Advances in Understanding Renin–Angiotensin System-Mediated Anti-Tumor Activity of Natural Polyphenols
Abstract
1. Introduction
2. The Impact of RAS on Cancer
2.1. The Dysregulation of RAS Components in Cancer
2.2. Progress in Targeted RAS Therapy for Cancer
2.3. Influence of the RASi in Cancer Biology
3. Natural Polyphenols and Their Derivatives on RAS Regulation
3.1. Classification and Structural Characteristics of Polyphenols and Their Derivatives
3.2. Natural Polyphenols and Their Derivatives Regulate RAS Biaxial Balance
3.3. Natural Polyphenol Oxidative Products Regulate RAS
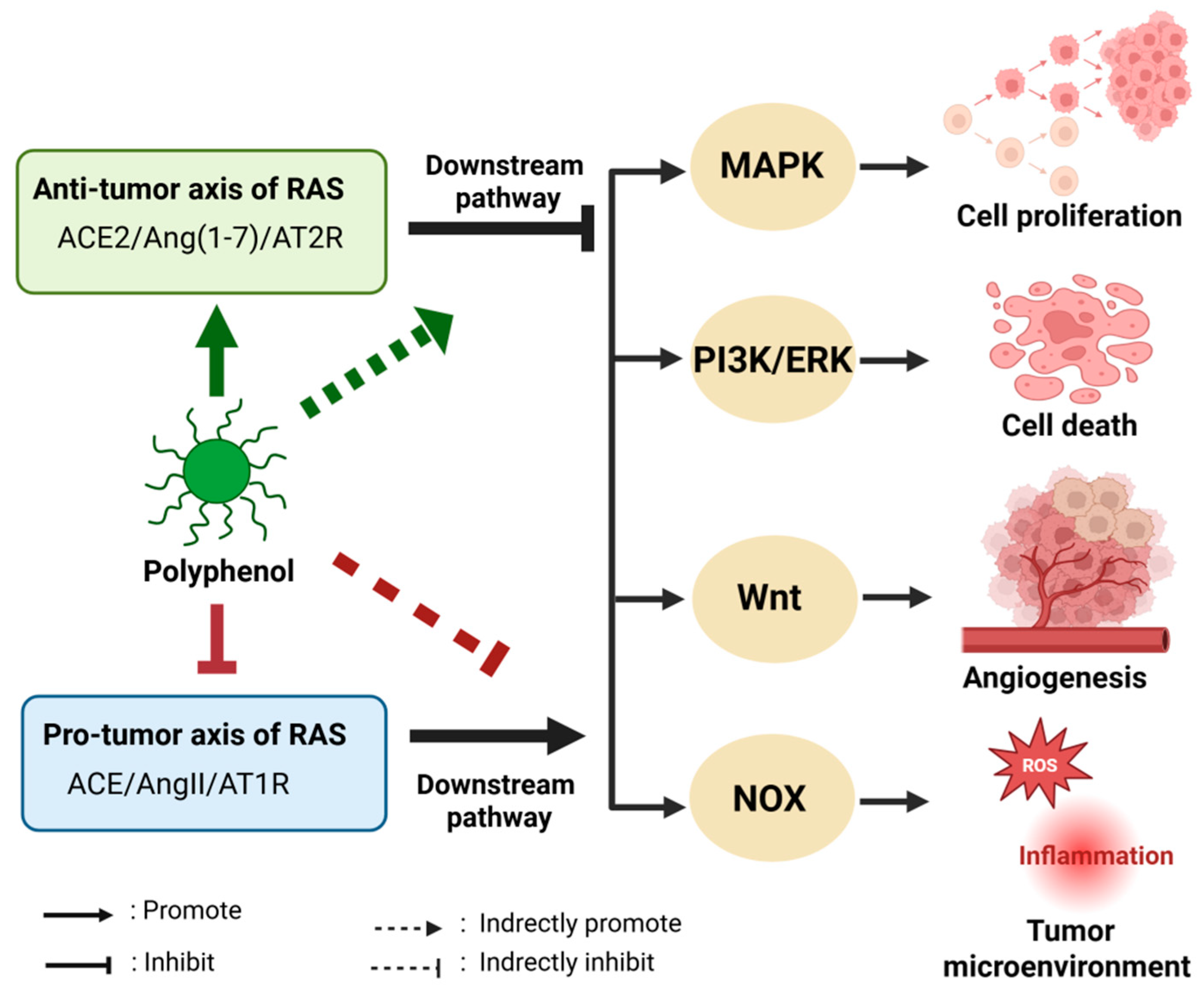
3.4. Anti-Tumor Effects of Polyphenols by Regulating RAS
4. Mechanisms of Natural Polyphenols in Regulating RAS
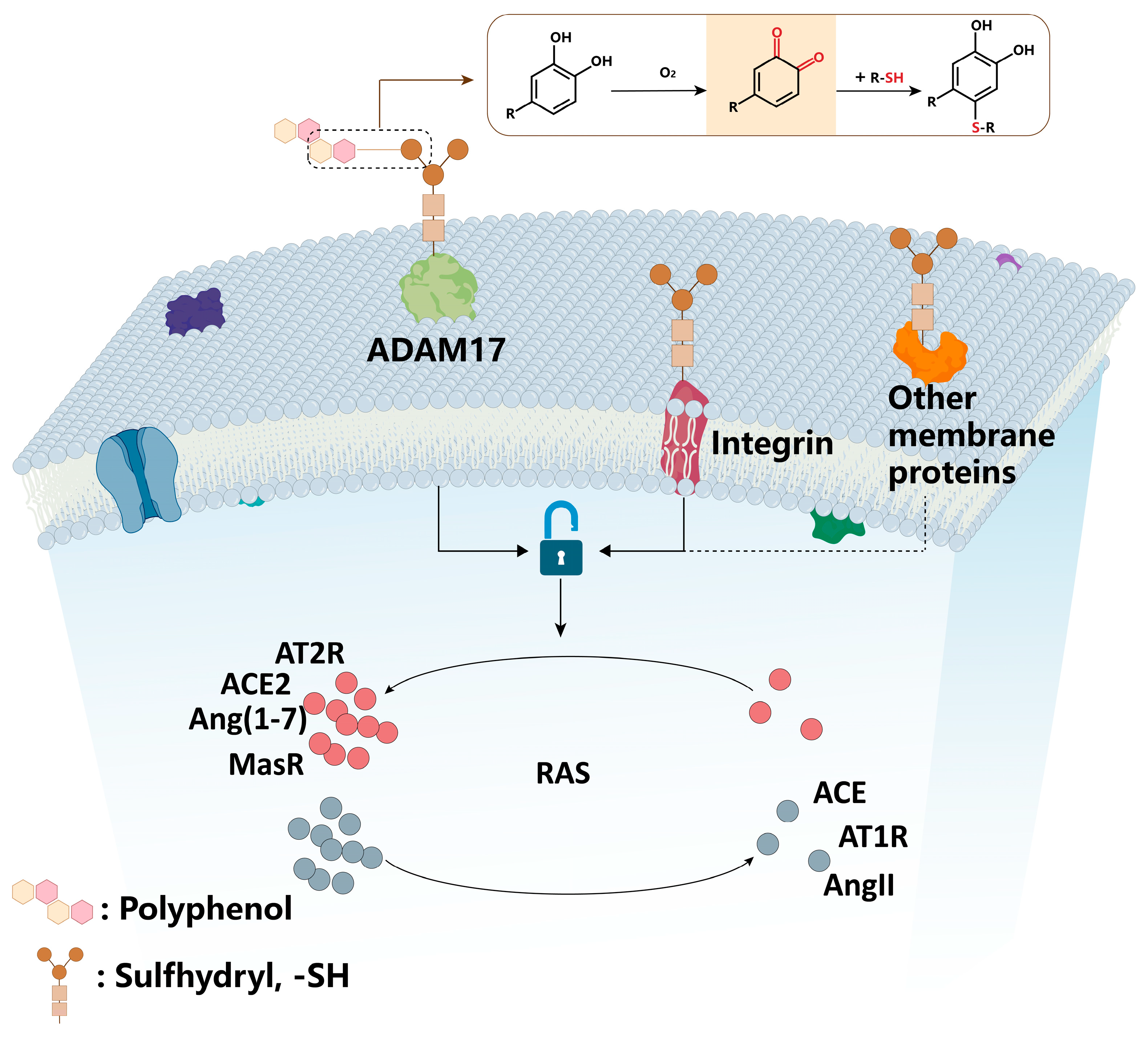
4.1. Polyphenols Regulate RAS by Modifying Thiol-Containing Transmembrane Proteins on the Cell Membrane
4.2. Direct Binding of Polyphenols to the Active Site of RAS Components
5. Clinical Research
5.1. Introduction to RAS-Targeted Clinical Evidence
5.2. Cancer-Specific Clinical/Preclinical Analyses of RAS Targeting
5.3. Translational Implications for Polyphenols
6. Challenges and Translational Opportunities for RAS-Targeted Cancer Therapy
6.1. Key Challenges to Translating RAS-Polyphenol Research
6.2. Patient-Centric Translational Opportunities
6.3. Polyphenol-Rich Dietary Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RAS | renin–angiotensin system |
| AngII | angiotensin II |
| ACE | angiotensin-converting enzyme |
| AT1R | angiotensin type 1 receptor |
| AT2R | angiotensin type 2 receptor |
| RASi | RAS inhibitors |
| ACE2 | angiotensin-converting enzyme 2 |
| Ang(1-7) | angiotensin 1-7 |
| ACEis | ACE inhibitors |
| ARBs | angiotensin receptor blockers |
| AGT | angiotensin |
| CC-RCC | clear cell renal cell carcinoma |
| CRC | colorectal cancer CRC |
| EGCG | epigallocatechin gallate |
| EAOP | EGCG autoxidation products |
| P-gp | P-glycoprotein |
| ROS | reactive oxygen species |
| NO | nitric oxide |
| NOX | NADPH oxidase |
| (P)RR | (Pro)renin receptor |
| PFS | progression-free survival |
| DKK3 | dickkopf-3 |
| QSOX1 | Quiescin Sulfhydryl Oxidase 1 |
| LPS | lipopolysaccharide |
| TNBC | triple-negative breast cancer |
| CSM | cancer-specific mortality |
| OM | overall mortality |
| VEGF | vascular endothelial growth factor |
| NSCLC | non-small cell lung cancer |
| ORR | objective response rate |
| OS | overall survival |
| ICI | immune checkpoint inhibitors |
| EPIC | the European Prospective Investigation into Cancer and Nutrition |
| DFS | disease-free survival |
| AEs | adverse events |
| ADAM17 | a disintegrin and metalloproteinase 17 |
| MAPK | mitogen-activated protein kinase |
| NF-κB | NF-kappaB |
| STATs | signal transducers and activators of transcription |
References
- Hassani, B.; Attar, Z.; Firouzabadi, N. The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: Foes versus allies. Cancer Cell Int. 2023, 23, 254. [Google Scholar] [CrossRef]
- Jiang, H.; Tai, Z.; Chen, Z.; Zhu, Q.; Bao, L. Clinical applicability of renin-angiotensin system inhibitors in cancer treatment. Am. J. Cancer Res. 2021, 11, 318–336. [Google Scholar] [PubMed] [PubMed Central]
- Ishikane, S.; Takahashi-Yanaga, F. The role of angiotensin II in cancer metastasis: Potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem. Pharmacol. 2018, 151, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Nichols, P.; Lohse, C.M.; Kosari, F.; Kattah, A.G.; Harris, F.R.; Karagouga, G.; Mehra, R.; Fine, S.W.; Reuter, V.E.; et al. Renin Production by Juxtaglomerular Cell Tumors and Clear Cell Renal Cell Carcinoma and the Role of Angiotensin Signaling Inhibitors. Mayo Clin. Proc. 2022, 97, 2050–2064. [Google Scholar] [CrossRef]
- Yin, L.; Mao, L.; Yin, R.; Lv, C.; Shi, X.; Yue, C.; Chen, Y.; Lu, C.; Wu, Z.; Xu, K.; et al. ACE Loss Drives Renal Cell Carcinoma Growth and Invasion by Modulating AKT-FOXO1. Biologics 2024, 18, 397–412. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Li, D.; Zhu, N.; Nishiyama, A.; Yuan, Y. (Pro)renin receptor promotes colorectal cancer progression through inhibiting the NEDD4L-mediated Wnt3 ubiquitination and modulating gut microbiota. Cell Commun. Signal. CCS 2023, 21, 2. [Google Scholar] [CrossRef]
- Shibayama, Y.; Takahashi, K.; Yamaguchi, H.; Yasuda, J.; Yamazaki, D.; Rahman, A.; Fujimori, T.; Fujisawa, Y.; Takai, S.; Furukawa, T.; et al. Aberrant (pro)renin receptor expression induces genomic instability in pancreatic ductal adenocarcinoma through upregulation of SMARCA5/SNF2H. Commun. Biol. 2020, 3, 724. [Google Scholar] [CrossRef]
- Siljee, S.; Buchanan, O.; Brasch, H.D.; Bockett, N.; Patel, J.; Paterson, E.; Purdie, G.L.; Davis, P.F.; Itinteang, T.; Tan, S.T. Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System. Cells 2021, 10, 243. [Google Scholar] [CrossRef]
- Lozinski, M.; Lumbers, E.R.; Bowden, N.A.; Martin, J.H.; Fay, M.F.; Pringle, K.G.; Tooney, P.A. Upregulation of the Renin–Angiotensin System Is Associated with Patient Survival and the Tumour Microenvironment in Glioblastoma. Cells 2024, 13, 634. [Google Scholar] [CrossRef]
- Khoshghamat, N.; Jafari, N.; Toloue-Pouya, V.; Azami, S.; Mirnourbakhsh, S.H.; Khazaei, M.; Ferns, G.A.; Rajabian, M.; Avan, A. The therapeutic potential of renin-angiotensin system inhibitors in the treatment of pancreatic cancer. Life Sci. 2021, 270, 119118. [Google Scholar] [CrossRef]
- Pinter, M.; Jain, R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 2017, 9, eaan5616. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fang, Y.; He, Y.; Zhang, J. (−)-Epigallocatechin-3-Gallate and Quercetin Inhibit Quiescin Sulfhydryl Oxidase 1 Secretion from Hepatocellular Carcinoma Cells. Antioxidants 2025, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wang, L.; Ma, J.; Yue, X.; Wang, K.; Ma, X.; Yu, X.; Xiao, Z. Different effects of polyphenols on hydration, pasting and rheological properties of rice starch under extrusion condition: From the alterations in starch structure. Food Chem. 2025, 465 Pt 2, 142002. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, X.Y.; Xue, R.Y.; Yang, J.L.; Zhang, Y.S.; Zhou, D.; Li, H.B. Mechanistic Insights into Effects of Different Dietary Polyphenol Supplements on Arsenic Bioavailability, Biotransformation, and Toxicity Based on a Mouse Model. Environ. Sci. Technol. 2023, 57, 15422–15431. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Oishi, Y.; Isemura, M.; Miyoshi, N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants 2022, 11, 2352. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between beta-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Ren, T.; Zheng, P.; Zhang, K.; Liao, J.; Xiong, F.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X. Effects of GABA on the polyphenol accumulation and antioxidant activities in tea plants (Camellia sinensis L.) under heat-stress conditions. Plant Physiol. Biochem. PPB 2021, 159, 363–371. [Google Scholar] [CrossRef]
- Xin, J.; Song, M.; Liu, X.; Zou, H.; Wang, J.; Xiao, L.; Jia, Y.; Zhang, G.; Jiang, W.; Lei, M.; et al. A new strategy of using low-dose caffeic acid carbon nanodots for high resistance to poorly differentiated human papillary thyroid cancer. J. Nanobiotechnol. 2024, 22, 571. [Google Scholar] [CrossRef]
- Ressaissi, A.; Pacheco, R.; Serralheiro, M.L.M. Molecular-level changes induced by hydroxycinnamic acid derivatives in HepG2 cell line: Comparison with pravastatin. Life Sci. 2021, 283, 119846. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomed. Int. J. Phytother. Phytopharm. 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Ossikbayeva, S.; Khanin, M.; Sharoni, Y.; Trachtenberg, A.; Tuleukhanov, S.; Sensenig, R.; Rom, S.; Danilenko, M.; Orynbayeva, Z. Curcumin and Carnosic Acid Cooperate to Inhibit Proliferation and Alter Mitochondrial Function of Metastatic Prostate Cancer Cells. Antioxidants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Ekrami, E.M.; Aghdas, S.A.M.; Mihanfar, A.; Hallaj, S.; Yousefi, B.; Safa, A.; Majidinia, M. Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: Implication for cancer therapy. Life Sci. 2020, 255, 117481. [Google Scholar] [CrossRef]
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T.B.; Islam, F.; Rauf, A.; Hossain, M.J.; Aljohani, A.S.M.; et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022, 184, 106398. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Gupta, S.C.; Nabavizadeh, A.; Aggarwal, B.B. Regulation of cell signaling pathways by dietary agents for cancer prevention and treatment. Semin. Cancer Biol. 2017, 46, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- NavaneethaKrishnan, S.J.L.; Rosales, J.L.; Lee, K.-Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxidative Med. Cell. Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Nelen, J.; Naponelli, V.; Villalgordo-Soto, J.M.; Falasca, M.; Pérez-Sánchez, H. Targeting Drug Resistance in Cancer: Dimethoxycurcumin as a Functional Antioxidant Targeting ABCC3. Antioxidants 2025, 14, 599. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, Z.; Karimi-Soureh, R.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Luo, W.; Han, S.; Sun, M.; Lin, M.; Wu, C.; Gao, L.; Xie, T.; Kong, N. Integration of active ingredients from traditional Chinese medicine with nano-delivery systems for tumor immunotherapy. J. Nanobiotechnol. 2025, 23, 357. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhang, H. Dietary polyphenols for tumor therapy: Bioactivities, nano-therapeutic systems and delivery strategies. Food Funct. 2025, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.T.; Sacinti, K.G.; Turkoglu, I.; Sanlier, N. Some Polyphenolic Compounds as Potential Therapeutic Agents in Cervical Cancer: The Most Recent Advances and Future Prospects. Nutr. Rev. 2025, 83, 880–896. [Google Scholar] [CrossRef]
- Losada-Echeberría, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E. Polyphenols as Promising Drugs against Main Breast Cancer Signatures. Antioxidants 2017, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomed. Int. J. Phytother. Phytopharm. 2023, 119, 154979. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.L.; Najjar, R.S.; Danh, J.P.; Knapp, D.; Wanders, D.; Feresin, R.G. Berry consumption mitigates the hypertensive effects of a high-fat, high-sucrose diet via attenuation of renal and aortic AT(1)R expression resulting in improved endothelium-derived NO bioavailability. J. Nutr. Biochem. 2023, 112, 109225. [Google Scholar] [CrossRef]
- Eff, A.R.Y.; Yenhart, I.T.; Eden, Y.; Rahayu, S.T.; Mahayasih, P.G.M. Investigation renin inhibitor activity from flavonoids derivates by in silico study. J. Adv. Pharm. Technol. Res. 2023, 14, 82–88. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The renin-angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 2010, 10, 745–759. [Google Scholar] [CrossRef]
- Pei, N.; Mao, Y.; Wan, P.; Chen, X.; Li, A.; Chen, H.; Li, J.; Wan, R.; Zhang, Y.; Du, H.; et al. Angiotensin II type 2 receptor promotes apoptosis and inhibits angiogenesis in bladder cancer. J. Exp. Clin. Cancer Res. CR 2017, 36, 77. [Google Scholar] [CrossRef]
- Shibata, K.; Kikkawa, F.; Mizokami, Y.; Kajiyama, H.; Ino, K.; Nomura, S.; Mizutani, S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumour Biol. 2005, 26, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Hashiguchi, Y.; Yagi, T.; Fukushima, Y.; Shimada, R.; Hayama, T.; Tsuchiya, T.; Nozawa, K.; Iinuma, H.; Ishihara, S.; et al. Angiotensin I-converting enzyme inhibitors/angiotensin II receptor blockers may reduce tumor recurrence in left-sided and early colorectal cancers. Int. J. Color. Dis. 2019, 34, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Carl-McGrath, S.; Ebert, M.P.; Lendeckel, U.; Röcken, C. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol. Ther. 2007, 6, 1218–1226. [Google Scholar] [CrossRef][Green Version]
- Rhodes, D.R.; Ateeq, B.; Cao, Q.; Tomlins, S.A.; Mehra, R.; Laxman, B.; Kalyana-Sundaram, S.; Lonigro, R.J.; Helgeson, B.E.; Bhojani, M.S.; et al. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc. Natl. Acad. Sci. USA 2009, 106, 10284–10289. [Google Scholar] [CrossRef]
- Röcken, C.; Lendeckel, U.; Dierkes, J.; Westphal, S.; Carl-McGrath, S.; Peters, B.; Krüger, S.; Malfertheiner, P.; Roessner, A.; Ebert, M.P. The number of lymph node metastases in gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin. Cancer Res. 2005, 11, 2526–2530. [Google Scholar] [CrossRef]
- Suganuma, T.; Ino, K.; Shibata, K.; Kajiyama, H.; Nagasaka, T.; Mizutani, S.; Kikkawa, F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin. Cancer Res. 2005, 11, 2686–2694. [Google Scholar] [CrossRef]
- Uemura, H.; Hasumi, H.; Ishiguro, H.; Teranishi, J.; Miyoshi, Y.; Kubota, Y. Renin-angiotensin system is an important factor in hormone refractory prostate cancer. Prostate 2006, 66, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Petty, W.J.; Miller, A.A.; McCoy, T.; Gallagher, P.E.; Tallant, E.A.; Torti, F.M. Phase I and pharmacokinetic study of angiotensin-(1-7), an endogenous antiangiogenic hormone. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7398–7404. [Google Scholar] [CrossRef]
- Xu, J.; Fan, J.; Wu, F.; Huang, Q.; Guo, M.; Lv, Z.; Han, J.; Duan, L.; Hu, G.; Chen, L.; et al. The ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Pleiotropic Roles in Cancer. Front. Physiol. 2017, 8, 276. [Google Scholar] [CrossRef]
- Zong, H.; Yin, B.; Zhou, H.; Cai, D.; Ma, B.; Xiang, Y. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 5171–5177. [Google Scholar] [CrossRef]
- Perryman, R.; Renziehausen, A.; Shaye, H.; Kostagianni, A.D.; Tsiailanis, A.D.; Thorne, T.; Chatziathanasiadou, M.V.; Sivolapenko, G.B.; El Mubarak, M.A.; Han, G.W.; et al. Inhibition of the angiotensin II type 2 receptor AT(2)R is a novel therapeutic strategy for glioblastoma. Proc. Natl. Acad. Sci. USA 2022, 119, e2116289119. [Google Scholar] [CrossRef]
- Nguyen, N.T.H.; Nguyen, P.-A.; Huang, C.-W.; Wang, C.-H.; Lin, M.-C.; Hsu, M.-H.; Bao, H.B.; Chien, S.-C.; Yang, H.-C. Renin-Angiotensin-Aldosterone System Inhibitors and Development of Gynecologic Cancers: A 23 Million Individual Population-Based Study. Int. J. Mol. Sci. 2023, 24, 3814. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.S.; Saha, S.; Magnuson, W.J.; Morris, B.A.; Borkenhagen, J.F.; Ching, A.; Hirose, G.; McMurry, V.; Francis, D.M.; Harari, P.M.; et al. Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Cancer 2016, 122, 2487–2495. [Google Scholar] [CrossRef]
- Zeman, M.; Skałba, W.; Wilk, A.M.; Cortez, A.J.; Maciejewski, A.; Czarniecka, A. Impact of renin-angiotensin system inhibitors on the survival of patients with rectal cancer. BMC Cancer 2022, 22, 815. [Google Scholar] [CrossRef]
- Shen, J.; Hou, H.; Liang, B.; Guo, X.; Chen, L.; Yang, Y.; Wang, Y. Effect of renin-angiotensin-aldosterone system inhibitors on survival outcomes in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1155104. [Google Scholar] [CrossRef] [PubMed]
- Mandilaras, V.; Bouganim, N.; Yin, H.; Asselah, J.; Azoulay, L. The use of drugs acting on the renin-angiotensin system and the incidence of pancreatic cancer. Br. J. Cancer 2017, 116, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, X.; He, Y.; Li, X.; Yang, L.; Song, T.; Wang, F.; Yang, C.S.; Zhang, J. EGCG oxidation-derived polymers induce apoptosis in digestive tract cancer cells via regulating the renin-angiotensin system. Food Funct. 2024, 15, 2052–2063. [Google Scholar] [CrossRef]
- Norambuena-Soto, I.; Lopez-Crisosto, C.; Martinez-Bilbao, J.; Hernandez-Fuentes, C.; Parra, V.; Lavandero, S.; Chiong, M. Angiotensin-(1-9) in hypertension. Biochem. Pharmacol. 2022, 203, 115183. [Google Scholar] [CrossRef]
- Liau, Y.; Chua, I.; Kennedy, M.A.; Maggo, S. Pharmacogenetics of angiotensin-converting enzyme inhibitor-induced angioedema. Clin. Exp. Allergy 2019, 49, 142–154. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, Z.; Wang, F.; Liu, P.; Tao, J.; Li, C.; Han, Z.; Li, Z.; Ma, Y.; Gu, Y. AT1R-Specific Ligand Angiotensin II as a Novel SPECT Agent for Hepatocellular Carcinoma Diagnosis. ACS Sens. 2020, 5, 4072–4080. [Google Scholar] [CrossRef]
- Pinter, M.; Kwanten, W.J.; Jain, R.K. Renin-Angiotensin System Inhibitors to Mitigate Cancer Treatment-Related Adverse Events. Clin. Cancer Res. 2018, 24, 3803–3812. [Google Scholar] [CrossRef]
- Berlo, B.V.; Civati, C.; Esposito, P.; De Keulenaer, G.W.; Guns, P.J.D.F.; Segers, V.F.M. Angiotensin II as a linking factor in cardiovascular disease enhanced cancer growth. Eur. Heart J. 2024, 45, ehae666.3205. [Google Scholar] [CrossRef]
- Fan, F.; Tian, C.; Tao, L.; Wu, H.; Liu, Z.; Shen, C.; Jiang, G.; Lu, Y. Candesartan attenuates angiogenesis in hepatocellular carcinoma via downregulating AT1R/VEGF pathway. Biomed. Pharmacother. 2016, 83, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Perini, M.V.; Dmello, R.S.; Nero, T.L.; Chand, A.L. Evaluating the benefits of renin-angiotensin system inhibitors as cancer treatments. Pharmacol. Ther. 2020, 211, 107527. [Google Scholar] [CrossRef]
- Wang, S.; Du, Q.; Meng, X.; Zhang, Y. Natural polyphenols: A potential prevention and treatment strategy for metabolic syndrome. Food Funct. 2022, 13, 9734–9753. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. PTR 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Reboredo-Rodriguez, P.; Suntar, I.; Sureda, A.; Belwal, T.; Loizzo, M.R.; Tundis, R.; Sobarzo-Sanchez, E.; Rastrelli, L.; Forbes-Hernandez, T.Y.; et al. Evaluation of the status quo of polyphenols analysis: Part I-phytochemistry, bioactivity, interactions, and industrial uses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3191–3218. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Suhail, M.; Khan, M.K.; Zughaibi, T.A.; Alserihi, R.F.; Zaidi, S.K.; Tabrez, S. Polyphenols as anticancer agents: Toxicological concern to healthy cells. Phytother. Res. PTR 2021, 35, 6063–6079. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ma, C.; Huang, D.; Chen, S.; Zhu, S.; Wang, H. Enzymatic molecular modification of water-soluble polyphenols: Synthesis, structure, bioactivity and application. Crit. Rev. Food Sci. Nutr. 2023, 63, 12637–12651. [Google Scholar] [CrossRef]
- Wang, S.; Mo, L.; Wu, B.; Ma, C.; Wang, H. Effect of structural stability of lipase in acetonitrile on its catalytic activity in EGCG esterification reaction: FTIR and MD simulation. Int. J. Biol. Macromol. 2024, 255, 128266. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Chakraborty, R.; Roy, S. Angiotensin-converting enzyme inhibitors from plants: A review of their diversity, modes of action, prospects, and concerns in the management of diabetes-centric complications. J. Integr. Med. 2021, 19, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Iijima, K.; Okuzawa, R.; Kawata, R.; Kimura, A.; Shinohara, Y.; Shimada, A.; Yamanaka, M.; Youda, A.; Iwamoto, T. Augmented Intrarenal and Urinary Angiotensinogen in Diabetic Nephropathy: The Role of Isoflavones. Int. J. Mol. Sci. 2025, 26, 1443. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Shu, G.; Li, J. Antihypertensive Potential of Plant Foods: Research Progress and Prospect of Plant-Derived Angiotensin-Converting Enzyme Inhibition Compounds. J. Agric. Food Chem. 2021, 69, 5297–5305. [Google Scholar] [CrossRef]
- Moran, C.S.; Biros, E.; Krishna, S.M.; Wang, Y.; Tikellis, C.; Morton, S.K.; Moxon, J.V.; Cooper, M.E.; Norman, P.E.; Burrell, L.M.; et al. Resveratrol Inhibits Growth of Experimental Abdominal Aortic Aneurysm Associated With Upregulation of Angiotensin-Converting Enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2195–2203. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, J.C.; Feng, Y.; Wei, W.T.; Li, S.Z.; Wu, M.D.; Mo, C.J.; Huang, J.W.; Yang, C.W.; Hu, S.Q.; et al. Carnosic acid, a novel food-source AT1R antagonist and its anti-hypertension mechanism. Int. J. Biol. Macromol. 2024, 278 Pt 3, 135012. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.R.; Shahien, M.M.; Ibrahim, S.; Alenazi, F.S.; Hussein, W.; Abdallah, M.H.; Aljadani, A.; Alreshidi, F.; El-Horany, H.E.; Elhussein, G.E.M.O.; et al. Novel Insights in the Hypertension Treatment & Type 2 Diabetics Induced by Angiotensin Receptor Blockers: MD Simulation Studies & Molecular Docking of Some Promising Natural Therapies. ACS Omega 2024, 9, 21234–21244. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Mu, S.; Feresin, R.G. Blueberry Polyphenols Increase Nitric Oxide and Attenuate Angiotensin II-Induced Oxidative Stress and Inflammatory Signaling in Human Aortic Endothelial Cells. Antioxidants 2022, 11, 616. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Ma, X.; Yang, S.; Hu, X.; Tao, J.; Hou, Y.; Bai, G. Glycyrrhetinic acid binds to the conserved P-loop region and interferes with the interaction of RAS-effector proteins. Acta Pharm. Sinica. B 2019, 9, 294–303. [Google Scholar] [CrossRef]
- Tiwari, P.; Tiwari, V.; Gupta, S.; Shukla, S.; Hanif, K. Activation of Angiotensin-converting Enzyme 2 Protects Against Lipopolysaccharide-induced Glial Activation by Modulating Angiotensin-converting Enzyme 2/Angiotensin (1-7)/Mas Receptor Axis. Mol. Neurobiol. 2023, 60, 203–227. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, F.; Cao, Y.; Cai, G.; Wei, Q.; Shi, S.; Guo, Y. Screening of potent alpha-glucosidase inhibitory and antioxidant polyphenols in Prunella vulgaris L. by bioreaction-HPLC-quadrupole-time-of-flight-MS/MS and in silico analysis. J. Sep. Sci. 2022, 45, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, M.; He, Y.; Wang, F.; Kong, Y.; Ling, T.J.; Zhang, J. EGCG-derived polymeric oxidation products enhance insulin sensitivity in db/db mice. Redox Biol. 2022, 51, 102259. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Tan, S.T. Cancer Stem Cells and the Renin–Angiotensin System in the Tumor Microenvironment of Melanoma: Implications on Current Therapies. Int. J. Mol. Sci. 2025, 26, 1389. [Google Scholar] [CrossRef]
- Khan, S.H.; Alhumaydhi, F.A.; Khan, M.A.; Younus, H. Therapeutic Potential of Polyphenols and their Nanoformulations in the Treatment of Colorectal Cancer. Anti-Cancer Agents Med. Chem. 2021, 21, 2117–2129. [Google Scholar] [CrossRef]
- Kandhavelu, J.; Subramanian, K.; Naidoo, V.; Sebastianelli, G.; Doan, P.; Konda Mani, S.; Yapislar, H.; Haciosmanoglu, E.; Arslan, L.; Ozer, S.; et al. A novel EGFR inhibitor, HNPMI, regulates apoptosis and oncogenesis by modulating BCL-2/BAX and p53 in colon cancer. Br. J. Pharmacol. 2024, 181, 107–124. [Google Scholar] [CrossRef]
- Shrestha, S.; Adib, E.; Imani, J.; Aguiar, D.J.; Lamattina, A.M.; Tassew, D.D.; Henske, E.P.; Perrella, M.A.; Priolo, C.; El-Chemaly, S. Angiotensin II receptor type 1 blockade regulates Klotho expression to induce TSC2-deficient cell death. J. Biol. Chem. 2022, 298, 102580. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sameri, S.; Liskova, A.; Zhai, K.; Varghese, E.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers 2021, 13, 188. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, H.C.; Chuang, H.C.; Hsu, Y.C.; Yang, M.Y.; Chien, C.Y. Pre-treatment with angiotensin-(1-7) inhibits tumor growth via autophagy by downregulating PI3K/Akt/mTOR signaling in human nasopharyngeal carcinoma xenografts. J. Mol. Med. 2018, 96, 1407–1418. [Google Scholar] [CrossRef]
- Lee, C.; Pratap, K.; Zhang, L.; Chen, H.D.; Gautam, S.; Arnaoutova, I.; Raghavankutty, M.; Starost, M.F.; Kahn, M.; Mansfield, B.C.; et al. Inhibition of Wnt/beta-catenin signaling reduces renal fibrosis in murine glycogen storage disease type Ia, Biochimica et biophysica acta. Mol. Basis Dis. 2024, 1870, 166874. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Ichiki, T.; Funakoshi, Y.; Ito, K.; Takeshita, A. Downregulation of angiotensin II type 1 receptor by all-trans retinoic acid in vascular smooth muscle cells. Hypertension 2000, 35 Pt 2, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Thapa, P.; Prajapati, A. New insights into prostate Cancer from the renin-angiotensin-aldosterone system. Cell. Signal. 2024, 124, 111442. [Google Scholar] [CrossRef] [PubMed]
- de Paula Gonzaga, A.; Palmeira, V.A.; Ribeiro, T.F.S.; Costa, L.B.; de Sa Rodrigues, K.E.; Simoes, E.S.A.C. ACE2/Angiotensin-(1-7)/Mas Receptor Axis in Human Cancer: Potential Role for Pediatric Tumors. Curr. Drug Targets 2020, 21, 892–901. [Google Scholar] [CrossRef]
- Savoia, C.; Arrabito, E.; Parente, R.; Nicoletti, C.; Madaro, L.; Battistoni, A.; Filippini, A.; Steckelings, U.M.; Touyz, R.M.; Volpe, M. Mas Receptor Activation Contributes to the Improvement of Nitric Oxide Bioavailability and Vascular Remodeling During Chronic AT1R (Angiotensin Type-1 Receptor) Blockade in Experimental Hypertension. Hypertension 2020, 76, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Malek, V.; Mulay, S.R.; Gaikwad, A.B. Angiotensin II type 2 receptor and angiotensin-converting enzyme 2 mediate ischemic renal injury in diabetic and non-diabetic rats. Life Sci. 2019, 235, 116796. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xue, F.; Cheng, C.; Sui, W.; Zhang, M.; Qiao, L.; Ma, J.; Ji, X.; Chen, W.; Yu, X.; et al. ADAM17 knockdown mitigates while ADAM17 overexpression aggravates cardiac fibrosis and dysfunction via regulating ACE2 shedding and myofibroblast transformation. Front. Pharmacol. 2022, 13, 997916. [Google Scholar] [CrossRef]
- Zhai, C.G.; Xu, Y.Y.; Tie, Y.Y.; Zhang, Y.; Chen, W.Q.; Ji, X.P.; Mao, Y.; Qiao, L.; Cheng, J.; Xu, Q.B.; et al. DKK3 overexpression attenuates cardiac hypertrophy and fibrosis in an angiotensin-perfused animal model by regulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways. J. Mol. Cell. Cardiol. 2018, 114, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.S.; Mia, M.S.; Sun, D.; Mathew, S. Integrin α2β1 inhibition decreases angiotensin-converting enzyme (ACE) expression in renal cancer. Physiology 2023, 38 (Suppl. S1), 5729494. [Google Scholar] [CrossRef]
- Lorenzen, I.; Eble, J.A.; Hanschmann, E.M. Thiol switches in membrane proteins—Extracellular redox regulation in cell biology. Biol. Chem. 2021, 402, 253–269. [Google Scholar] [CrossRef]
- Mia, M.S.; Hossain, D.; Woodbury, E.; Kelleher, S.; Palamuttam, R.J.; Rao, R.; Steen, P.; Jarajapu, Y.P.; Mathew, S. Integrin β1 is a key determinant of the expression of angiotensin-converting enzyme 2 (ACE2) in the kidney epithelial cells. Eur. J. Cell Biol. 2023, 102, 151316. [Google Scholar] [CrossRef]
- An, T.T.; Feng, S.; Zeng, C.M. Oxidized epigallocatechin gallate inhibited lysozyme fibrillation more strongly than the native form. Redox Biol. 2017, 11, 315–321. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.J.; Feng, S.; Zeng, C.M. Quinopeptide formation associated with the disruptive effect of epigallocatechin-gallate on lysozyme fibrils. Int. J. Biol. Macromol. 2015, 78, 389–395. [Google Scholar] [CrossRef]
- Shibata, S.; Yamada, K.; Kon, S. Carnosic acid inhibits integrin expression and prevents pulmonary metastasis of melanoma. Biosci. Biotechnol. Biochem. 2025, 89, 284–293. [Google Scholar] [CrossRef]
- Shafaei, A.; Sultan Khan, M.S.; Aisha, A.F.A.; Abdul Majid, A.M.S.; Hamdan, M.R.; Mordi, M.N.; Ismail, Z. Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study. Molecules 2016, 21, 1500. [Google Scholar] [CrossRef]
- Looi, D.; Goh, B.H.; Khan, S.U.; Ahemad, N.; Palanisamy, U.D. Metabolites of the ellagitannin, geraniin inhibit human ACE; in vitro and in silico evidence. Int. J. Food Sci. Nutr. 2021, 72, 470–477. [Google Scholar] [CrossRef]
- Jenis, J.; Kim, J.Y.; Uddin, Z.; Song, Y.H.; Lee, H.H.; Park, K.H. Phytochemical profile and angiotensin I converting enzyme (ACE) inhibitory activity of Limonium michelsonii Lincz. J. Nat. Med. 2017, 71, 650–658. [Google Scholar] [CrossRef]
- Santos, M.C.; Toson, N.S.B.; Pimentel, M.C.B.; Bordignon, S.A.L.; Mendez, A.S.L.; Henriques, A.T. Polyphenols composition from leaves of Cuphea spp. and inhibitor potential, in vitro, of angiotensin I-converting enzyme (ACE). J. Ethnopharmacol. 2020, 255, 112781. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ling, Q.; Bao, G.; Xie, L.; Shi, Y.; Wang, X. Effect of Various Fruit Extracts on Angiotensin I-Converting Enzyme (ACE) and Kallikrein (KLK) Activities. Plant Foods Hum. Nutr. 2024, 79, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Wang, D.; Cheng, Y.; Wu, M.; Saleem, M.Z.; Wei, L.; Xie, Y.; Yan, M.; Chu, J.; Yang, Y.; et al. Quercetin attenuates angiotensin II-induced proliferation of vascular smooth muscle cells and p53 pathway activation in vitro and in vivo. BioFactors 2023, 49, 956–970. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W.; Kim, S.-A.; Ju, W.-T.; Kim, S.-R.; Kim, H.-B.; Cha, I.-S.; Kim, S.-W.; Park, J.-W.; Kang, S.-K. Modulatory Effects of the Kuwanon-Rich Fraction from Mulberry Root Bark on the Renin–Angiotensin System. Foods 2024, 13, 1547. [Google Scholar] [CrossRef]
- Yoshida, T.; Kinoshita, H.; Fukui, K.; Matsuzaki, T.; Yoshida, K.; Mishima, T.; Yanishi, M.; Komai, Y.; Sugi, M.; Inoue, T.; et al. Prognostic Impact of Renin-Angiotensin Inhibitors in Patients with Bladder Cancer Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2017, 24, 823–831. [Google Scholar] [CrossRef]
- Dougherty, U.; Mustafi, R.; Haider, H.I.; Khalil, A.; Souris, J.S.; Joseph, L.; Hart, J.; Konda, V.J.; Zhang, W.; Pekow, J.; et al. Losartan and Vitamin D Inhibit Colonic Tumor Development in a Conditional Apc-Deleted Mouse Model of Sporadic Colon Cancer. Cancer Prev. Res. 2019, 12, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Vallejo Ardila, D.L.; Walsh, K.A.; Fifis, T.; Paolini, R.; Kastrappis, G.; Christophi, C.; Perini, M.V. Immunomodulatory effects of renin-angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J. Immunother. Cancer 2020, 8, e000487. [Google Scholar] [CrossRef]
- Renziehausen, A.; Wang, H.; Rao, B.; Weir, L.; Nigro, C.L.; Lattanzio, L.; Merlano, M.; Vega-Rioja, A.; Del Carmen Fernandez-Carranco, M.; Hajji, N.; et al. The renin angiotensin system (RAS) mediates bifunctional growth regulation in melanoma and is a novel target for therapeutic intervention. Oncogene 2019, 38, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, F.S.; Guimaraes, J.P.T.; Menikdiwela, K.R.; Mabry, B.; Dhakal, R.; Rahman, R.L.; Moussa, H.; Moustaid-Moussa, N. Breast cancer and the renin-angiotensin system (RAS): Therapeutic approaches and related metabolic diseases. Mol. Cell. Endocrinol. 2021, 528, 111245. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Wei, X.L.; Fang, Y.P.; Gan, R.Y.; Wang, M.; Ge, Y.Y.; Zhang, D.; Cheng, L.Z.; Corke, H. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 2020, 60, 1243–1264. [Google Scholar] [CrossRef]
- Yang, M.; He, Y.; Ni, Q.; Zhou, M.; Chen, H.; Li, G.; Yu, J.; Wu, X.; Zhang, X. Polyphenolic Nanomedicine Regulating Mitochondria REDOX for Innovative Cancer Treatment. Pharmaceutics 2024, 16, 972. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wang, D.; Wang, Z.; Yang, M.; Yang, L.; Wang, F.; Wang, W.; Zhang, X. Formation of EGCG oxidation self-assembled nanoparticles and their antioxidant activity in vitro and hepatic REDOX regulation activity in vivo. Food Funct. 2024, 15, 2181–2196. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Zhang, W.; Wu, W. Association of tea consumption and the risk of oral cancer: A meta-analysis. Oral Oncol. 2014, 50, 276–281. [Google Scholar] [CrossRef]
- Tang, N.; Wu, Y.; Zhou, B.; Wang, B.; Yu, R. Green tea, black tea consumption and risk of lung cancer: A meta-analysis. Lung Cancer 2009, 65, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Yuan, J.M.; Koh, W.P.; Yu, M.C. Green tea, black tea and colorectal cancer risk: A meta-analysis of epidemiologic studies. Carcinogenesis 2006, 27, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- BraakhuiBraakhuis, A.J.; Campion, P.; Bishop, K.S. Reducing Breast Cancer Recurrence: The Role of Dietary Polyphenolics. Nutrients 2016, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Ramirez, Y.; Cornelis, M.C.; Berrington de González, A.; Freedman, N.D.; Loftfield, E. Tea Consumption and All-Cause and Cause-Specific Mortality in the UK Biobank: A Prospective Cohort Study. Ann. Intern. Med. 2022, 175, 1201–1211. [Google Scholar] [CrossRef]


| Categories of Polyphenols | Representative Compound | IC50/Inhibition Ratio | Reference |
|---|---|---|---|
| Flavonoid | 3, 7-dihydroxyflavone | IC50 = 0.04 μmol/L | [78] |
| Fisetin | IC50 = 0.08 μmol/L | [78] | |
| Luteolin | IC50 = 7.1 μmol/L | [78] | |
| Quercetin | IC50 = 30.3 μM | [113] | |
| Eupatorin | IC50 = 15.35 ± 4.49 µg/mL | [111] | |
| Sinensetin | IC50 = 29.5 ± 0.3 µg/mL | [111] | |
| 3′-hydroxy-5,6,7,4′-tetramethoxyflavone | IC50 = 27.9 ± 2.4 µg/mL | [111] | |
| Flavonoid derivative/flavonoid glycosides | Acteoside | IC50 = 0.36 μmol/L | [78] |
| Isoacteoside | IC50 = 0.46 μmol/L | [78] | |
| Rutin | IC50 = 0.45 μmol/L | [78] | |
| Miquelianin | 32.41% (100 ng/mL) | [114] | |
| Myricitrin | 31.66% (100 ng/mL) | [114] | |
| Myricetin glycoside | IC50 = 10.2–14.5 μM | [113] | |
| Phenolic acid | Rosmarinic acid | IC50 = 0.05 μmol/L | [78] |
| Ethyl caffeate | 55.19% (10 μg/mL) | [81] | |
| Caffeic acid | IC50 = 2.10 mM | [81] | |
| Ferulic acid | IC50 = 4.40 mM | [81] | |
| Phenolic acid derivative | 3-feruloylquinic acid | 26.32% (100 ng/mL) | [114] |
| Rosmarinic acid | IC50 = 18.8 ± 0.2 µg/mL | [111] | |
| Geraniin | IC50 = 13.22 uM | [112] | |
| Anthocyanin | Delphinidin-3-O-sambur diglycoside | IC50 = 0.14 μmol/L | [78] |
| Cyanidin-3-o-sambu diglycoside | IC50 = 0.11 μmol/L | [78] | |
| Other phenolic derivative | (+)-trans-Decursidinol | IC50 = 0.00468 mM | [81] |
| Butein | IC50 = 8.44 μmol/L | [78] | |
| Polyphenol complexes | Coriandrum sativum flavonoid-rich fraction (containing pinocembrin, apigenin) | IC50 = 28.91 ± 13.42 μg/mL | [114] |
| Polyphenol extracts | Kiwifruit aqueous extracts | 47.71% (2.8 mg/g Dry weight) | [115] |
| Ocimum basilicum leaves extracts (containing Rutin, quercetin, and quercitrin, caffeic, chlorogenic, and gallic acids) | IC50 = 64.99 µg/mL | [112] | |
| O. Gratissimum leaves extracts (containing Rutin, quercitrin, luteolin; ellagic, chlorogenic acids) | IC50 = 29.44 µg/mL | [112] | |
| Mesembryanthemum crystallinum extracts (containing apigenin, diosmin and luteolin) | 90.5% (1 mg/mL) | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, M.; Zhang, H.; Yang, L.; He, Y.; Cheng, X.; Zhu, G. Advances in Understanding Renin–Angiotensin System-Mediated Anti-Tumor Activity of Natural Polyphenols. Biomolecules 2025, 15, 1541. https://doi.org/10.3390/biom15111541
Wu X, Yang M, Zhang H, Yang L, He Y, Cheng X, Zhu G. Advances in Understanding Renin–Angiotensin System-Mediated Anti-Tumor Activity of Natural Polyphenols. Biomolecules. 2025; 15(11):1541. https://doi.org/10.3390/biom15111541
Chicago/Turabian StyleWu, Ximing, Mingchuan Yang, Hailing Zhang, Lumin Yang, Yufeng He, Xiaozhong Cheng, and Guilan Zhu. 2025. "Advances in Understanding Renin–Angiotensin System-Mediated Anti-Tumor Activity of Natural Polyphenols" Biomolecules 15, no. 11: 1541. https://doi.org/10.3390/biom15111541
APA StyleWu, X., Yang, M., Zhang, H., Yang, L., He, Y., Cheng, X., & Zhu, G. (2025). Advances in Understanding Renin–Angiotensin System-Mediated Anti-Tumor Activity of Natural Polyphenols. Biomolecules, 15(11), 1541. https://doi.org/10.3390/biom15111541





