ZC3H12A: A Critical Mediator of Inflammation, Tumor Immunotherapy, and Metabolic–Immune Crosstalk—Implications for Disease Treatment
Abstract
1. Introduction
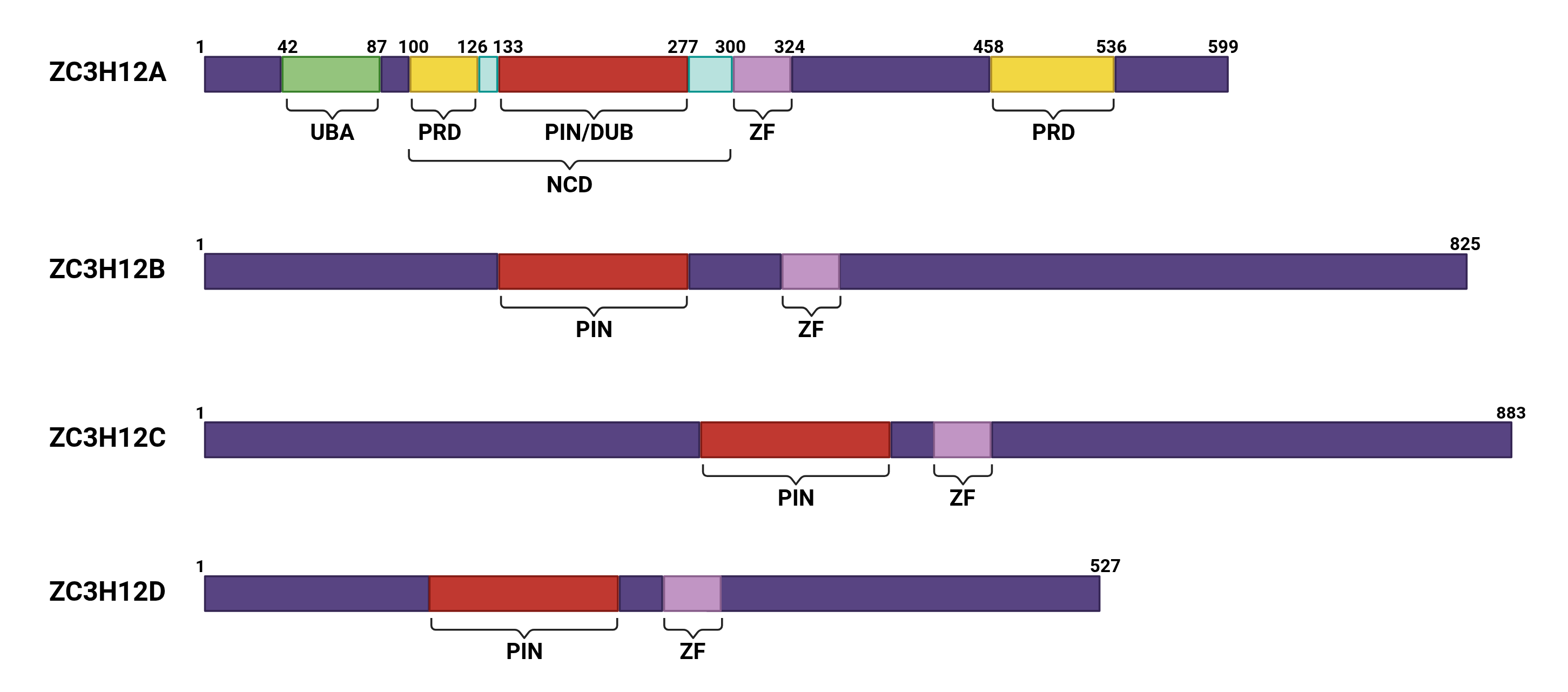
2. ZC3H12A as the “Master Switch” of Immune Homeostasis
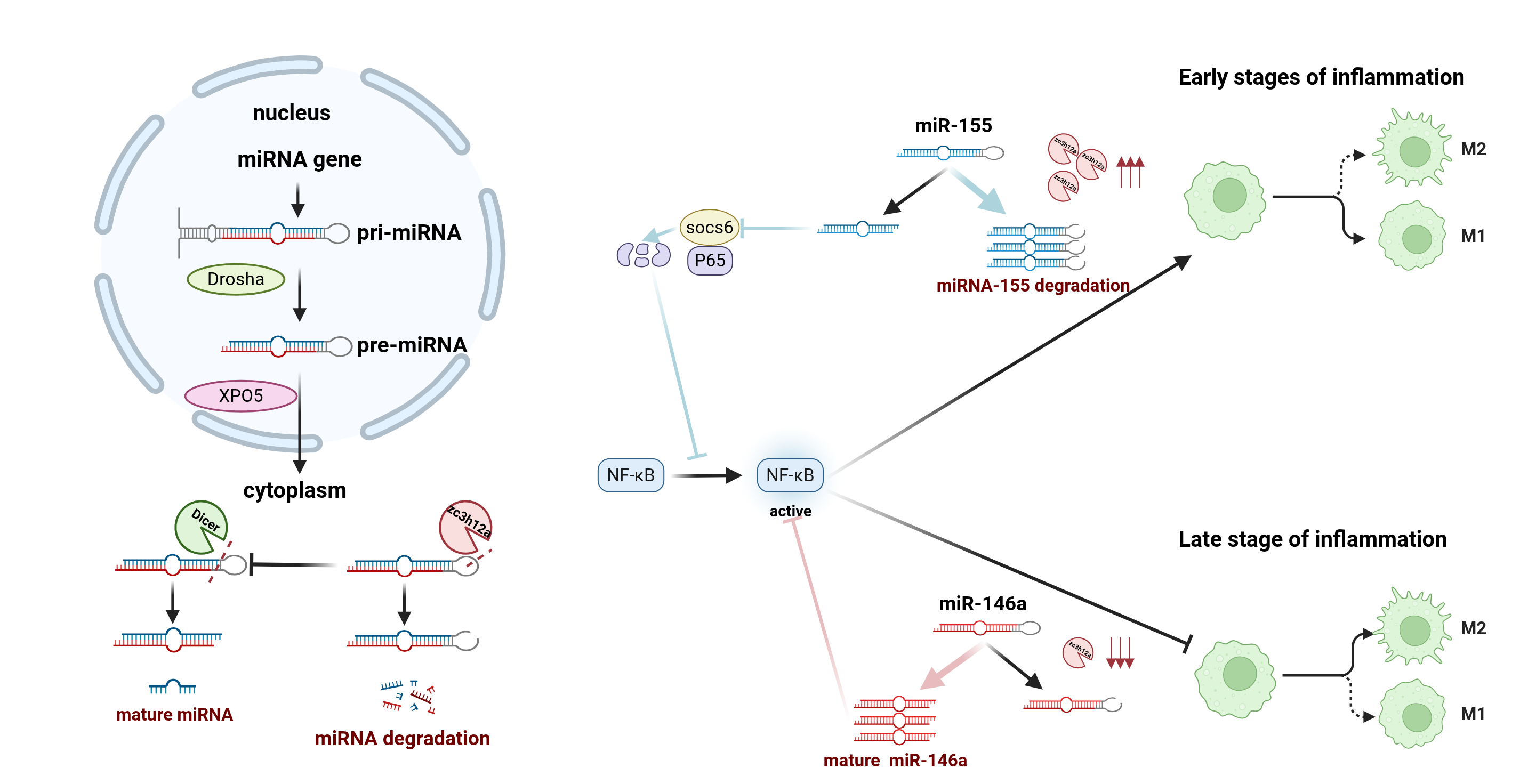
2.1. Regulatory Functions in Innate Immunity
2.2. “Brakes and Accelerator” of Adaptive Immunity
3. Bidirectional Regulation of ZC3H12A in Tumors: From “Tumor Suppressor Gene” to “Therapeutic Lever”
3.1. The Intrinsic “Environment-Dependent” Role of Tumor Cells
3.2. The “Architect” of the Tumor Immune Microenvironment
3.3. The “Game Changer” in CAR-T Immunotherapy
4. ZC3H12A-Mediated Systemic Metabolic–Immune Dialog
5. New Strategies for Treating Inflammatory and Infectious Diseases with ZC3H12A
5.1. Enhancing ZC3H12A as a Therapeutic Strategy in Inflammatory Diseases
5.2. The Dual Role of ZC3H12A in Infectious Diseases
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SL | stem loop |
| TLRs | Toll-like receptors |
| PRRs | pattern-recognition receptors |
| RBPs | RNA-binding proteins |
| miRNAs | microRNAs |
| 3′ UTR | 3′ untranslated region |
| DUB | deubiquitination enzyme |
| NF-κB | nuclear factor kappa-B |
| IHD | ischemic heart disease |
| MCP-1 | monocyte chemoattractant protein 1 |
| CCR2 | monocyte chemokine receptor 2 |
| ESTs | expressed sequence tags |
| MCPIP | MCP-inducible protein |
| LPS | lipopolysaccharide |
| SNPs | single nucleotide polymorphisms |
| PRDs | proline-rich structural domains |
| NCD | N-terminal conserved structural domain |
| UBA | ubiquitin-associated functional domain |
| JNK | c-Jun N-terminal kinase |
| TTP | tristetraprolin |
| ZF | zinc finger motif |
| DR5 | death receptor 5 |
| pri-miRNA | Primary miRNA |
| pre-miRNA | precursor miRNA |
| RORα | retinoid-related orphan receptor α |
| PAMPs | pathogen-associated molecular patterns |
| IBD | inflammatory bowel disease |
| NK | Natural killer |
| OCT2 | octamer binding protein 2 |
| TCR | T cell receptor |
| MOs | morpholino oligonucleotides |
| KP | Klebsiella pneumoniae |
| RCC | renal cell carcinoma |
| ccRCC | clear cell renal cell carcinoma |
| VEGF | vascular endothelial growth factor |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| CRC | colorectal cancer |
| TNF-α | tumor necrosis factor α |
| ACT | Apheresis cell therapy |
| CAR | chimeric antigen receptor |
| CVB3 | coxsackievirus B3 |
| ARE | AU-rich element |
| HCV | hepatitis C |
| TR | terminal redundant |
| DAMPs | damage-associated molecular patterns |
| XPO5 | Exportin-5 |
| T2DM | type 2 diabetes |
| DKD | diabetic kidney disease |
| TKIs | tyrosine kinase inhibitors |
| C/EBPβ | CCAAT/enhancer-binding protein β |
| EMT | epithelial–mesenchymal transition |
| TNBC | triple-negative breast cancer |
| SCLC | small cell lung cancer |
| TIF | tumor-immunological fitness |
| FGF21 | fibroblast growth factor 21 |
| ILC2s | type II innate lymphoid cells |
| PDAC | pancreatic ductal adenocarcinoma |
| GC | gastric cancer |
| CTLs | cytotoxic T lymphocytes |
References
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric cancer originating from bone marrow-derived cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Post-transcriptional control of cytokine production. Nat. Immunol. 2008, 9, 353–359. [Google Scholar] [CrossRef]
- Stoecklin, G.; Anderson, P. Posttranscriptional mechanisms regulating the inflammatory response. Adv. Immunol. 2006, 89, 1–37. [Google Scholar]
- Katsanou, V.; Dimitriou, M.; Kontoyiannis, D.L. Post-Transcriptional Regulators in Inflammation: Exploring New Avenues in Biological Therapeutics. Ernst Schering Foundation Symposium Proceedings; 2006; pp. 37–57. [Google Scholar] [CrossRef]
- Mao, R.; Yang, R.; Chen, X.; Harhaj, E.W.; Wang, X.; Fan, Y. Regnase-1, a rapid response ribonuclease regulating inflammation and stress responses. Cell. Mol. Immunol. 2017, 14, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, S.; Peng, W.; Rao, Z. MCP-1-induced protein-1, an immune regulator. Protein Cell 2012, 3, 903–910. [Google Scholar] [CrossRef]
- Mellado, M.; Rodríguez-Frade, J.M.; Aragay, A.; del Real, G.; Martín, A.M.; Vila-Coro, A.J.; Serrano, A.; Mayor, F.; Martínez-A, C. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 1998, 161, 805–813. [Google Scholar] [CrossRef]
- Zhou, L.; Azfer, A.; Niu, J.; Graham, S.; Choudhury, M.; Adamski, F.M.; Younce, C.; Binkley, P.F.; Kolattukudy, P.E. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006, 98, 1177–1185. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Zhelyabovska, O.; Fatma, S.; Kolattukudy, P.E. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J. Biol. Chem. 2008, 283, 14542–14551. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Azfer, A.; Song, W.; Tromp, G.; Kolattukudy, P.E.; Fu, M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J. Biol. Chem. 2008, 283, 6337–6346. [Google Scholar] [CrossRef]
- Matsushita, K.; Takeuchi, O.; Standley, D.M.; Kumagai, Y.; Kawagoe, T.; Miyake, T.; Satoh, T.; Kato, H.; Tsujimura, T.; Nakamura, H.; et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 2009, 458, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, R.A.; Cruz-Tapias, P.; Rojas-Villarraga, A.; Anaya, J.-M. ZC3H12A (MCPIP1): Molecular characteristics and clinical implications. Clin. Chim. Acta 2010, 411, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Sunde, M. Zinc fingers--folds for many occasions. IUBMB Life 2002, 54, 351–355. [Google Scholar] [CrossRef]
- Hall, T.M.T. Multiple modes of RNA recognition by zinc finger proteins. Curr. Opin. Struct. Biol. 2005, 15, 367–373. [Google Scholar] [CrossRef]
- Fu, M.; Blackshear, P.J. RNA-binding proteins in immune regulation: A focus on CCCH zinc finger proteins. Nat. Rev. Immunol. 2017, 17, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.S.; Carballo, E.; Strum, J.R.; Kennington, E.A.; Phillips, R.S.; Blackshear, P.J. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell. Biol. 1999, 19, 4311–4323. [Google Scholar] [CrossRef]
- Carrick, D.M.; Lai, W.S.; Blackshear, P.J. The tandem CCCH zinc finger protein tristetraprolin and its relevance to cytokine mRNA turnover and arthritis. Arthritis Res. Ther. 2004, 6, 248–264. [Google Scholar] [CrossRef]
- Brown, R.S. Zinc finger proteins: Getting a grip on RNA. Curr. Opin. Struct. Biol. 2005, 15, 94–98. [Google Scholar] [CrossRef]
- Xu, J.; Peng, W.; Sun, Y.; Wang, X.; Xu, Y.; Li, X.; Gao, G.; Rao, Z. Structural study of MCPIP1 N-terminal conserved domain reveals a PIN-like RNase. Nucleic Acids Res. 2012, 40, 6957–6965. [Google Scholar] [CrossRef] [PubMed]
- Jura, J.; Skalniak, L.; Koj, A. Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim. Biophys. Acta 2012, 1823, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Kasza, A.; Wyrzykowska, P.; Horwacik, I.; Tymoszuk, P.; Mizgalska, D.; Palmer, K.; Rokita, H.; Sharrocks, A.D.; Jura, J. Transcription factors Elk-1 and SRF are engaged in IL1-dependent regulation of ZC3H12A expression. BMC Mol. Biol. 2010, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Saad, Y.; Lei, T.; Wang, J.; Qi, D.; Yang, Q.; Kolattukudy, P.E.; Fu, M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J. Exp. Med. 2010, 207, 2959–2973. [Google Scholar] [CrossRef]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A genomic and functional inventory of deubiquitinating enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef]
- Reyes-Turcu, F.E.; Ventii, K.H.; Wilkinson, K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009, 78, 363–397. [Google Scholar] [CrossRef]
- Wang, W.; Huang, X.; Xin, H.-B.; Fu, M.; Xue, A.; Wu, Z.-H. TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase. J. Biol. Chem. 2015, 290, 13372–13385. [Google Scholar] [CrossRef]
- Liang, J.; Song, W.; Tromp, G.; Kolattukudy, P.E.; Fu, M. Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS ONE 2008, 3, e2880. [Google Scholar] [CrossRef]
- Musson, R.; Szukała, W.; Jura, J. MCPIP1 RNase and Its Multifaceted Role. Int. J. Mol. Sci. 2020, 21, 7183. [Google Scholar] [CrossRef]
- Mino, T.; Takeuchi, O. Post-transcriptional regulation of cytokine mRNA controls the initiation and resolution of inflammation. Biotechnol. Genet. Eng. Rev. 2013, 29, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Huang, S.; Miao, R.; She, Z.-G.; Quinn, T.; Chang, Y.; Liu, J.; Fan, D.; Chen, Y.E.; Fu, M. Monocyte chemotactic protein-induced protein 1 (MCPIP1) suppresses stress granule formation and determines apoptosis under stress. J. Biol. Chem. 2011, 286, 41692–41700. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, E.; Lorenz, G.; Ribeiro, A.; Würf, V.; Wadowska, M.; Kotlinowski, J.; Schmaderer, C.; Potempa, J.; Fu, M.; Koziel, J.; et al. Murine myeloid cell MCPIP1 suppresses autoimmunity by regulating B-cell expansion and differentiation. Dis. Models Mech. 2021, 14, dmm047589. [Google Scholar] [CrossRef]
- Mino, T.; Iwai, N.; Endo, M.; Inoue, K.; Akaki, K.; Hia, F.; Uehata, T.; Emura, T.; Hidaka, K.; Suzuki, Y.; et al. Translation-dependent unwinding of stem-loops by UPF1 licenses Regnase-1 to degrade inflammatory mRNAs. Nucleic Acids Res. 2019, 47, 8838–8859. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, T.; Jiang, L.; Gao, J.; Yu, D.; Ge, Y.; Lin, S. MCP-induced protein 1 attenuates sepsis-induced acute lung injury by modulating macrophage polarization via the JNK/c-Myc pathway. Int. Immunopharmacol. 2019, 75, 105741. [Google Scholar] [CrossRef]
- Dhamija, S.; Winzen, R.; Doerrie, A.; Behrens, G.; Kuehne, N.; Schauerte, C.; Neumann, E.; Dittrich-Breiholz, O.; Kracht, M.; Holtmann, H. Interleukin-17 (IL-17) and IL-1 activate translation of overlapping sets of mRNAs, including that of the negative regulator of inflammation, MCPIP1. J. Biol. Chem. 2013, 288, 19250–19259. [Google Scholar] [CrossRef]
- Garg, A.V.; Amatya, N.; Chen, K.; Cruz, J.A.; Grover, P.; Whibley, N.; Conti, H.R.; Hernandez Mir, G.; Sirakova, T.; Childs, E.C.; et al. MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity 2015, 43, 475–487. [Google Scholar] [CrossRef]
- Chen, X.-W.; Zhou, S.-F. Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug Des. Dev. Ther. 2015, 9, 2941–2946. [Google Scholar] [CrossRef]
- Dobosz, E.; Wadowska, M.; Kaminska, M.; Wilamowski, M.; Honarpisheh, M.; Bryzek, D.; Potempa, J.; Jura, J.; Lech, M.; Koziel, J. MCPIP-1 Restricts Inflammation via Promoting Apoptosis of Neutrophils. Front. Immunol. 2021, 12, 627922. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Xu, C.; Lu, H.; Zhang, C.; Pang, Z.; Liu, Z. Monocyte Chemotactic Protein 1-Induced Protein 1 Is Highly Expressed in Inflammatory Bowel Disease and Negatively Regulates Neutrophil Activities. Mediat. Inflamm. 2020, 2020, 8812020. [Google Scholar] [CrossRef]
- Lin, J.; Lu, Z.; Li, G.; Zhang, C.; Lu, H.; Gao, S.; Zhu, R.; Huang, H.; Aden, K.; Wang, J.; et al. MCPIP-1-Mediated Immunosuppression of Neutrophils Exacerbates Acute Bacterial Peritonitis and Liver Injury. J. Innate Immun. 2023, 15, 262–282. [Google Scholar] [CrossRef]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Sun, X.; Nagahama, Y.; Singh, S.K.; Kozakai, Y.; Nabeshima, H.; Fukushima, K.; Tanaka, H.; Motooka, D.; Fukui, E.; Vivier, E.; et al. Deletion of the mRNA endonuclease Regnase-1 promotes NK cell anti-tumor activity via OCT2-dependent transcription of Ifng. Immunity 2024, 57, 1360–1377.e13. [Google Scholar] [CrossRef]
- Nakatsuka, Y.; Yaku, A.; Handa, T.; Vandenbon, A.; Hikichi, Y.; Motomura, Y.; Sato, A.; Yoshinaga, M.; Tanizawa, K.; Watanabe, K.; et al. Profibrotic function of pulmonary group 2 innate lymphoid cells is controlled by regnase-1. Eur. Respir. J. 2021, 57, 2000018. [Google Scholar] [CrossRef] [PubMed]
- Szeto, A.C.H.; Clark, P.A.; Ferreira, A.C.F.; Heycock, M.; Griffiths, E.L.; Jou, E.; Mannion, J.; Luan, S.L.; Storrar, S.; Knolle, M.D.; et al. Mef2d potentiates type-2 immune responses and allergic lung inflammation. Science 2024, 384, eadl0370. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Virgen-Slane, R.; Ramezani-Rad, P.; Leung, C.R.; Chen, C.; Balsells, D.; Shukla, A.; Kao, E.; Apgar, J.R.; Fu, M.; et al. Regnase-1 is essential for B cell homeostasis to prevent immunopathology. J. Exp. Med. 2021, 218, e20200971. [Google Scholar] [CrossRef] [PubMed]
- Uehata, T.; Iwasaki, H.; Vandenbon, A.; Matsushita, K.; Hernandez-Cuellar, E.; Kuniyoshi, K.; Satoh, T.; Mino, T.; Suzuki, Y.; Standley, D.M.; et al. Malt1-induced cleavage of regnase-1 in CD4+ helper T cells regulates immune activation. Cell 2013, 153, 1036–1049. [Google Scholar] [CrossRef]
- Behrens, G.; Edelmann, S.L.; Raj, T.; Kronbeck, N.; Monecke, T.; Davydova, E.; Wong, E.H.; Kifinger, L.; Giesert, F.; Kirmaier, M.E.; et al. Disrupting Roquin-1 interaction with Regnase-1 induces autoimmunity and enhances antitumor responses. Nat. Immunol. 2021, 22, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, K.M.; Hu, D.; Brenner, S.; Zöller, J.; Heinz, G.A.; Nagel, D.; Vogel, K.U.; Rehage, N.; Warth, S.C.; Edelmann, S.L.; et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat. Immunol. 2014, 15, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Dou, Y.; Xiao, X.; Wang, Y.; Ming, Y.; Li, X.C. Transgenic Expression of a Mutant Ribonuclease Regnase-1 in T Cells Disturbs T Cell Development and Functions. Front. Immunol. 2021, 12, 682220. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Liu, Y.; Qu, J.; Cao, W.; Zhang, E.; He, J.; Cai, Z. How are MCPIP1 and cytokines mutually regulated in cancer-related immunity? Protein Cell 2020, 11, 881–893. [Google Scholar] [CrossRef]
- Ligeza, J.; Marona, P.; Gach, N.; Lipert, B.; Miekus, K.; Wilk, W.; Jaszczynski, J.; Stelmach, A.; Loboda, A.; Dulak, J.; et al. MCPIP1 contributes to clear cell renal cell carcinomas development. Angiogenesis 2017, 20, 325–340. [Google Scholar] [CrossRef]
- Liang, Z.; Brooks, J.; Willard, M.; Liang, K.; Yoon, Y.; Kang, S.; Shim, H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007, 359, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Marona, P.; Górka, J.; Mazurek, Z.; Wilk, W.; Rys, J.; Majka, M.; Jura, J.; Miekus, K. MCPIP1 Downregulation in Clear Cell Renal Cell Carcinoma Promotes Vascularization and Metastatic Progression. Cancer Res. 2017, 77, 4905–4920. [Google Scholar] [CrossRef]
- Desar, I.M.E.; Mulder, S.F.; Stillebroer, A.B.; van Spronsen, D.-J.; van der Graaf, W.T.A.; Mulders, P.F.A.; van Herpen, C.M.L. The reverse side of the victory: Flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol. 2009, 48, 927–931. [Google Scholar] [CrossRef]
- Wolter, P.; Beuselinck, B.; Pans, S.; Schöffski, P. Flare-up: An often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol. 2009, 48, 621–624. [Google Scholar] [CrossRef]
- Marona, P.; Górka, J.; Kwapisz, O.; Jura, J.; Rys, J.; Hoffman, R.M.; Miekus, K. Resistance to tyrosine kinase inhibitors promotes renal cancer progression through MCPIP1 tumor-suppressor downregulation and c-Met activation. Cell Death Dis. 2022, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Marona, P.; Myrczek, R.; Piasecka, I.; Gorka, J.; Kwapisz, O.; Pospiech, E.; Rys, J.; Jura, J.; Miekus, K. The endonuclease activity of MCPIP1 controls the neoplastic transformation of epithelial cells via the c-Met/CD44 axis. Cell Commun. Signal. 2025, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Gorka, J.; Marona, P.; Kwapisz, O.; Waligórska, A.; Pospiech, E.; Dobrucki, J.W.; Rys, J.; Jura, J.; Miekus, K. MCPIP1 inhibits Wnt/β-catenin signaling pathway activity and modulates epithelial-mesenchymal transition during clear cell renal cell carcinoma progression by targeting miRNAs. Oncogene 2021, 40, 6720–6735. [Google Scholar] [CrossRef]
- Gorka, J.; Marona, P.; Kwapisz, O.; Rys, J.; Jura, J.; Miekus, K. The anti-inflammatory protein MCPIP1 inhibits the development of ccRCC by maintaining high levels of tumour suppressors. Eur. J. Pharmacol. 2020, 888, 173591. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; He, L.; Guo, Y.; Wang, J.; Liu, H.; Li, Z. MCPIP1 Elicits a Therapeutic Effect on Cervical Cancer by Facilitating XIAP mRNA Decay via Its Endoribonuclease Activity. Int. J. Mol. Sci. 2024, 25, 10285. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, F.; Liu, K.; Ding, Z. ZC3H12A inhibits tumor growth and metastasis of breast cancer under hypoxic condition via the inactivation of IL-17 signaling pathway. Cell Cycle 2024, 23, 188–204. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Q.; Yu, X.; Yang, N.; Wang, Y.; Zeng, Y.; Zheng, Z.; Zhou, F.; Zhou, Y. MCPIP1-mediated NFIC alternative splicing inhibits proliferation of triple-negative breast cancer via cyclin D1-Rb-E2F1 axis. Cell Death Dis. 2021, 12, 370. [Google Scholar] [CrossRef]
- Wang, R.; Sun, S.; Wang, Z.; Xu, X.; Jiang, T.; Liu, H.; Li, X.; Ren, Z. MCPIP1 promotes cell proliferation, migration and angiogenesis of glioma via VEGFA-mediated ERK pathway. Exp. Cell Res. 2022, 418, 113267. [Google Scholar] [CrossRef]
- Oh, Y.-T.; Qian, G.; Deng, J.; Sun, S.-Y. Monocyte chemotactic protein-induced protein-1 enhances DR5 degradation and negatively regulates DR5 activation-induced apoptosis through its deubiquitinase function. Oncogene 2018, 37, 3415–3425. [Google Scholar] [CrossRef]
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s path in the immune system. Immunology 2009, 127, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Cui, Y.; Rong, J.; Huang, W.; Zheng, Z.; Li, A.; Li, Y. MCPIP1 Suppresses the NF-κB Signaling Pathway Through Negative Regulation of K63-Linked Ubiquitylation of TRAF6 in Colorectal Cancer. Cancer Gene Ther. 2023, 30, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, X.; Wang, D.; Lu, L.; Lin, H.; Zhang, Z.; Jia, Y.; Nie, X.; Liu, T.; Fu, W. CD40×HER2 bispecific antibody overcomes the CCL2-induced trastuzumab resistance in HER2-positive gastric cancer. J. Immunother. Cancer 2022, 10, e005063. [Google Scholar] [CrossRef] [PubMed]
- Okabe, J.; Kodama, T.; Sato, Y.; Shigeno, S.; Matsumae, T.; Daiku, K.; Sato, K.; Yoshioka, T.; Shigekawa, M.; Higashiguchi, M.; et al. Regnase-1 downregulation promotes pancreatic cancer through myeloid-derived suppressor cell-mediated evasion of anticancer immunity. J. Exp. Clin. Cancer Res. 2023, 42, 262. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Zhao, H.; Liu, G.; Mao, H.; Liu, Y. Elevated linc00936 or silenced microRNA-425-3p inhibits immune escape of gastric cancer cells via elevation of ZC3H12A. Int. Immunopharmacol. 2021, 95, 107559. [Google Scholar] [CrossRef]
- Iguchi, E.; Takai, A.; Oe, N.; Fujii, Y.; Omatsu, M.; Takeda, H.; Shimizu, T.; Maruno, T.; Nakanishi, Y.; Yoshinaga, M.; et al. Epithelial Regnase-1 inhibits colorectal tumor growth by regulating IL-17 signaling via degradation of NFKBIZ mRNA. Proc. Natl. Acad. Sci. USA 2025, 122, e2500820122. [Google Scholar] [CrossRef]
- Zhou, W.; Li, S.; Zhang, X.; Li, C.; Zhang, J. miR-143-3p shuttled by M2 macrophage-derived extracellular vesicles induces progression of colorectal cancer through a ZC3H12A/C/EBPβ axis-dependent mechanism. Int. Immunopharmacol. 2023, 119, 110137. [Google Scholar] [CrossRef]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef]
- Wei, J.; Long, L.; Zheng, W.; Dhungana, Y.; Lim, S.A.; Guy, C.; Wang, Y.; Wang, Y.-D.; Qian, C.; Xu, B.; et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 2019, 576, 471–476. [Google Scholar] [CrossRef]
- Zheng, W.; Wei, J.; Zebley, C.C.; Jones, L.L.; Dhungana, Y.; Wang, Y.D.; Mavuluri, J.; Long, L.; Fan, Y.; Youngblood, B.; et al. Regnase-1 suppresses TCF-1+ precursor exhausted T-cell formation to limit CAR-T-cell responses against ALL. Blood 2021, 138, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, G.; Zhou, Q.; Liu, Y.; Zhao, X.; Li, Z.; Yin, N.; Peng, M. Induction of immortal-like and functional CAR T cells by defined factors. J. Exp. Med. 2024, 221, e20232368. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, Z.; Zhao, X.; Wang, L.; Jin, G.; Li, Z.; Yin, N.; Li, Y.; Peng, M. BCOR and ZC3H12A suppress a core stemness program in exhausted CD8+ T cells. J. Exp. Med. 2025, 222, e20241133. [Google Scholar] [CrossRef]
- Zhang, G.; Yin, N.; Peng, M. GD2T(IF) cells as a platform for single-dose and long-term delivery of biologics. Nat. Commun. 2025, 16, 8088. [Google Scholar] [CrossRef]
- Haapaniemi, E.; Botla, S.; Persson, J.; Schmierer, B.; Taipale, J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018, 24, 927–930. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Tsuchida, C.A.; Brandes, N.; Bueno, R.; Trinidad, M.; Mazumder, T.; Yu, B.; Hwang, B.; Chang, C.; Liu, J.; Sun, Y.; et al. Mitigation of chromosome loss in clinical CRISPR-Cas9-engineered T cells. Cell 2023, 186, 4567–4582.e20. [Google Scholar] [CrossRef]
- Mikkelsen, N.S.; Ravendran, S.; Broksø, A.D.; Skjelbostad, S.F.; Pedersen, M.G.; Fang, H.; Terkelsen, T.; Thomsen, M.K.; Bak, R.O. Orthogonal CRISPR systems for targeted integration and multiplex base editing enable nonviral engineering of allogeneic CAR-T cells. Mol. Ther. 2025. [Google Scholar] [CrossRef]
- Pal, S.K.; Tran, B.; Haanen, J.; Hurwitz, M.E.; Sacher, A.; Tannir, N.M.; Budde, L.E.; Harrison, S.J.; Klobuch, S.; Patel, S.S.; et al. CD70-Targeted Allogeneic CAR T-Cell Therapy for Advanced Clear Cell Renal Cell Carcinoma. Cancer Discov. 2024, 14, 1176–1189. [Google Scholar] [CrossRef]
- Mai, D.; Johnson, O.; Reff, J.; Fan, T.-J.; Scholler, J.; Sheppard, N.C.; June, C.H. Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2218632120. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Liu, Y.; Wang, L.; He, Z.; Zhao, X.; Ma, Y.; Jia, Y.; Li, Z.; Yin, N.; Peng, M. A single infusion of engineered long-lived and multifunctional T cells confers durable remission of asthma in mice. Nat. Immunol. 2024, 25, 1059–1072. [Google Scholar] [CrossRef]
- Knudsen, N.H.; Escobar, G.; Korell, F.; Kienka, T.; Nobrega, C.; Anderson, S.; Cheng, A.Y.; Zschummel, M.; Armstrong, A.; Bouffard, A.; et al. In vivo CRISPR screens identify modifiers of CAR T cell function in myeloma. Nature 2025. [Google Scholar] [CrossRef]
- Mai, D.; Harro, C.; Sanyal, A.; Rommel, P.C.; Sheppard, N.C.; June, C.H. Stem Loop Mediated Transgene Modulation in Human T Cells. ACS Synth. Biol. 2024, 13, 3897–3907. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596. [Google Scholar] [CrossRef]
- Losko, M.; Dolicka, D.; Pydyn, N.; Jankowska, U.; Kedracka-Krok, S.; Kulecka, M.; Paziewska, A.; Mikula, M.; Major, P.; Winiarski, M.; et al. Integrative genomics reveal a role for MCPIP1 in adipogenesis and adipocyte metabolism. Cell. Mol. Life Sci. 2020, 77, 4899–4919. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, C.; Li, P.; Shao, Y.; Wang, M.; Wang, F.; Niu, G.; Sun, K.; Zhang, Q.; Gou, Z.; et al. FGF21 protects against doxorubicin-induced cardiotoxicity by inhibiting connexin 43 ubiquitination. Free Radic. Biol. Med. 2023, 208, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Tyka, K.; Jörns, A.; Dunst, A.; Tang, Y.; Bryde, T.H.; Mehmeti, I.; Walentinsson, A.; Marselli, L.; Cnop, M.; Tyrberg, B.; et al. MCPIP1 is a novel link between diabetogenic conditions and impaired insulin secretory capacity. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166199. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xu, Y.; Tan, Y.; Zhang, J.; Yu, J.; Zhao, J.; Da, Q.; Yu, F.; Zha, Y. Central administration of Dapagliflozin alleviates a hypothalamic neuroinflammatory signature and changing tubular lipid metabolism in type 2 diabetic nephropathy by upregulating MCPIP1. Biomed. Pharmacother. 2023, 168, 115840. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Kotlinowski, J.; Blacharczyk, O.; Giergiel, M.; Szymanowski, K.; Metwally, S.; Wojnar-Lason, K.; Dobosz, E.; Koziel, J.; Lekka, M.; et al. Early and late phases of liver sinusoidal endothelial cell (LSEC) defenestration in mouse model of systemic inflammation. Cell. Mol. Biol. Lett. 2024, 29, 139. [Google Scholar] [CrossRef]
- Żurawek, D.; Pydyn, N.; Major, P.; Szade, K.; Trzos, K.; Kuś, E.; Pośpiech, E.; Małczak, P.; Radkowiak, D.; Budzyński, A.; et al. Diosmetin alleviates TNFα-induced liver inflammation by improving liver sinusoidal endothelial cell dysfunction. Biomed. Pharmacother. 2025, 183, 117843. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Arase, M.; Matsuyama, H.; Choi, Y.L.; Ueno, T.; Mano, H.; Sugimoto, K.; Miyazono, K. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol. Cell 2011, 44, 424–436. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Wu, M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct. Target Ther. 2018, 3, 14. [Google Scholar] [CrossRef]
- Jin, W.; Wang, J.; Liu, C.-P.; Wang, H.-W.; Xu, R.-M. Structural Basis for pri-miRNA Recognition by Drosha. Mol. Cell 2020, 78, 423–433.e5. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Kim, H.; Kim, V.N. Sequence determinant of small RNA production by DICER. Nature 2023, 615, 323–330. [Google Scholar] [CrossRef]
- Li, Z.; Han, S.; Jia, Y.; Yang, Y.; Han, F.; Wu, G.; Li, X.; Zhang, W.; Jia, W.; He, X.; et al. MCPIP1 regulates RORα expression to protect against liver injury induced by lipopolysaccharide via modulation of miR-155. J. Cell. Physiol. 2019, 234, 16562–16572. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Cao, J.; Zhang, F.; Cui, H.; Teng, J.; Li, J.; Liu, Z.; Morehouse, C.; Jallal, B.; Tang, Y.; et al. Type I Interferon Inhibition of MicroRNA-146a Maturation Through Up-Regulation of Monocyte Chemotactic Protein-Induced Protein 1 in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liao, Z.; Li, Z.; Li, H.; Wu, Z.; Chen, C.; Wang, H. Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury. Exp. Neurol. 2023, 362, 114295. [Google Scholar] [CrossRef] [PubMed]
- Omiya, S.; Omori, Y.; Taneike, M.; Murakawa, T.; Ito, J.; Tanada, Y.; Nishida, K.; Yamaguchi, O.; Satoh, T.; Shah, A.M.; et al. Cytokine mRNA Degradation in Cardiomyocytes Restrains Sterile Inflammation in Pressure-Overloaded Hearts. Circulation 2020, 141, 667–677. [Google Scholar] [CrossRef]
- Kotlinowski, J.; Hutsch, T.; Czyzynska-Cichon, I.; Wadowska, M.; Pydyn, N.; Jasztal, A.; Kij, A.; Dobosz, E.; Lech, M.; Miekus, K.; et al. Deletion of Mcpip1 in Mcpip1(fl/fl)Alb(Cre) mice recapitulates the phenotype of human primary biliary cholangitis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166086. [Google Scholar] [CrossRef]
- Ribeiro, A.; Dobosz, E.; Krill, M.; Köhler, P.; Wadowska, M.; Steiger, S.; Schmaderer, C.; Koziel, J.; Lech, M. Macrophage-Specific MCPIP1/Regnase-1 Attenuates Kidney Ischemia-Reperfusion Injury by Shaping the Local Inflammatory Response and Tissue Regeneration. Cells 2022, 11, 397. [Google Scholar] [CrossRef]
- Yaku, A.; Inagaki, T.; Asano, R.; Okazawa, M.; Mori, H.; Sato, A.; Hia, F.; Masaki, T.; Manabe, Y.; Ishibashi, T.; et al. Regnase-1 Prevents Pulmonary Arterial Hypertension Through mRNA Degradation of Interleukin-6 and Platelet-Derived Growth Factor in Alveolar Macrophages. Circulation 2022, 146, 1006–1022. [Google Scholar] [CrossRef]
- Yang, G.; Lee, H.E.; Trzeciak, M.; Pawelczyk, T.; Takeuchi, O.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Regnase-1 plays an essential role in maintaining skin immune homeostasis via regulation of chemokine expression. Biomed. Pharmacother. 2023, 162, 114558. [Google Scholar] [CrossRef] [PubMed]
- Pydyn, N.; Ferenc, A.; Trzos, K.; Pospiech, E.; Wilamowski, M.; Mucha, O.; Major, P.; Kadluczka, J.; Rodrigues, P.M.; Banales, J.M.; et al. MCPIP1 Inhibits Hepatic Stellate Cell Activation in Autocrine and Paracrine Manners, Preventing Liver Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, C.; Wu, W.; Chen, H.; Lin, R.; Sun, R.; Gao, X.; Li, G.; He, Q.; Gao, H.; et al. MCPIP1 restrains mucosal inflammation by orchestrating the intestinal monocyte to macrophage maturation via an ATF3-AP1S2 axis. Gut 2023, 72, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Takeuchi, O.; Teraguchi, S.; Matsushita, K.; Uehata, T.; Kuniyoshi, K.; Satoh, T.; Saitoh, T.; Matsushita, M.; Standley, D.M.; et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat. Immunol. 2011, 12, 1167–1175. [Google Scholar] [CrossRef]
- Tse, K.M.; Vandenbon, A.; Cui, X.; Mino, T.; Uehata, T.; Yasuda, K.; Sato, A.; Tsujimura, T.; Hia, F.; Yoshinaga, M.; et al. Enhancement of Regnase-1 expression with stem loop-targeting antisense oligonucleotides alleviates inflammatory diseases. Sci. Transl. Med. 2022, 14, eabo2137. [Google Scholar] [CrossRef]
- Trevejo-Nuñez, G.; Lin, B.; Fan, L.; Aggor, F.E.Y.; Biswas, P.S.; Chen, K.; Gaffen, S.L. Regnase-1 Deficiency Restrains Klebsiella pneumoniae Infection by Regulation of a Type I Interferon Response. MBio 2021, 13, e0379221. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, C.; Miao, R.; Zhou, J.; Lee, A.; Liu, B.; Lester, S.N.; Fu, W.; Zhu, L.; Zhang, L.; et al. MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, 19083–19088. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Lv, F.; Wang, X.; Ji, X.; Xu, Y.; Sun, J.; Wu, L.; Zheng, Y.-T.; Gao, G. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl. Acad. Sci. USA 2011, 108, 15834–15839. [Google Scholar] [CrossRef]
- Li, M.; Yan, K.; Wei, L.; Yang, Y.; Qian, Q.; Xu, W. MCPIP1 inhibits coxsackievirus B3 replication by targeting viral RNA and negatively regulates virus-induced inflammation. Med. Microbiol. Immunol. 2018, 207, 27–38. [Google Scholar] [CrossRef]
- Kook, I.; Ziegelbauer, J.M. Monocyte chemoattractant protein-induced protein 1 directly degrades viral miRNAs with a specific motif and inhibits KSHV infection. Nucleic Acids Res. 2021, 49, 4456–4471. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Chu, J.-S.; Chien, H.-L.; Tseng, C.-H.; Ko, P.-C.; Mei, Y.-Y.; Tang, W.-C.; Kao, Y.-T.; Cheng, H.-Y.; Liang, Y.-C.; et al. MCPIP1 suppresses hepatitis C virus replication and negatively regulates virus-induced proinflammatory cytokine responses. J. Immunol. 2014, 193, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, J.; Zhao, Y.; Song, Y.; Yin, S.; Guo, J.; Zhang, H.; Wang, K.; Wei, L.; Li, S.; et al. MCPIP1 inhibits Hepatitis B virus replication by destabilizing viral RNA and negatively regulates the virus-induced innate inflammatory responses. Antiviral Res. 2020, 174, 104705. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Gao, Q.; Jin, R.; Gu, M.; Wang, Z.; Li, X.; Li, W.; Wang, J.; Ma, T. The Ribonuclease ZC3H12A is required for self-inflicted DNA breaks after DNA damage in small cell lung cancer cells. Cell. Oncol. 2024, 47, 1497–1502. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Guo, J.; Wang, C.; Wan, B.; Ma, T. ZC3H12A: A Critical Mediator of Inflammation, Tumor Immunotherapy, and Metabolic–Immune Crosstalk—Implications for Disease Treatment. Biomolecules 2025, 15, 1473. https://doi.org/10.3390/biom15101473
Lu M, Guo J, Wang C, Wan B, Ma T. ZC3H12A: A Critical Mediator of Inflammation, Tumor Immunotherapy, and Metabolic–Immune Crosstalk—Implications for Disease Treatment. Biomolecules. 2025; 15(10):1473. https://doi.org/10.3390/biom15101473
Chicago/Turabian StyleLu, Mingjun, Jingwei Guo, Chenyang Wang, Bingbing Wan, and Teng Ma. 2025. "ZC3H12A: A Critical Mediator of Inflammation, Tumor Immunotherapy, and Metabolic–Immune Crosstalk—Implications for Disease Treatment" Biomolecules 15, no. 10: 1473. https://doi.org/10.3390/biom15101473
APA StyleLu, M., Guo, J., Wang, C., Wan, B., & Ma, T. (2025). ZC3H12A: A Critical Mediator of Inflammation, Tumor Immunotherapy, and Metabolic–Immune Crosstalk—Implications for Disease Treatment. Biomolecules, 15(10), 1473. https://doi.org/10.3390/biom15101473




