Mallmann, R.T.; Grossmann, S.; Sleman, F.; Klugbauer, N. Two-pore channel protein TPC1 is a determining factor for the adaptation of proximal tubular phosphate handling. Acta Physiol. Oxf. 2023, 237, e13914. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.M.; Rao, L.G.; Divieti, P.; Bringhurst, F.R. Parathyroid hormone secretion and action: Evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands. Endocr. Rev. 2005, 26, 78–113. [Google Scholar] [CrossRef]
- Nabeshima, Y. Discovery of alpha-Klotho unveiled new insights into calcium and phosphate homeostasis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 125–141. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, J.R. The regulation of parathyroid hormone secretion and synthesis. J. Am. Soc. Nephrol. 2011, 22, 216–224. [Google Scholar] [CrossRef]
- Hemmati, H.R. Interactions of vitamin D with parathyroid glands; an updated mini-review. J. Parathyr. Dis. 2023, 11, e11228. [Google Scholar] [CrossRef]
- Kowalski, G.J.; Buła, G.; Żądło, D.; Gawrychowska, A.; Gawrychowski, J. Primary hyperparathyroidism. Endokrynol. Pol. 2020, 71, 260–270. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross , A.C., Taylor , C.L., Yaktine , A.L., Del Valle , H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [PubMed]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- van der Plas, W.Y.; Noltes, M.E.; van Ginhoven, T.M.; Kruijff, S. Secondary and Tertiary Hyperparathyroidism: A Narrative Review. Scand. J. Surg. 2020, 109, 271–278. [Google Scholar] [CrossRef]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nissenson, R.A. PTH differentially regulates expression of RANKL and OPG. J. Bone Miner. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Lee, E.Y.; Lezcano, V.; Ronda, A.C.; Condon, K.W.; Allen, M.R.; Plotkin, L.I.; Bellido, T. Resorption controls bone anabolism driven by parathyroid hormone (PTH) receptor signaling in osteocytes. J. Biol. Chem. 2013, 288, 29809–29820. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.; Cooper, C.; Dennison, E. Epidemiology of osteoporosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Di Somma, C.; Rubino, M.; Faggiano, A.; Vuolo, L.; Guerra, E.; Contaldi, P.; Savastano, S.; Colao, A. The roles of parathyroid hormone in bone remodeling: Prospects for novel therapeutics. J. Endocrinol. Investig. 2011, 34, 18–22. [Google Scholar]
- Brandi, M.L.; Falchetti, A. Genetics of primary hyperparathyroidism. Urol. Int. 2004, 72 (Suppl. 1), 11–16. [Google Scholar] [CrossRef]
- Simonds, W.F. Clinical and Molecular Genetics of Primary Hyperparathyroidism. Horm. Metab. Res. 2020, 52, 578–587. [Google Scholar] [CrossRef]
- Association, C.O. Diagnosis and treatment of osteoporotic fractures. Orthop. Surg. 2009, 1, 251–257. [Google Scholar] [CrossRef]
- Brown, S.J.; Ruppe, M.D.; Tabatabai, L.S. The Parathyroid Gland and Heart Disease. Methodist. Debakey Cardiovasc. J. 2017, 13, 49–54. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef]
- Cusano, N.E.; Cetani, F. Normocalcemic primary hyperparathyroidism. Arch. Endocrinol. Metab. 2022, 66, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef]
- Walker, M.D.; Cong, E.; Lee, J.A.; Kepley, A.; Zhang, C.; McMahon, D.J.; Silverberg, S.J. Vitamin D in Primary Hyperparathyroidism: Effects on Clinical, Biochemical, and Densitometric Presentation. J. Clin. Endocrinol. Metab. 2015, 100, 3443–3451. [Google Scholar] [CrossRef]
- Kolcsar, M.; Szabó, L.; Dénes, O.M.; Gáll, Z. Assessment of Vitamin D Status in Primary Hyperparathyroidism Patients: A Retrospective Study. Cureus 2024, 16, e64988. [Google Scholar] [CrossRef]
- Walker, M.D.; Bilezikian, J.P. Vitamin D and primary hyperparathyroidism: More insights into a complex relationship. Endocrine 2017, 55, 3–5. [Google Scholar] [CrossRef]
- Tassone, F.; Gianotti, L.; Baffoni, C.; Visconti, G.; Pellegrino, M.; Cassibba, S.; Croce, C.G.; Magro, G.; Cesario, F.; Attanasio, R.; et al. Vitamin D status in primary hyperparathyroidism: A Southern European perspective. Clin. Endocrinol. 2013, 79, 784–790. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Bioletto, F.; Barale, M.; Maiorino, F.; Pusterla, A.; Fraire, F.; Arvat, E.; Ghigo, E.; Procopio, M. Trabecular Bone Score as a Marker of Skeletal Fragility Across the Spectrum of Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2024, 109, e1534–e1543. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; Bilezikian, J.P.; Canalis, E.; Terenzi, U.; Giustina, A. New insights into the vitamin D/PTH axis in endocrine-driven metabolic bone diseases. Endocrine 2024, 85, 1007–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yang, Y.; Deng, J.; Zhang, Z. Vitamin D and bone health: From physiological function to disease association. Nutr. Metab. 2025, 22, 113. [Google Scholar] [CrossRef]
- Cusack, S.; Mølgaard, C.; Michaelsen, K.F.; Jakobsen, J.; Lamberg-Allardt, C.J.; Cashman, K.D. Vitamin D and estrogen receptor-alpha genotype and indices of bone mass and bone turnover in Danish girls. J. Bone Miner. Metab. 2006, 24, 329–336. [Google Scholar] [CrossRef]
- Wang, J.; Shu, B.; Li, C.G.; Xie, X.W.; Liang, D.; Chen, B.L.; Lin, X.C.; Wei, X.; Wang, L.; Leng, X.Y.; et al. Polymorphisms of genes related to vitamin D metabolism and transportation and its relationship with the risk of osteoporosis: Protocol for a multicentre prospective cohort study in China. BMJ Open 2019, 9, e028084. [Google Scholar] [CrossRef]
- Fang, Y.; van Meurs, J.B.; Arp, P.; van Leeuwen, J.P.; Hofman, A.; Pols, H.A.; Uitterlinden, A.G. Vitamin D binding protein genotype and osteoporosis. Calcif. Tissue Int. 2009, 85, 85–93. [Google Scholar] [CrossRef]
- Garnero, P.; Vassy, V.; Bertholin, A.; Riou, J.P.; Delmas, P.D. Markers of bone turnover in hyperthyroidism and the effects of treatment. J. Clin. Endocrinol. Metab. 1994, 78, 955–959. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Aziz, I.; Yakout, S.; Aljohani, N.J.; Al-Saleh, Y.; Amer, O.E.; Sheshah, E.; Younis, G.Z.; Al-Badr, F.B.M. Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine 2017, 96, e5780. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, C.; Grippaudo, C.; Dell’Aquila, M.; Cimmino, P.; D’Addona, A.; De Angelis, P.; Ottaiano, M.P.; Costagliola, D.; Benincasa, G.; Micera, A.; et al. Association between Vitamin D Receptor Gene Polymorphisms and Periodontal Bacteria: A Clinical Pilot Study. Biomolecules 2022, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Baldini, A.; Nota, A.; Fanti, E.; Martelli, F.S.; Ottomano, C.; Lippi, G. Association between periodontal disease and Interleukin-1β +3953 and vitamin D receptor Taq1 genetic polymorphisms in an Italian caucasian population. Ann. Stomatol. Roma 2013, 4, 191–195. [Google Scholar]
- Martelli, F.S.; Mengoni, A.; Martelli, M.; Rosati, C.; Fanti, E. VDR TaqI polymorphism is associated with chronic periodontitis in Italian population. Arch. Oral. Biol. 2011, 56, 1494–1498. [Google Scholar] [CrossRef]
- Martelli, F.S.; Martelli, M.; Rosati, C.; Fanti, E. Vitamin D: Relevance in dental practice. Clin. Cases Miner. Bone Metab. 2014, 11, 15–19. [Google Scholar] [CrossRef]
- Piirainen, R.; Englund, E.; Henriksson, A.E. The impact of seasonal variation of 25-hydroxyvitamin D and parathyroid hormone on calcium levels. Clin. Biochem. 2016, 49, 850–853. [Google Scholar] [CrossRef]
- Nevo-Shor, A.; Kogan, S.; Joshua, B.Z.; Bahat-Dinur, A.; Novack, V.; Fraenkel, M. Seasonal changes in serum calcium, PTH and vitamin D levels in patients with primary hyperparathyroidism. Bone 2016, 89, 59–63. [Google Scholar] [CrossRef]
- Hegarty, V.; Woodhouse, P.; Khaw, K.-T. Seasonal Variation in 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations in Healthy Elderly People. Age Ageing 1994, 23, 478–482. [Google Scholar] [CrossRef]

| Variables | Mean Values | Standard Deviations (SD) | Reference Values |
|---|---|---|---|
| Age/years | 61.5 | ±12.1 | |
| PTH (pg/mL) | 51.08 | ±27.4 | 10–65 pg/mL |
| 25(OH)D (ng/mL) | 35.1 | ±17.9 | >30 ng/mL |
| Serum Calcium | 9.2 | ±1.7 | 9–11 mg/dL |
| Serum Phosphate | 3.4 | ±0.6 | 2.5–4.5 mg/dL |
| Urinary Ca/24 h (mg/dL) | 222.8 | 168.5 | 100–250 mg/24 h |
| Urinary P/24 h (mg/dL) | 492.5 | 267.5 | 400–1300 mg/24 h |
| Homocysteine (µmol/L) | 13.4 | ±6.7 | <15 µmol/L |
| Uric acid (mg/dL) | 4.6 | ±0.6 | 2.6–7.2 mg/dL |
| Glycemia (mg/dL) | 89.2 | ±17.6 | 70–100 mg/dL |
| Total cholesterol (mg/dL) | 188.7 | ±79.6 | <200 mg/dL |
| LDL (mg/dL) | 139.7 * | ±53.3 | <100 mg/dL |
| FT3 (pmol/L) | 2.9 * | ±0.5 | 3–8 pmol/L |
| FT4 (pmol/L) | 5.05 * | ±4.8 | 9–23 pmol/L |
| TSH (µIU/mL) | 5.2 | ±9.1 | 0.4–5.5 µIU/mL |
| Bone alkaline phosphatase (B-ALP (U/L) | 19.2 * | ±10.6 | 50–220 U/L |
| Lumbar spine (L1-L4) T-score | −1.38 * | ±1.56 | >−1.0 |
| Femoral neck T-score | −1.65 * | ±0.93 | >−1.0 |
| Femoral trochanter T-score | −1.36 * | ±1.02 | >−1.0 |
| Feet T-score | −2.02 * | ±0.67 | >−1.0 |
| PTH > 40 | PTH ≤ 40 | |
|---|---|---|
| N (patients) | 146 | 115 |
| PTH (Mean) | 62.8 ± 23.5 | 27.7 ± 7.8 |
| 25(OH)D (Mean) | 39.3 ± 24.8 | 32.8 ± 15.4 |
| T-score Mean (Mean) | −1.6 ± 0.8 | −1.3 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marroncini, G.; Martinelli, S.; Petrelli, F.; Bombardiere, F.; Sarnataro, A.; Martelli, F.S. Serum PTH ≥ 40 pg/mL as a Marker of Bone Fragility and Vitamin D Deficiency in Periodontitis Patients: Biochemical, Densitometric and Genetic Evidence. Biomolecules 2025, 15, 1600. https://doi.org/10.3390/biom15111600
Marroncini G, Martinelli S, Petrelli F, Bombardiere F, Sarnataro A, Martelli FS. Serum PTH ≥ 40 pg/mL as a Marker of Bone Fragility and Vitamin D Deficiency in Periodontitis Patients: Biochemical, Densitometric and Genetic Evidence. Biomolecules. 2025; 15(11):1600. https://doi.org/10.3390/biom15111600
Chicago/Turabian StyleMarroncini, Giada, Serena Martinelli, Francesco Petrelli, Francesco Bombardiere, Antonio Sarnataro, and Francesco Saverio Martelli. 2025. "Serum PTH ≥ 40 pg/mL as a Marker of Bone Fragility and Vitamin D Deficiency in Periodontitis Patients: Biochemical, Densitometric and Genetic Evidence" Biomolecules 15, no. 11: 1600. https://doi.org/10.3390/biom15111600
APA StyleMarroncini, G., Martinelli, S., Petrelli, F., Bombardiere, F., Sarnataro, A., & Martelli, F. S. (2025). Serum PTH ≥ 40 pg/mL as a Marker of Bone Fragility and Vitamin D Deficiency in Periodontitis Patients: Biochemical, Densitometric and Genetic Evidence. Biomolecules, 15(11), 1600. https://doi.org/10.3390/biom15111600






