High PEEP Activates ITGB1, Inducing Diaphragm Fibrosis During Prolonged Mechanical Ventilation
Abstract
1. Introduction
2. Materials and Methods
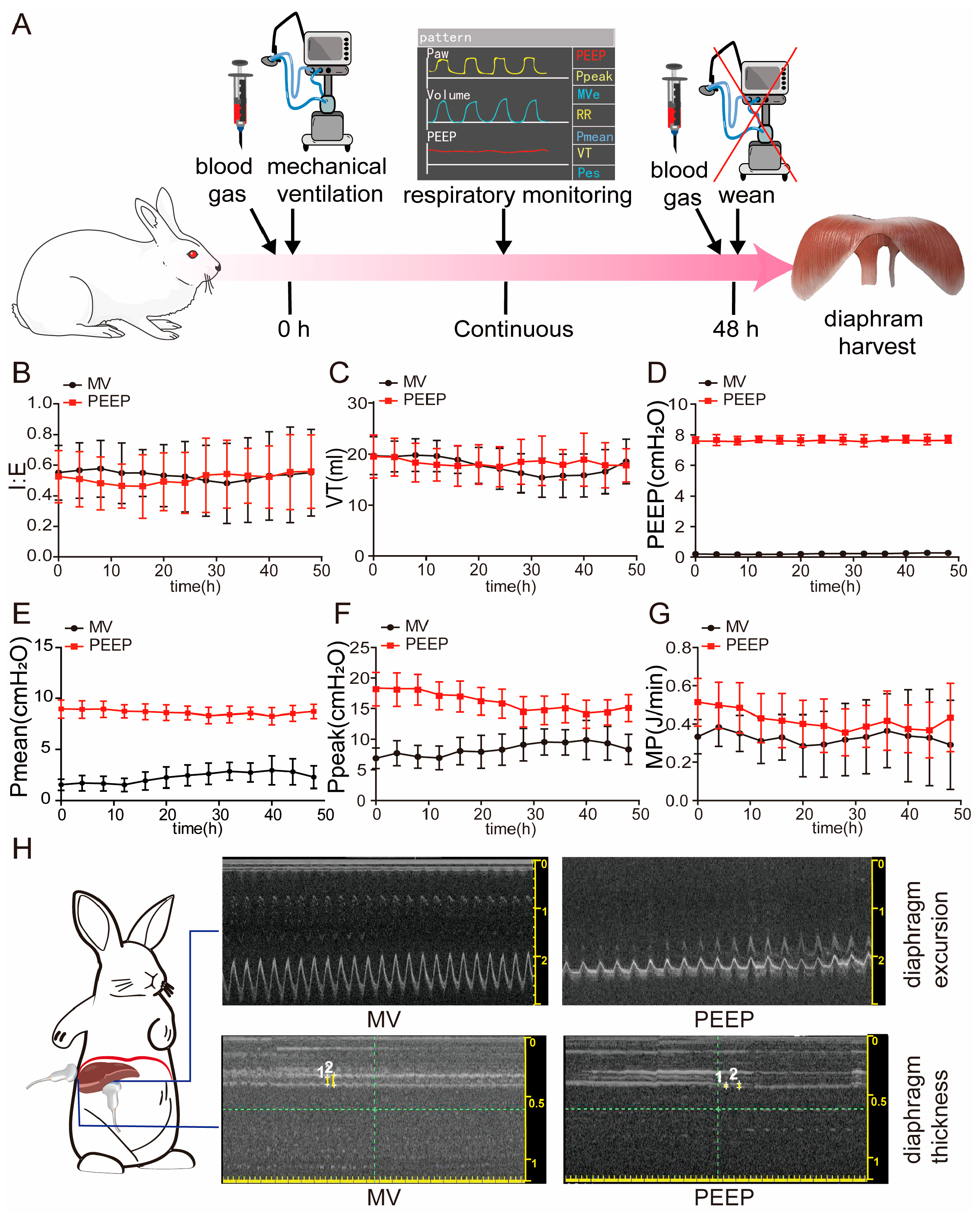
2.1. Mechanically Ventilated Animal Mode
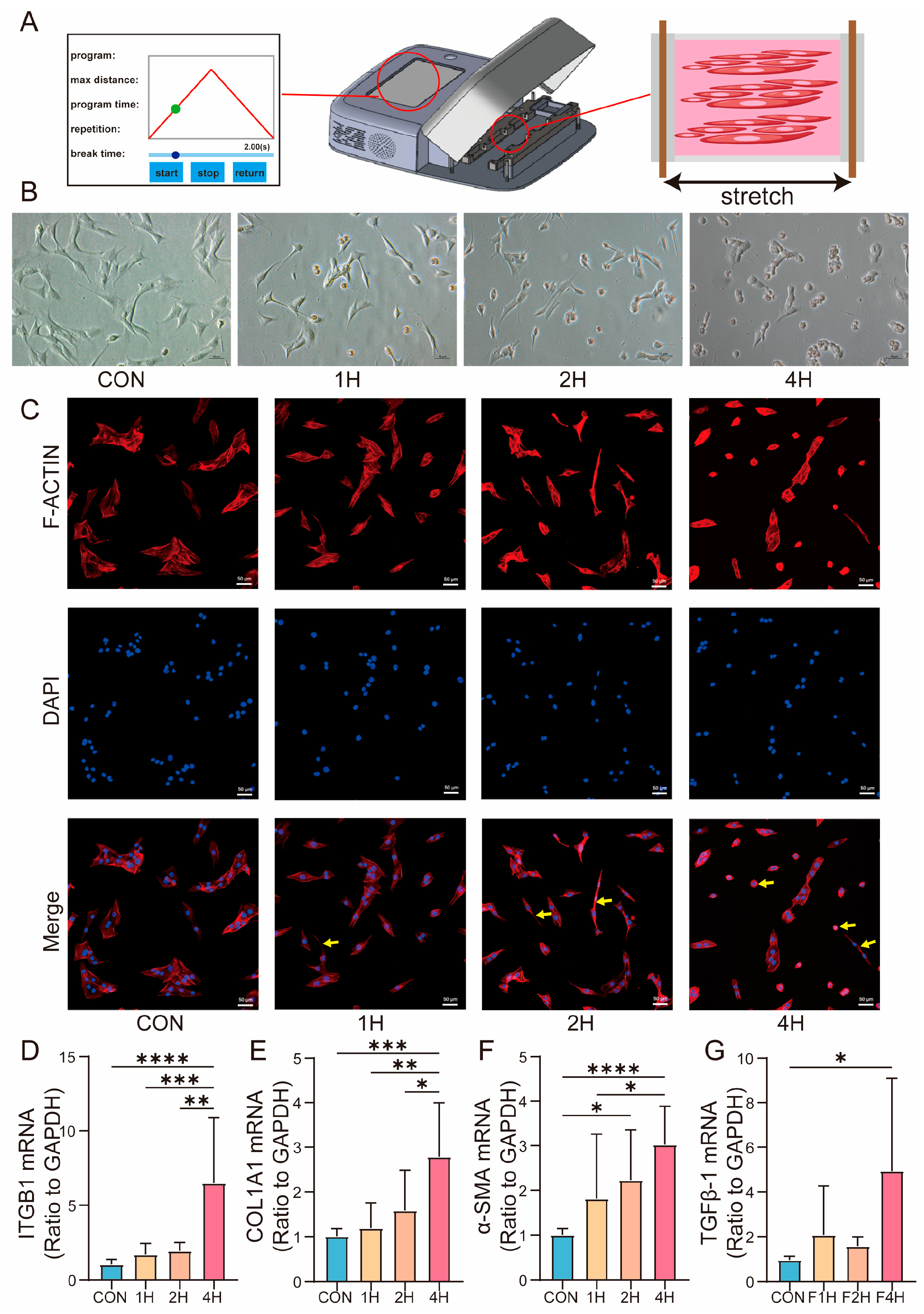
2.2. Cell Culture and Mechanical Stimulation
2.3. Knockdown of ITGB1 Expression by siRNA
2.4. Diaphragm Ultrasonic
2.5. Ultrastructural Observations
2.6. Immunohistochemistry Staining
2.7. Confocal Microscopic Imaging of F-Actin Staining
2.8. Sirius Red Staining
2.9. RNA-Seq Analysis
2.10. Quantitative RT-qPCR
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. High PEEP During Mechanical Ventilation Leads to Changes in Respiratory Mechanics and Diaphragmatic Dysfunction in Rabbits
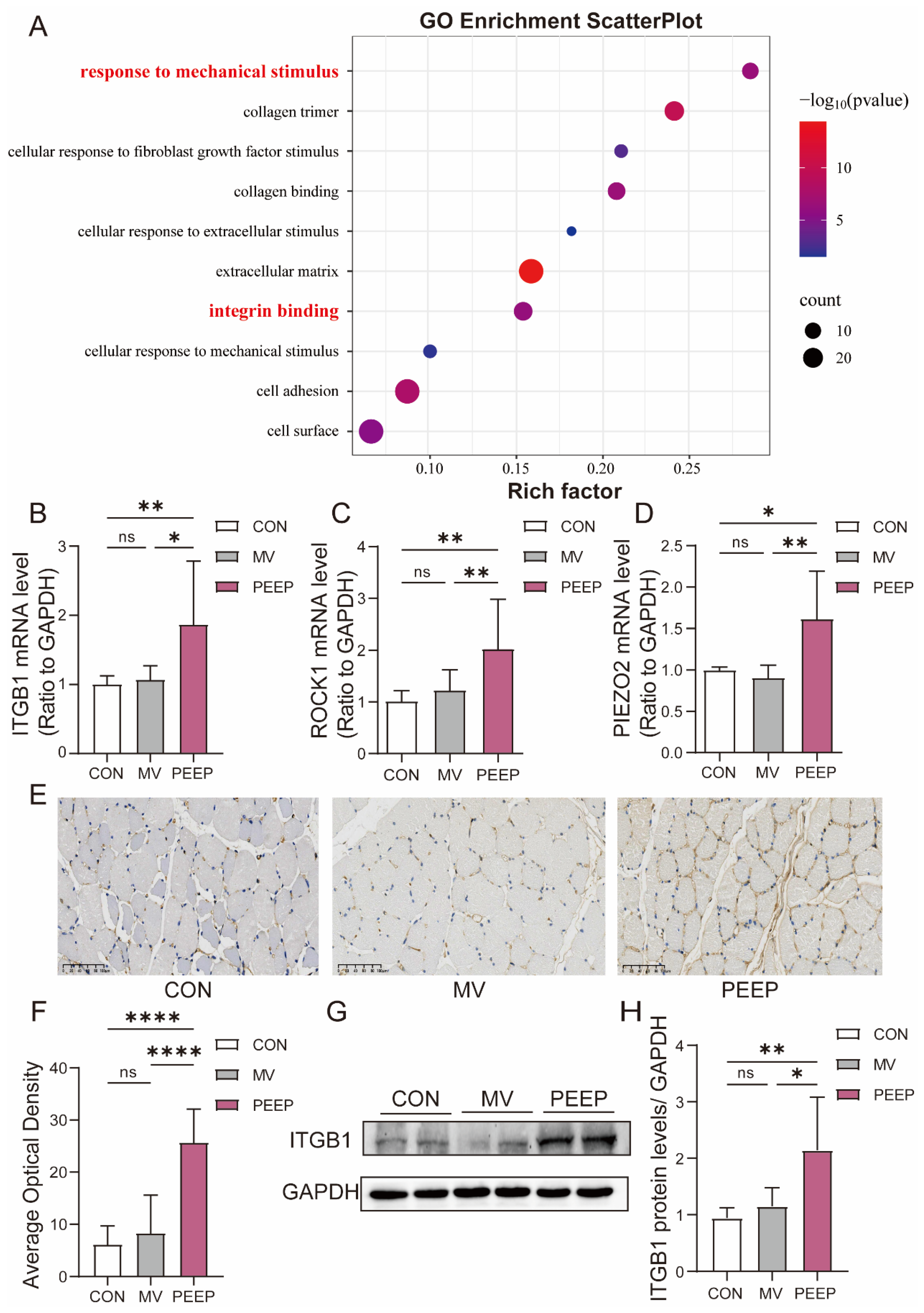
3.2. Diaphragmatic Fibrosis Was Found in Rabbits After Mechanical Ventilation with PEEP for 48 h
3.3. Diaphragm Fibrosis in Mechanical Ventilation with PEEP Application Is Associated with Mechanical Force Stimulation and Activated ITGB1
3.4. ITGB1 Expression and Cellular Morphological Changes Under Mechanical Stimulation
3.5. ITGB1-Dependent Modulation of TGFβ-1 Signaling Alters Fibrosis Expression Under Long-Term Mechanical Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITGB1 | Integrin beta-1 |
| MV | Mechanical ventilation |
| PEEP | Positive end-expiratory pressure |
| VIDD | Ventilation-induced diaphragm dysfunction |
| TGFβ-1 | Transforming growth factor-β1 |
| ROCK1 | Rho-associated, coiled-coil containing protein kinase 1 |
| α-SMA | α-smooth muscle actin |
| EELV | End-expiratory lung volume |
| LAP | Latency-Associated Peptide |
| PETCO2 | End tidal CO2 pressure |
| SBT | Spontaneous breathing trial |
| RR | Respiratory rate |
| VT | Tidal volume |
| VE | Total minute ventilation |
| FBS | Fetal bovine serum |
| TEM | Transmission electron microscope |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| RT-qPCR | Real-time reverse transcriptase polymerase chain reaction |
| ECL | Enhanced chemiluminescence |
| ANOVA | One-way analysis of variance |
| Pmean | Mean airway pressure |
| Ppeak | Peak inspiratory pressure |
| MP | Mechanical power |
| ECM | Extracellular matrix |
| VILI | Ventilator-induced lung injury |
| ARDS | Acute respiratory distress syndrome |
References
- Dres, M.; Goligher, E.C.; Heunks, L.M.A.; Brochard, L.J. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017, 43, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K. Ventilator-induced diaphragm dysfunction: Phenomenology and mechanism(s) of pathogenesis. J. Physiol. 2024, 602, 4729–4752. [Google Scholar] [CrossRef]
- Shi, Z.; van den Berg, M.; Bogaards, S.; Conijn, S.; Paul, M.; Beishuizen, A.; Heunks, L.; Ottenheijm, C.A.C. Replacement Fibrosis in the Diaphragm of Mechanically Ventilated Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2023, 207, 351–354. [Google Scholar] [CrossRef]
- Jansen, D.; Jonkman, A.H.; Vries, H.J.; Wennen, M.; Elshof, J.; Hoofs, M.A.; van den Berg, M.; Man, A.M.E.; Keijzer, C.; Scheffer, G.J.; et al. Positive end-expiratory pressure affects geometry and function of the human diaphragm. J. Appl. Physiol. 2021, 131, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Muresan, C.; Shang, X.; Huet-Calderwood, C.; Schwartz, M.A.; Calderwood, D.A.; Murrell, M. Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton. Nat. Commun. 2023, 14, 8011. [Google Scholar] [CrossRef]
- Lindqvist, J.; van den Berg, M.; van der Pijl, R.; Hooijman, P.E.; Beishuizen, A.; Elshof, J.; de Waard, M.; Girbes, A.; Spoelstra-de Man, A.; Shi, Z.H.; et al. Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers. Am. J. Respir. Crit. Care Med. 2018, 198, 472–485. [Google Scholar] [CrossRef]
- Formenti, P.; Miori, S.; Galimberti, A.; Umbrello, M. The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening. Diagnostics 2023, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Xanthis, I.; Souilhol, C.; Serbanovic-Canic, J.; Roddie, H.; Kalli, A.C.; Fragiadaki, M.; Wong, R.; Shah, D.R.; Askari, J.A.; Canham, L.; et al. β1 integrin is a sensor of blood flow direction. J. Cell Sci. 2019, 132, jcs229542. [Google Scholar] [CrossRef]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Panelli, A.; Grimm, A.M.; Krause, S.; Verfuß, M.A.; Ulm, B.; Grunow, J.J.; Bartels, H.G.; Carbon, N.M.; Niederhauser, T.; Weber-Carstens, S.; et al. Noninvasive Electromagnetic Phrenic Nerve Stimulation in Critically Ill Patients: A Feasibility Study. Chest 2024, 166, 502–510. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Trombetta-Lima, M. Mechanotransduction and Skeletal Muscle Atrophy: The Interplay Between Focal Adhesions and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 2802. [Google Scholar] [CrossRef]
- Shi, Z.; Bogaards, S.J.P.; Conijn, S.; Onderwater, Y.; Espinosa, P.; Bink, D.I.; van den Berg, M.; van de Locht, M.; Bugiani, M.; van der Hoeven, H.; et al. COVID-19 is associated with distinct myopathic features in the diaphragm of critically ill patients. BMJ Open Respir. Res. 2021, 8, e001052. [Google Scholar] [CrossRef]
- Qian, X.; Jiang, Y.; Jia, J.; Shen, W.; Ding, Y.; He, Y.; Xu, P.; Pan, Q.; Xu, Y.; Ge, H. PEEP application during mechanical ventilation contributes to fibrosis in the diaphragm. Respir. Res. 2023, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Ngo, U.; Shi, Y.; Woodruff, P.; Shokat, K.; DeGrado, W.; Jo, H.; Sheppard, D.; Sundaram, A.B. IL-13 and IL-17A activate β1 integrin through an NF-kB/Rho kinase/PIP5K1γ pathway to enhance force transmission in airway smooth muscle. Proc. Natl. Acad. Sci. USA 2024, 121, e2401251121. [Google Scholar] [CrossRef]
- Katoh, K. Integrin and Its Associated Proteins as a Mediator for Mechano-Signal Transduction. Biomolecules 2025, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Massuto, D.A.; Kneese, E.C.; Johnson, G.A.; Burghardt, R.C.; Hooper, R.N.; Ing, N.H.; Jaeger, L.A. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: Proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 2010, 139, 465–478. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Li, B.; Hong, S.; Min, J.; Hu, M.; Tang, J.; Wang, T.; Yang, L.; Hong, L. Electrical stimulation activates calpain 2 and subsequently upregulates collagens via the integrin β1/TGF-β1 signaling pathway. Cell. Signal. 2019, 59, 141–151. [Google Scholar] [CrossRef]
- Masuzaki, R.; Ray, K.C.; Roland, J.; Zent, R.; Lee, Y.A.; Karp, S.J. Integrin β1 Establishes Liver Microstructure and Modulates Transforming Growth Factor β during Liver Development and Regeneration. Am. J. Pathol. 2021, 191, 309–319. [Google Scholar] [CrossRef]
- Suraneni, P.K.; Corey, S.J.; Hession, M.J.; Ishaq, R.; Awomolo, A.; Hasan, S.; Shah, C.; Liu, H.; Wickrema, A.; Debili, N.; et al. Dynamins 2 and 3 control the migration of human megakaryocytes by regulating CXCR4 surface expression and ITGB1 activity. Blood Adv. 2018, 2, 3540–3552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, H.; Lit, L.C.; Grothey, A.; Athanasiadou, M.; Kiritsi, M.; Lombardo, Y.; Frampton, A.E.; Green, A.R.; Ellis, I.O.; et al. The kinase LMTK3 promotes invasion in breast cancer through GRB2-mediated induction of integrin β1. Sci. Signal. 2014, 7, ra58. [Google Scholar] [CrossRef]
- Han, T.; Jiang, Y.; Wang, X.; Deng, S.; Hu, Y.; Jin, Q.; Long, D.; Liu, K. 3D matrix promotes cell dedifferentiation into colorectal cancer stem cells via integrin/cytoskeleton/glycolysis signaling. Cancer Sci. 2022, 113, 3826–3837. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.H.; Kang, Y.J.; Hwangbo, H.; Yoon, A.; Kim, H.S.; Lee, D.; Lee, S.Y.; Choi, B.H.; Kim, J.J.; et al. CD82 attenuates TGF-β1-mediated epithelial-mesenchymal transition by blocking smad-dependent signaling in ARPE-19 cells. Front. Pharmacol. 2022, 13, 991056. [Google Scholar] [CrossRef]
- Tonelli, R.; Grasso, S.; Cortegiani, A.; Ball, L.; Castaniere, I.; Tabbì, L.; Fantini, R.; Andrisani, D.; Gozzi, F.; Moretti, A.; et al. Physiological effects of lung-protective ventilation in patients with lung fibrosis and usual interstitial pneumonia pattern versus primary ARDS: A matched-control study. Crit. Care 2023, 27, 398. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.; Gazdhar, A.; Lopez-Rodriguez, E.; Ruppert, C.; Mahavadi, P.; Günther, A.; Klepetko, W.; Bates, J.H.; Smith, B.; Geiser, T.; et al. Alveolar derecruitment and collapse induration as crucial mechanisms in lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Benítez, N.E.; Parotto, M.; Post, M.; Han, B.; Spieth, P.M.; Cheng, W.E.; Valladares, F.; Villar, J.; Liu, M.; Sato, M.; et al. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition. Crit. Care Med. 2012, 40, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.A.F.; Padilha, G.A.; Moraes, L.; Santos, C.L.; Maia, L.A.; Braga, C.L.; Duarte, M.; Andrade, L.B.; Schanaider, A.; Capellozzi, V.L.; et al. Effects of pressure support ventilation on ventilator-induced lung injury in mild acute respiratory distress syndrome depend on level of positive end-expiratory pressure: A randomised animal study. Eur. J. Anaesthesiol. 2018, 35, 298–306. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, M.; Lu, Z.; Zhong, Q.; Tan, M.; Wei, Q.; Zheng, L. Role of integrin β1 and tenascin C mediate TGF-SMAD2/3 signaling in chondrogenic differentiation of BMSCs induced by type I collagen hydrogel. Regen. Biomater. 2024, 11, rbae017. [Google Scholar] [CrossRef]
- Kong, M.; Wang, Z.; Hao, Y.; Shi, Y.; Yang, X.; Djurist, N.R.; Li, Y. Role of the integrin-β1/TGF-β1 signaling pathway in the pathogenesis of pelvic organ prolapse: A study on vaginal wall tissue alterations and molecular dysfunction. Mol. Med. Rep. 2025, 31, 104. [Google Scholar] [CrossRef] [PubMed]
- Bellani, S.; Molyneaux, P.L.; Maher, T.M.; Spagnolo, P. Potential of αvβ6 and αvβ1 integrin inhibition for treatment of idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets 2024, 28, 575–585. [Google Scholar] [CrossRef] [PubMed]
- García-Valdés, P.; Fernández, T.; Jalil, Y.; Peñailillo, L.; Damiani, L.F. Eccentric Contractions of the Diaphragm During Mechanical Ventilation. Respir. Care 2023, 68, 1757–1762. [Google Scholar] [CrossRef] [PubMed]


| Rabbit Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TGF-β1 | CTGGAACGGGCTCAACATCT | CAGGTCCTTGCGGAAGTCAA |
| COL1A1 | ACCACTGCAAGAACAGCGTA | TCGTGGAGGACAGTGTAGGT |
| COL3A1 | AACAATGGTAGTCCTGGCGG | CACCGTTCTTACCGGGTTCA |
| Piezo2 | CTCAACGTGGGAAAAGACA | CCAGACTCGCAATGAACA |
| GAPDH | TAAGAGCCCTCAAACCACCG | AAGAGGGGCAGATTCTCAGC |
| ITGB1-F | CCAGTGCCGAGCCTTCAATA | CAGCAGTCGTCCACATCCTT |
| ROCK1-F | CTCGCGGAGTAAGTTGGTTGA | CAAAGCATCCAATCCATCCAGC |
| C2C12 Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TGF-β1 | CCTGAGTGGCTGTCTTTTGA | CGTGGAGTTTGTTATCTTTGCTG |
| ACTA2 | TGAGCGTGGCTATTCCTTCG | AGCGTTCGTTTCCAATGGTG |
| COL1A1 | ACAGTCGCTTCACCTACAGC | GGGTGGAGGGAGTTTACACG |
| COL3A1 | ACGTAAGCACTGGTGGACAG | CAGGAGGGCCATAGCTGAAC |
| GAPDH | TGTCAAGCTCATTTCCTGGT | TAGGGCCTCTCTTGCTCAGT |
| ITGB1 | TTCAGACTTCCGCATTGGCT | CAGCCAATCAGCGATCCACA |
| CON | MV | PEEP | p | |
|---|---|---|---|---|
| pH | 7.39 ± 0.02 | 7.39 ± 0.04 | 7.37 ± 0.08 | 0.70 |
| PaCO2 (mmHg) | 38.99 ± 3.49 | 36.68 ± 5.50 | 36.65 ± 7.00 | 0.99 |
| PaO2 (mmHg) | 89.49 ± 12.98 | 85.77 ± 22.38 | 75.60 ± 15.13 | 0.42 |
| Lac (mmol/L) | 2.40 ± 0.71 | 4.40 ± 3.26 | 4.90 ± 2.10 | 0.78 |
| HCO3− (mmol/L) | 22.65 ± 3.48 | 21.80 ± 1.85 | 20.92 ± 2.83 | 0.57 |
| PaO2/FiO2 (mmHg) | 426.15 ± 61.80 | 408.41 ± 106.57 | 360.00 ± 72.03 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Jia, J.; He, R.; Yu, X.; Jiang, Y.; Shen, W.; Qian, X.; Xu, P.; Xu, Y.; Ge, H. High PEEP Activates ITGB1, Inducing Diaphragm Fibrosis During Prolonged Mechanical Ventilation. Biomolecules 2025, 15, 1466. https://doi.org/10.3390/biom15101466
Gong J, Jia J, He R, Yu X, Jiang Y, Shen W, Qian X, Xu P, Xu Y, Ge H. High PEEP Activates ITGB1, Inducing Diaphragm Fibrosis During Prolonged Mechanical Ventilation. Biomolecules. 2025; 15(10):1466. https://doi.org/10.3390/biom15101466
Chicago/Turabian StyleGong, Jiahong, Jianwei Jia, Runze He, Xiaolan Yu, Ye Jiang, Weimin Shen, Xiaoli Qian, Peifeng Xu, Ying Xu, and Huiqing Ge. 2025. "High PEEP Activates ITGB1, Inducing Diaphragm Fibrosis During Prolonged Mechanical Ventilation" Biomolecules 15, no. 10: 1466. https://doi.org/10.3390/biom15101466
APA StyleGong, J., Jia, J., He, R., Yu, X., Jiang, Y., Shen, W., Qian, X., Xu, P., Xu, Y., & Ge, H. (2025). High PEEP Activates ITGB1, Inducing Diaphragm Fibrosis During Prolonged Mechanical Ventilation. Biomolecules, 15(10), 1466. https://doi.org/10.3390/biom15101466






