FGF22 Secreted by Hair Papilla Cells Regulates Hair Follicle Stem Cell Proliferation and Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
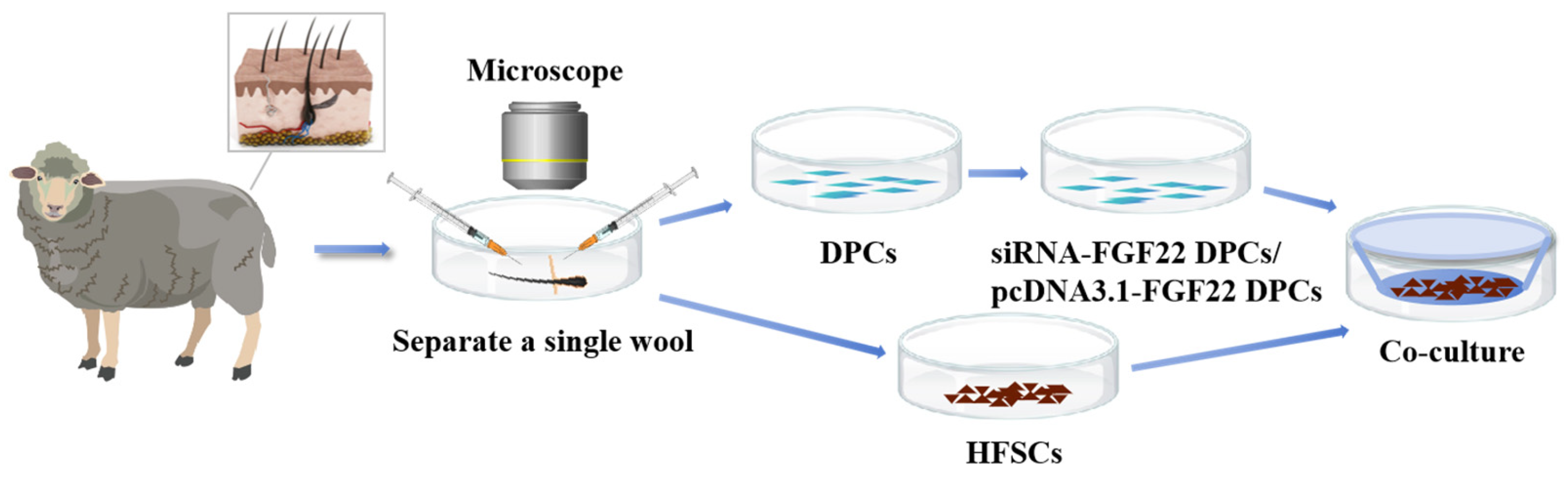
2.2. Animals and Sample Preparation
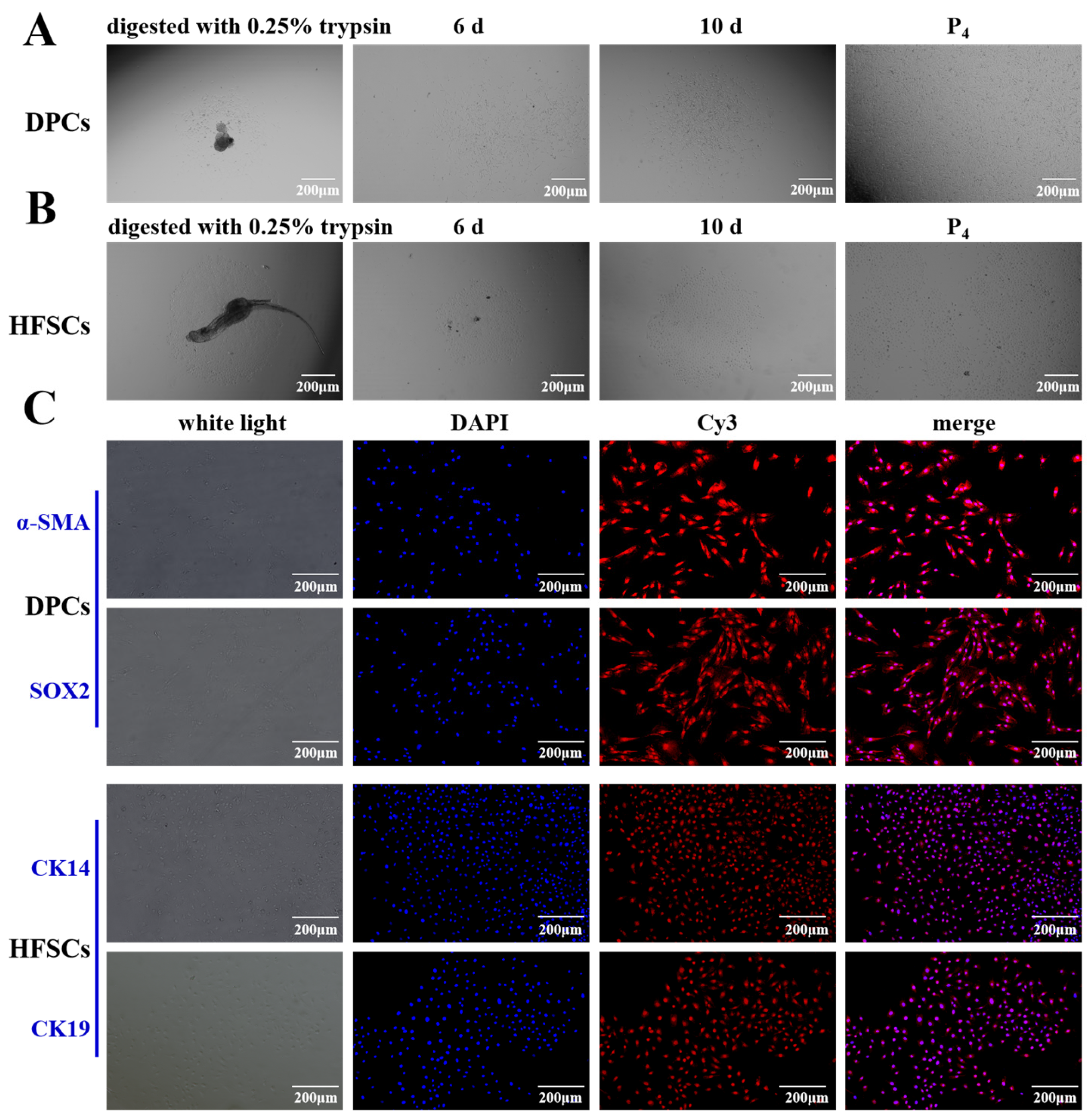
2.3. Isolation, Purification and Identification of Primary DPCs and HFSCs
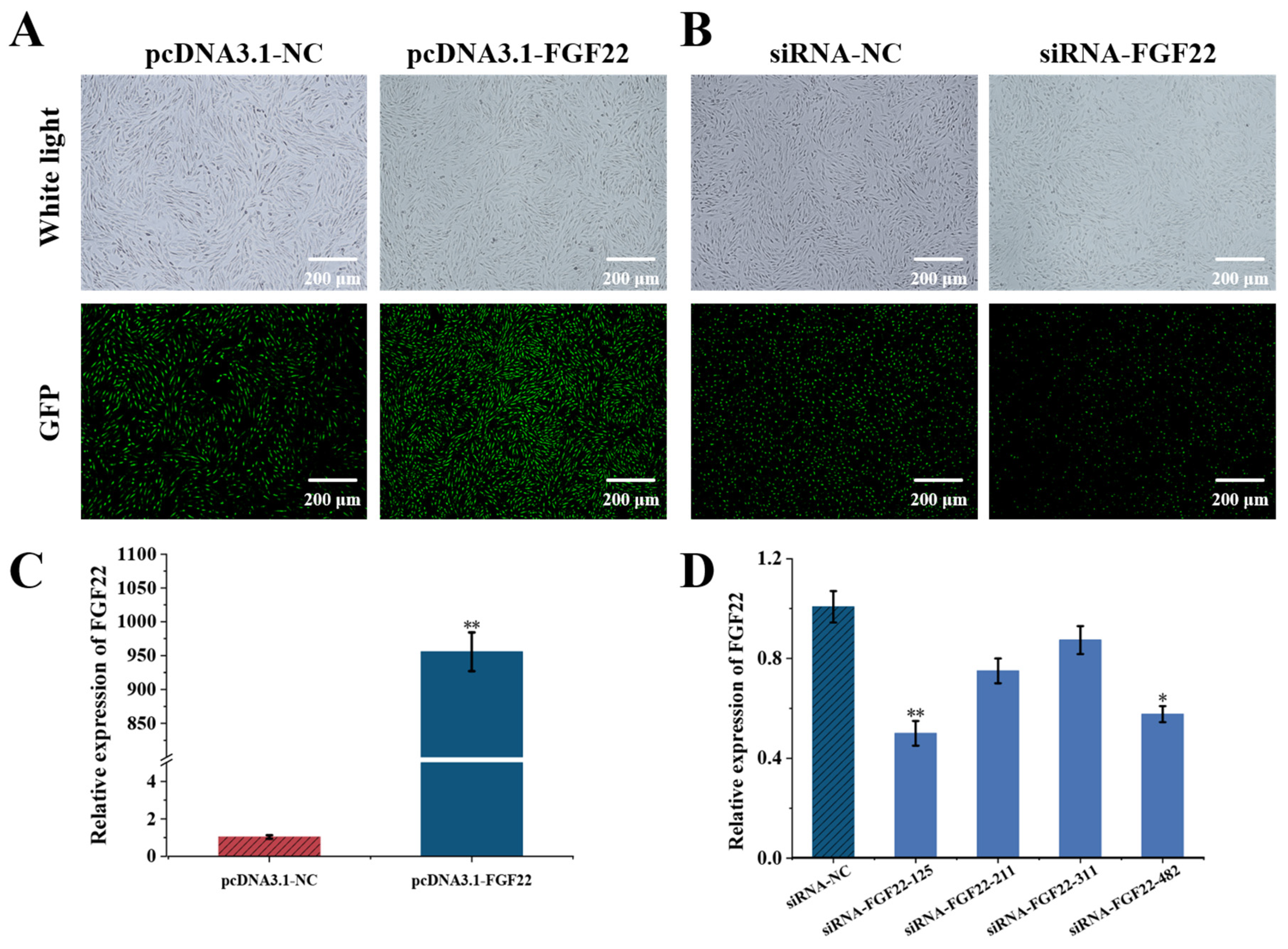
2.4. Overexpression and Knockdown of FGF22
2.5. DPC Transfection and Efficiency Test
2.6. Construction of DPC and HFSC Co-Culture Model
2.7. Total RNA Extraction and qRT-PCR
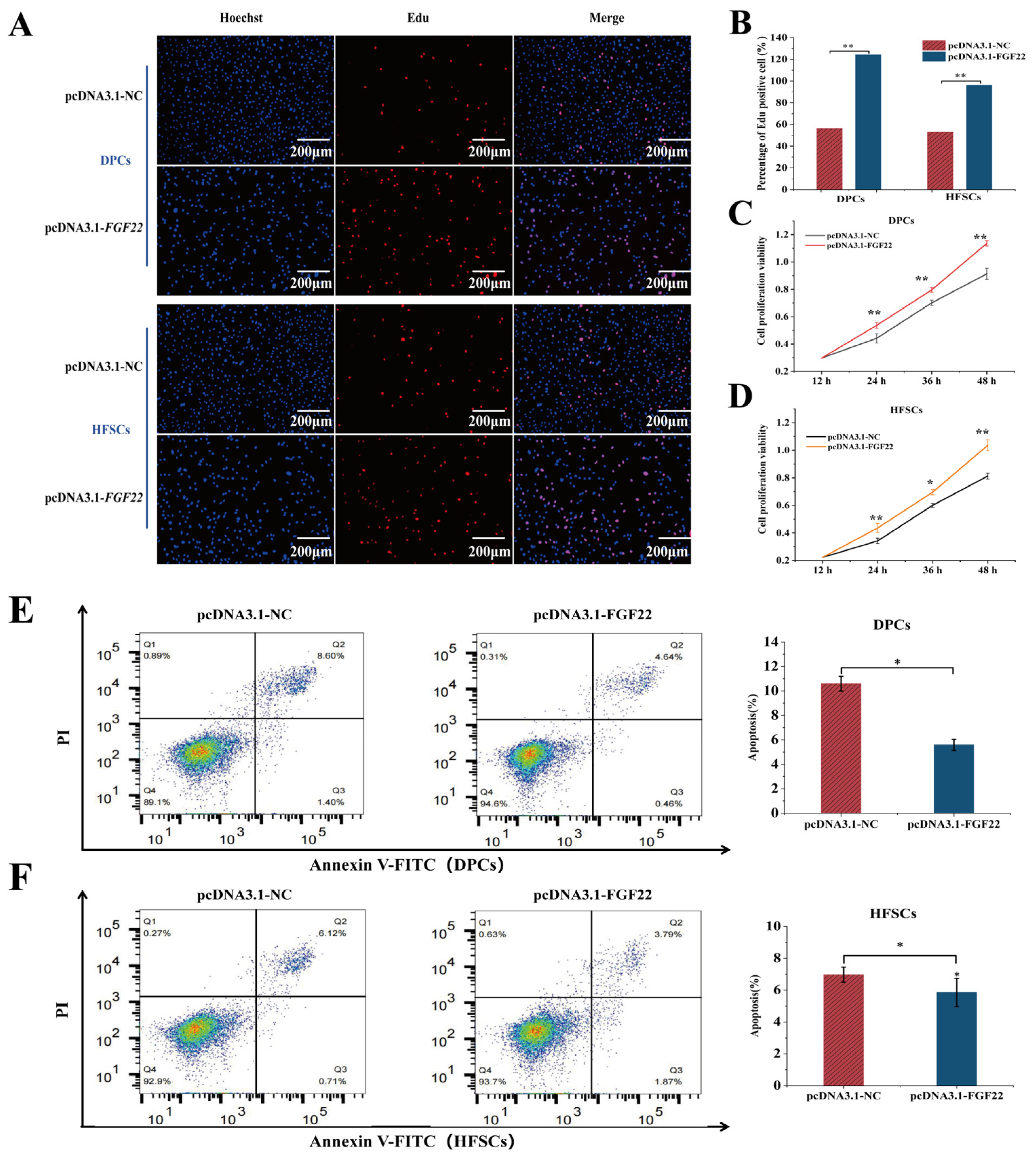
2.8. Proliferation and Viability Assessment in the DPC and HFSC Co-Culture Model
2.9. Flow Cytometry Analysis of Apoptosis
2.10. Statistical Analysis
3. Results
3.1. Morphology and Identification of the Primary DPCs and HFSCs
3.2. Detecting the Transfection Efficiency of FGF22 in DPC
3.3. The Effect of FGF22 Overexpression on the Expression Levels of FGF22-Related Receptor Genes and Differentiation-Related Pathway Marker Genes in HFSCs
3.4. Effects of FGF22 Knockout on FGF22-Related Receptor Gene Expression Levels and Differentiation-Related Pathway Marker Genes in HFSCs
3.5. Overexpression of FGF22 in DPCs Promoted the Proliferation of DPCs and HFSCs
3.6. Knockout of FGF22 in DPCs Reduced the Proliferation of DPCs and HFSCs
4. Discussion
4.1. Hair Follicle Cycle Regulatory Networks and Cellular Interactions Mechanisms
4.2. Functional Validation of FGF22 as a Key Factor in DPCs-HFSCs Interactions
4.3. Molecular Mechanisms of FGF22 in the Multipathway Coordinated Regulation of HFSCs Fate
4.4. Limitations and Future Directions of the Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFSC | Hair follicle stem cells |
| HFs | Hair follicles |
| DPC | Dermal papilla cells |
References
- Chuong, C.M.; Nickoloff, B.; Elias, P.; Goldsmith, L.; Macher, E.; Maderson, P.; Sundberg, J.; Tagami, H.; Plonka, P.; Thestrup, P.K. What is the’true’function of skin? Exp. Dermatol. 2002, 11, 159. [Google Scholar] [PubMed]
- Yesudian, P. Human Hair–An Evolutionary Relic? Int. J. Trichol. 2011, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Liu, N.; Nanba, D.; Ichinose, S.; Takada, A.; Kurata, S.; Morinaga, H.; Mohri, Y.; Arcangelis, A.; Ohno, S. Distinct types of stem cell divisions determine organ regeneration and aging in hair follicles. Nat. Aging 2021, 1, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, X.; Zhang, S.; Jin, M.; Wang, M.; Deng, Z.; Liu, Z.; Qian, M.; Shi, W.; Wang, Z. SIRT 7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 2020, 39, e104365. [Google Scholar] [CrossRef]
- Morgan, B.A. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef]
- Wang, E.C.; Dai, Z.; Ferrante, A.W.; Drake, C.G.; Christiano, A.M. A subset of TREM2+ dermal macrophages secretes oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 2019, 24, 654. [Google Scholar] [CrossRef]
- Lorz, C.; García, E.R.; Segrelles, C.; Garín, M.I.; Ariza, J.M.; Santos, M.; Ruiz, S.; Lara, M.F.; Martínez, A.B.; Costa, C.A. functional role of RB-dependent pathway in the control of quiescence in adult epidermal stem cells revealed by genomic profiling. Stem Cell Rev. Rep. 2010, 6, 162–177. [Google Scholar] [CrossRef]
- Oshima, H.; Rochat, A.; Kedzia, C.; Kobayashi, K.; Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001, 104, 233. [Google Scholar] [CrossRef]
- Hu, X.M.; Li, Z.X.; Zhang, D.Y.; Yang, Y.C.; Fu, S.A.; Zhang, Z.Q.; Yang, R.H.; Xiong, K. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res. Ther. 2021, 12, 453. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Li, L.; Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014, 157, 935. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017, 169, 1119. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Basilico, C.; Moscatelli, D. The FGF family of growth factors and oncogenes. Adv. Cancer Res. 1992, 59, 115. [Google Scholar]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, 1. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004, 20, 563. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996, 10, 165–175. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, W.D.; Zhong, Z.Y.; Zhou, X.B.; Shi, X.R.; Xin, W. FGF7 secreted from dermal papillae cell regulates the proliferation and differentiation of hair follicle stem cell1. J. Integr. Agric. 2025, 24, 3583–3597. [Google Scholar]
- Yang, M.; Li, Y.; Liang, Q.; Dong, H.; Ma, Y.; Andersson, G.; Bongcam-Rudloff, E.; Ahmad, H.I.; Fu, X.; Han, J. Identification of lncRNAs involved in the hair follicle cycle transition of cashmere goats in response to photoperiod change. BMC Genom. 2025, 26, 487. [Google Scholar] [CrossRef]
- Wang, X.; Cai, B.; Zhou, J.; Zhu, H.; Niu, Y.; Ma, B.; Yu, H.; Lei, A.; Yan, H.; Shen, Q.; et al. Disruption of FGF5 in Cashmere Goats Using CRISPR/Cas9 Results in More Secondary Hair Follicles and Longer Fibers. PLoS ONE 2016, 11, e0164640. [Google Scholar]
- Liu, X.; Zhang, P.; Zhang, X.; Li, X.; Bai, Y.; Ao, Y.; Hexig, B.; Guo, X.; Liu, D. Fgf21 knockout mice generated using CRISPR/Cas9 reveal genetic alterations that may affect hair growth. Gene 2020, 733, 144242. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Lu, Z.; Yue, L.; Xiao, T.; Yang, B.; Liu, J.; Yuan, C.; Guo, T. FGF20 Secreted from Dermal Papilla Cells Regulate the Proliferation and Differentiation of Hair Follicle Stem Cells in Fine-Wool Sheep. J. Anim. Physiol. Anim. Nutr. 2024, 109, 655. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, H.; Xu, X.; Weinberg, W.; Deng, C.X. Fibroblast growth factor receptor 2 (Fgfr2) plays an important role in eyelid and skin formation and patterning. Dev. Dyn. 2001, 222, 471. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, 132–142. [Google Scholar] [CrossRef]
- Hunt, D.P.; Morris, P.N.; Sterling, J.; Anderson, J.A.; Joannides, A.; Jahoda, C.; Compston, A.; Chandran, S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells 2008, 26, 163. [Google Scholar] [CrossRef]
- Lavker, R.M.; Sun, T.T.; Oshima, H.; Barrandon, Y.; Akiyama, M.; Ferraris, C.; Chevalier, G.; Favier, B.; Jahoda, C.A.; Dhouailly, D. Hair follicle stem cells. J. Investig. Dermatol. Symp. Proc. 2003, 8, 28–38. [Google Scholar] [CrossRef]
- Beyer, T.A.; Werner, S.; Dickson, C.; Grose, R. Fibroblast growth factor 22 and its potential role during skin development and repair. Exp. Cell Res. 2003, 287, 228. [Google Scholar] [CrossRef]
- Bratka, C.B.; Mitteregger, G.; Aichinger, A.; Egerbacher, M.; Helmreich, M.; Bamberg, E. Primary cell culture and morphological characterization of canine dermal papilla cells and dermal fibroblasts. Vet. Dermatol. 2002, 13, 1. [Google Scholar] [CrossRef]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Grisanti, L.; Zemla, R.; Rezza, A.; Barros, R.; Sennett, R.; Mazloom, A.R.; Chung, C.Y.; Cai, X.; Cai, C.L. Sox2 in the Dermal Papilla Niche Controls Hair Growth by Fine-Tuning BMP Signaling in Differentiating Hair Shaft Progenitors. Dev. Cell 2012, 23, 981. [Google Scholar] [CrossRef] [PubMed]
- Keita, I.; Noriyuki, A.; Takahiro, S.; Yuji, Y.; Hirotaka, S.; Hitomi, E.; Harunosuke, K.; Jun, A.; Kotaro, Y. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009, 89, 844. [Google Scholar]
- Elisabeth, K.J.; Stephan, T.; Jürgen, B.; Peter, R.D.; Wilfried, M.; Reinhard, F.; Ralf, P. Immunophenotyping of the human bulge region: The quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp. Dermatol. 2008, 17, 592. [Google Scholar] [CrossRef]
- Ma, D.R.; Yang, E.N.; Lee, S.T. A review: The location, molecular characterisation and multipotency of hair follicle epidermal stem cells. Ann. Acad. Med. Singap. 2004, 33, 784. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, S.; Wang, D.; Liu, J.; Luo, X.; Liu, Y.; Li, X.; Sun, F.; Xia, G.; Zhang, L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes 2023, 14, 1106. [Google Scholar] [CrossRef]
- Petiot, A.; Conti, J.A.; Grose, R.; Revest, J.M.; Hodivala, K.M.; Dickson, C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development 2003, 130, 5493. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hori, Y.; Yamasaki, M.; Harada, H.; Sekine, K.; Kato, S.; Itoh, N. FGF10 Acts as a Major Ligand for FGF Receptor 2 IIIb in Mouse Multi-Organ Development. Biochem. Biophys. Res. Commun. 2000, 277, 643. [Google Scholar] [CrossRef]
- Heydarkhan, H.S.; Helenius, G.; Johansson, B.R.; Li, J.Y.; Mattsson, E.; Risberg, B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem. 2003, 89, 1250. [Google Scholar] [CrossRef]
- Khodarev, N.N.; Yu, J.; Labay, E.; Darga, T.; Brown, C.K.; Mauceri, H.J.; Yassari, R.; Gupta, N.; Weichselbaum, R.R. Tumour-endothelium interactions in co-culture: Coordinated changes of gene expression profiles and phenotypic properties of endothelial cells. J. Cell Sci. 2003, 116, 1013. [Google Scholar] [CrossRef]
- Kaji, H.; Camci, U.G.; Langer, R.; Khademhosseini, A. Engineering systems for the generation of patterned co-cultures for controlling cell–cell interactions. BBA Gen. Subj. 2010, 1810, 239. [Google Scholar] [CrossRef]
- Orwin, E.J.; Hubel, A. In vitro culture characteristics of corneal epithelial, endothelial, and keratocyte cells in a native collagen matrix. Tissue Eng. 2000, 6, 307. [Google Scholar] [CrossRef]
- Ding, Y.; Xue, X.; Liu, Z.; Ye, Y.; Xiao, P.; Pu, Y.; Guan, W.; Mwacharo, J.M.; Ma, Y.; Zhao, Q. Expression Profiling and Functional Characterization of miR-26a and miR-130a in Regulating Zhongwei Goat Hair Development via the TGF-β/SMAD Pathway. Int. J. Mol. Sci. 2020, 21, 5076. [Google Scholar] [CrossRef] [PubMed]
- Sarina, H.S.; Hila, D.; Christof, N.; Emil, A.; David, E.S. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114. [Google Scholar]
- Bai, X.; Lei, M.; Shi, J.; Yu, Y.; Qiu, W.; Lai, X.; Liu, Y.; Yang, T.; Yang, L.; Widelitz, R.B.; et al. Roles of GasderminA3 in Catagen–Telogen Transition During Hair Cycling. J. Investig. Dermatol. 2015, 135, 2162. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 547. [Google Scholar] [CrossRef]
- Ritusree, B.; Avinanda, B.; Sergio, L.; Zhihai, Z.; Vairavan, L.; Ryan, L.; Shimin, L.; Manando, N.; Vassily, K.; Graham, W.; et al. Mechanical instability of adherens junctions overrides intrinsic quiescence of hair follicle stem cells. Dev. Cell 2021, 56, 761–780. [Google Scholar] [CrossRef]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair Follicle Stem Cells Are Specified and Function in Early Skin Morphogenesis. Cell Stem Cell 2008, 3, 33. [Google Scholar] [CrossRef]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.L.; Pasolli, H.A.; Sheu, S.H.; Fan, M.Y.; Shamsi, F.; Assaad, S.; Lin, E.T.; Zhang, B. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 2020, 182, 578. [Google Scholar] [CrossRef]
- Andreu, C.; Gary, S. Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound Patched protein. Nature 2004, 431, 76. [Google Scholar] [CrossRef]
- Jeng, K.S.; Chang, C.F.; Lin, S.-S. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int. J. Mol. Sci. 2020, 21, 758. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Liu, Z.Y.; Shapiro, R.; Yang, J.; Sizing, I.; Rayhorn, P.; Garber, E.A.; Benjamin, C.D.; Williams, K.P.; Taylor, F.R. Conditional Disruption of Hedgehog Signaling Pathway Defines its Critical Role in Hair Development and Regeneration. J. Investig. Dermatol. 2000, 114, 901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chang, J.; Zhang, R.; Liu, Z.; Liang, J.; Wang, D.; Feng, J.; Zhao, W.; Xiao, H. Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats. Animals 2024, 14, 2049. [Google Scholar] [CrossRef] [PubMed]
- Maryanovich, M.; Frenette, P.S. T-Regulating Hair Follicle Stem Cells. Immunity 2017, 46, 979. [Google Scholar] [CrossRef]
- Xing, W.; Yang, J.; Zheng, Y.; Yao, L.; Peng, X.; Chen, Y.; Yang, C. The Role of the Notch Signaling Pathway in the Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Front. Biosci. (Landmark Ed.) 2024, 29, 74. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Hu, Y.; Zhou, F.; Zhang, L. TGFβ family signaling in human stem cell self-renewal and differentiation. Cell Regen. 2024, 13, 26. [Google Scholar] [CrossRef]
- Michael, R.; Lisa, P.; Elaine, F. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef]
- Hata, A.; Lagna, G.; Massagué, J.; Hemmati, A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998, 12, 186. [Google Scholar] [CrossRef]
- Marcin, K.; Ulrich, V.; Rosita, B.; Henrik, H.; Aristidis, M. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell Biol. 2004, 24, 4241. [Google Scholar]



| DMEM/F12 Medium | 10% FBS | 5 µg/mL Insulin | 10 µg/mL Epidermal Growth Factor | 0.5 µg/mL Hydrocortisone | |
|---|---|---|---|---|---|
| DPCs | √ | √ | √ | √ | |
| HFSCs | √ | √ | √ | √ | √ |
| Gene Name | Sequence (5′-3′) |
|---|---|
| siRNA-FGF22-125 | F: CCUAUGGCUGGGCCUGGUGTT |
| R: CACCAGGCCCAGCCAUAGGTT | |
| siRNA-FGF22-211 | F: ACCCGCACCUGGAGGGCGATT |
| R: UCGCCCUCCAGGUGCGGGUTT | |
| siRNA-FGF22-311 | F: GCGCGACAACCCUGACAGCTT |
| R: GCUGUCAGGGUUGUCGCGCTT | |
| siRNA-FGF22-482 | F: CUACAAUACCUACGCGUCATT |
| R: UGACGCGUAGGUAUUGUAGTT | |
| siRNA-NC | F: UUCUCCGAACGUGUCACGUTT |
| R: ACGUGACACGUUCGGAGAATT |
| Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| FGF22 | GAGCCCGTGTCCAGTTACTC | ACCACCCCTCCAACTCAGT |
| FGFR1 | ACTCTACCCCAGCCCTAAGG | ACCATCCATTCACACGACCC |
| FGFR2 | AGAGTGATGTCTGGTCCTTCG | GAAGAGCAAGAACTCCTGGTG |
| β-Catenin | ACACAGTTCGATGCTGCTCA | GATTGCACGTGTGGCAAGTT |
| SOX9 | CGAAACTGGACTGGAAACCT | GTTCTCTCTGCCTGTTTGGA |
| c-myc | CGGAAGAGGCGAGAACAGTT | ATCCAGCCAAGGTTGTGAGG |
| APC | TCCTCCCGACTCAGTGTTCT | GTCCCCGTCGCTATACTTGG |
| Shh | TGGTCTCCTCGCTGTTGATG | CTCGTATCTTCCACTGGCCC |
| GLI1 | CCTCGTGGCCTTCATCAACT | GGTGGTTCATCTGGGGTGAG |
| LGR5 | TTCACTTTCGGCAGCTTTGC | CCAGGGTGAGCAGGAAAACT |
| Smo | CTCCCTTGGTTCGGACTGAC | GTAGCTGTGCATGTCCTGGT |
| NOTCH1 | ACAACGCCTACCTCTGCTTC | ACACATGCTCCCTGTGTAGC |
| NOTCH3 | CTTGGGTCCTGTGGTGAGTC | AGCAGGAGGAGTGAGAGAGG |
| HES1 | GACCCAGATCAACGCCATGA | GAACACCTTAGCCGCCTCTC |
| DLL1 | GCACGGACCTCAAGTACTCC | ACTTTCTCCCCTCTCTCCCC |
| BMP2 | CACACCCTACCCGAGATTGG | CTGAGTCCCCAGTAATCCGC |
| BMP4 | CTTCAGTCTGGGGAGGAGGA | AGAGTTTTCGCTGGTCCCTG |
| SMAD6 | AACCCCTACCATTTCAGCCG | GGAGTTGGTGGCCTCTGTTT |
| ID1 | AAGGTGAGCAGGGTGGAGAT | TCGCCATTGAGAGTGCTGAG |
| β-actin | CAGTCGGTTGGATGGAGCAT | AGGCAGGGACTTCCTGTAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xiao, T.; Xi, B.; Song, Y.; Lu, Z.; Yuan, C.; Liu, J.; Guo, T. FGF22 Secreted by Hair Papilla Cells Regulates Hair Follicle Stem Cell Proliferation and Differentiation. Biomolecules 2025, 15, 1560. https://doi.org/10.3390/biom15111560
Luo Y, Xiao T, Xi B, Song Y, Lu Z, Yuan C, Liu J, Guo T. FGF22 Secreted by Hair Papilla Cells Regulates Hair Follicle Stem Cell Proliferation and Differentiation. Biomolecules. 2025; 15(11):1560. https://doi.org/10.3390/biom15111560
Chicago/Turabian StyleLuo, Yu, Tong Xiao, Binpeng Xi, Yufang Song, Zengkui Lu, Chao Yuan, Jianbin Liu, and Tingting Guo. 2025. "FGF22 Secreted by Hair Papilla Cells Regulates Hair Follicle Stem Cell Proliferation and Differentiation" Biomolecules 15, no. 11: 1560. https://doi.org/10.3390/biom15111560
APA StyleLuo, Y., Xiao, T., Xi, B., Song, Y., Lu, Z., Yuan, C., Liu, J., & Guo, T. (2025). FGF22 Secreted by Hair Papilla Cells Regulates Hair Follicle Stem Cell Proliferation and Differentiation. Biomolecules, 15(11), 1560. https://doi.org/10.3390/biom15111560






