Stigmasterol Protects Against Dexamethasone-Induced Muscle Atrophy by Modulating the FoxO3–MuRF1/MAFbx Signaling Pathway in C2C12 Myotubes and Mouse Skeletal Muscle
Abstract
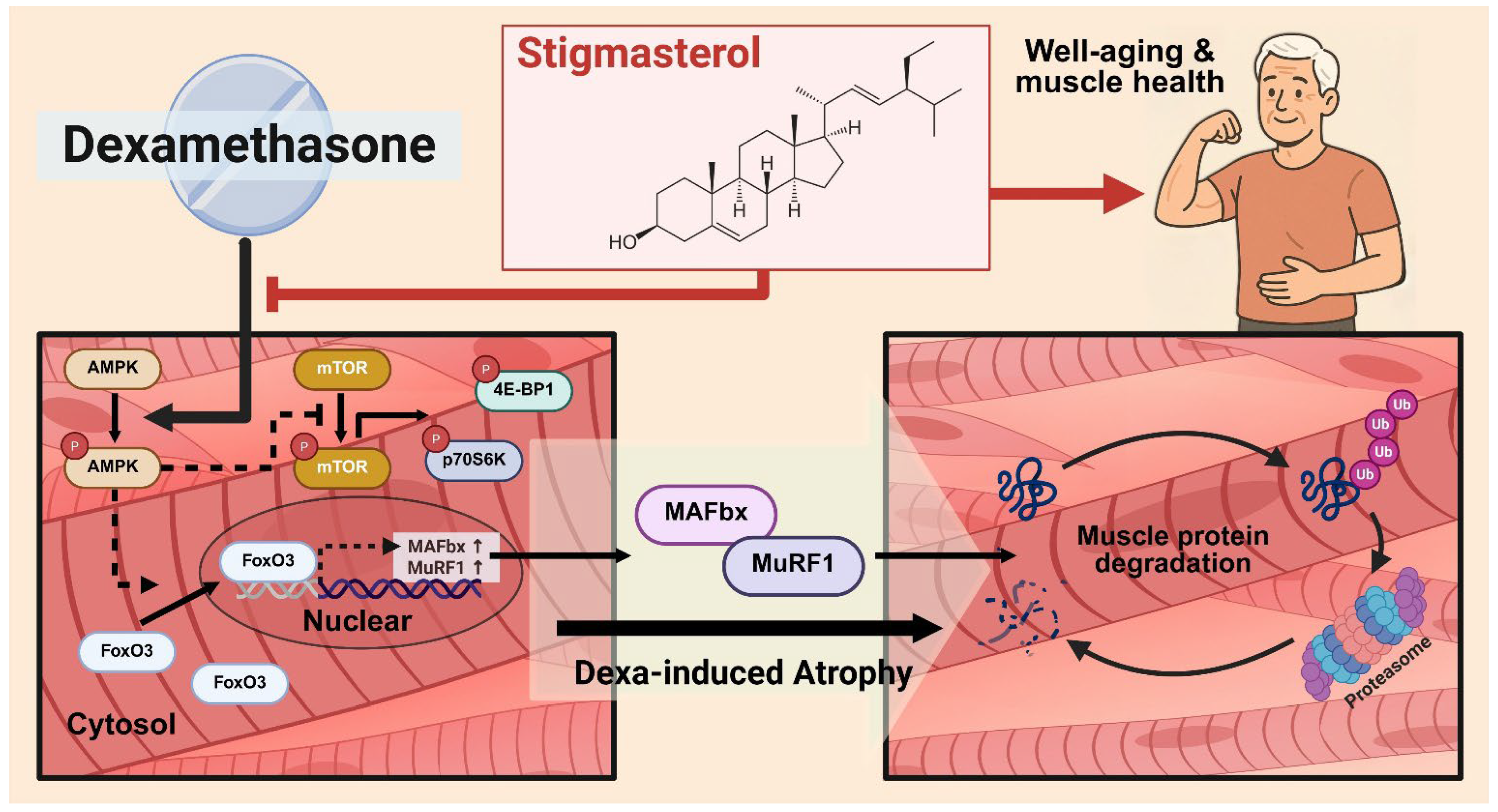
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment
2.2.1. Cell Maintenance and Differentiation
2.2.2. Dose–Response Study
2.2.3. Dexamethasone-Induced Atrophy and Stigmasterol Treatment
2.2.4. Giemsa and May–Grunwald Staining
2.2.5. Fusion Index Quantification
2.3. Cytoplasmic and Nuclear Protein Fractionation
2.4. Western Blot Analysis
2.5. Animal Model and Experimental Design
2.5.1. Animals
2.5.2. Induction of Muscle Atrophy and Treatment
2.5.3. Body Weight and Muscle Mass Measurements
2.5.4. Bone Mineral Density (BMD) Measurement
2.5.5. Immunofluorescence and Cross-Sectional Area (CSA) Analysis
2.6. Statistical Analysis
3. Results
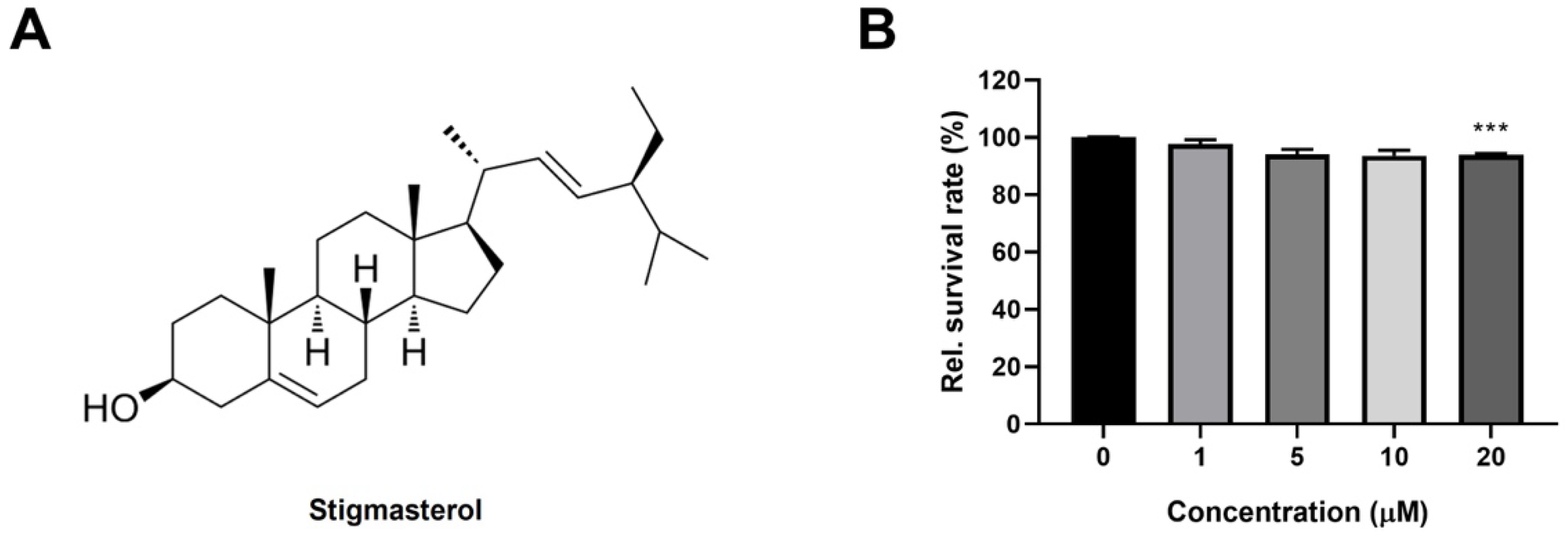
3.1. Stigmasterol Exhibits Minimal Toxicity on C2C12 Myoblasts at Low Concentrations
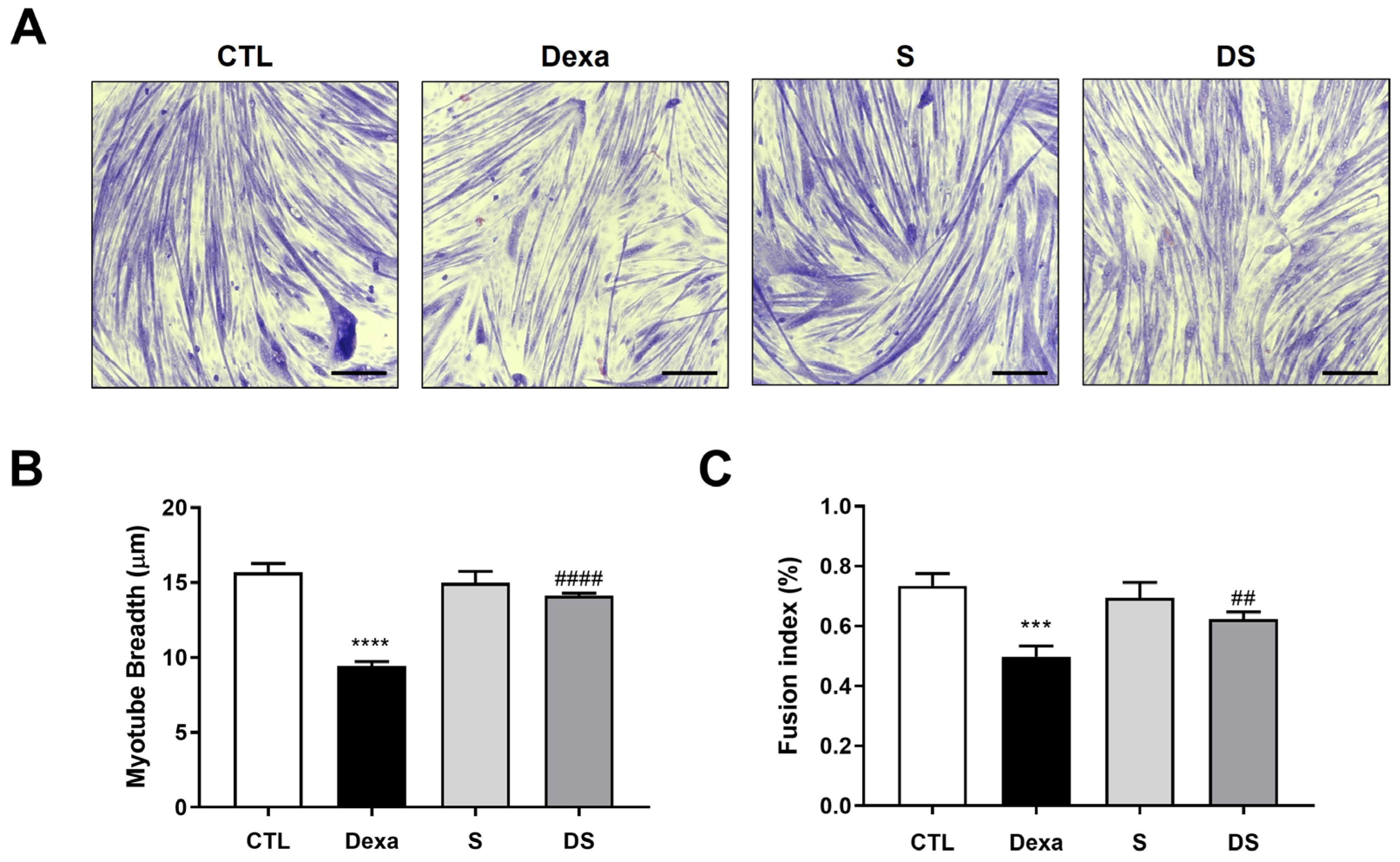
3.2. Stigmasterol Mitigates Dexamethasone-Induced Atrophy in C2C12 Myotubes
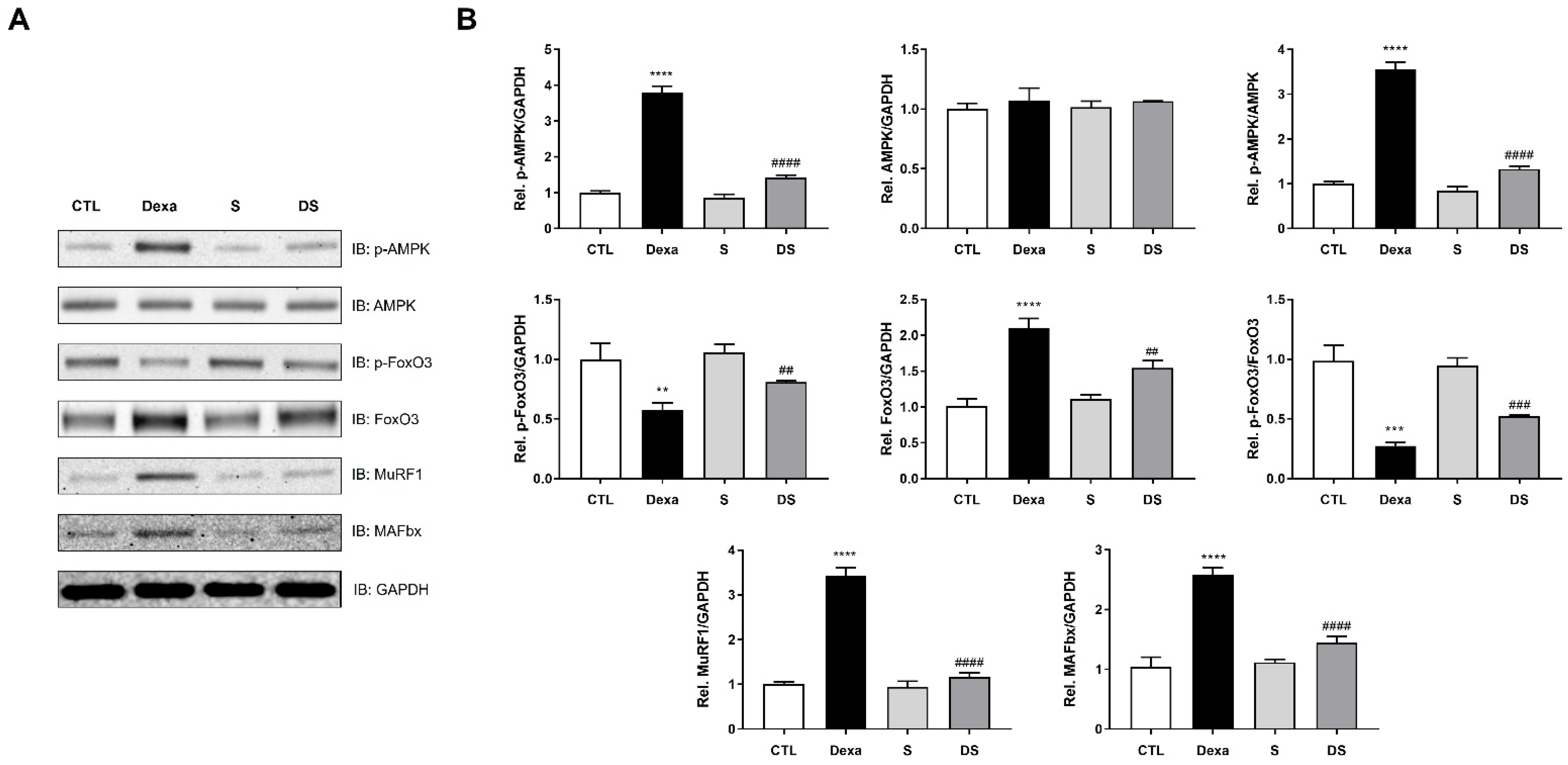
3.3. Stigmasterol Downregulates the AMPK–FoxO3–MuRF1-MAFbx Pathway in Dexamethasone-Treated C2C12 Myotubes
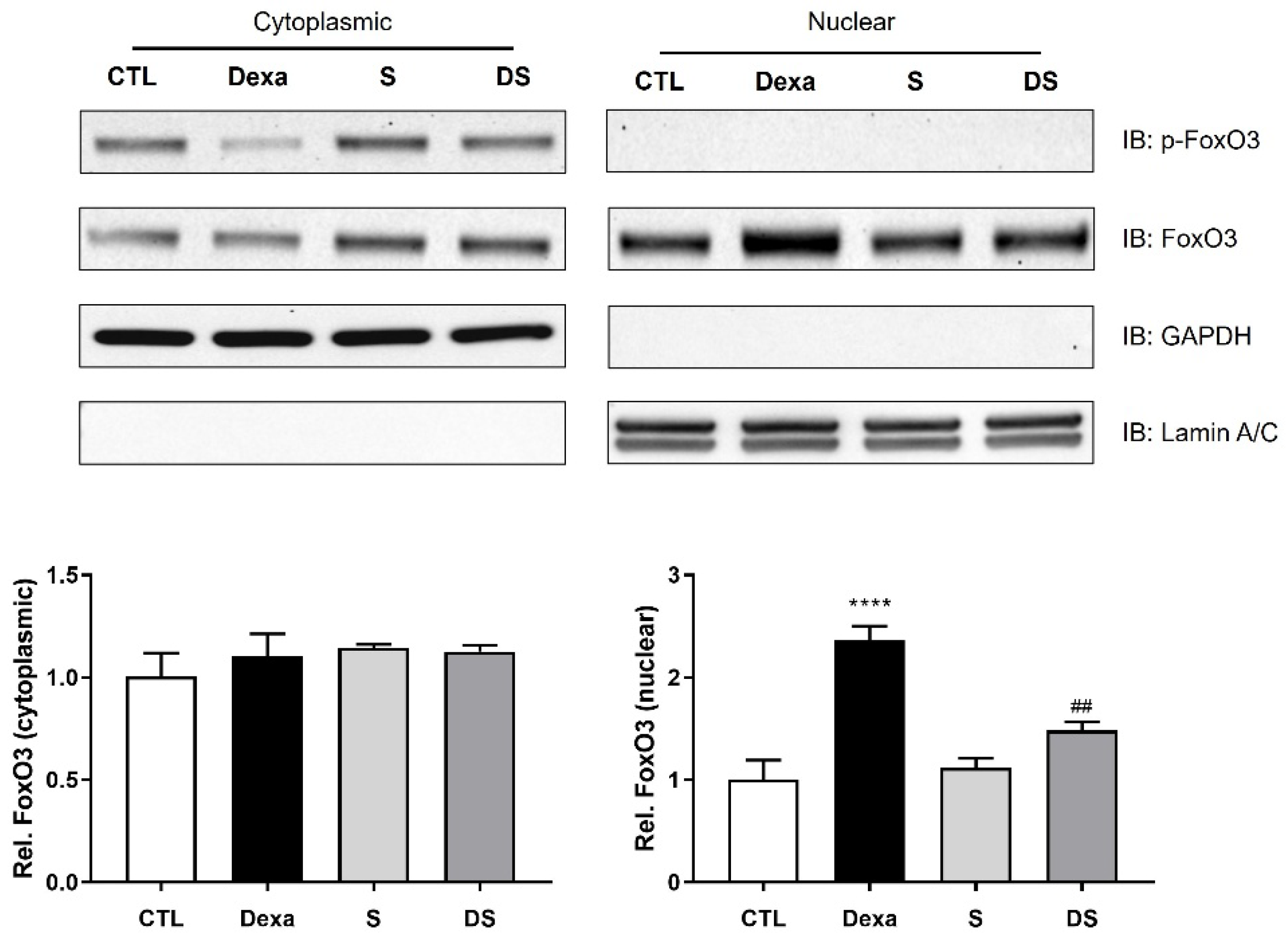
3.4. Stigmasterol Inhibits the Nuclear Translocation of FoxO3 Protein in Dexamethasone-Treated C2C12 Myotubes
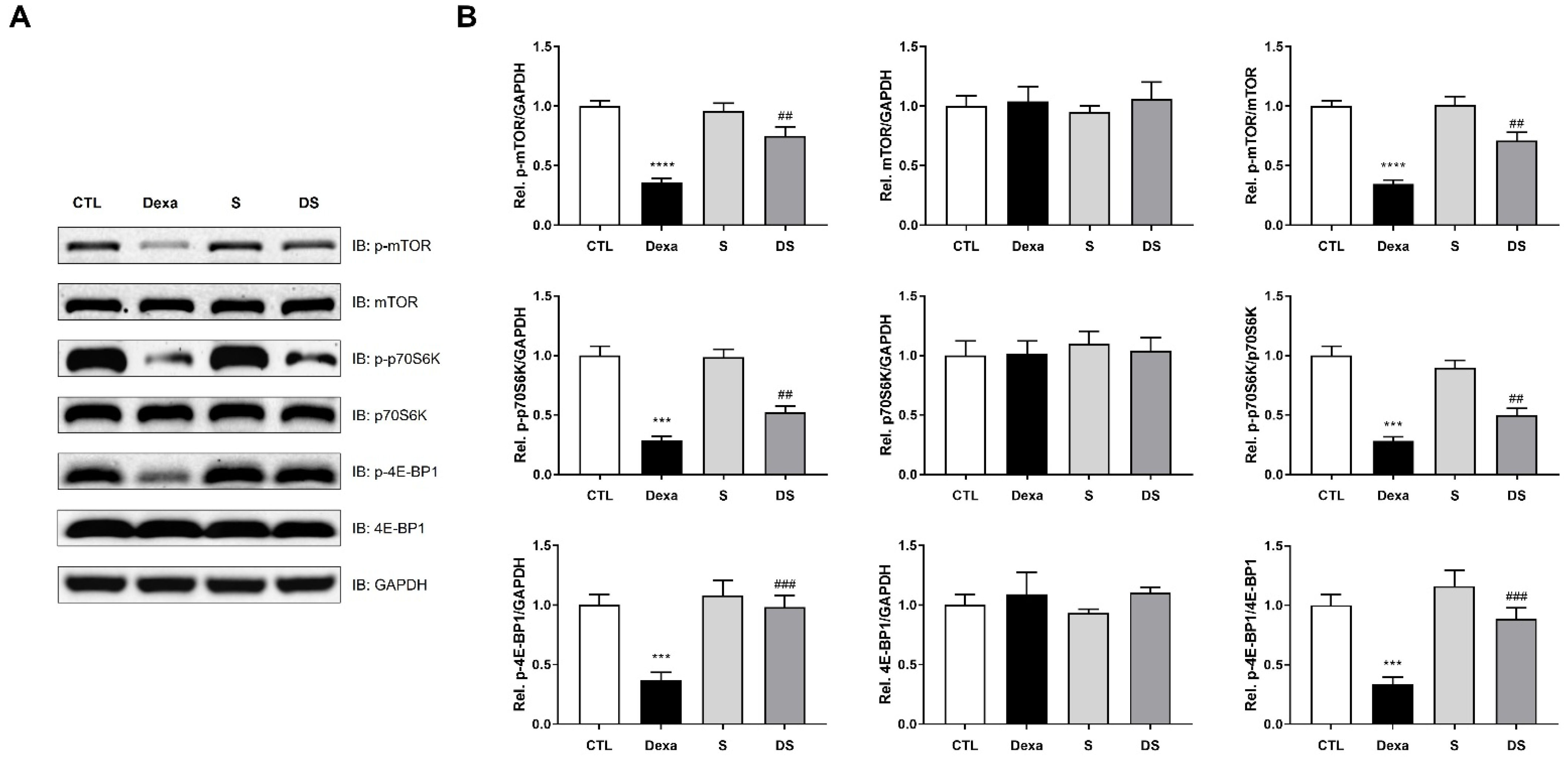
3.5. Stigmasterol Prevents Dexamethasone-Induced Inactivation of the mTOR/p70S6K/4E-BP1 Pathway in C2C12 Myotubes
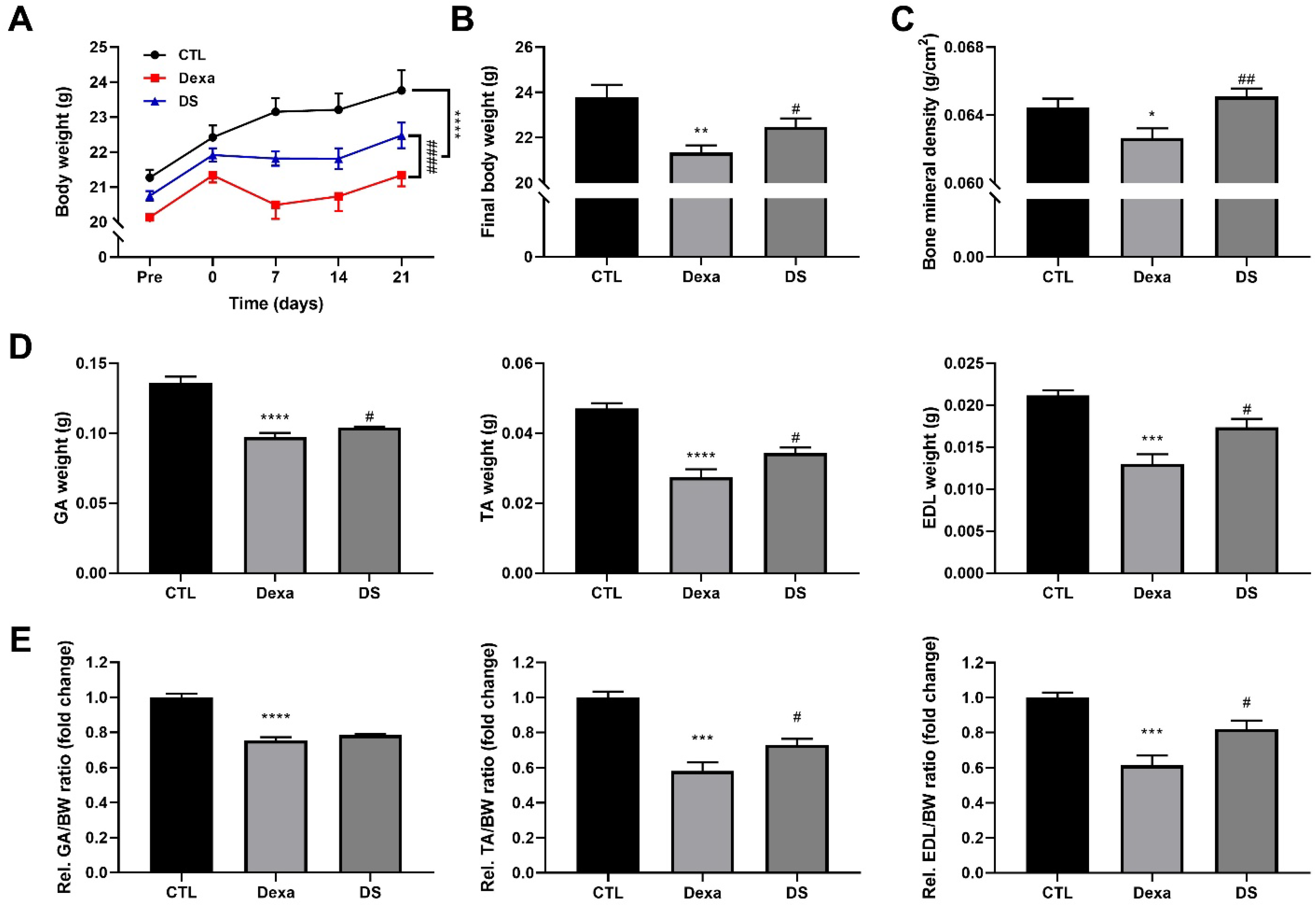
3.6. Stigmasterol Prevents Dexamethasone-Induced Muscle Atrophy in Mice
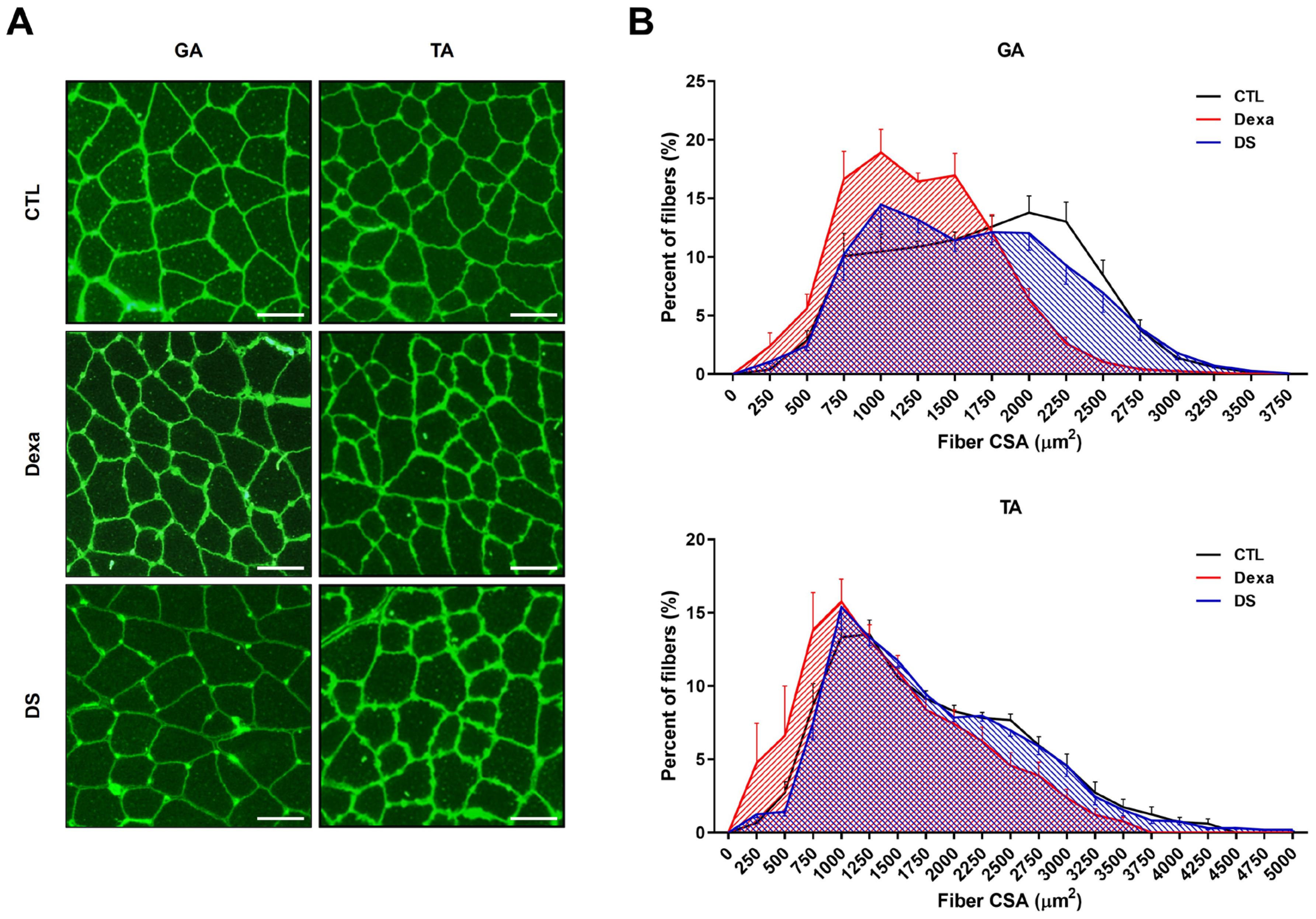
3.7. Stigmasterol Restores Muscle Fiber Cross-Sectional Area in Dexamethasone-Treated Mice
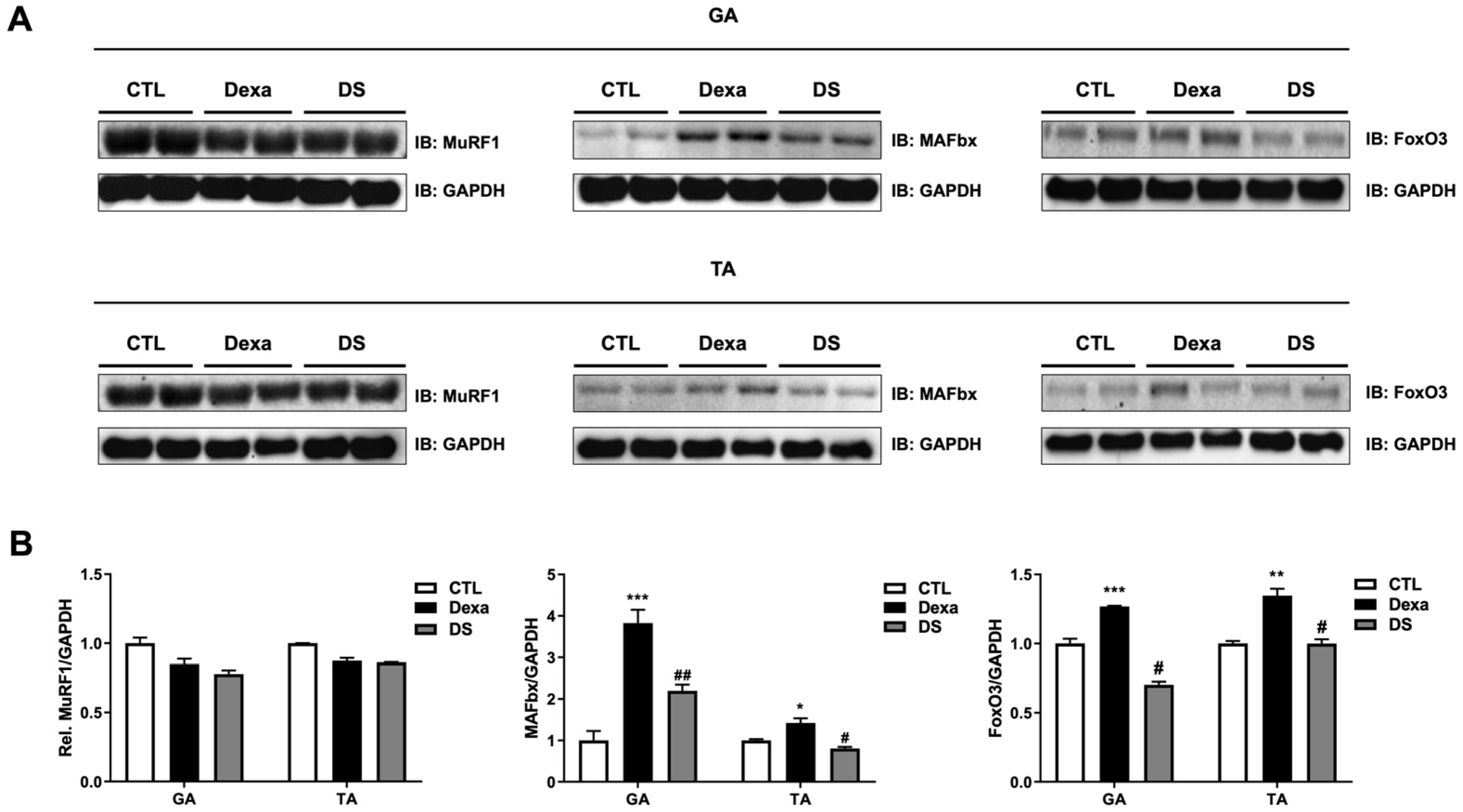
3.8. Stigmasterol Reduces Expression of Atrophy-Related Proteins in Mouse Muscle Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Go, H.; Sung, N.J.; Choi, J.; Kim, L.; Park, E.J. 6’-sialyllactose prevents dexamethasone-induced muscle atrophy by controlling the muscle protein degradation pathway. Biochem. Biophys. Res. Commun. 2024, 736, 150892. [Google Scholar] [CrossRef]
- Wang, B.Y.; Hsiao, A.W.; Wong, N.; Chen, Y.F.; Lee, C.W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Transl. 2023, 39, 12–20. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Prevalence of Sarcopenia Among the Elderly in Korea: A Meta-Analysis. J. Prev. Med. Public Health 2021, 54, 96–102. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Glass, D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 2003, 5, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. Embo J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.S.; Milan, G.; Ham, D.J.; Lin, S.; Oliveri, F.; Chojnowska, K.; Tintignac, L.A.; Mittal, N.; Zimmerli, C.E.; Glass, D.J.; et al. Dual roles of mTORC1-dependent activation of the ubiquitin-proteasome system in muscle proteostasis. Commun. Biol. 2022, 5, 1141. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef]
- Mazucanti, C.H.; Cabral-Costa, J.V.; Vasconcelos, A.R.; Andreotti, D.Z.; Scavone, C.; Kawamoto, E.M. Longevity Pathways (mTOR, SIRT, Insulin/IGF-1) as Key Modulatory Targets on Aging and Neurodegeneration. Curr. Top. Med. Chem. 2015, 15, 2116–2138. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Zanchi, N.E.; Lancha, A.H., Jr. Mechanical stimuli of skeletal muscle: Implications on mTOR/p70s6k and protein synthesis. Eur. J. Appl. Physiol. 2008, 102, 253–263. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Fang, H.; Zou, M.; Yin, J.; Zhong, W.; Luo, Z.; Hu, C.; He, D.; Wang, X. High-oleic rapeseed oil quality indicators and endogenous antioxidant substances under different processing methods. Food Chem. X 2023, 19, 100804. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2020, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Lee, W.K.; Lee, S.; Kim, E.J.; Lee, J.H.; Lee, S.J.; Ji, Y.H.; Kim, S.G.; Lee, H.H.; Hong, S.Y.; et al. β-Sitosterol Attenuates Dexamethasone-Induced Muscle Atrophy via Regulating FoxO1-Dependent Signaling in C2C12 Cell and Mice Model. Nutrients 2022, 14, 2894. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Jiang, J.; Cui, X.; Cheng, S.; Liu, L.; Huang, Z.; Liao, R.; Zhao, P.; Yu, J.; et al. Stigmasterol attenuates hepatic steatosis in rats by strengthening the intestinal barrier and improving bile acid metabolism. NPJ Sci. Food 2022, 6, 38. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- Cadot, B.; Gache, V.; Vasyutina, E.; Falcone, S.; Birchmeier, C.; Gomes, E.R. Nuclear movement during myotube formation is microtubule and dynein dependent and is regulated by Cdc42, Par6 and Par3. EMBO Rep. 2012, 13, 741–749. [Google Scholar] [CrossRef]
- Suzuki, K.; Bose, P.; Leong-Quong, R.Y.; Fujita, D.J.; Riabowol, K. REAP: A two minute cell fractionation method. BMC Res. Notes 2010, 3, 294. [Google Scholar] [CrossRef]
- Wen, Y.; Murach, K.A.; Vechetti, I.J., Jr.; Fry, C.S.; Vickery, C.; Peterson, C.A.; McCarthy, J.J.; Campbell, K.S. MyoVision: Software for automated high-content analysis of skeletal muscle immunohistochemistry. J. Appl. Physiol. (1985) 2018, 124, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, P.; Li, Z.; Dai, A.; Yang, M.; Chen, S.; Su, J.; Deng, Z.; Li, L. Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy. Am. J. Pathol. 2022, 192, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Wang, X.; Fan, Y.; Qin, C.; Shi, L.; Tang, Y.; Cao, K.; Li, H.; Long, J.; et al. Mitochondrial Dysfunction Launches Dexamethasone-Induced Skeletal Muscle Atrophy via AMPK/FOXO3 Signaling. Mol. Pharm. 2016, 13, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 2006, 116, 1776–1783. [Google Scholar] [CrossRef]
- Ross, F.A.; MacKintosh, C.; Hardie, D.G. AMP-activated protein kinase: A cellular energy sensor that comes in 12 flavours. Febs J. 2016, 283, 2987–3001. [Google Scholar] [CrossRef]
- Egawa, T.; Goto, A.; Ohno, Y.; Yokoyama, S.; Ikuta, A.; Suzuki, M.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Hayashi, T.; et al. Involvement of AMPK in regulating slow-twitch muscle atrophy during hindlimb unloading in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E651–E662. [Google Scholar] [CrossRef]
- Van Nostrand, J.L.; Hellberg, K.; Luo, E.C.; Van Nostrand, E.L.; Dayn, A.; Yu, J.; Shokhirev, M.N.; Dayn, Y.; Yeo, G.W.; Shaw, R.J. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes. Dev. 2020, 34, 1330–1344. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Baehr, L.M.; Furlow, J.D.; Bodine, S.C. Muscle sparing in muscle RING finger 1 null mice: Response to synthetic glucocorticoids. J. Physiol. 2011, 589 Pt 19, 4759–4776. [Google Scholar] [CrossRef]
- Baehr, L.M.; West, D.W.D.; Marshall, A.G.; Marcotte, G.R.; Baar, K.; Bodine, S.C. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J. Appl. Physiol. (1985) 2017, 122, 1336–1350. [Google Scholar] [CrossRef]
- Hughes, D.C.; Goodman, C.A.; Baehr, L.M.; Gregorevic, P.; Bodine, S.C. A critical discussion on the relationship between E3 ubiquitin ligases, protein degradation, and skeletal muscle wasting: It’s not that simple. Am. J. Physiol. Cell Physiol. 2023, 325, C1567–C1582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hah, Y.-S.; Lee, S.-J.; Ji, Y.-H.; Hwang, J.; Kim, H.-G.; Ju, Y.-T.; Yoo, J.-I.; Kwag, S.-J. Stigmasterol Protects Against Dexamethasone-Induced Muscle Atrophy by Modulating the FoxO3–MuRF1/MAFbx Signaling Pathway in C2C12 Myotubes and Mouse Skeletal Muscle. Biomolecules 2025, 15, 1551. https://doi.org/10.3390/biom15111551
Hah Y-S, Lee S-J, Ji Y-H, Hwang J, Kim H-G, Ju Y-T, Yoo J-I, Kwag S-J. Stigmasterol Protects Against Dexamethasone-Induced Muscle Atrophy by Modulating the FoxO3–MuRF1/MAFbx Signaling Pathway in C2C12 Myotubes and Mouse Skeletal Muscle. Biomolecules. 2025; 15(11):1551. https://doi.org/10.3390/biom15111551
Chicago/Turabian StyleHah, Young-Sool, Seung-Jun Lee, Yeung-Ho Ji, Jeongyun Hwang, Han-Gil Kim, Young-Tae Ju, Jun-Il Yoo, and Seung-Jin Kwag. 2025. "Stigmasterol Protects Against Dexamethasone-Induced Muscle Atrophy by Modulating the FoxO3–MuRF1/MAFbx Signaling Pathway in C2C12 Myotubes and Mouse Skeletal Muscle" Biomolecules 15, no. 11: 1551. https://doi.org/10.3390/biom15111551
APA StyleHah, Y.-S., Lee, S.-J., Ji, Y.-H., Hwang, J., Kim, H.-G., Ju, Y.-T., Yoo, J.-I., & Kwag, S.-J. (2025). Stigmasterol Protects Against Dexamethasone-Induced Muscle Atrophy by Modulating the FoxO3–MuRF1/MAFbx Signaling Pathway in C2C12 Myotubes and Mouse Skeletal Muscle. Biomolecules, 15(11), 1551. https://doi.org/10.3390/biom15111551







