RYR1-Related Myopathies Involve More than Calcium Dysregulation: Insights from Transcriptomic Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Ethics Statement
2.2. RNA Extraction and Quantification
2.3. Sequencing
2.4. Differential Expression Analysis
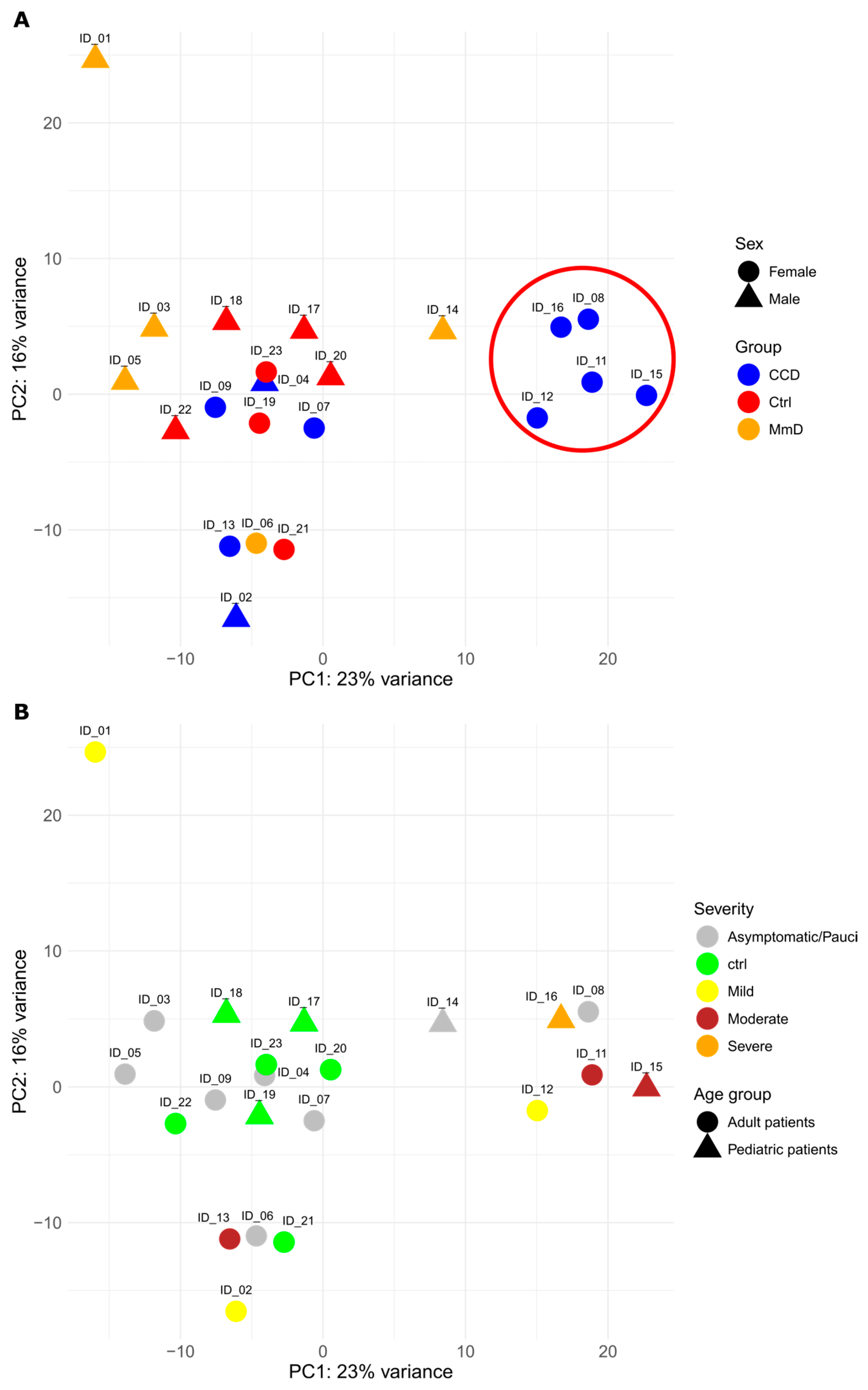
2.5. Principal Component Analysis
2.6. Functional Pathway Analysis
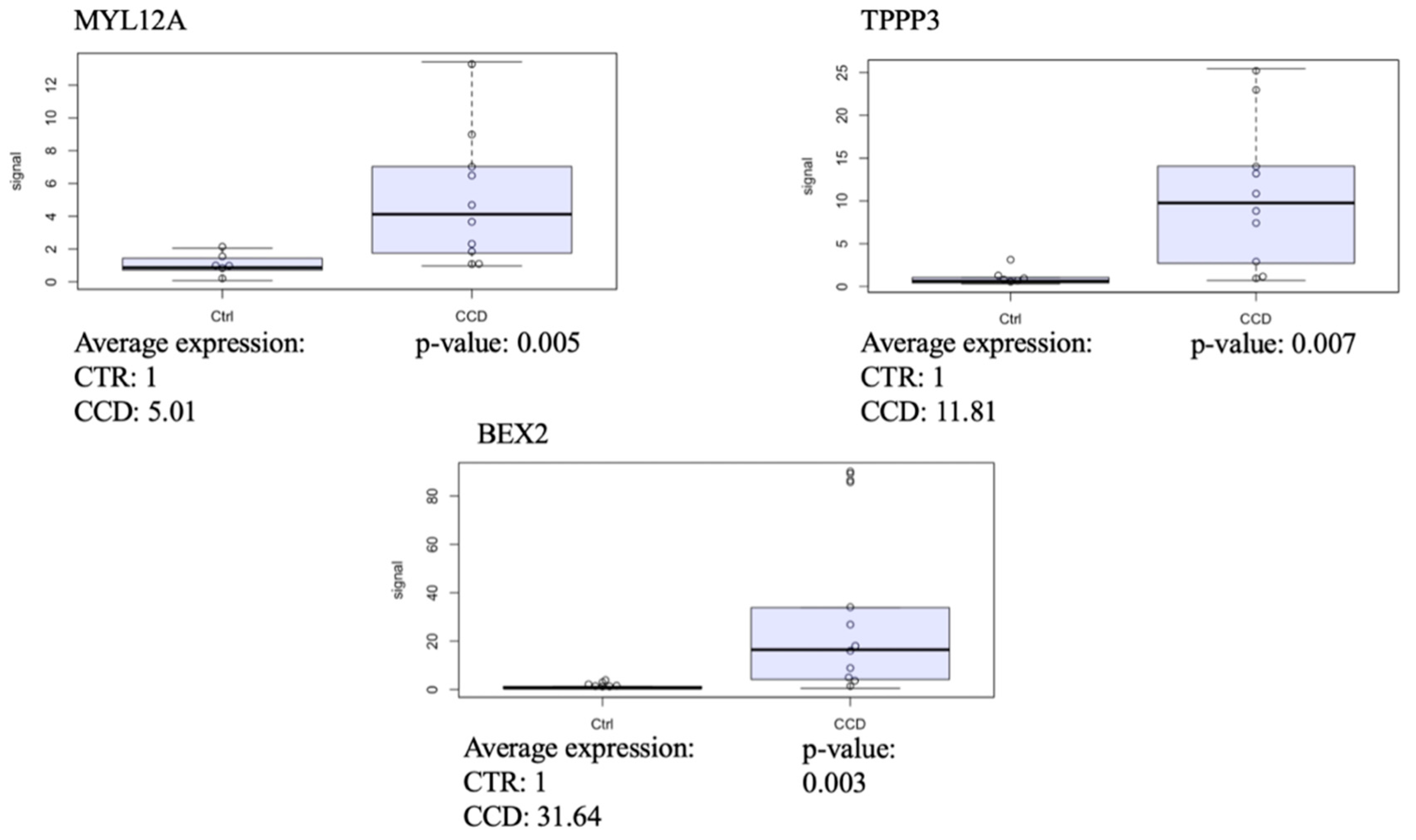
2.7. Gene Validation
3. Results
3.1. Patient Cohort
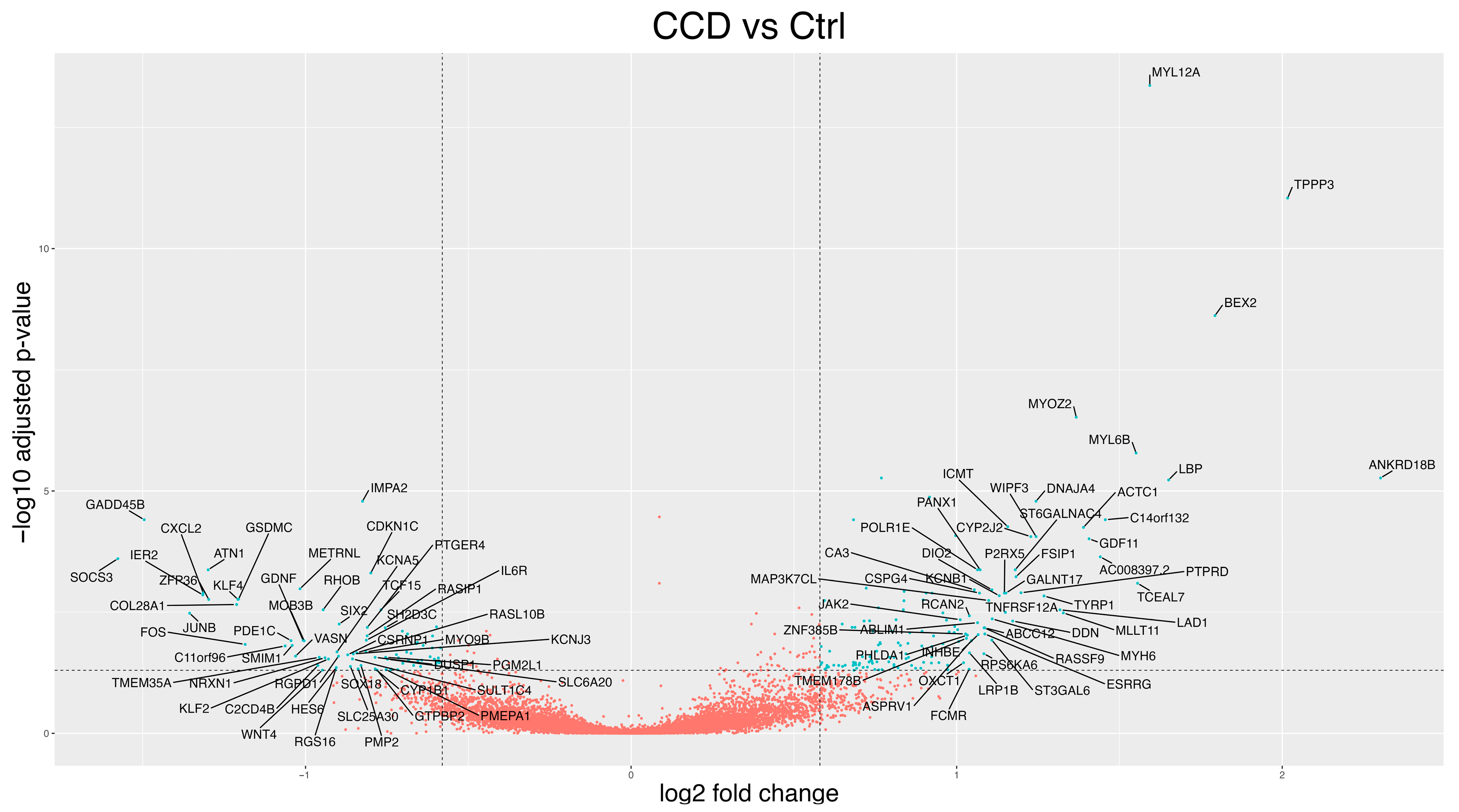
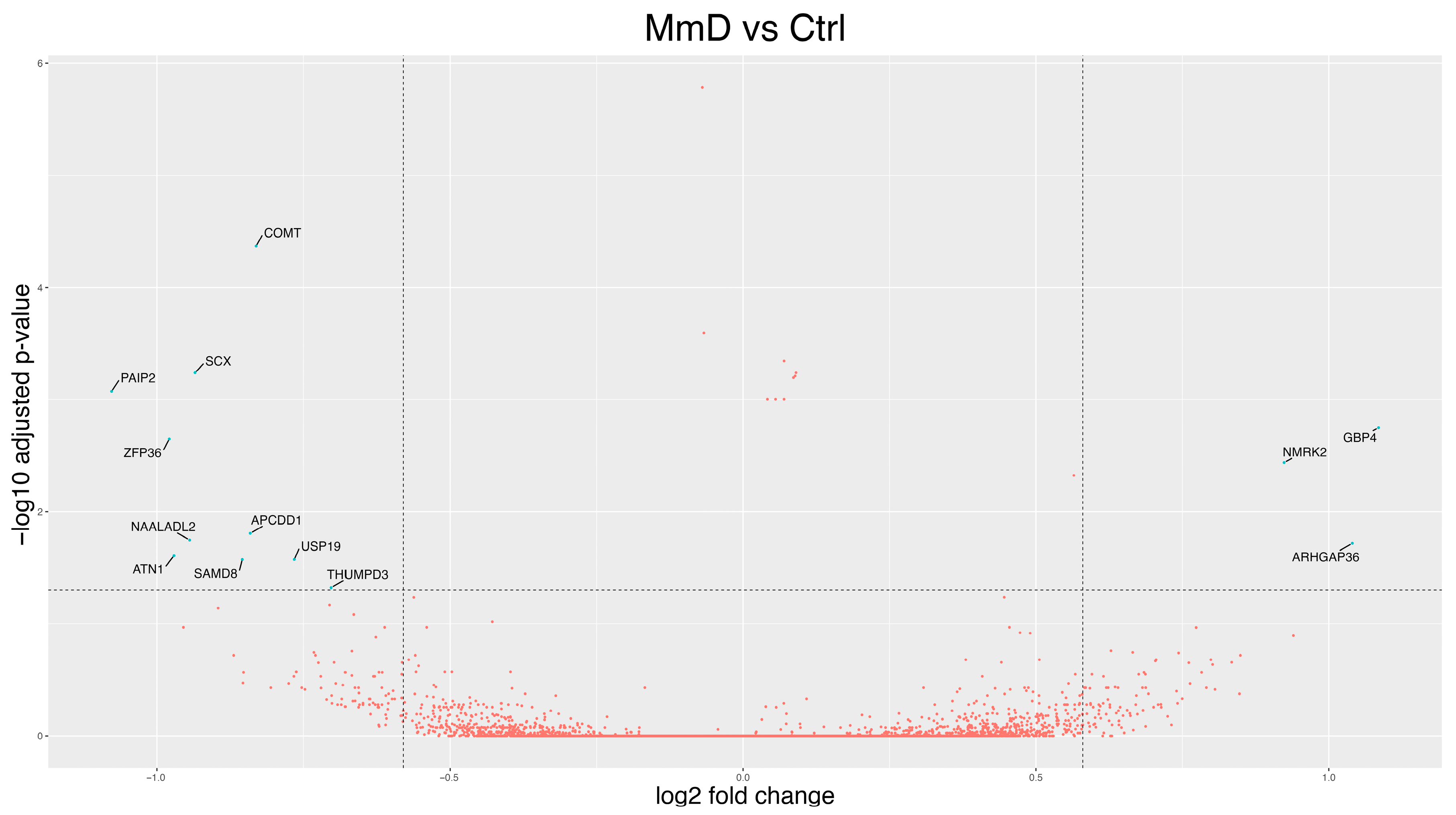
3.2. Differentially Expressed Genes (DEGs)
3.2.1. CCD Versus Controls
3.2.2. MmD Versus Controls
3.2.3. All RYR1-RM Patients vs. Controls
3.2.4. Symptomatic-Only Analyses
3.3. Analyses of Gene Function
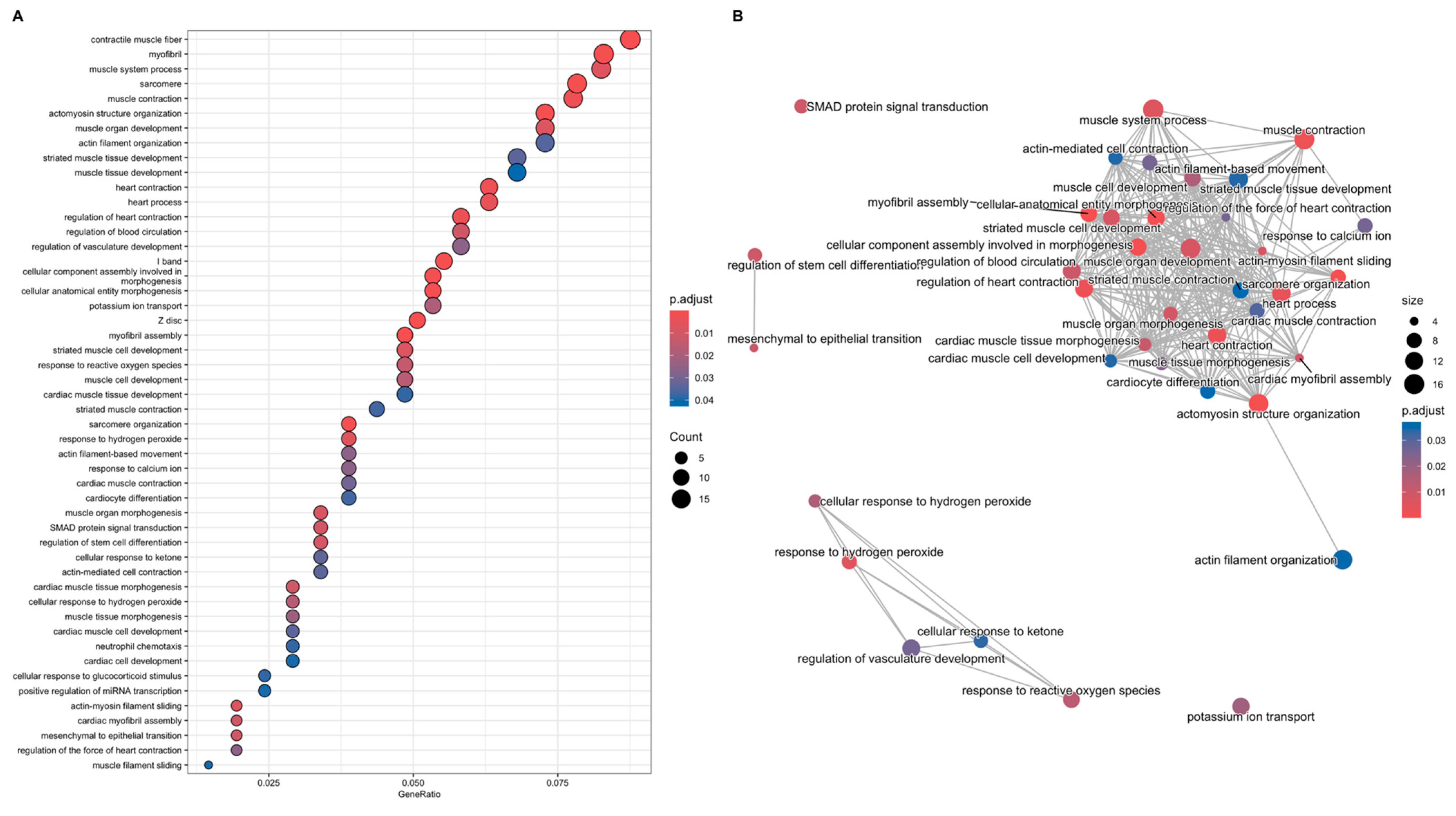
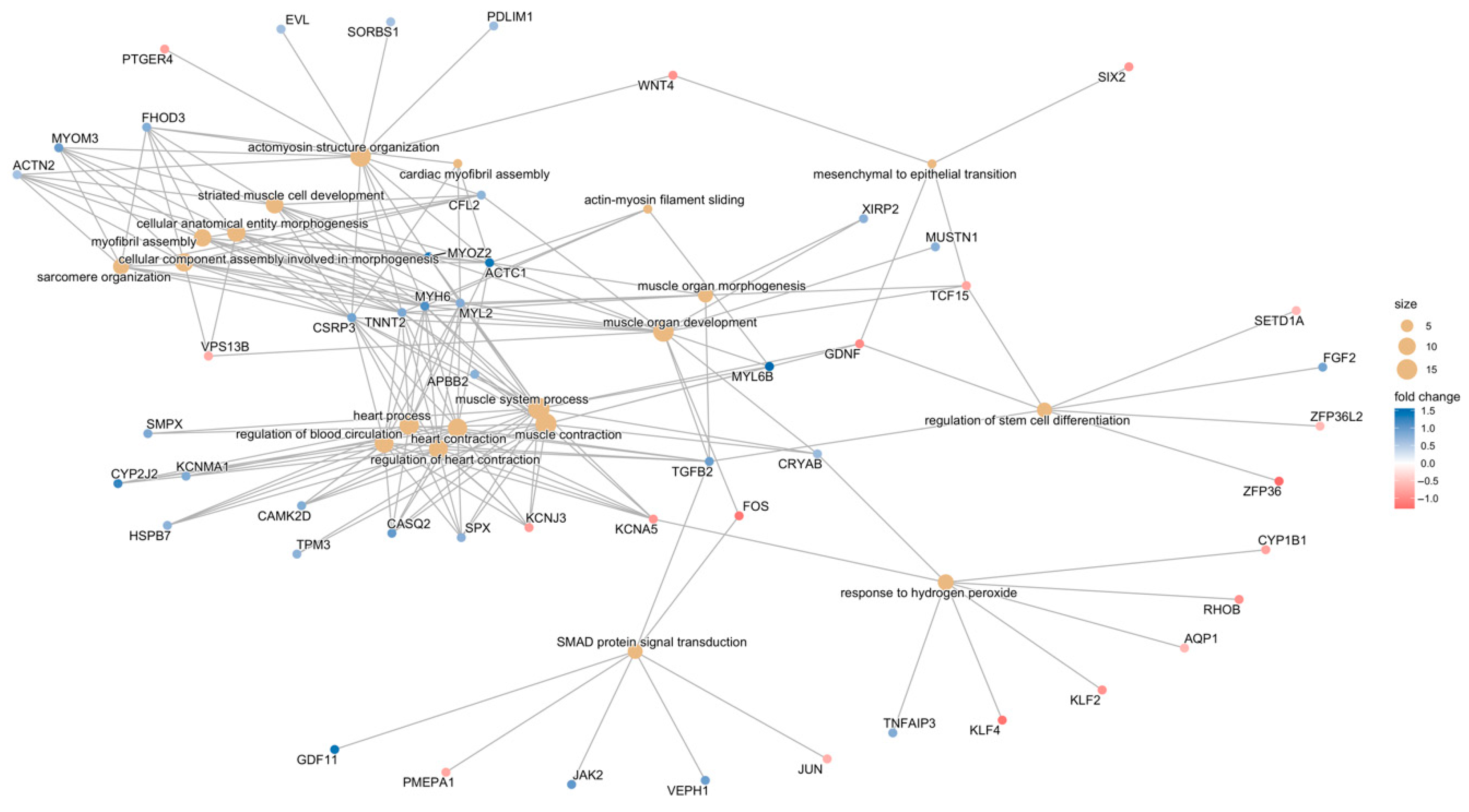
3.3.1. Gene-Function Analyses: CCD Versus Controls
3.3.2. Gene-Function Analyses: MmD Versus Controls
3.3.3. Gene-Function Analyses: All RYR1-RM Patients Versus Controls
3.3.4. Symptomatic-Only Gene-Function Analyses
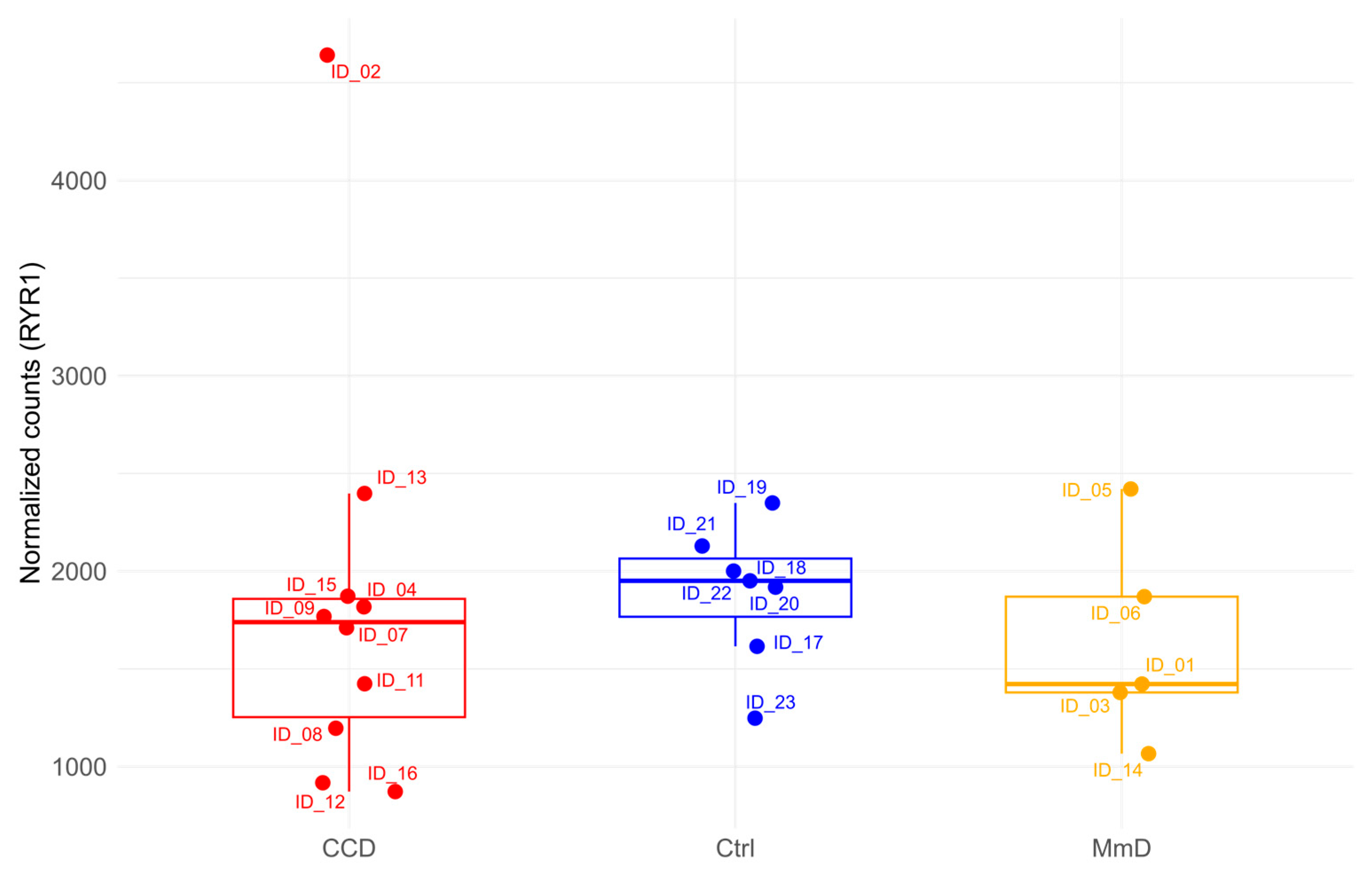
3.4. Analysis of RYR1 Expression
4. Discussion
4.1. Transcriptomic Distinction Between CCD and MmD
4.2. Pathway Dysregulation in CCD: Muscle Function and Beyond
4.3. Evidence for Apoptosis in CCD
4.4. Transcriptomic Profile of MmD and Shared Mechanisms
4.5. Symptomatic-Only Analysis—Rationale and Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RYR1-RM | Ryanodine Receptor 1-Related Myopathies |
| CCD | Central Core Disease |
| MmD | Multi-Mini Core Disease |
| MAPK | Mitogen-Activated Protein Kinase |
| ZFP36 | Zinc Finger Protein 36 |
| ATN1 | Ataxin 1 |
| DuCD | Dusty Core Disease |
| SR | Sarcoplasmic Reticulum |
| GLM | Generalised Linear Model |
| log2FC | Log2FoldChange |
| HB | Benjamini and Hochberg |
| ORA | Over-Representation Analysis |
| GO | Gene Ontology |
| DE | Differential expression |
| GEA | Gene Enrichment Analysis |
| DEGs | Differentially Expressed Genes |
| PCA | Principal Component Analysis |
| MYL12A | Myosin Light Chain 12A |
| TPPP3 | Tubulin Polymerisation Promoting Protein Family Member 3 |
| BEX2 | Brain Expressed X-Linked 2 |
| SOCS3 | Cytokine Signalling 3 |
| GADD45B | Growth Arrest and DNA Damage Inducible Beta |
| CL5 CCD | Clustered Group of 5 CCD |
| PAIP2 | Poly(A) Binding Protein Interacting Protein 2 |
| ATN1 | Atrophin 1 |
| ARHGAP36 | Rho Gtpase Activating Protein 36 |
| GBP1 | Guanylate-Binding Protein 1 |
| GBP4 | Guanylate-Binding Protein 4 |
| TGF-β | Transforming Growth Factor-β |
| UPR | Unfolded Protein Response |
| ER | Endoplasmic Reticulum |
| SOCS3 | Suppressor Of Cytokine Signalling 3 |
| GRP78 | Glucose Regulatory Protein 78 |
| AATF | Antiapoptotic Transcription Factor |
| MH | Malignant Hyperthermia |
References
- Amburgey, K.; McNamara, N.; Bennett, L.R.; McCormick, M.E.; Acsadi, G.; Dowling, J.J. Prevalence of Congenital Myopathies in a Representative Pediatric United States Population. Ann. Neurol. 2011, 70, 662–665. [Google Scholar] [CrossRef]
- Snoeck, M.; van Engelen, B.G.M.M.; Küsters, B.; Lammens, M.; Meijer, R.; Molenaar, J.P.F.F.; Raaphorst, J.; Verschuuren-Bemelmans, C.C.; Straathof, C.S.M.M.; Sie, L.T.L.L.; et al. RYR1-Related Myopathies: A Wide Spectrum of Phenotypes throughout Life. Eur. J. Neurol. 2015, 22, 1094–1112. [Google Scholar] [CrossRef]
- Fusto, A.; Cassandrini, D.; Fiorillo, C.; Codemo, V.; Astrea, G.; D’Amico, A.; Maggi, L.; Magri, F.; Pane, M.; Tasca, G.; et al. Expanding the Clinical-Pathological and Genetic Spectrum of RYR1-Related Congenital Myopathies with Cores and Minicores: An Italian Population Study. Acta Neuropathol. Commun. 2022, 10, 54. [Google Scholar] [CrossRef]
- Shy, G.M.; Magee, K.R. A New Congenital Non-Progressice Myopathy. Brain 1956, 79, 610–621. [Google Scholar] [CrossRef]
- De Cauwer, H.; Heytens, L.; Martin, J.-J. Workshop Report of the 89th ENMC International Workshop: Central Core Disease, 19th–20th January 2001, Hilversum, The Netherlands. Neuromuscul. Disord. 2002, 12, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Sewry, C.A.; Muntoni, F. Core Myopathies. Semin. Pediatr. Neurol. 2011, 18, 239–249. [Google Scholar] [CrossRef]
- Scacheri, P.C.; Hoffman, E.P.; Fratkin, J.D.; Semino-Mora, C.; Senchak, A.; Davis, M.R.; Laing, N.G.; Vedanarayanan, V.; Subramony, S.H. A Novel Ryanodine Receptor Gene Mutation Causing Both Cores and Rods in Congenital Myopathy. Neurology 2000, 55, 1689–1696. [Google Scholar] [CrossRef]
- Monnier, N.; Romero, N.B.; Lerale, J.; Nivoche, Y.; Qi, D.; MacLennan, D.H.; Fardeau, M.; Lunardi, J. An Autosomal Dominant Congenital Myopathy with Cores and Rods Is Associated with a Neomutation in the RYR1 Gene Encoding the Skeletal Muscle Ryanodine Receptor. Human Mol. Genet. 2000, 9, 2599–2608. [Google Scholar] [CrossRef]
- Sewry, C.A.; Müller, C.; Davis, M.; Dwyer, J.S.M.; Dove, J.; Evans, G.; Schröder, R.; Fürst, D.; Helliwell, T.; Laing, N.; et al. The Spectrum of Pathology in Central Core Disease. Neuromuscul. Disord. 2002, 12, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, M.; Rendu, J.; Brocard, J.; Lacene, E.; Fauré, J.; Brochier, G.; Beuvin, M.; Labasse, C.; Madelaine, A.; Malfatti, E.; et al. “Dusty Core Disease” (DuCD): Expanding Morphological Spectrum of RYR1 Recessive Myopathies. Acta Neuropathol. Commun. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, A.; Quijano-Roy, S.; Pichereau, C.; Moghadaszadeh, B.; Goemans, N.; Bönnemann, C.; Jungbluth, H.; Straub, V.; Villanova, M.; Leroy, J.-P.; et al. Mutations of the Selenoprotein N Gene, Which Is Implicated in Rigid Spine Muscular Dystrophy, Cause the Classical Phenotype of Multiminicore Disease: Reassessing the Nosology of Early-Onset Myopathies. Am. J. Human Genet. 2002, 71, 739–749. [Google Scholar] [CrossRef]
- Tajsharghi, H.; Hammans, S.; Lindberg, C.; Lossos, A.; Clarke, N.F.; Mazanti, I.; Waddell, L.B.; Fellig, Y.; Foulds, N.; Katifi, H.; et al. Recessive Myosin Myopathy with External Ophthalmoplegia Associated with MYH2 Mutations. Eur. J. Human Genet. 2014, 22, 801. [Google Scholar] [CrossRef]
- Lossos, A.; Baala, L.; Soffer, D.; Averbuch-Heller, L.; Dotan, S.; Munnich, A.; Lyonnet, S.; Gomori, J.M.; Genem, A.; Neufeld, M.; et al. A Novel Autosomal Recessive Myopathy with External Ophthalmoplegia Linked to Chromosome 17p13.1-P12. Brain 2005, 128, 42–51. [Google Scholar] [CrossRef]
- Donkervoort, S.; Kutzner, C.E.; Hu, Y.; Lornage, X.; Rendu, J.; Stojkovic, T.; Baets, J.; Neuhaus, S.B.; Tanboon, J.; Maroofian, R.; et al. Pathogenic Variants in the Myosin Chaperone UNC-45B Cause Progressive Myopathy with Eccentric Cores. Am. J. Human Genet. 2020, 107, 1078–1095. [Google Scholar] [CrossRef] [PubMed]
- Cullup, T.; Lamont, P.J.; Cirak, S.; Damian, M.S.; Wallefeld, W.; Gooding, R.; Tan, S.V.; Sheehan, J.; Muntoni, F.; Abbs, S.; et al. Mutations in MYH7 Cause Multi-Minicore Disease (MmD) with Variable Cardiac Involvement. Neuromuscul. Disord. 2012, 22, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, C.; Bonnemann, C.G.; Julien, C.; Kho, A.L.; Marks, H.; Talim, B.; Maury, P.; Arne-Bes, M.C.; Uro-Coste, E.; Alexandrovich, A.; et al. Recessive TTN Truncating Mutations Define Novel Forms of Core Myopathy with Heart Disease. Human Mol. Genet. 2014, 23, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S.E.; Mahoney, L.J.; Kawahara, G.; Myers, J.A.; Mitsuhashi, S.; Estrella, E.A.; Duncan, A.R.; Dey, F.; DeChene, E.T.; Blasko-Goehringer, J.M.; et al. Mutations in the Satellite Cell Gene MEGF10 Cause a Recessive Congenital Myopathy with Minicores. Neurogenetics 2012, 13, 115–124. [Google Scholar] [CrossRef]
- Kazamel, M.; Milone, M. Congenital Myopathy with a Novel SELN Missense Mutation and the Challenge to Differentiate It from Congenital Muscular Dystrophy. J. Clin. Neurosci. 2019, 62, 238–239. [Google Scholar] [CrossRef]
- Estañ, M.C.; Fernández-Núñez, E.; Zaki, M.S.; Esteban, M.I.; Donkervoort, S.; Hawkins, C.; Caparros-Martin, J.A.; Saade, D.; Hu, Y.; Bolduc, V.; et al. Recessive Mutations in Muscle-Specific Isoforms of FXR1 Cause Congenital Multi-Minicore Myopathy. Nat. Commun. 2019, 10, 797. [Google Scholar] [CrossRef]
- Jungbluth, H.; Müller, C.R.; Halliger–Keller, B.; Brockington, M.; Brown, S.C.; Feng, L.; Chattopadhyay, A.; Mercuri, E.; Manzur, A.Y.; Ferreiro, A.; et al. Autosomal Recessive Inheritance of RYR1 Mutations in a Congenital Myopathy with Cores. Neurology 2002, 59, 284–287. [Google Scholar] [CrossRef]
- Ferreiro, A.; Monnier, N.; Romero, N.B.N.B.; Leroy, J.-P.J.P.; Bönnemann, C.; Haenggeli, C.-A.C.A.; Straub, V.; Voss, W.D.W.D.; Nivoche, Y.; Jungbluth, H.; et al. A Recessive Form of Central Core Disease, Transiently Presenting as Multi-Minicore Disease, Is Associated with a Homozygous Mutation in the Ryanodine Receptor Type 1 Gene. Ann. Neurol. 2002, 51, 750–759. [Google Scholar] [CrossRef]
- Jungbluth, H.; Dowling, J.J.; Ferreiro, A.; Muntoni, F. 182nd ENMC International Workshop: RYR1-Related Myopathies, 15–17th April 2011, Naarden, The Netherlands. Neuromuscul. Disord. 2012, 22, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H. Multi-Minicore Disease. Orphanet J. Rare Dis. 2007, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Witherspoon, J.W.; Meilleur, K.G. Review of RyR1 Pathway and Associated Pathomechanisms. Acta Neuropathol. Commun. 2016, 4, 121. [Google Scholar] [CrossRef]
- Lawal, T.A.; Todd, J.J.; Witherspoon, J.W.; Bönnemann, C.G.; Dowling, J.J.; Hamilton, S.L.; Meilleur, K.G.; Dirksen, R.T. Ryanodine Receptor 1-Related Disorders: An Historical Perspective and Proposal for a Unified Nomenclature. Skelet. Muscle 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wei, R.; Wei, C.; Liu, J.; Qin, L.; Yan, H.; Ma, Y.; Wang, Z.; Xiong, H. Correlation of Phenotype–Genotype and Protein Structure in RYR1-Related Myopathy. Front. Neurol. 2022, 13, 870285. [Google Scholar] [CrossRef] [PubMed]
- Treves, S.; Anderson, A.A.; Ducreux, S.; Divet, A.; Bleunven, C.; Grasso, C.; Paesante, S.; Zorzato, F. Ryanodine Receptor 1 Mutations, Dysregulation of Calcium Homeostasis and Neuromuscular Disorders. Neuromuscul. Disord. 2005, 15, 577–587. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Rainer, J.; Gatto, L.; Weichenberger, C.X. Ensembldb: An R Package to Create and Use Ensembl-Based Annotation Resources. Bioinformatics 2019, 35, 3151–3153. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284. [Google Scholar] [CrossRef]
- Bioconductor—Enrichplot. Available online: https://www.bioconductor.org/packages/release/bioc/html/enrichplot.html (accessed on 26 April 2022).
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Galli, L.; Orrico, A.; Cozzolino, S.; Pietrini, V.; Tegazzin, V.; Sorrentino, V. Mutations in the RYR1 Gene in Italian Patients at Risk for Malignant Hyperthermia: Evidence for a Cluster of Novel Mutations in the C-Terminal Region. Cell Calcium 2002, 32, 143–151. [Google Scholar] [CrossRef]
- Johannsen, S.; Treves, S.; Müller, C.R.; Mögele, S.; Schneiderbanger, D.; Roewer, N.; Schuster, F. Functional Characterization of the RYR1 Mutation p.Arg4737Trp Associated with Susceptibility to Malignant Hyperthermia. Neuromuscul. Disord. 2016, 26, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Sambuughin, N.; Nelson, T.E.; Jankovic, J.; Xin, C.; Meissner, G.; Mullakandov, M.; Ji, J.; Rosenberg, H.; Sivakumar, K.; Goldfarb, L.G. Identification and Functional Characterization of a Novel Ryanodine Receptor Mutation Causing Malignant Hyperthermia in North American and South American Families. Neuromuscul. Disord. 2001, 11, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Monnier, N.; Kozak-Ribbens, G.; Krivosic-Horber, R.; Nivoche, Y.; Qi, D.; Kraev, N.; Loke, J.; Sharma, P.; Tegazzin, V.; Figarella-Branger, D.; et al. Correlations between Genotype and Pharmacological, Histological, Functional, and Clinical Phenotypes in Malignant Hyperthermia Susceptibility. Human Mutat. 2005, 26, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and Localization of Mutations in the 106 Exons of the RYR1 Gene in 50 Individuals with Malignant Hyperthermia. Human Mutat. 2006, 27, 830. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.-A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in Malignant Hyperthermia and Central Core Disease. Human Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef]
- Wu, S.; Ibarra, M.C.A.; Malicdan, M.C.V.; Murayama, K.; Ichihara, Y.; Kikuchi, H.; Nonaka, I.; Noguchi, S.; Hayashi, Y.K.; Nishino, I. Central Core Disease Is Due to RYR1 Mutations in More than 90% of Patients. Brain 2006, 129, 1470–1480. [Google Scholar] [CrossRef]
- Yang, T.; Riehl, J.; Esteve, E.; Matthaei, K.I.; Goth, S.; Allen, P.D.; Pessah, I.N.; Lopez, J.R. Pharmacologic and Functional Characterization of Malignant Hyperthermia in the R163C RyR1 Knock-in Mouse. Anesthesiology 2006, 105, 1164–1175. [Google Scholar] [CrossRef]
- Robinson, R.L.; Brooks, C.; Brown, S.L.; Ellis, F.R.; Halsall, P.J.; Quinnell, R.J.; Shaw, M.-A.; Hopkins, P.M. RYR1 Mutations Causing Central Core Disease Are Associated with More Severe Malignant Hyperthermia in Vitro Contracture Test Phenotypes. Human Mutat. 2002, 20, 88–97. [Google Scholar] [CrossRef]
- Malandrini, A.; Orrico, A.; Gaudiano, C.; Gambelli, S.; Galli, L.; Berti, G.; Tegazzin, V.; Dotti, M.T.; Federico, A.; Sorrentino, V. Muscle Biopsy and in Vitro Contracture Test in Subjects with Idiopathic HyperCKemia. Anesthesiology 2008, 109, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Groom, L.; Muldoon, S.M.; Tang, Z.Z.; Brandom, B.W.; Bayarsaikhan, M.; Bina, S.; Lee, H.-S.; Qiu, X.; Sambuughin, N.; Dirksen, R.T. Identical de Novo Mutation in the Type 1 Ryanodine Receptor Gene Associated with Fatal, Stress-Induced Malignant Hyperthermia in Two Unrelated Families. Anesthesiology 2011, 115, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Kraeva, N.; Heytens, L.; Jungbluth, H.; Treves, S.; Voermans, N.; Kamsteeg, E.; Ceuterick-de Groote, C.; Baets, J.; Riazi, S. Compound RYR1 Heterozygosity Resulting in a Complex Phenotype of Malignant Hyperthermia Susceptibility and a Core Myopathy. Neuromuscul. Disord. 2015, 25, 567–576. [Google Scholar] [CrossRef]
- Johnston, J.J.; Dirksen, R.T.; Girard, T.; Hopkins, P.M.; Kraeva, N.; Ognoon, M.; Radenbaugh, K.B.; Riazi, S.; Robinson, R.L.; Saddic Iii, L.A.; et al. Updated Variant Curation Expert Panel Criteria and Pathogenicity Classifications for 251 Variants for RYR1-Related Malignant Hyperthermia Susceptibility. Hum. Mol. Genet. 2022, 31, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, S.; Hu, J.; Yuan, Y.; Wang, Z.; Da, Y.; Wu, S. Novel RYR1 Missense Mutations in Six Chinese Patients with Central Core Disease. Neurosci. Lett. 2014, 566, 32–35. [Google Scholar] [CrossRef]
- Kage, F.; Vicente-Manzanares, M.; McEwan, B.C.; Kettenbach, A.N.; Higgs, H.N. Myosin II Proteins Are Required for Organization of Calcium-Induced Actin Networks Upstream of Mitochondrial Division. Mol. Biol. Cell 2022, 33, ar63. [Google Scholar] [CrossRef]
- Mu, N.; Wang, Y.; Li, X.; Du, Z.; Wu, Y.; Su, M.; Wang, Y.; Sun, X.; Su, L.; Liu, X. Crotonylated BEX2 Interacts with NDP52 and Enhances Mitophagy to Modulate Chemotherapeutic Agent-Induced Apoptosis in Non-Small-Cell Lung Cancer Cells. Cell Death Dis. 2023, 14, 645. [Google Scholar] [CrossRef]
- Son, Y.-O.; Heo, J.-S.; Kim, T.-G.; Jeon, Y.-M.; Kim, J.-G.; Lee, J.-C. Over-Expression of JunB Inhibits Mitochondrial Stress and Cytotoxicity in Human Lymphoma Cells Exposed to Chronic Oxidative Stress. BMB Rep. 2010, 43, 57–61. [Google Scholar] [CrossRef]
- Deng, K.; Fan, Y.; Liang, Y.; Cai, Y.; Zhang, G.; Deng, M.; Wang, Z.; Lu, J.; Shi, J.; Wang, F.; et al. FTO-Mediated Demethylation of GADD45B Promotes Myogenesis through the Activation of P38 MAPK Pathway. Mol. Ther. Nucleic Acids 2021, 26, 34–48. [Google Scholar] [CrossRef]
- Wu, F.; Huang, W.; Tan, Q.; Guo, Y.; Cao, Y.; Shang, J.; Ping, F.; Wang, W.; Li, Y. ZFP36L2 Regulates Myocardial Ischemia/Reperfusion Injury and Attenuates Mitochondrial Fusion and Fission by LncRNA PVT1. Cell Death Dis. 2021, 12, 614. [Google Scholar] [CrossRef]
- Rizk, J.; Sahu, R.; Duteil, D. An Overview on Androgen-Mediated Actions in Skeletal Muscle and Adipose Tissue. Steroids 2023, 199, 109306. [Google Scholar] [CrossRef]
- Pataky, M.W.; Dasari, S.; Michie, K.L.; Sevits, K.J.; Kumar, A.A.; Klaus, K.A.; Heppelmann, C.J.; Robinson, M.M.; Carter, R.E.; Lanza, I.R.; et al. Impact of Biological Sex and Sex Hormones on Molecular Signatures of Skeletal Muscle at Rest and in Response to Distinct Exercise Training Modes. Cell Metab. 2023, 35, 1996–2010.e6. [Google Scholar] [CrossRef]
- Ogasawara, M.; Ogawa, M.; Nonaka, I.; Hayashi, S.; Noguchi, S.; Nishino, I. Evaluation of the Core Formation Process in Congenital Neuromuscular Disease with Uniform Type 1 Fiber and Central Core Disease. J. Neuropathol. Exp. Neurol. 2020, 79, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Franzini-Armstrong, C. Myology: Basic and Clinical; McGraw-Hill: Columbus, OH, USA, 1994; ISBN 0070195587. [Google Scholar]
- Filipova, D.; Henry, M.; Rotshteyn, T.; Brunn, A.; Carstov, M.; Deckert, M.; Hescheler, J.; Sachinidis, A.; Pfitzer, G.; Papadopoulos, S. Distinct Transcriptomic Changes in E14.5 Mouse Skeletal Muscle Lacking RYR1 or Cav1.1 Converge at E18.5. PLoS ONE 2018, 13, e0194428. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.G.; Goonasekera, S.A.; Durham, W.J.; Tang, W.; Lueck, J.D.; Riehl, J.; Pessah, I.N.; Zhang, P.; Bhattacharjee, M.B.; Dirksen, R.T.; et al. Heat- and Anesthesia-induced Malignant Hyperthermia in an RyR1 Knock-in Mouse. FASEB J. 2006, 20, 329–330. [Google Scholar] [CrossRef]
- Lopez, J.R.; Kaura, V.; Diggle, C.P.; Hopkins, P.M.; Allen, P.D. Malignant Hyperthermia, Environmental Heat Stress, and Intracellular Calcium Dysregulation in a Mouse Model Expressing the p.G2435R Variant of RYR1. Br. J. Anaesth. 2018, 121, 953–961. [Google Scholar] [CrossRef]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-Nitrosylation Underlies Environmental Heat Stroke and Sudden Death in Y522S RyR1 Knockin Mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef]
- Michelucci, A.; De Marco, A.; Guarnier, F.A.; Protasi, F.; Boncompagni, S. Antioxidant Treatment Reduces Formation of Structural Cores and Improves Muscle Function in RYR1Y522S/WT Mice. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria Are Linked to Calcium Stores in Striated Muscle by Developmentally Regulated Tethering Structures. Mol. Biol. Cell 2009, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Ross-Inta, C.; Omanska-Klusek, A.; Napoli, E.; Sakaguchi, D.; Barrientos, G.; Allen, P.D.; Pessah, I.N. Basal Bioenergetic Abnormalities in Skeletal Muscle from Ryanodine Receptor Malignant Hyperthermia-Susceptible R163C Knock-in Mice. J. Biol. Chem. 2011, 286, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Liu, X.; Diggle, C.P.; Boyle, J.P.; Hopkins, P.M.; Shaw, M.-A.; Allen, P.D. Bioenergetic Defects in Muscle Fibers of RYR1 Mutant Knock-in Mice Associated with Malignant Hyperthermia. J. Biol. Chem. 2020, 295, 15226–15235. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Motley, R.; Daly, C.L.; Diggle, C.P.; Hopkins, P.M.; Shaw, M.-A. An Association between OXPHOS-Related Gene Expression and Malignant Hyperthermia Susceptibility in Human Skeletal Muscle Biopsies. Int. J. Mol. Sci. 2024, 25, 3489. [Google Scholar] [CrossRef]
- Yoo, J.; Ghiassi, M.; Jirmanova, L.; Balliet, A.G.; Hoffman, B.; Fornace, A.J.; Liebermann, D.A.; Böttinger, E.P.; Roberts, A.B. Transforming Growth Factor-β-Induced Apoptosis Is Mediated by Smad-Dependent Expression of GADD45b through P38 Activation. J. Biol. Chem. 2003, 278, 43001–43007. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A. New Roles for Smad Signaling and Phosphatidic Acid in the Regulation of Skeletal Muscle Mass. F1000Prime Rep. 2014, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pan, C.C.; Shah, N.; Wheeler, S.E.; Hoyt, K.R.; Hempel, N.; Mythreye, K.; Lee, N.Y. Activation of Mitofusin2 by Smad2-RIN1 Complex during Mitochondrial Fusion. Mol. Cell 2016, 62, 520–531. [Google Scholar] [CrossRef]
- Yamada, A.K.; Verlengia, R.; Bueno Junior, C.R. Mechanotransduction Pathways in Skeletal Muscle Hypertrophy. J. Recept. Signal Transduct. 2012, 32, 42–44. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanical Signal Transduction in Skeletal Muscle Growth and Adaptation. J. Appl. Physiol. 2005, 98, 1900–1908. [Google Scholar] [CrossRef]
- Martineau, L.C.; Gardiner, P.F. Insight into Skeletal Muscle Mechanotransduction: MAPK Activation Is Quantitatively Related to Tension. J. Appl. Physiol. 2001, 91, 693–702. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-Dependent Regulations and Signaling in Skeletal Muscle: From Electro-Mechanical Coupling to Adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- Tang, M.; Lu, G.; Shen, H.-M. SMAD3 and PINK1 Constitute a New Positive Feedback Loop in Regulation of Mitophagy. Autophagy 2025, 21, 2074–2076. [Google Scholar] [CrossRef]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell Death, Clearance and Immunity in the Skeletal Muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, Y.; Xu, D.; He, L.; Zhang, X.; Yan, E.; Wang, L.; Yin, J. Ryanodine Receptor RyR1-Mediated Elevation of Ca2+ Concentration Is Required for the Late Stage of Myogenic Differentiation and Fusion. J. Anim. Sci. Biotechnol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Ozcan, L.; Tabas, I. Role of Endoplasmic Reticulum Stress in Metabolic Disease and Other Disorders. Annu. Rev. Med. 2012, 63, 317–328. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Reich, S.; Nguyen, C.D.L.; Has, C.; Steltgens, S.; Soni, H.; Coman, C.; Freyberg, M.; Bichler, A.; Seifert, N.; Conrad, D.; et al. A Multi-Omics Analysis Reveals the Unfolded Protein Response Regulon and Stress-Induced Resistance to Folate-Based Antimetabolites. Nat. Commun. 2020, 11, 2936. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Urano, F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Methods Enzym. 2011, 490, 71. [Google Scholar] [CrossRef]
- Vincze, O.; Tökési, N.; Oláh, J.; Hlavanda, E.; Zotter, A.; Horváth, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; et al. Tubulin Polymerization Promoting Proteins (TPPPs): Members of a New Family with Distinct Structures and Functions. Biochemistry 2006, 45, 13818–13826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, X.; Li, L.; Feng, X.; Yang, Z.; Zhang, W.; Hu, R. Depletion of Tubulin Polymerization Promoting Protein Family Member 3 Suppresses HeLa Cell Proliferation. Mol. Cell. Biochem. 2010, 333, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Liu, J.; Hughes-Davies, L. BEX2 Has a Functional Interplay with C-Jun/JNK and P65/RelA in Breast Cancer. Mol. Cancer 2010, 9, 111. [Google Scholar] [CrossRef]
- Naderi, A. Molecular Functions of Brain Expressed X-Linked 2 (BEX2) in Malignancies. Exp. Cell Res. 2019, 376, 221–226. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhou, Z.; Jin, W.; Sun, C. SOCS3 Promotes Inflammation and Apoptosis via Inhibiting JAK2/STAT3 Signaling Pathway in 3T3-L1 Adipocyte. Immunobiology 2015, 220, 947–953. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, S.-M.; Hwang, E.M.; Baek, K.E.; Kim, I.-K.; Nam, I.-K.; Im, M.-J.; Park, S.-H.; Bae, S.; Park, J.-Y.; et al. Gadd45b Mediates Fas-Induced Apoptosis by Enhancing the Interaction between P38 and Retinoblastoma Tumor Suppressor. J. Biol. Chem. 2010, 285, 25500–25505. [Google Scholar] [CrossRef] [PubMed]
- Gurzov, E.N.; Ortis, F.; Bakiri, L.; Wagner, E.F.; Eizirik, D.L.; Jun, B. Inhibits ER Stress and Apoptosis in Pancreatic Beta Cells. PLoS ONE 2008, 3, e3030. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, W.C.; Zhang, Y.L.; Lin, Q.Y.; Liao, J.W.; Song, G.R.; Ma, X.L.; Li, H.H.; Zhang, B. SOCS3 Negatively Regulates Cardiac Hypertrophy via Targeting GRP78-Mediated ER Stress During Pressure Overload. Front. Cell Dev. Biol. 2021, 9, 629932. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.A.; Lteif, C.; Huang, Y.; Flohr, R.M.; Nogueira, A.C.; Gawronski, B.E.; Duarte, J.D. Genetic Variation in RYR1 Is Associated with Heart Failure Progression and Mortality in a Diverse Patient Population. Front. Cardiovasc. Med. 2025, 12, 1529114. [Google Scholar] [CrossRef]
- Beiter, T.; Hoene, M.; Prenzler, F.; Mooren, F.C.; Steinacker, J.M.; Weigert, C.; LastNameNieß, A.M.; Munz, B. Exercise, Skeletal Muscle and Inflammation: ARE-Binding Proteins as Key Regulators in Inflammatory and Adaptive Networks. Exerc. Immunol. Rev. 2015, 21, 42–57. [Google Scholar]
- Schichl, Y.M.; Resch, U.; Hofer-Warbinek, R.; de Martin, R. Tristetraprolin Impairs NF-KappaB/P65 Nuclear Translocation. J. Biol. Chem. 2009, 284, 29571–29581. [Google Scholar] [CrossRef]
- Liang, J.; Lei, T.; Song, Y.; Yanes, N.; Qi, Y.; Fu, M. RNA-Destabilizing Factor Tristetraprolin Negatively Regulates NF-KappaB Signaling. J. Biol. Chem. 2009, 284, 29383–29390. [Google Scholar] [CrossRef]
- Engel, W.K.; Foster, J.B.; Hughes, B.P.; Huxley, H.E.; Mahler, R. Central Core Disease-an Investigation of a Rare Muscle Cell Abnormality. Brain 1961, 84, 167–185. [Google Scholar] [CrossRef]
| Sample ID | Sex | Age at Biopsy | Nucleotide Change | Amino Acid Change | Exon | Histological Diagnosis | Protein Domain | Structural Effect | Disease Severity | References |
|---|---|---|---|---|---|---|---|---|---|---|
| ID01 | M | 55 | c.14209C>T | p.Arg4737Trp | 98 | MmD | pVSD | Impairs a salt bridge with Glu4736. Impairs apoCAM binding. | Mild myopathy | [34,35] |
| ID02 | M | 53 | c.7048G>A | p.Ala2350Thr | 44 | CCD | Bsol | New interaction with Ser2345. Loss of interaction with calstabin 1. | Mild myopathy | [36] |
| ID03 | M | 35 | c.6617C>T | p.Thr2206Met | 40 | MmD | Bsol | Hydrophobic residue inside a polar pocket. Increases caffeine sensitivity. | Asymptomatic | [37] |
| c.10537A>G | p.Thr3513Ala | 71 | Bsol | Hydrophobic residue inside a polar pocket. Impairs apoCAM binding. | [3] | |||||
| ID04 | M | 24 | c.467G>A | p.Arg156Lys | 6 | CCD | NTD-A | Impairs an exposed salt bridge with Glu160. Destabilises MIR folding domain. | Paucisymptomatic | [38,39] |
| ID05 | M | 33 | c.14209C>T | p.Arg4737Trp | 98 | MmD | pVSD | Impairs a salt bridge with Glu4736. Impairs apoCAM binding. | Paucisymptomatic | [34,35] |
| ID06 | F | 41 | c.3901C>T | p.Arg1301Cys | 28 | MmD | SPRY2/SPRY3 | No local modification. Interferes with DHPR regulation. | Asymptomatic | dbSNP: rs745920741 |
| c.5360C>T | p.Pro1787Leu | 34 | Jsol | No local modification. Likely benign. | [39] | |||||
| ID07 | F | 46 | c.7304G>A | p.Arg2435His | 45 | CCD | Bsol | Weaker electrostatic interaction with Asp2431. Inactivates the channel gating. | Paucisymptomatic | [37] |
| ID08 | F | 29 | c.7523G>A | p.Arg2508His | 47 | CCD | Bsol | Impairs a salt bridge with Glu42439. Loss of interaction with calstabin. | Paucisymptomatic | [38,40] |
| ID09 | F | 33 | c.487C>T | p.Arg163Cys | 6 | CCD | NTD-A | Impairs electrostatic interactions with Glu160 and Glu2088. Destabilises MIR folding domain. | Paucisymptomatic | [41,42] |
| ID11 | F | 37 | c.487C>T | p.Arg163Cys | 6 | CCD | NTD-A | Impairs electrostatic interactions with Glu160 and Glu2088. Destabilises MIR folding domain. | Moderate myopathy | [41,42] |
| ID12 | F | 41 | c.14510delA | p.Gln4837ArgfsX3 | 100 | CCD | pore | Possible local misfolding. Alters quantitative level of RyR1. | Mild myopathy | [39] |
| ID13 | F | 22 | c.7085A>G | p.Glu2362Gly | 44 | CCD | Bsol | Putative loss of interaction with ions. Inactivates the channel gating. | Moderate myopathy | [38] |
| c.13513G>C | p.Asp4505His | 92 | pVSD | Possible local unfolding. Impairs apoCAM binding. | [43,44] | |||||
| ID14 | M | 6 | c.4711A>G | p.Ile1571Val | 33 | MmD | RY2/SPRY3 | Hydrophobic residue inside a polar pocket. Likely benign. | Paucisymptomatic | [45,46] |
| c.9407delT | p.Leu3136ArgfsX3 | 63 | Bsol | Possible local misfolding. Alters quantitative level of RyR1. | [3] | |||||
| ID15 | F | 12 | c.14693T>C | p.Ile4898Thr | 102 | CCD | pore | Polar residue inside a hydrophobic pocket. Disrupts the channel activity. | Moderate myopathy | [27,47] |
| ID16 | F | 12 | c.472_474delGAA | p.Glu158del | 6 | CCD | NTD-A | Possible local misfolding. Reduces MIR2 folding domain. | Severe myopathy | [3] |
| ID17 | M | 3 | - | - | - | Ctrl | - | - | ||
| ID18 | M | 8 | - | - | Ctrl | - | - | |||
| ID19 | F | 6 | - | - | Ctrl | - | - | |||
| ID20 | M | 37 | - | - | Ctrl | - | - | |||
| ID21 | F | 35 | - | - | Ctrl | - | - | |||
| ID22 | M | 57 | - | - | Ctrl | - | - | |||
| ID23 | F | 30 | - | - | Ctrl | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, D.; Gorgoglione, D.; Minervini, G.; Fusto, A.; Suman, M.; Romualdi, C.; Vianello, S.; Capece, G.; Sorarù, G.; Marchioretti, C.; et al. RYR1-Related Myopathies Involve More than Calcium Dysregulation: Insights from Transcriptomic Profiling. Biomolecules 2025, 15, 1599. https://doi.org/10.3390/biom15111599
Sabbatini D, Gorgoglione D, Minervini G, Fusto A, Suman M, Romualdi C, Vianello S, Capece G, Sorarù G, Marchioretti C, et al. RYR1-Related Myopathies Involve More than Calcium Dysregulation: Insights from Transcriptomic Profiling. Biomolecules. 2025; 15(11):1599. https://doi.org/10.3390/biom15111599
Chicago/Turabian StyleSabbatini, Daniele, Domenico Gorgoglione, Giovanni Minervini, Aurora Fusto, Matteo Suman, Chiara Romualdi, Sara Vianello, Giuliana Capece, Gianni Sorarù, Caterina Marchioretti, and et al. 2025. "RYR1-Related Myopathies Involve More than Calcium Dysregulation: Insights from Transcriptomic Profiling" Biomolecules 15, no. 11: 1599. https://doi.org/10.3390/biom15111599
APA StyleSabbatini, D., Gorgoglione, D., Minervini, G., Fusto, A., Suman, M., Romualdi, C., Vianello, S., Capece, G., Sorarù, G., Marchioretti, C., Pennuto, M., Vedovelli, L., Szabadkai, G., Bello, L., & Pegoraro, E. (2025). RYR1-Related Myopathies Involve More than Calcium Dysregulation: Insights from Transcriptomic Profiling. Biomolecules, 15(11), 1599. https://doi.org/10.3390/biom15111599











