Screening and Characterization of TAT-Fused Nanobodies Targeting Bovine Viral Diarrhea Virus NS3/NS5A for Antiviral Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Virus
2.2. Expression and Purification of BVDV NS3 and NS5A Recombinant Proteins
2.3. Alpaca Immunization and Construction of the VHH Phage Display Library
2.4. Preparation and Titration of Helper Phage
2.5. Screening and Identification of NS3- and NS5A-Specific Nanobodies
2.6. Expression of TAT-Nb Recombinant Proteins
2.7. Cell Viability Assay
2.8. IFA Membrane Permeabilization Assay
2.9. Western Blot Membrane Permeabilization Assay
2.10. RT-qPCR Neutralization Assay
2.11. Western Blot Assay for Evaluating BVDV Replication
2.12. Molecular Docking of Nanobodies with Their Target Antigen Proteins
2.13. Statistical Analysis
3. Results
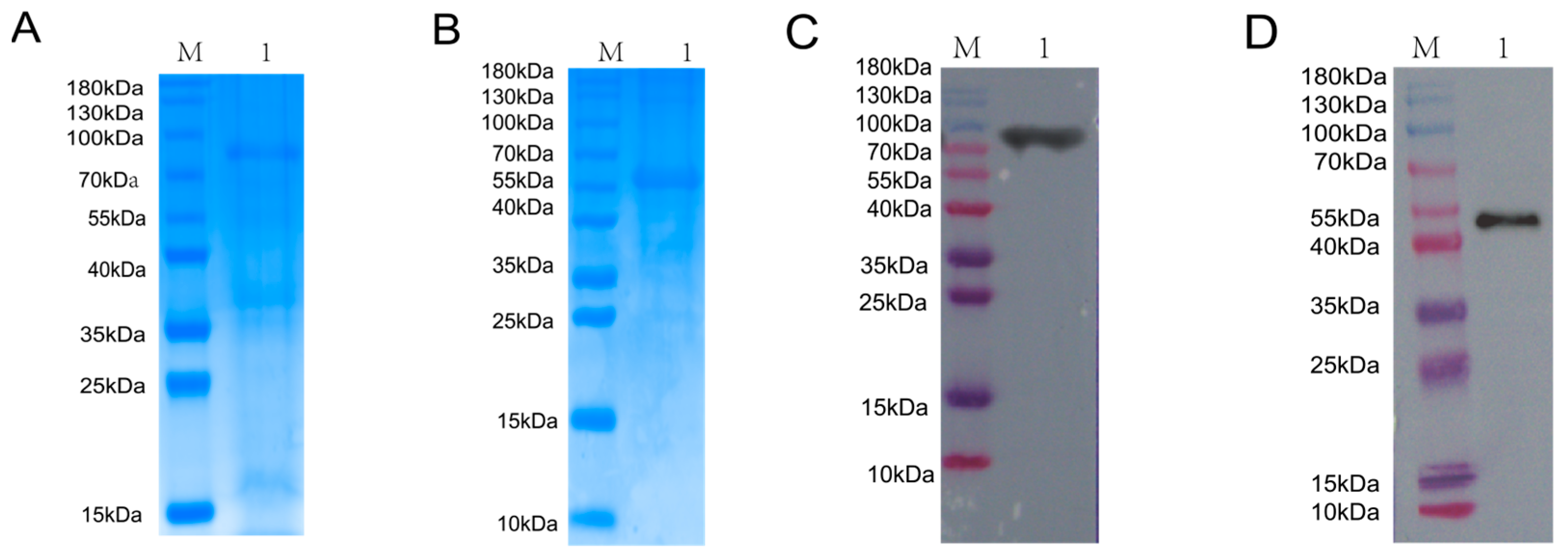
3.1. Expression and Purification of BVDV NS3 and NS5A Recombinant Proteins
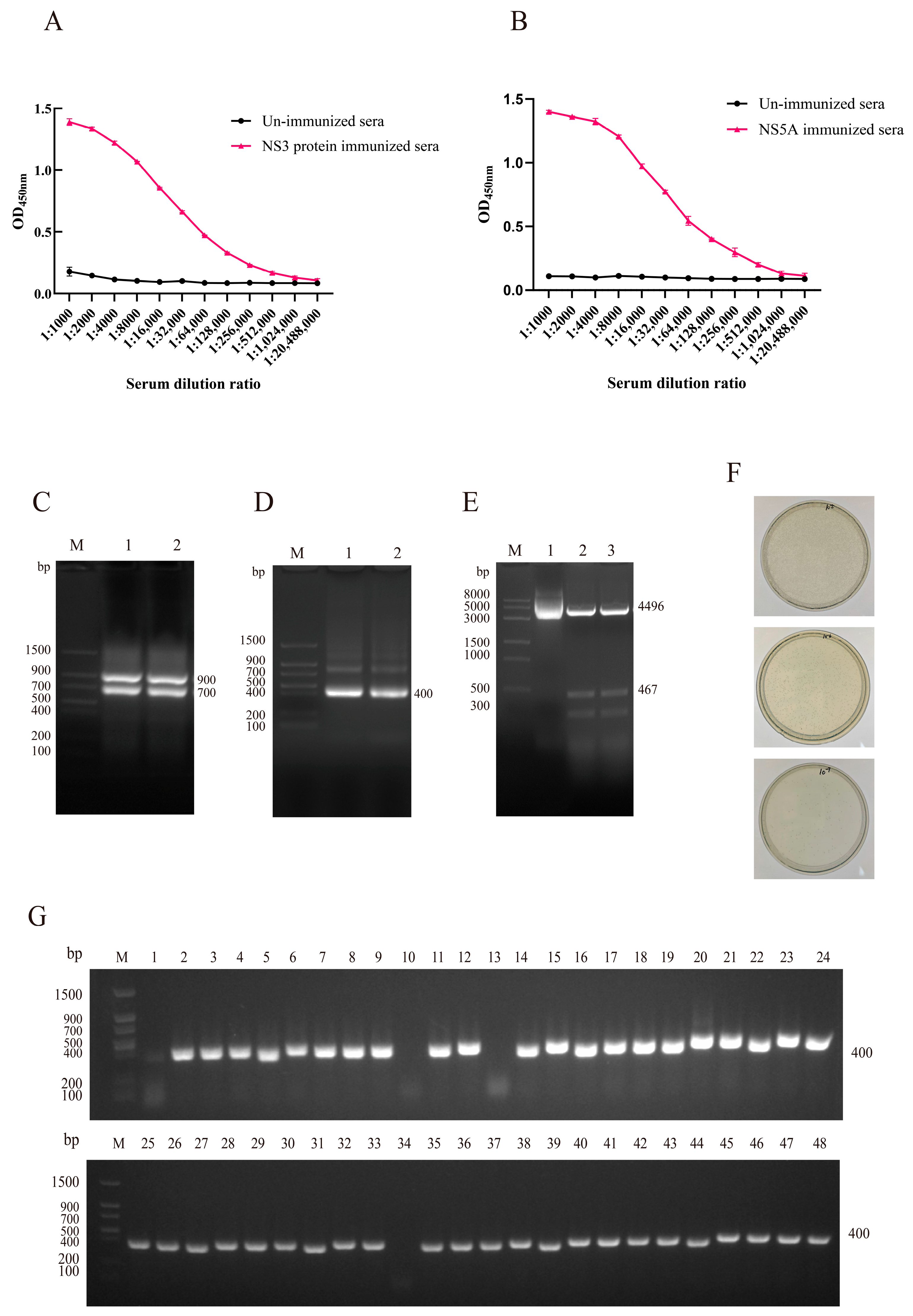
3.2. Construction and Characterization of VHH Phage Display Library
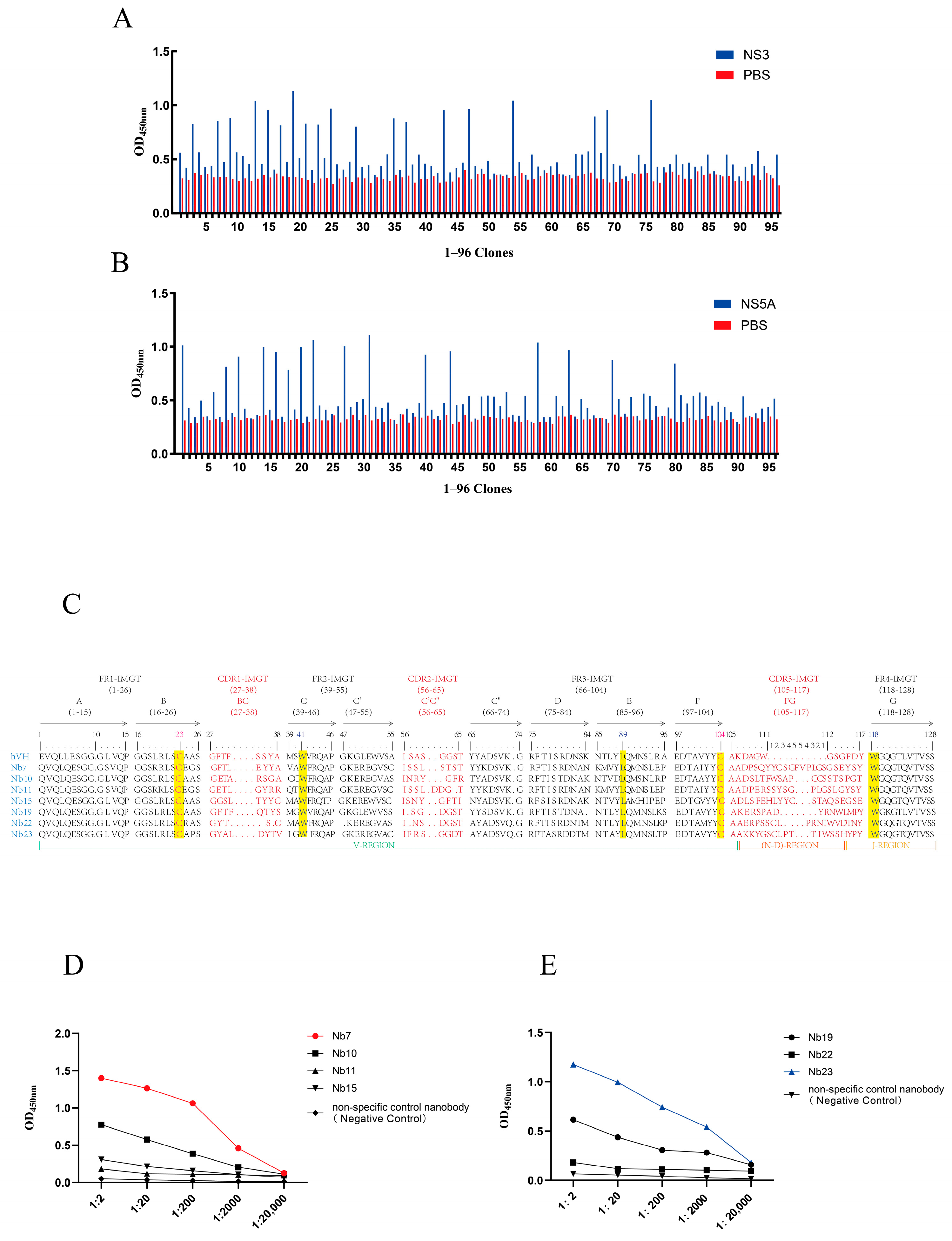
3.3. Screening and Identification of Specific Nanobodies
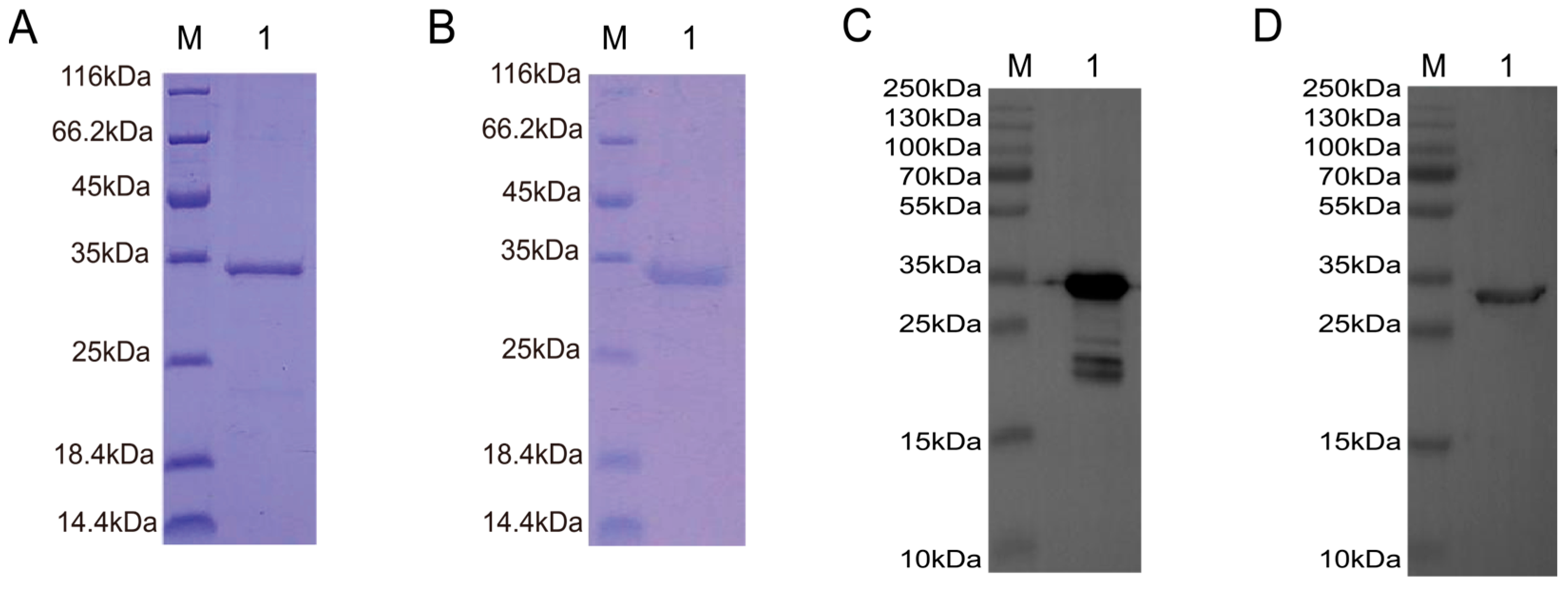
3.4. Expression and Purification of TAT-Nb7 and TAT-Nb23
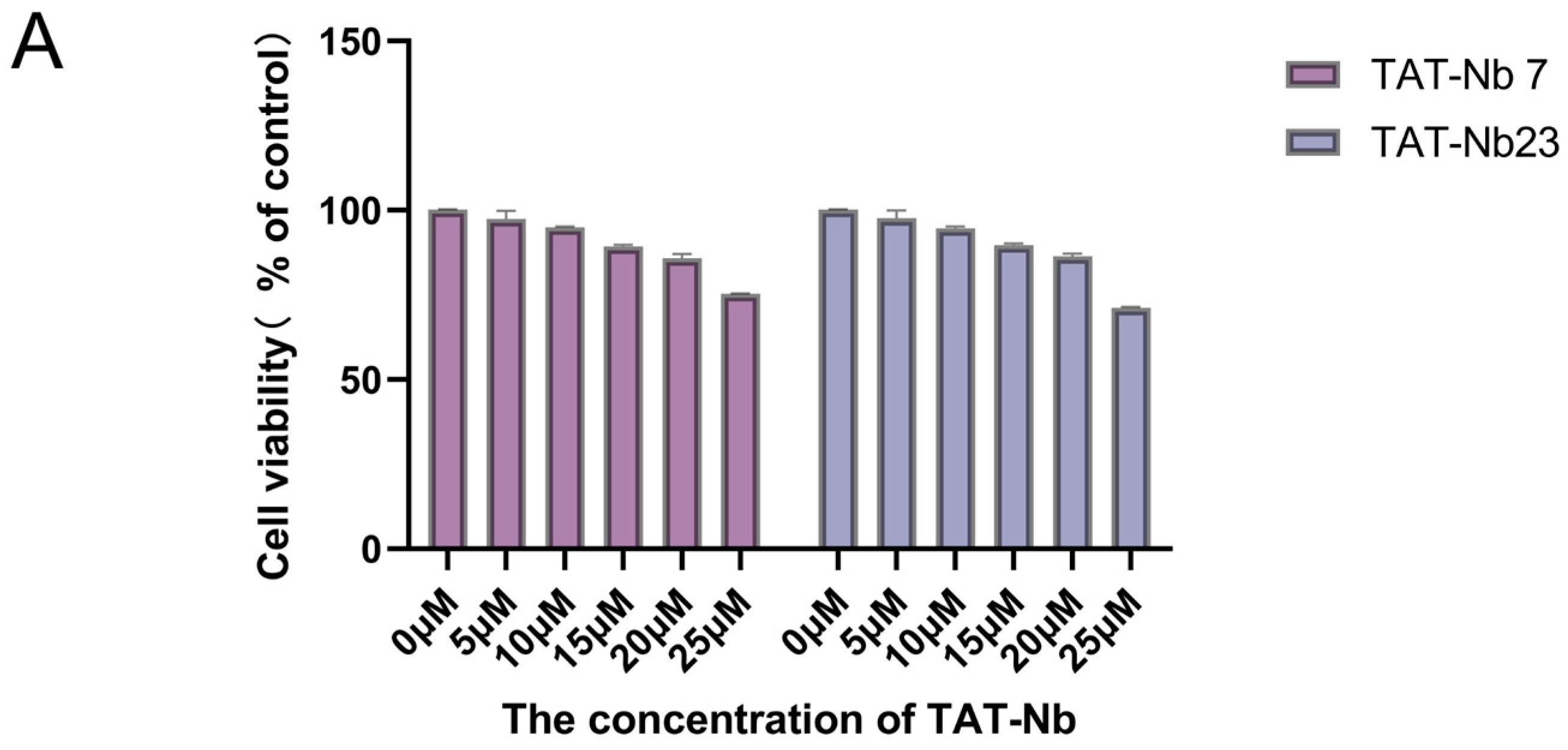
3.5. Cytotoxicity Assay and Transmembrane Verification
3.6. Evaluation of TAT-Nb7 and TAT-Nb23 on BVDV Replication in MDBK Cells
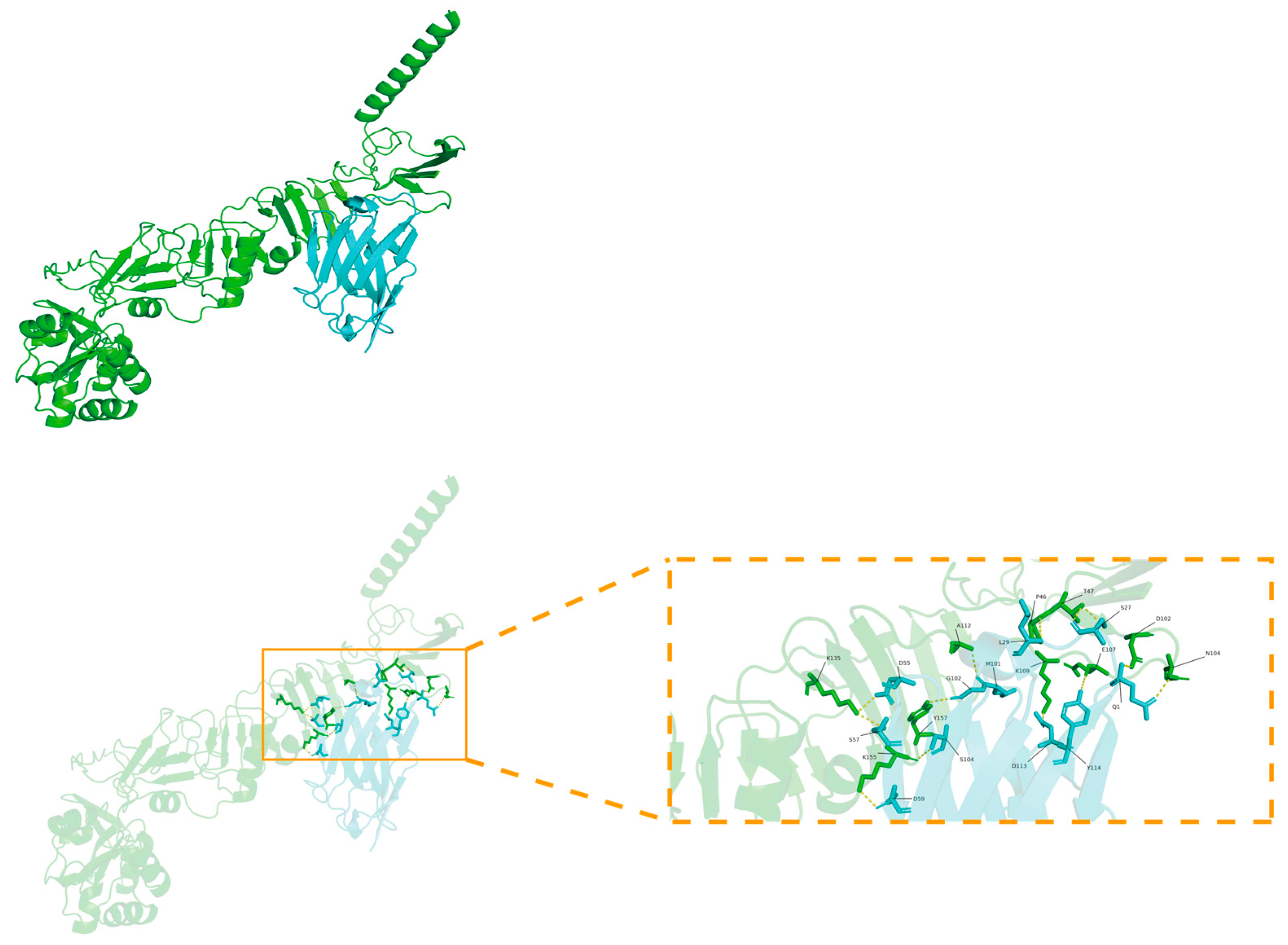
3.7. Molecular Docking Analysis of TAT-Nb7 with NS5A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, A.; Fu, Y.; Meens, J.; Yang, W.; Meng, F.; Herrler, G.; Becher, P. Infection of polarized bovine respiratory epithelial cells by bovine viral diarrhea virus (BVDV). Virulence 2021, 12, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Yaegashi, G.; Fukunari, K.; Suzuki, T. Clinical Analysis for Long-Term Sporadic Bovine Viral Diarrhea Transmitted by Calves with an Acute Infection of Bovine Viral Diarrhea Virus 2. Viruses 2021, 13, 621. [Google Scholar] [CrossRef]
- Baker, J.C. The clinical manifestations of bovine viral diarrhea infection. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 425–445. [Google Scholar] [CrossRef]
- Brock, K.V. The persistence of bovine viral diarrhea virus. Biologicals 2003, 31, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.D.; Duprau, J.L.; Wolff, P.L.; Evermann, J.F. Persistent Bovine Viral Diarrhea Virus Infection in Domestic and Wild Small Ruminants and Camelids Including the Mountain Goat (Oreamnos americanus). Front. Microbiol. 2015, 6, 1415. [Google Scholar] [CrossRef]
- Pecora, A.; Perez Aguirreburualde, M.S.; Ridpath, J.F.; Dus Santos, M.J. Molecular Characterization of Pestiviruses in Fetal Bovine Sera Originating from Argentina: Evidence of Circulation of HoBi-like Viruses. Front. Vet. Sci. 2019, 6, 359. [Google Scholar] [CrossRef]
- Giangaspero, M.; Vacirca, G.; Harasawa, R.; Büttner, M.; Panuccio, A.; De Giuli Morghen, C.; Zanetti, A.; Belloli, A.; Verhulst, A. Genotypes of pestivirus RNA detected in live virus vaccines for human use. J. Vet. Med. Sci. 2001, 63, 723–733. [Google Scholar] [CrossRef][Green Version]
- Larghi, M. Comparative study in the control of bovine viral diarrhea. Anim. Health Res. Rev. 2018, 19, 125–133. [Google Scholar] [CrossRef]
- Chi, S.; Chen, S.; Jia, W.; He, Y.; Ren, L.; Wang, X. Non-structural proteins of bovine viral diarrhea virus. Virus Genes 2022, 58, 491–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; Liu, W.; Zhou, L.; Yang, C.; Li, Y.; Du, E. Identification of three novel B cell epitopes targeting the bovine viral diarrhea virus NS3 protein for use in diagnostics and vaccine development. Int. J. Biol. Macromol. 2025, 308, 142767. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Kaiser, A.; Thiel, H.J. NS3 serine protease of bovine viral diarrhea virus: Characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology 2000, 273, 351–363. [Google Scholar] [CrossRef]
- Warrener, P.; Collett, M.S. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J. Virol. 1995, 69, 1720–1726. [Google Scholar] [CrossRef]
- Reed, K.E.; Gorbalenya, A.E.; Rice, C.M. The NS5A/NS5 proteins of viruses from three genera of the family Flaviviridae are phosphorylated by associated serine/threonine kinases. J. Virol. 1998, 72, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Sapay, N.; Montserret, R.; Chipot, C.; Brass, V.; Moradpour, D.; Deléage, G.; Penin, F. NMR structure and molecular dynamics of the in-plane membrane anchor of nonstructural protein 5A from bovine viral diarrhea virus. Biochemistry 2006, 45, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, J.; Xiao, J.; Sheng, C.; Wang, J.; Jia, L.; Zhi, Y.; Li, G.; Chen, J.; Xiao, M. Classical swine fever virus NS5A regulates viral RNA replication through binding to NS5B and 3′UTR. Virology 2012, 432, 376–388. [Google Scholar] [CrossRef]
- Grassmann, C.W.; Isken, O.; Tautz, N.; Behrens, S.E. Genetic analysis of the pestivirus nonstructural coding region: Defects in the NS5A unit can be complemented in trans. J. Virol. 2001, 75, 7791–7802. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.A.; Yamane, D.; Mohamed, Y.M.; Kobayashi, K.; Kato, K.; Tohya, Y.; Akashi, H. Characterization and application of monoclonal antibodies to bovine viral diarrhea virus nonstructural protein 5A. Arch. Virol. 2009, 154, 1745–1754. [Google Scholar] [CrossRef]
- de Oliveira, P.S.B.; Silva Júnior, J.V.J.; Weiblen, R.; Flores, E.F. Subtyping bovine viral diarrhea virus (BVDV): Which viral gene to choose? Infect. Genet. Evol. 2021, 92, 104891. [Google Scholar] [CrossRef]
- Tesfaye Melkamsew, A.; Sisay Tessema, T.; Paeshuyse, J. Host Immune Response to Bovine Viral Diarrhea Virus (BVDV): Insights and Strategies for Effective Vaccine Design. Vaccines 2025, 13, 456. [Google Scholar] [CrossRef]
- Duan, Q.; Ai, T.; Ma, Y.; Li, R.; Jin, H.; Chen, X.; Zhang, R.; Bao, K.; Chen, Q. Research Progress on the Application of Neutralizing Nanobodies in the Prevention and Treatment of Viral Infections. Microorganisms 2025, 13, 1352. [Google Scholar] [CrossRef]
- Caljon, G.; Caveliers, V.; Lahoutte, T.; Stijlemans, B.; Ghassabeh, G.H.; Van Den Abbeele, J.; Smolders, I.; De Baetselier, P.; Michotte, Y.; Muyldermans, S.; et al. Using microdialysis to analyse the passage of monovalent nanobodies through the blood-brain barrier. Br. J. Pharmacol. 2012, 165, 2341–2353. [Google Scholar] [CrossRef]
- Mohseni, A.; Molakarimi, M.; Taghdir, M.; Sajedi, R.H.; Hasannia, S. Exploring single-domain antibody thermostability by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2019, 37, 3686–3696. [Google Scholar] [CrossRef]
- Liu, W.; Song, H.; Chen, Q.; Yu, J.; Xian, M.; Nian, R.; Feng, D. Recent advances in the selection and identification of antigen-specific nanobodies. Mol. Immunol. 2018, 96, 37–47. [Google Scholar] [CrossRef]
- Ren, J.; Duan, H.; Dong, H.; Wu, S.; Du, Y.; Zhang, G.; Zhang, A. TAT Nanobody Exerts Antiviral Effect Against PRRSV In Vitro by Targeting Viral Nucleocapsid Protein. Int. J. Mol. Sci. 2023, 24, 1905. [Google Scholar] [CrossRef]
- de Beer, M.A.; Giepmans, B.N.G. Nanobody-Based Probes for Subcellular Protein Identification and Visualization. Front. Cell. Neurosci. 2020, 14, 573278. [Google Scholar] [CrossRef]
- Zou, L.; Peng, Q.; Wang, P.; Zhou, B. Progress in Research and Application of HIV-1 TAT-Derived Cell-Penetrating Peptide. J. Membr. Biol. 2017, 250, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ehrenmann, F.; Kaas, Q.; Lefranc, M.P. IMGT/3Dstructure-DB and IMGT/DomainGapAlign: A database and a tool for immunoglobulins or antibodies, T cell receptors, MHC, IgSF and MhcSF. Nucleic Acids Res. 2010, 38, D301–D307. [Google Scholar] [CrossRef] [PubMed]
- Khodakaram-Tafti, A.; Farjanikish, G.H. Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran. J. Vet. Res. 2017, 18, 154–163. [Google Scholar]
- Fulton, R.W.; Cook, B.J.; Payton, M.E.; Burge, L.J.; Step, D.L. Immune response to bovine viral diarrhea virus (BVDV) vaccines detecting antibodies to BVDV subtypes 1a, 1b, 2a, and 2c. Vaccine 2020, 38, 4032–4037. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Jin, D.; Liu, Y. Nanobody-A versatile tool for cancer diagnosis and therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1697. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Deng, Q.; Guo, C.; Wu, Q.; Li, Q. A High-Affinity and Potently Neutralized Nanobody against Zika Virus. ACS Infect. Dis. 2025, 11, 1975–1982. [Google Scholar] [CrossRef]
- Rossotti, M.A.; van Faassen, H.; Tran, A.T.; Sheff, J.; Sandhu, J.K.; Duque, D.; Hewitt, M.; Wen, X.; Bavananthasivam, J.; Beitari, S.; et al. Arsenal of nanobodies shows broad-spectrum neutralization against SARS-CoV-2 variants of concern in vitro and in vivo in hamster models. Commun. Biol. 2022, 5, 933. [Google Scholar] [CrossRef]
- Ibañez, L.I.; De Filette, M.; Hultberg, A.; Verrips, T.; Temperton, N.; Weiss, R.A.; Vandevelde, W.; Schepens, B.; Vanlandschoot, P.; Saelens, X. Nanobodies with in vitro neutralizing activity protect mice against H5N1 influenza virus infection. J. Infect. Dis. 2011, 203, 1063–1072. [Google Scholar] [CrossRef]
- Gao, M.; Nettles, R.E.; Belema, M.; Snyder, L.B.; Nguyen, V.N.; Fridell, R.A.; Serrano-Wu, M.H.; Langley, D.R.; Sun, J.H.; O’Boyle, D.R., 2nd; et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010, 465, 96–100. [Google Scholar] [CrossRef]
- Poordad, F.; McCone, J., Jr.; Bacon, B.R.; Bruno, S.; Manns, M.P.; Sulkowski, M.S.; Jacobson, I.M.; Reddy, K.R.; Goodman, Z.D.; Boparai, N.; et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 364, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Lawitz, E.; Zeuzem, S.; Gane, E.; Bronowicki, J.P.; Andreone, P.; Horban, A.; Brown, A.; Peeters, M.; Lenz, O.; et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: A phase 3 trial. Gastroenterology 2014, 146, 1669–1679.e1663. [Google Scholar] [CrossRef]
- Grassmann, C.W.; Isken, O.; Behrens, S.E. Assignment of the multifunctional NS3 protein of bovine viral diarrhea virus during RNA replication: An in vivo and in vitro study. J. Virol. 1999, 73, 9196–9205. [Google Scholar] [CrossRef] [PubMed]
- Schaut, R.G.; McGill, J.L.; Neill, J.D.; Ridpath, J.F.; Sacco, R.E. Bovine viral diarrhea virus type 2 in vivo infection modulates TLR4 responsiveness in differentiated myeloid cells which is associated with decreased MyD88 expression. Virus Res. 2015, 208, 44–55. [Google Scholar] [CrossRef]
- Gao, S.; Zuo, W.; Kang, C.; Zou, Z.; Zhang, K.; Qiu, J.; Shang, X.; Li, J.; Zhang, Y.; Zuo, Q.; et al. Saccharomyces cerevisiae oral immunization in mice using multi-antigen of the African swine fever virus elicits a robust immune response. Front. Immunol. 2024, 15, 1373656. [Google Scholar] [CrossRef]
- Throsby, M.; van den Brink, E.; Jongeneelen, M.; Poon, L.L.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Liang, Y.; Feng, W.; Yu, H.; Tong, C.; Song, B. Advancement in the development of single chain antibodies using phage display technology. PeerJ 2024, 12, e17143. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Ljungars, A.; Rimbault, C.; Sørensen, C.V.; Tulika, T.; Wade, J.; Wouters, Y.; McCafferty, J.; Laustsen, A.H. Advances in antibody phage display technology. Drug Discov. Today 2022, 27, 2151–2169. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, F.; Amini, S.M.; Kharrazi, S.; Rasaee, M.J.; Mazlomi, M.A.; Asadi-Ghalehni, M.; Rajabibazl, M.; Sadroddiny, E. Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 ScFv antibody. Colloids Surf. B Biointerfaces 2017, 159, 770–780. [Google Scholar] [CrossRef]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef]
- Almagro, J.C.; Pedraza-Escalona, M.; Arrieta, H.I.; Pérez-Tapia, S.M. Phage Display Libraries for Antibody Therapeutic Discovery and Development. Antibodies 2019, 8, 44. [Google Scholar] [CrossRef]
- Miersch, S.; Sidhu, S.S. Synthetic antibodies: Concepts, potential and practical considerations. Methods 2012, 57, 486–498. [Google Scholar] [CrossRef]
- Hawkins, R.E.; Russell, S.J.; Winter, G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J. Mol. Biol. 1992, 226, 889–896. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Wang, Y.; Brooks Iii, C.L. Electrostatic Forces Control the Negative Allosteric Regulation in a Disordered Protein Switch. J. Phys. Chem. Lett. 2020, 11, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, J.; Kang, G.; Hu, M.; Yuan, B.; Zhang, Y.; Huang, H. Homology Modeling-Based in Silico Affinity Maturation Improves the Affinity of a Nanobody. Int. J. Mol. Sci. 2019, 20, 4187. [Google Scholar] [CrossRef]
- Igawa, T.; Tsunoda, H.; Kuramochi, T.; Sampei, Z.; Ishii, S.; Hattori, K. Engineering the variable region of therapeutic IgG antibodies. MAbs 2011, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Z.; Yang, T.; Zhao, X.; Zheng, C.; Li, Y.; Li, Y.; Guo, X.; Xu, L.; Zheng, Z.; et al. A cell-penetrating NS5B-specific nanobody inhibits bovine viral diarrhea virus replication. Microb. Pathog. 2024, 197, 107107. [Google Scholar] [CrossRef]
- Osorio, J.S.; Bionaz, M. Plasmid transfection in bovine cells: Optimization using a realtime monitoring of green fluorescent protein and effect on gene reporter assay. Gene 2017, 626, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Munyendo, W.L.; Lv, H.; Benza-Ingoula, H.; Baraza, L.D.; Zhou, J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules 2012, 2, 187–202. [Google Scholar] [CrossRef]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The Use of TAT Peptide-Functionalized Graphene as a Highly Nuclear-Targeting Carrier System for Suppression of Choroidal Melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef]
- Ziu, T.; Sambur, E.; Ruzsics, Z.; Hengel, H.; Grabherr, R.; Höfinger, S.; Harant, H. In Vitro Profiling of the Antiviral Peptide TAT-I24. Int. J. Mol. Sci. 2024, 25, 10463. [Google Scholar] [CrossRef]
- Silva, F.S.R.; Santos, S.P.O.; Meyer, R.; Silva, E.S.; Pinheiro, C.S.; Alcantara-Neves, N.M.; Pacheco, L.G.C. In vivo cleavage of solubility tags as a tool to enhance the levels of soluble recombinant proteins in Escherichia coli. Biotechnol. Bioeng. 2021, 118, 4159–4167. [Google Scholar] [CrossRef]
- Vives, E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J. Control. Release 2005, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]


| Primers | Primer Sequences (5′–3′) | Digestion Site |
|---|---|---|
| AIPVh-LD | CTTGGTGGTCCTGGCTGC | |
| CH2-R | GGTACGTGCTGTTGAACTGTTCC | |
| VHH-Forward | TCGCGGCCCAGCCGGCCCAGGTCCAACTGCAGGAGTCTGGGG | Sfi I |
| VHH-Reverse | ATAAGAATGCGGCCGCTGAGGAGACGGTGACCTGGGTCCCC | Not I |
| Primers | Primer Sequences (5′–3′) |
|---|---|
| β-actin F | TGCTGTCCCTGTATGCCTCT |
| β-actin R | TGTCACGCACGATTTCCC |
| 5′UTR-F | CCTAGCCATGCCCTTAGTAGGACT |
| 5′UTR-R | GGAACTCCATGTGCCATGTACA |
| Elution Rounds | Phage Input (PFU/mL) | P Output (PFU/mL) | N Output (PFU/mL) | Recovery (P/Input) | P/N |
|---|---|---|---|---|---|
| First Round | 3.5 × 1012 | 2.4 × 104 | 3.6 × 103 | 6.86 × 10−9 | 6.7 |
| Second Round | 3.5 × 1012 | 7.9 × 105 | 2.7 × 104 | 2.3 × 10−7 | 29.2 |
| Third Round | 3.5 × 1012 | 3.01 × 106 | 3.6 × 104 | 8.6 × 10−7 | 83.6 |
| Elution Rounds | Phage Input (PFU/mL) | P Output (PFU/mL) | N Output (PFU/mL) | Recovery (P/Input) | P/N |
|---|---|---|---|---|---|
| First Round | 4.5 × 1012 | 1.88 × 105 | 5.5 × 104 | 4.18 × 10−8 | 3.4 |
| Second Round | 4.5 × 1012 | 6.4 × 106 | 9.1 × 104 | 1.42 × 10−6 | 70.3 |
| Third Round | 4.5 × 1012 | 8.5 × 107 | 7.5 × 105 | 1.89 × 10−5 | 113.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Xiao, Y.; Liu, Z.; Zhang, W.; Wu, A.; Zhang, H.; Sheng, J. Screening and Characterization of TAT-Fused Nanobodies Targeting Bovine Viral Diarrhea Virus NS3/NS5A for Antiviral Application. Biomolecules 2025, 15, 1593. https://doi.org/10.3390/biom15111593
Dong Q, Xiao Y, Liu Z, Zhang W, Wu A, Zhang H, Sheng J. Screening and Characterization of TAT-Fused Nanobodies Targeting Bovine Viral Diarrhea Virus NS3/NS5A for Antiviral Application. Biomolecules. 2025; 15(11):1593. https://doi.org/10.3390/biom15111593
Chicago/Turabian StyleDong, Qianqian, Yangyang Xiao, Zhao Liu, Wenxiang Zhang, Aodi Wu, Hanwen Zhang, and Jinliang Sheng. 2025. "Screening and Characterization of TAT-Fused Nanobodies Targeting Bovine Viral Diarrhea Virus NS3/NS5A for Antiviral Application" Biomolecules 15, no. 11: 1593. https://doi.org/10.3390/biom15111593
APA StyleDong, Q., Xiao, Y., Liu, Z., Zhang, W., Wu, A., Zhang, H., & Sheng, J. (2025). Screening and Characterization of TAT-Fused Nanobodies Targeting Bovine Viral Diarrhea Virus NS3/NS5A for Antiviral Application. Biomolecules, 15(11), 1593. https://doi.org/10.3390/biom15111593







