Abstract
Preeclampsia is a severe complication affecting both maternal and neonatal health and is becoming a significant global public health issue. As a vital steroid hormone, progesterone (P4) plays a crucial role during pregnancy and in regulating various physiological processes. Recent studies have indicated that P4 is not only involved in pregnancy maintenance, but may also be closely related to preeclampsia pathogenesis and prevention. Previous research has suggested that P4 may participate in the mechanism of preeclampsia by regulating vascular function, immune responses, and placental function. Moreover, key enzymes and metabolites involved in the synthesis and metabolism of P4 are also associated with preeclampsia onset. Additionally, the potential value of clinically applying P4 in preventing and treating preeclampsia has been shown; however, the corresponding clinical practices require further validation and optimization. This study aimed to review the physiological effects, pathological functions, and clinical applications of P4 in preeclampsia, providing evidence for future research and clinical practice.
1. Introduction
Preeclampsia (PE) is defined as the onset of new hypertension after 20 weeks of gestation with or without proteinuria. In severe cases, preeclampsia may also be associated with renal, cardiac, pulmonary, hepatic, and neurological dysfunction, as well as hematological disorders, fetal growth restriction (FGR), stillbirth, and even maternal mortality [1]. PE affects 2–8% of pregnant women worldwide and is a major cause of morbidity and mortality for both mothers and fetuses; however, the only known cure for PE is delivery [2,3].
Progesterone (P4) is a cholesterol-derived hormone that is essential for establishing and maintaining pregnancy [4]. A systematic review and meta-analysis showed that the use of vaginal micronized P4 in the first trimester may reduce the risk of hypertensive disorders of pregnancy (HDPs) and PE in singleton pregnancies [5,6]. The oral P4 preparation dydrogesterone significantly reduced PE incidence in women with higher-risk pregnancies and reduced hypertension, proteinuria, FGR, and preterm labor when supplemented in the first and second trimester (from 6 to 20 weeks of gestation) [7,8]. This evidence implies the important role of P4 in preventing PE, the mechanism of which is crucial to explore.
This review aims to characterize the potential mechanisms by which P4 is involved in PE, including its effects on vascular function, immune response, and placental function. By regulating P4 levels, the key enzymes and metabolites involved in P4 synthesis and metabolism are also associated with PE onset. In addition, we discuss the application potential of P4 in clinical management and PE-like animal models. Through a comprehensive analysis of the literature, we hope to provide new insights and evidence regarding the mechanisms, prevention, and treatment of PE, thereby improving maternal and infant health.
2. P4 Synthesis and Metabolism
2.1. P4 Synthesis Process
As a steroid hormone synthesized by the ovarian corpus luteum, placenta, and adrenal glands, P4 plays a crucial role during pregnancy and other physiological activities [9]. Its synthesis requires the cytochrome P450 side-chain cleavage enzyme (P450scc) to convert cholesterol inside the mitochondria to pregnenolone (PG), followed by the conversion of PG to P4 by 3β-hydroxysteroid dehydrogenase (3β-HSD) in the endoplasmic reticulum. The synthesis of P4 and its key enzymes is illustrated in Figure 1. Synthesized P4 can be released into the extracellular space through simple diffusion before it enters the bloodstream and exerts its physiological effects.
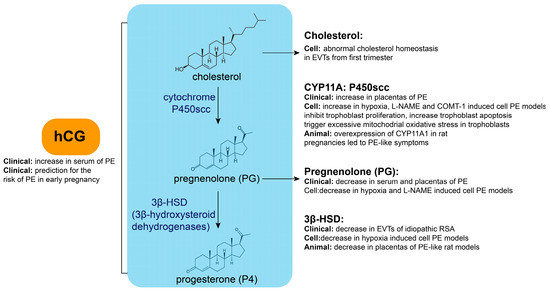
Figure 1.
Key enzyme or metabolite abnormalities during P4 synthesis in PE.
2.2. Key Enzyme or Metabolite Abnormalities During P4 Synthesis in PE
Abnormalities in the key enzymes or metabolites during P4 synthesis are closely related to PE (Figure 1). Cholesterol is the raw material in progesterone synthesis. Cellular cholesterol homeostasis is differentially regulated in extravillous trophoblast cells (EVTs) compared with villous cytotrophoblast cells (VCTs) using primary human trophoblasts isolated from placental tissues in the first trimester [10]. EVTs display increased levels of free and esterified cholesterol. Additionally, PG concentrations tended to decrease in the serum and placentas of PE [11], as well as in cellular PE models induced by hypoxia and N nitro L arginine methyl ester hydrochloride (L-NAME) [12]. Moreover, 3β-HSD levels are decreased in EVTs of idiopathic recurrent spontaneous abortions [10], while its expression is decreased in cellular hypoxia-induced PE models [12]. Maternal cadmium exposure contributes to PE onset in pregnant rats by disrupting local P4 synthesis in the placenta and inhibiting 3β-HSD expression [13]. Additionally, it has been reported that the expression of cytochrome P450 family 11 subfamily A member 1 (CYP11A) is significantly increased in placentas of severe PE (sPE) compared with normal pregnancy. An abnormally high expression of CYP11A inhibits trophoblastic proliferation, triggers excessive mitochondrial oxidative stress, and increases apoptosis [14,15]. CYP11A expression was increased in cellular PE models induced by hypoxia, L-NAME, and catechol-O-methyltransferase inhibitor (COMT-1) [12]. CYP11A1 overexpression in rat pregnancies leads to PE-like symptoms [16]. This evidence indicates that abnormalities in key enzymes or metabolites during P4 synthesis are closely related to PE.
2.3. P4 Synthesis Regulation
P4 synthesis and secretion are regulated by various factors, including the action of luteinizing hormone (LH) prior to ovulation, which prompts the ovarian corpus luteum to synthesize a substantial amount of P4 [17]. If the egg is successfully fertilized, the ovarian corpus luteum transforms into a pregnancy corpus luteum, which, under the influence of human chorionic gonadotropin (hCG) secreted by the placenta, maintains its function and continues to secrete P4. At approximately 10 weeks of gestation, the placenta begins to synthesize P4 on its own to support pregnancy and ensure proper embryonic development.
2.4. hCG Abnormalities in PE
Abnormalities in hCG may be related to PE development (Figure 1). The serum hCG concentration is significantly increased in patients with PE and is associated with its severity in early, mid-, and late pregnancy. Additionally, in early pregnancy, the hCG concentration can be used as a marker to predict PE risk with better sensitivity and specificity [18,19,20,21,22]. It has been reported that every sigma (standard deviation) increase in β-hCG level leads to a 2.73-fold increase in PE risk [23,24,25,26,27,28,29]. This evidence shows that abnormal P4 synthesis regulation may also be involved in PE pathogenesis.
2.5. P4 Metabolism
P4 metabolism mainly occurs in the liver, where it is converted into water-soluble metabolites through reduction, hydroxylation, and conjugation reactions for excretion (Figure 2) [30,31]. First, P4 is catalyzed by 5α-reductase or 5β-reductase to form dihydroprogesterone (DHP), which is then converted by 3α-hydroxysteroid dehydrogenase (3α-HSD) into pregnanedione and further reduced by 20α-hydroxysteroid dehydrogenase (20α-HSD) to yield pregnanetriol. In the alternative pathway, P4 undergoes hydroxylation by 17α-hydroxylase (CYP17A1) to generate 17α-hydroxyprogesterone (17-OHP). Additionally, a small portion of P4 is hydroxylated by CYP450 enzymes, such as aromatase (CYP19A1), to form less active metabolites, such as 6β-hydroxyprogesterone or 16α-hydroxyprogesterone. Finally, these metabolites (e.g., pregnanetriol and pregnanedione) are conjugated with glucuronic acid or sulfate via UDP-glucuronosyltransferase (UGT) or sulfotransferase (SULT), forming water-soluble conjugates, such as pregnanediol glucuronide (P3G), which are primarily excreted in urine or bile. Individual variations in metabolic pathways are influenced by genetics, liver and kidney function, and hormonal status (e.g., pregnancy).
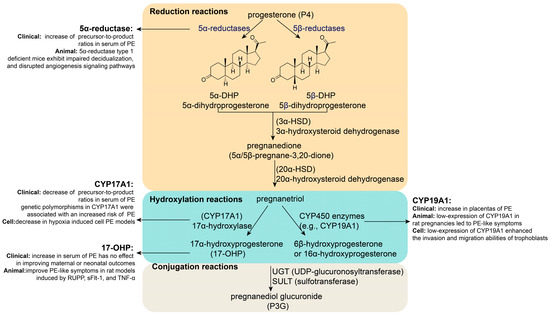
Figure 2.
Key enzyme or metabolite abnormalities during P4 metabolism in PE.
2.6. Key Enzyme or Metabolite Abnormalities During P4 Metabolism in PE
The abnormal expression of key enzymes or metabolites in P4 metabolism is associated with PE (Figure 2). Research has shown that the precursor-to-product ratio of 5α-reductase is significantly increased in the serum of women with PE [32]. 5α-reductase type 1-deficient mice exhibit impaired decidualization and disrupted angiogenesis signaling pathways [33]. Additionally, it has been reported that the precursor-to-product ratios of CYP17A1 were significantly decreased in the serum of women with PE [32]. The expression of CYP17A1 was significantly decreased in a hypoxia-induced cellular PE model [12]. Interestingly, genetic polymorphisms in CYP17A1 might play a crucial role in PE, especially in early-onset sPE [34]. For example, CYP17A1 rs4919690 and rs4919687 are associated with an increased risk of sPE [34], while CYP17A1 rs1004467 and rs3824755 seem to be closely associated with mild PE in Han women [35]. Furthermore, research has shown that CYP19A1 expression is relatively low in the placentas of PE patients [36]. Animal experiments have shown that low CYP19A1 expression in rat pregnancies leads to PE-like symptoms [36]. Research has found that low CYP19A1 expression enhances the invasion and migration abilities of trophoblasts [36]. In addition, 17-OHP levels were significantly higher in the serum of patients with PE than in normal women [37]. A randomized open-label controlled study showed that 17-OHP has no effect on improving maternal or neonatal outcomes in conservatively managed early-onset PE (EOPE); however, it does alter the levels of inflammatory markers such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which could reduce PE pathogenesis [38]. Animal experiments have shown that the intraperitoneal administration of 17-hydroxyprogesterone caproate (17-OHPC) can reduce hypertension, fetal demise, T cells, and NK cells in response to placental ischemia in PE-like rat models induced by reduced uterine perfusion pressure (RUPP), soluble fms-like tyrosine kinase-1 (sFlt-1), and TNF-α [39,40,41,42,43]. This evidence indicates a relationship between the key P4 metabolism enzymes or metabolites and PE.
In the above, we reviewed the P4 synthesis and metabolism processes, as well as the key enzymes and metabolic products involved, and their correlation with PE pathogenesis; this was validated using cell, animal, and clinical specimens. This evidence suggests that P4 may play an important role in PE pathogenesis and may be involved in its early prediction and prevention. Next, we summarize the preventive effects of P4 itself on PE, as well as the animal model studies and clinical research.
3. The Potential of P4 Supplementation in Preventing PE
3.1. Animal Research
Animal experiments show that P4 levels were lower in the urine and blood of PE-like models [11,44,45]. In addition, P4 can prevent PE-like symptoms in several animal models induced by L-NAME, sirtuin 1 (SIRT1) knockdown, RUPP, and cadmium (Table 1) [4,41,44,46]. Additionally, progesterone-induced blocking factor (PIBF) can reduce hypertension, inflammation, and fetal growth restriction in a RUPP rat model of PE [47], while PIBF blockade causes hypertension, inflammation, and signs of endothelial dysfunction, all of which are associated with PE [48]. Furthermore, impaired luteal phase P4 signaling via the administration of the P4 antagonist RU486 (mifepristone) causes fetal loss and FGR during late gestation [49]. This evidence demonstrates that P4 supplementation during pregnancy might play a role in PE treatment and prevention and may be involved in its pathogenesis.

Table 1.
Animal research showing the potential of P4 support in preventing PE.
3.2. Clinical Research
Insufficient P4 leads to PE development, and supplementation may prevent its onset (Table 2). P4 levels are lower in the urine and blood of PE patients than in normal control patients during pregnancy [4,11,41,44,45,50,51,52,53,54]. A systematic review and meta-analysis including several RCTs showed that the use of vaginal micronized P4 in the first trimester may reduce the risk of PE (RR, 0.61; 95%CI, 0.41–0.92; 3RCTs, n = 5267, moderate-certainty evidence; OR, 0.64; 95%CI, 0.42–0.98; 3RCTs, n = 3982, moderate-certainty evidence) in singleton pregnancies [5,6]. However, a retrospective cohort study including 2056 twin pregnancies showed that micronized vaginal P4, or its combination with aspirin, was not superior to aspirin alone in reducing the risk of PE or HDP in twin pregnancies [55]. This evidence showed the beneficial effect of vaginal P4 supplementation in early pregnancy to prevent PE in singleton pregnancies.
Additionally, several previous reviews have shown the potential benefits of dydrogesterone in preventing PE and HDP [56,57]. A comparative retrospective study showed that dydrogesterone supplementation in the first and second periods of pregnancy (from 6 to 20 weeks of gestation) significantly reduced PE incidence [study group (n = 169) vs. control group (n = 237): 13.1% vs. 71.4%, p < 0.001] and reduced the risk of hypertension, proteinuria, FGR, uterus–placenta velocity destruction, and preterm labor in women with higher-risk pregnancies [7]. Furthermore, a clinical case showed that a patient with early sPE during the first pregnancy—to prevent PE in a second pregnancy—received dydrogesterone (30 mg) from 6 to 37 weeks of gestation, and had no clinical signs of PE, including blood pressure <120–130/70–80 mmHg, no proteinuria, no edema, and no fetal or placental abnormalities [8]. These reports indicated that oral dydrogesterone (30 mg, in first and second trimesters) may be the preferred form and dosage for P4 supplementation to prevent PE.
Moreover, in pregnant women with assisted reproductive technology (ART), it is reported that early dydrogesterone supplementation can also decrease PE incidence and outcomes [study group (n = 570) vs. control group (n = 570): 8.4% vs. 14.2%, p < 0.05, a retrospective comparative analysis], HDP and fetal destress [study group (n = 116) vs. control group (n = 116): 1.7% vs. 12.9%, p = 0.001; 4.3% vs. 18.1%, p = 0.001; a prospective cross-sectional comparative study], and fetal weight [study group (n = 136) vs. control group (n = 91): 82.4% vs. 70.3%, p = 0.033, secondary data analysis study based on two randomized control trials] [58,59,60]. However, women supplemented with dydrogesterone only showed a lower PE incidence compared with women who received a combination of dydrogesterone and hydroxyprogesterone caproate. This was, nevertheless, not statistically significant [59]. However, it has also been reported that early vaginal P4 supplementation (from embryo transfer to 8 weeks of gestation) in pregnancies with natural cycle frozen embryo transfer (NC-FET) decreased the incidence of PE and HDP, but the difference was not statistically significant [60]. Furthermore, Conrad et al. [61] reported that the risk of developing PE and sPE increased specifically in women undergoing autologous frozen embryo transfer (FET) in artificial cycles without the formation of a corpus luteum relative to natural, modified natural, stimulated, or controlled ovarian stimulation cycles and spontaneous pregnancies. This evidence demonstrated that P4 supplementation is also beneficial for preventing PE in ART.
This evidence suggests that P4 may play an important role in PE pathogenesis and may be involved in its early prediction and prevention. Next, we focused on the possible mechanism by which P4 affects PE progression, mainly by activating nuclear and membrane P4 receptors.

Table 2.
Clinical research showing the potential of P4 support in preventing PE.
Table 2.
Clinical research showing the potential of P4 support in preventing PE.
| PMID | Study Design | Results |
|---|---|---|
| 37941309 [5] | A systematic review and meta-analysis: Meta-analyses with random-effects model MEDLINE, PubMed, CENTRAL, Embase, and ClinicalTrials.gov (until 20230620) 11 RCTs (3 single center, 8 multiple centers) 11640 patients (5267 patients in 3 RCTs initiated vaginal P4 in the first trimester; 6373 patients in 8 RCTs initiated vaginal P4 in the second and third trimester) Vaginal micronized P4 (100–800 mg/d) Subgroup analysis: 400 mg/bid vs. 400 mg/qd | Vaginal P4 in the first trimester: HDP (RR 0.71, 95%CI 0.53–0.93, 2 RCTs, n = 4431, moderate-certainty evidence) Subgroup analysis: a benefit in using the 400 mg/bid regimen (RR 0.74, 95%CI 0.55–0.99, 1 RCT, n = 4153) PE (RR 0.61, 95%CI 0.41–0.92, 3 RCTs, n = 5267, moderate-certainty evidence) Subgroup analysis: a benefit in using the 400 mg/bid regimen (RR 0.65, 95%CI 0.42–0.99, 2 RCTs, n = 4989) Vaginal P4 in the second or third trimesters: HDP (RR 1.19, 95%CI 0.67–2.12, 3 RCTs, n = 1602, low-certainty evidence) PE (RR 0.97, 95%CI 0.71–1.31, 5 RCTs, n = 4274, low-certainty evidence) |
| 34732210 [6] | A systematic review and meta-analysis: Pairwise meta-analyses with random-effects model MEDLINE, Cochrane Library, Embase and ClinicalTrials.gov (until 20210403) 9 RCTs, 6125 singleton pregnancies Study group: 3055 women treated with P4 before 20 weeks gestation (8 RCTs used oral or vaginal P4; 1 RCTs used rectal P4) Control group: 3070 women unexposed | P4 group: PE (OR 0.64, 95%CI 0.42–0.98, 3 RCTs, n = 3982, moderate-certainty evidence) Subgroup analysis: a benefit in using vaginal P4 (OR 0.62, 95%CI 0.40–0.96) |
| 38621482 [55] | A retrospective cohort study: 1800 twin pregnancies managed at a tertiary referral center in the UK (200001–202311) P4 group: 69 treated with P4 only P4+aspirin group: 105 treated with P4 and aspirin in the first trimester Aspirin group: 1156 treated with aspirin only Control group: 470 treated with no medication | PE incidence: Control group vs. Aspirin group vs. P4 group vs. P4+aspirin group: 7% vs. 4.6% vs. 5.8% vs. 7.6% HR for PE: Aspirin group: 0.62, 95%CI 0.40–0.97, p = 0.036 P4 group: 0.83, 95%CI 0.37–1.83, p = 0.637 P4+aspirin group: 0.71, 95%CI 0.25–2.03, p = 0.527 |
| 31876197 [7] | Comparative retrospective study: 406 pregnancies with risk factors of PE Study group: 169 pregnancies with dydrogesterone supplementation (30 mg/d) at 6–20 weeks gestation Control group: 237 pregnancies without dydrogesterone supplementation | PE: 13.1% and 71.4%, p < 0.001 Hypertension: 3.2% and 71.2%, p < 0.001 Proteinuria: 0.0% vs. 66.18%, p < 0.001 FGR: 2.2% vs. 21.58%, p < 0.001 Destroy of uteri-placenta velocity: 3.2% vs. 21.58%, p < 0.001 PL: 8.6% vs. 53.95%, p < 0.001 |
| 39722356 [60] | A secondary data analysis study based on 2 randomized control trials (2008–2011 and 2013–2018) at 2 university hospitals in Sweden 227 singleton pregnancies of NC-FET Study group: 136 pregnancies with luteal phase vaginal P4 supplementation from ET to 8 weeks gestation 56 pregnancies, 2008–2011, micronized P4, 400 mg/bid, as a vaginal suppository 80 pregnancies, 2013–2018, P4, 100 mg/bid, as a vaginal tablet Control group: 91 pregnancies, receive standard of care, without P4 supplementation | Study group vs. Control group: Birth weights: 82.4% vs. 70.3%, p = 0.033 HDP: 4.4% vs. 11.1%, p = 0.058 Mild/moderate PE: 1.5% vs. 3.3% Severe PE: 1.5% vs. 2.2% |
| 24552449 [58] | A prospective cross-sectional comparative study: 232 primigravidae in a tertiary center (201001-201012) Study group: 116 primigravidae with dydrogesterone supplementation (10 mg/tid) following ART or IUI up to 16 weeks gestation Control group: 116 primigravidae, age and race matched spontaneous pregnancies at 16 weeks, without dydrogesterone supplementation | Study group vs. Control group: HDP: 1.7% vs. 12.9%, p = 0.001 fetal distress: 4.3% vs. 18.1%, p = 0.001 |
| 26910749 [59] | A retrospective comparative analysis: 1140 pregnancies in a tertiary center (200601–201503) Study group: 570 with P4 support following ART or IUI until 14–16 weeks gestation Control group: 570 without P4 support | Study group vs. Control group: PE: 8.4% vs. 14.2%, p < 0.05 Subgroup analysis: Dydrogesterone only (10 mg, n = 276) vs. Dydrogesterone+hydroxyprogesterone (intramuscular injection of 500 mg, n = 294): PE: 6.9% vs. 9.9%, p = 0.2 |
4. P4’s Genomic and Non-Genomic Mechanisms of Action
4.1. P4 Classical Signaling Pathway: Genomic Receptor Mechanism (Nuclear)
P4 is a lipophilic molecule that readily crosses cell membranes by diffusion and interacts at the nuclear level with nuclear P4 receptors (nPGRs), PGR-A (94 kDa), and PGR-B (120 kDa) [62]; thus, this activates co-regulators that act on ribosomal RNA, resulting in the production of corresponding proteins [63,64]. Notably, higher P4 levels downregulated PGR levels, which in turn induced further P4 secretion [65]. A feedback loop exists between P4 and PGR. The unbound receptor exists in both subcellular compartments and resides in complexes with heat-shock protein (HSP) chaperone molecules, such as HSP70 and HSP90 [66]. Ligand binding to PGR induces dissociation from HSPs, the retention of dimerized PGR in the nucleus, and transcription activation. The genomic receptors PGR-A and PGR-B are encoded by a single gene but are transcribed from different promoters to generate distinct subclasses of PGR mRNAs [67]. These PGR-A and PGR-B isoforms share the transcription-activating function (AF) domains AF-1 and AF-2; the PGR-B isoform contains a specific N-terminal segment, AF-3, as well as the B-upstream segment that is absent from the PGR-A isoform. Both PGR-A and PGR-B contain a C-terminal ligand-binding domain and hinge region [64].
Since there are various cell types at the maternal–fetal interface, including trophoblasts, immune cells, and decidual stromal cells, we first discuss the distribution of PGRs in humans and other mammalian species. In human and primate placentas, PGR is expressed in stromal cells, spiral arteries, and/or the myometrium from early to late pregnancy [68]. However, whether PGR is expressed in trophoblasts remains controversial. Research has shown that PGR-A/B is also expressed in trophoblasts, as well as spatial differences in PGR-B dominance in early and PGR-A in late trophoblasts from legal abortion [68]. PGRs are expressed in several trophoblast cell lines, such as HTR-8/SVneo, JEG3, and BeWo cells [69,70]. However, research has also shown that JEG3 and EVTs from primary trophoblasts do not express PGR for P4 [71,72,73,74]. In other mammals, the PGR expression range differs. In canines, decidual stromal cells are the only placental cell population that expresses PGR [75], which has been detected at the mRNA and protein levels in several placental compartments during early pregnancy in sheep [76,77]. In pigs, PGR is expressed in the uterine stroma and myometrium throughout pregnancy [78]. Due to the varying PGR expression levels in different cell types, P4 may affect different cell types, thereby having different impacts on cell functions and overall maternal–fetal interface function.
Research showed that the abnormal expression of PGR in the placenta is associated with PE development (Table 3). It is reported that PGR-B gene expression and protein abundance are remarkably disrupted in the decidua of sPE pregnancies [79]. Transcription factors PGR and cAMP-responsive element binding protein 1 (CREB1) jointly regulate the transcription of downstream genes in response to decidual signals, influencing the decidualization of endometrial stroma [80]. Additionally, it has been reported that PGR levels were elevated in preeclamptic placentas [70], and that this can influence trophoblast function. For example, P4 can promote trophoblast cell proliferation and invasion [44], and PGR deficiency downregulates cyclin D1 protein levels in trophoblasts, which plays an important role in cell proliferation [65]. This evidence indicates that P4 influences PGR expression at the maternal–fetal interface, thus leading to PE development.

Table 3.
The expression and function of several P4 receptors.
4.2. P4 Non-Classical Signaling Pathway: Non-Genomic Receptor Mechanism (Extranuclear)
4.2.1. mPRs
Specific membrane-bound P4 receptors (mPRs), also called progestin and adipoQ receptors (PAQRs), have been described as having seven transmembrane domains, similar to G protein-coupled receptors [88]. There are currently five mPR subtypes (mPRα, mPRβ, mPRγ, mPRδ, and mPRε). Initial studies have indicated that mPR expression is highly tissue-specific, with mPRα being the predominant isoform in reproductive tissues, mPRβ in neural tissues, and mPRγ in the gastrointestinal tract. After P4 activates mPRs, it can affect the biological functions of cells by activating downstream cascade reactions, including stimulating extracellular signal-regulated kinases 1/2 (ERK1/2 or p42/44), p38 mitogen-activated protein kinase (MAPK), or intracellular Ca2+ mobilization [64].
Research has shown that mPRα is highly expressed in the placenta, and that the tissue expression levels of mPRα genes are negatively correlated with PGR expression, suggesting important functional roles for mPRα in specific reproductive tissues, particularly those that express low levels of nuclear PGR [86]. P4 stimulates mPRα and involves signaling through the phosphatidylinositol 3-kinases/protein kinase B (PI3K/AKT) and MAPK pathways to produce nitrogen oxide (NO) in human umbilical vein endothelial cells (HUVECs); this is important in vasodilatation [87]. Additionally, P4 binds to mPR in macrophages and upregulates the mRNA expression of cyclooxygenase 2 (COX2), TNF-α, and prostaglandin-endoperoxide synthase 2 (PTGS2), leading to the activation of the MAPK and protein kinase A (PKA) pathways. This thus contributes to the inflammatory responses in macrophages with subsequent labor regulation [89]. The above content is presented in Table 3.
4.2.2. PGRMC
Another membrane-bound P4 receptor, P4 receptor membrane component (PGRMC), was isolated from neural tissue and is distinct from known mPRs and nPGRs [90]. Within this protein family, PGRMC1 and PGRMC2 are the best characterized, as they mediate some of P4’s actions. Additionally, PGRMCs have both P4-dependent and -independent functions, and may more broadly serve as promiscuous receptors for selecting sterols [91,92]. This is supported by recent findings in breast cancer cell lines showing that different synthetic progestins cause many of the same cellular outcomes when signaling through the classical or nuclear P4 receptor, but have seemingly distinct PGRMC1-dependent actions [93,94]. For example, medroxyprogesterone acetate, norethisterone, and dienogest—but not nomegestrol acetate—induce proliferation in MCF7 and T47D breast cancer cells through a mechanism dependent on the phosphorylation of a casein kinase 2 domain within PGRMC1 [94,95,96].
- PGRMC1
PGRMC1 plays a crucial role in placental function (Table 3). Research has shown that PGRs are absent in EVTs and JEG3, whereas PGRMC1 and PGRMC2 are highly expressed in all trophoblasts [71,72,73,74]. It has been reported that JEG3 invasion is inhibited by P4 antagonist RU486 (mifepristone) in a PGRMC1-dependent manner [74]. Additionally, PGRMC1 downregulation accelerates the differentiation and fusion of BeWo cells [81]. In addition, it significantly promotes differentiation, decidualization, and cellular senescence in endometrial stromal cells (ESCs) [82,83].
- PGRMC2
PGRMC2 may also affect placental function (Table 3). Its attenuation plays a role in placentation by promoting cell proliferation, invasion, and angiogenesis in EVTs via the activation of hypoxia-inducible factor 1 alpha (HIF-1α) signaling [84]. An organ-on-chip model of the maternal–fetal membrane interface revealed that HLA-G and PGRMC2 produced by chorionic trophoblasts modulate inflammation, thus serving as an immunological barrier preventing membrane compromise [97]. Furthermore, PGRMC2 knockdown significantly compromised the ability of decidualized ESCs to support trophoblast expansion in an outgrowth model [85].
The above evidence suggests that P4 exerts its downstream functions at the maternal–fetal interface, mainly through activating nuclear and membrane P4 receptors. Next, we review the effects of P4 and the activation of its receptors on cellular function at the maternal–fetal interface, focusing primarily on three aspects: vascular function, immune response, and placental function.
5. P4 Might Protect Against PE by Regulating Vascular Endothelial Function
Vascular endothelial dysfunction is a typical characteristic of PE, characterized by oxidative stress and inflammatory response [98]. Research has shown that P4 plays an important role in vascular remodeling by regulating vascular endothelial function [99,100,101], thus likely contributing to its protected role in PE.
5.1. P4 Might Protect Against PE by Regulating VEGF Signaling
Vascular endothelial growth factor (VEGF) has emerged as a significant biomarker in the context of PE. Research indicates that altered levels of VEGF can be associated with the onset and severity of this condition, making it a focal point for both diagnostic and prognostic assessments [102,103].
P4 plays an important role in protecting vascular endothelial function in vascular remodeling by regulating VEGF signaling; it is thus involved in PE (Figure 3). On one hand, P4 levels are correlated with VEGFA expression. It has been reported that the glandular epithelial expression of VEGFA, VEGFC, and placental growth factor (PlGF) is higher in women with elevated P4 levels than in those with normal levels. Moreover, a significantly higher stromal expression of VEGFA and PLGF was found in women with elevated P4 levels [104]. On the other hand, P4 promotes VEGF expression to protect vascular endothelial function. Previous research has shown that ovarian stimulation and P4 administration enhance endometrial angiogenesis by upregulating VEGF expression [105,106]. Additionally, research has shown that P4 governs uterine angiogenesis and vascular remodeling via VEGFA/VEGFR2 signaling, especially in the anti-mesometrial region where the embryo resides during pregnancy [107,108,109]. Moreover, P4 alleviates dexamethasone-induced intrauterine growth retardation (IUGR), probably by promoting placental VEGF and angiogenesis [110]. Furthermore, in PE, P4 blunts the vascular endothelial cell secretion of endothelin-1, a partner of VEGF, in response to placental ischemia [41].
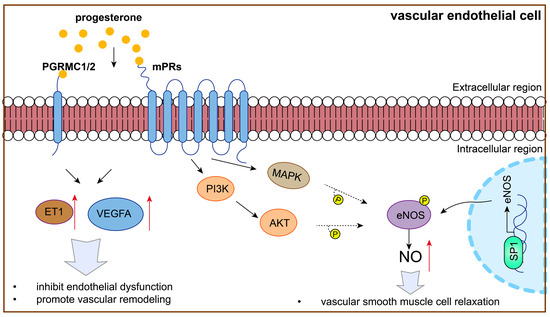
Figure 3.
P4 might protect against PE by regulating vascular endothelial function.
Notably, P4 is involved in regulating VEGF signaling and vascular endothelial function, which is dependent on the P4 receptor (Figure 3). P4 improves the angiogenic potential of endothelial progenitor cells, including tube formation, adhesion, migration, and VEGF secretion, in a dose-dependent manner, which can be reversed by a P4 receptor antagonist [111]. In addition, PGRMC1 overexpression significantly promoted the cellular processes associated with endothelial cell proliferation, migration, and angiogenesis [112]. Additionally, high mPRα expression enhances the activation of cAMP-Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling and increases HIF-1α-induced VEGF secretion into the tumor microenvironment, promoting HUVEC migration and tube formation under hypoxia in lung adenocarcinoma [113].
This evidence showed that, by regulating VEGF signaling and vascular remodeling, P4 and its receptor might be involved in PE development.
5.2. P4 Might Protect Against PE by Regulating eNOS/NO Expression
Preeclampsia (PE) is a complex pregnancy disorder characterized by hypertension and proteinuria [1]. One of the critical pathways implicated in the pathophysiology of PE is the endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signaling pathway [114,115]. Changes in this pathway can lead to impaired endothelial function, contributing to the vascular complications associated with PE [114,115]. Research indicates that eNOS expression and activity are significantly altered in preeclamptic patients, leading to reduced NO bioavailability. This reduction in NO is associated with increased vascular resistance and hypertension, hallmark features of preeclampsia [114,115].
P4 promotes NO production to protect vascular endothelial cells and is thus involved in PE (Figure 3). For example, P4 promotes NO production, inhibiting cellular antioxidant effects and increasing oxidative stress in arterial endothelial cells [116]. Additionally, P4 stimulates NO production in HUVECs and is involved in the PI3K/AKT and MAPK pathways by regulating by mPRα [87,117].
P4 promotes NO production by increasing eNOS expression. Studies have shown that P4 promotes vasodilation by regulating transcription factor specificity protein 1 (SP1) in endothelial cells, enhancing eNOS expression and further increasing NO synthesis [118]. Importantly, P4 may be important in increasing eNOS expression through mPRα and subsequent PI3K/AKT, as well as MAPK pathways, exerting beneficial vascular function effects [87,119,120,121]. This evidence showed that P4 and its receptor might be involved in PE development via regulating eNOS and NO expression.
In the above discussion, P4 might protect against PE by regulating vascular endothelial function, as well as VEGF signaling and eNOS and NO expression.
6. P4 Might Protect Against PE by Regulating Immune Response at the Maternal–Fetal Interface
Immune dysfunction at the maternal–fetal interface is also a key factor involved in PE pathophysiology. On one hand, immune cells at this interface in patients with PE tend to convert to a pro-inflammatory phenotype and secrete pro-inflammatory cytokines, accelerating PE onset and progression. Studies have found that, in patients with PE, there is an increase in the Th1/Th2 ratio [122,123,124], pro-inflammatory M1-type macrophages [125,126,127,128], cytolytic NK cells [129,130,131], and the pro-inflammatory cytokines TNF-α and interferon-gamma (IFN-γ), alongside a decrease in Treg cells [132,133], mature dendritic cells (DCs) [134,135,136], and anti-inflammatory cytokines. These changes lead to impaired immune suppression at the maternal–fetal interface and impaired uterine spiral artery remodeling, contributing to PE development. On the other hand, in patients with PE, human leukocyte antigen-C (HLA-C) and HLA-G expression is reduced in trophoblasts [137,138], which impairs their immunosuppressive function on immune cells, undermining immune tolerance at the maternal–fetal interface and further exacerbating PE development.
Research has shown that P4 plays an important immunomodulatory role during pregnancy, mainly promoting immune tolerance at the maternal–fetal interface by influencing immune cell [139,140] and trophoblast function, and thus protecting against PE. In addition, in the presence of P4, PGR-positive pregnancy lymphocytes produce a protein called P4-induced blocking factor (PIBF), which mediates some of P4’s immunological effects [141,142].
6.1. P4 Might Protect Against PE by Regulating Immune Cells at Maternal–Fetal Interface
6.1.1. Th1/Th2
P4 can promote PIBF production, which inhibits the Th1/Th2 ratio at the maternal–fetal interface (Figure 4). In humans, P4-treated human pregnancy lymphocytes produce PIBF, followed by decreased Th1-type cytokine and increased Th2-type cytokine production, which can promote T cell differentiation into Th2 [143,144]. Similarly, PIBF-treated spleen cells from non-pregnant female mice produce significantly more Th2-type cytokines, interleukin-4 (IL-4) and interleukin-10 (IL-10), than those without PIBF [145]. Furthermore, T cells from PIBF-deficient pregnant mice differentiate into Th1 [146].
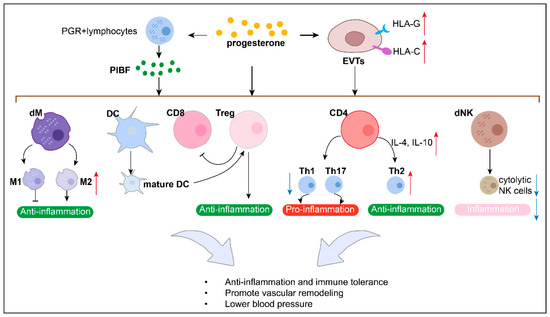
Figure 4.
P4 might protect against PE by regulating immune response in maternal–fetal interface.
P4 protects against PE by inhibiting the Th1/Th2 ratio at the maternal–fetal interface. For example, PIBF treatment normalized the Th1/Th2 ratio, reduced inflammation, corrected blood pressure, and prevented FGR in a rat model of PE [147].
6.1.2. Treg Cells
P4 promotes Treg cells at the maternal–fetal interface (Figure 4). Research has shown that P4 supports Treg expansion and suppressive function at this interface during pregnancy, as well as a skew toward an anti-inflammatory cytokine profile and CD8+T cell cytotoxicity suppression [49,139,148,149,150,151,152]. Impaired luteal phase P4 signaling following the administration of the P4 antagonist RU486 (mifepristone) causes Treg cell deficiency in a mouse model [49].
P4 might protect against PE by expanding the number of Treg cells at the maternal–fetal interface. In mice, PGR deletion on DCs promotes a non-tolerogenic, mature DC phenotype, along with failure to generate CD4+Tregs and CD8+CD122+Treg cells and impaired placental and fetal development [151].
6.1.3. CD8+T Cells
P4 decreases the number of CD8+T cells at the maternal–fetal interface (Figure 4). It has been reported that vaginal P4 decreases the proportion of decidual CD8+CD25+Foxp3+T cells at this interface in a mouse model [152]. Therefore, we suppose that P4 might protect against PE by decreasing the number of CD8+T cells at this site.
6.1.4. Macrophage and DCs
P4 promotes a tolerogenic profile of macrophages and dendritic cells (DCs) (Figure 4). For example, in vitro stimulation with P4 induces the maturation of macrophages with an M2-type profile [139,153,154] and prevents dendritic cell differentiation into a mature phenotype [155]. In addition, it has been reported that vaginal P4 decreases the proportion of decidual macrophages at the murine maternal–fetal interface [152].
P4 promotes a tolerogenic profile of macrophages and DCs, which are essential for successful uterine tissue remodeling, pregnancy maintenance, and reducing pregnancy complications such as PE [134,135,136]. Recent evidence has revealed that, in mice, the targeted deletion of PGR on DCs promotes a non-tolerogenic, mature DC phenotype, along with failure to generate CD4+Tregs and CD8+CD122+Treg cells and impaired placental and fetal development [151].
6.1.5. NK Cell
P4 can promote PIBF production, which inhibits dNK cell activity at the maternal–fetal interface (Figure 4). Research has shown that the PIBF present in the cytoplasmic granules of dNK cells contributes to the low dNK activity by inhibiting the release of perforin and other cytotoxic molecules [156].
P4 protects against PE by inhibiting dNK cell activity at the maternal–fetal interface. PIBF has been shown to decrease inflammation and cytolytic NK cells to prevent hypertension in a RUPP rat model of PE [47]. The adoptive transfer of spleen cells with high NK activity to pregnant mice increases fetal loss, which is counteracted by PIBF treatment [157]. In mice, PIBF depletion during the peri-implantation period results in reduced implantation and increased resorption rates, together with increased decidual and peripheral NK cell activity [146]. By contrast, the increased resorption rates observed in PIBF-depleted mice were corrected by treating them with anti-NK antibodies [158], suggesting that PIBF contributes to successful murine gestation by controlling NK activity.
6.1.6. Cytokine
In addition, P4 promotes the production of anti-inflammatory cytokines while inhibiting that of pro-inflammatory cytokines, thus hindering the immune response at the maternal–fetal interface [142,154]. P4 and its metabolites reduce TNF-α and IFN-γ production in CD4+ and CD8+T cells [159], which are important for immune tolerance at the maternal–fetal interface. Additionally, P4 can enhance the immunosuppressive environment by upregulating specific cytokines such as IL-10, which is crucial for protecting the fetus from maternal immune attacks [154].
P4 protects against PE by regulating pro- and anti-cytokines at the maternal–fetal interface. For example, in PE women, after P4 administration, TNF-α and interleukin-1beta (IL-1β) levels were decreased, while those of IL-4, IL-10, interleukin-13 (IL-13), cyclin D1, and proliferating cell nuclear antigen (PCNA) were increased [44]. P4 can significantly reduce interleukin-18 (IL-18) expression and increase CD56+CD16-NK cell growth, suggesting that these activities may underlie the mechanism by which P4 improves pregnancy outcomes [160]. Moreover, P4 treatment significantly reduced the secretion of inflammatory cytokines in the serum, macrophages, and trophoblasts of B. abortus-infected mice, leading to decreased placentitis and enhanced pup viability [161]. Insufficient P4 may lead to the activation of inflammatory responses in the placenta, thereby triggering PE [44].
Therefore, P4 can inhibit the activation of various pro-inflammatory cells (e.g., Th1 cells, CD8+T cells, M1-type macrophages, and cytotoxic NK cells) and cytokines (e.g., TNF-α and IFN-γ), and increase anti-inflammatory cells (e.g., Th2 cells, M2-type macrophages, and Treg cells) and cytokines (e.g., IL-10 and IL-4) at the maternal–fetal interface, exhibiting a net anti-inflammatory effect supporting successful pregnancy.
6.2. P4 Might Protect Against PE by Regulating Trophoblasts for Immune Tolerance
P4 stimulation has been reported to enhance the expression of HLA-C and HLA-G, which are key molecules involved in immune tolerance [74]. An organ-on-chip model of the maternal–fetal membrane interface revealed that HLA-G and PGRMC2 produced by chorionic trophoblasts modulate decidual immune cell inflammation, thus serving as an immunological barrier preventing membrane compromise [97]. In addition, in the placenta, P4 not only regulates immune cell activity, but also influences the secretory function of placental cells and regulates the placental response to the maternal immune system, thereby ensuring smooth pregnancy progression [162]. P4-driven B7-H4 (VTCN1), an immunosuppressive protein in trophoblasts, contributes to maternal–fetal immune tolerance by inhibiting CD8+T cell activity [163]. This evidence demonstrates the effect of P4 on trophoblasts for immune tolerance, which might influence immune cell function to protect against PE (Figure 4).
7. P4 Might Protect Against PE by Promoting Trophoblast Proliferation and Adhesion
Trophoblast cells, the primary cell type in the placenta, are crucial for successful implantation and placental development. In normal pregnancies, trophoblasts undergo a process of differentiation and invasion that allows them to remodel maternal blood vessels and establish a healthy placental environment. However, when these processes are disrupted, preeclampsia develops, leading to inadequate placental ischemia and elevated blood pressure in the mother [164,165,166,167]. The ability of trophoblasts to proliferate and adhere is essential for their invasive capabilities, and any impairment in these functions can trigger the cascade of events that culminate in preeclampsia [168,169].
Research has shown that P4 treatment can promote trophoblast proliferation and adhesion and inhibit trophoblast apoptosis [44,46,170]. P4 upregulates protein O-fucosyltransferase 1 (poFUT1) expression via the specific transcription factor AP-1 family members (c-Fos/c-Jun) and then increases trophoblast cell proliferation and adhesion potential [170]. Research has also shown that PGR deficiency downregulates cyclin D1 protein levels, which plays an important role in JEG3 cell proliferation [65]. In mouse trophoblast giant cells (TGCs), P4 protects against lamin B1 loss and prolongs the life and function of TGCs [171]. This evidence indicated that P4 might protect against PE by regulating proliferation and adhesion (Figure 5).
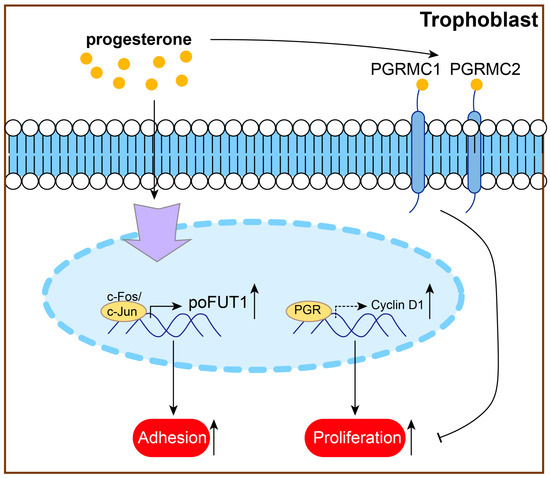
Figure 5.
P4 might protect against PE by promoting trophoblast proliferation and adhesion.
8. Conclusions
In this review, we summarize the clinical implications of progesterone in preeclampsia. Current RCT studies, systematic reviews, and meta-analyses suggest that P4 supplementation has a preventive effect on PE incidence in patients with singleton pregnancies, both in normal conception and assisted reproductive technology. On one hand, key enzyme or metabolite abnormalities during P4 synthesis and metabolism lead to PE development. On the other, P4 might protect against PE by regulating immune response at the maternal–fetal interface, inhibiting vascular function, and diminishing trophoblast proliferation and adhesion. However, research should also focus on the comprehensive mechanisms by which P4 is involved in PE pathogenesis at the maternal–fetal interface. Additionally, despite preliminary positive results, large-scale randomized controlled trials are required to validate the efficacy and safety of P4 interventions. Future research should focus on optimal administration routes, dosages, and the combined use of P4 with other therapeutic approaches to provide more effective strategies for managing gestational hypertension.
Author Contributions
Conceptualization, Z.L. and W.G.; methodology, Z.L.; software, Z.L.; validation, Z.L. and W.G.; formal analysis, Z.L.; investigation, Z.L.; resources, Z.L.; data curation, Z.L.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L. and W.G.; visualization, Z.L.; supervision, W.G.; project administration, W.G.; funding acquisition, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanghai Municipal Health Commission, grant number 202240082; and Science and Technology Commission of Shanghai Municipality (STCSM), grant number 21Y11907800.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Maski, M.R.; Bajracharya, S.; Wenger, J.B.; Zhang, D.; Salahuddin, S.; Shahul, S.S.; Thadhani, R.; Seely, E.W.; Karumanchi, S.A.; et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation 2015, 132, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Zhang, K.; Yan, Y.; Wu, J.; Wang, F.; Zhao, Y.; Xu, H.; Jiang, W.; Yu, D.; et al. Progesterone attenuates hypertension and autoantibody levels to the angiotensin II type 1 receptor in response to elevated cadmium during pregnancy. Placenta 2018, 62, 16–24. [Google Scholar] [CrossRef]
- Melo, P.; Devall, A.; Shennan, A.H.; Vatish, M.; Becker, C.M.; Granne, I.; Papageorghiou, A.T.; Mol, B.W.; Coomarasamy, A. Vaginal micronised progesterone for the prevention of hypertensive disorders of pregnancy: A systematic review and meta-analysis. Int. J. Obstet. Gynaecol. 2024, 131, 727–739. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Lin, X.; He, J.; Wang, S.; Zhou, P. Pregnancy-related complications and perinatal outcomes following progesterone supplementation before 20 weeks of pregnancy in spontaneously achieved singleton pregnancies: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 165. [Google Scholar] [CrossRef]
- Tskhay, V.; Schindler, A.; Shestakova, M.; Klimova, O.; Narkevich, A. The role of progestogen supplementation (dydrogesterone) in the prevention of preeclampsia. Gynecol. Endocrinol. 2020, 36, 698–701. [Google Scholar] [CrossRef]
- Tskhay, V.B.; Kovtun, N.M.; Schindler, A.E. Successful prevention of preeclampsia in a high-risk pregnancy using progestogen dydrogesterone: A clinical case. Horm. Mol. Biol. Clin. Investig. 2016, 27, 85–88. [Google Scholar] [CrossRef]
- Bencker, C.; Gschwandtner, L.; Nayman, S.; Grikšienė, R.; Nguyen, B.; Nater, U.M.; Guennoun, R.; Sundström-Poromaa, I.; Pletzer, B.; Bixo, M.; et al. Progestagens and progesterone receptor modulation: Effects on the brain, mood, stress, and cognition in females. Front. Neuroendocrinol. 2025, 76, 101160. [Google Scholar] [CrossRef]
- Vondra, S.; Kunihs, V.; Eberhart, T.; Eigner, K.; Bauer, R.; Haslinger, P.; Haider, S.; Windsperger, K.; Klambauer, G.; Schütz, B.; et al. Metabolism of cholesterol and progesterone is differentially regulated in primary trophoblastic subtypes and might be disturbed in recurrent miscarriages. J. Lipid Res. 2019, 60, 1922–1934. [Google Scholar] [CrossRef]
- Shin, Y.Y.; Jeong, J.S.; Park, M.; Lee, J.; An, S.; Cho, W.; Kim, S.C.; An, B.; Lee, K. Regulation of steroid hormones in the placenta and serum of women with preeclampsia. Mol. Med. Rep. 2018, 17, 2681–2688. [Google Scholar] [CrossRef]
- Shin, Y.Y.; An, S.-M.; Jeong, J.S.; Yang, S.Y.; Lee, G.-S.; Hong, E.-J.; Jeung, E.-B.; Kim, S.C.; An, B.-S. Comparison of steroid hormones in three different preeclamptic models. Mol. Med. Rep. 2021, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.; Meng, Z.; Jia, R.; Lian, F.; Lin, F. Cadmium-induced preeclampsia-like phenotype in the rat is related to decreased progesterone synthesis in the placenta. Xenobiotica 2022, 52, 625–632. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Xu, W.; Chen, Y.; Liu, X.; Xi, M. Abnormal apoptosis of trophoblastic cells is related to the up-regulation of CYP11A gene in placenta of preeclampsia patients. PLoS ONE 2013, 8, e59609. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Zhang, X.; Li, Y.; He, G.; Dinnyés, A.; Sun, Q.; Xu, W. CYP11A1 Upregulation Leads to Trophoblast Oxidative Stress and Fetal Neurodevelopmental Toxicity That can be Rescued by Vitamin D. Front. Mol. Biosci. 2020, 7, 608447. [Google Scholar] [CrossRef]
- Pan, T.; He, G.; Chen, M.; Bao, C.; Chen, Y.; Liu, G.; Zhou, M.; Li, S.; Xu, W.; Liu, X. Abnormal CYP11A1 gene expression induces excessive autophagy, contributing to the pathogenesis of preeclampsia. Oncotarget 2017, 8, 89824–89836. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Bhinderwala, F.; Powers, R.; McFee, R.M.; Cupp, A.S.; Wood, J.R.; Davis, J.S. Metabolic control of luteinizing hormone-responsive ovarian steroidogenesis. J. Biol. Chem. 2025, 301, 108042. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Korevaar, T.I.M.; Jaddoe, V.W.V.; de Rijke, Y.B.; Peeters, R.P.; Steegers, E.A.P. Human chorionic gonadotropin and risk of pre-eclampsia: Prospective population-based cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 477–483. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, X.; Wu, B.; Jiang, C.; Yin, Y. Relationship between increased maternal serum free human chorionic gonadotropin levels in the second trimester and adverse pregnancy outcomes: A retrospective cohort study. BMC Womens Health 2024, 24, 323. [Google Scholar] [CrossRef]
- Hasija, A.; Balyan, K.; Debnath, E.; V., R.; Kumar, M. Prediction of hypertension in pregnancy in high risk women using maternal factors and serial placental profile in second and third trimester. Placenta 2021, 104, 236–242. [Google Scholar] [CrossRef]
- Norris, W.; Nevers, T.; Sharma, S.; Kalkunte, S. Review: hCG, preeclampsia and regulatory T cells. Placenta 2011, 32, S182–S185. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, L.; Wang, X.; Liu, Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc. Res. 2021, 135, 104130. [Google Scholar] [CrossRef] [PubMed]
- Elazab, R.; Alkhiary, M.; Bedairi, M.; Wageh, A. Simultaneous use of Tumor Necrosis Factor, Lipid Profile, and beta-hCG As Markers of Severity of Preeclampsia. J. Obstet. Gynaecol. India 2022, 72, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Khanam, Z.; Mittal, P.; Suri, J. Does the Addition of Serum PAPP-A and beta-hCG Improve the Predictive Value of Uterine Artery Pulsatility Index for Preeclampsia at 11-14 Weeks of Gestation? A Prospective Observational Study. J. Obstet. Gynaecol. India 2021, 71, 226–234. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, Z.; Li, W.; Mei, L.; Shen, L.; Shan, H. Prediction of twin pregnancy preeclampsia based on clinical risk factors, early pregnancy serum markers, and uterine artery pulsatility index. Pak. J. Med. Sci. 2021, 37, 1727–1733. [Google Scholar] [CrossRef]
- Murmu, S.; Dwivedi, J. Second-Trimester Maternal Serum Beta-Human Chorionic Gonadotropin and Lipid Profile as a Predictor of Gestational Hypertension, Preeclampsia, and Eclampsia: A Prospective Observational Study. Int. J. Appl. Basic Med. Res. 2020, 10, 49–53. [Google Scholar] [CrossRef]
- Skogler, J.; Moberg, T.; Tancredi, L.; Styrmisdóttir, L.; Hedayati, E.; Alarcon-Ruiz, C.A.; Khamis, A.; Persad, E.; Iskandarani, G.; Hansson, S.R.; et al. Association between human chorionic gonadotropin (hCG) levels and adverse pregnancy outcomes: A systematic review and meta-analysis. Pregnancy Hypertens. 2023, 34, 124–137. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, H. The role of serum markers PAPP-A beta-hCG, AFP, and uE3 in predicting the risk of preeclampsia in early, middle, and late pregnancy. Technol. Health Care 2023, 31, 1027–1037. [Google Scholar] [CrossRef]
- Zhang, X.; Huangfu, Z.; Shi, F.; Xiao, Z. Predictive Performance of Serum beta-hCG MoM Levels for Preeclampsia Screening: A Meta-Analysis. Front. Endocrinol. 2021, 12, 619530. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, H. Effects of menopausal hormone therapy-based on the role of estrogens, progestogens, and their metabolites in proliferation of breast cancer cells. Cancer Biol. Med. 2021, 19, 432–449. [Google Scholar] [CrossRef]
- Klein, J. Progesterone Metabolism in Digitalis and Other Plants-60 Years of Research and Recent Results. Plant Cell Physiol. 2024, 65, 1500–1514. [Google Scholar] [CrossRef]
- Trummer, O.; Stern, C.; Reintar, S.; Mayer-Pickel, K.; Cervar-Zivkovic, M.; Dischinger, U.; Kurlbaum, M.; Huppertz, B.; Fluhr, H.; Obermayer-Pietsch, B. Steroid Profiles and Precursor-to-Product Ratios Are Altered in Pregnant Women with Preeclampsia. Int. J. Mol. Sci. 2024, 25, 12704. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.W.; Kirkwood, P.M.; Rebourcet, D.; Cousins, F.L.; Ainslie, R.J.; Livingstone, D.E.W.; Smith, L.B.; Saunders, P.T.; Gibson, D.A. A role for steroid 5 alpha-reductase 1 in vascular remodeling during endometrial decidualization. Front. Endocrinol. 2022, 13, 1027164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, H.; Feng, Y.; Wu, W.; Li, S.; Thompson, B.; Xie, B.; Guo, P.; Li, M.; Wang, Y.; et al. Polymorphisms in Sex Hormone Metabolism Genes and Risk of Preeclampsia in Taiyuan, China. Gynecol. Obstet. Investig. 2018, 83, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Jia, X.; Deng, Z.; Liu, X.; Liu, H.; Yu, H.; Liu, S. Exploration of CYP21A2 and CYP17A1 polymorphisms and preeclampsia risk among Chinese Han population: A large-scale case-control study based on 5021 subjects. Hum. Genom. 2020, 14, 33. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, J.; Gu, X.; Li, L.; Han, J. Downregulation of aromatase plays a dual role in preeclampsia. Mol. Hum. Reprod. 2021, 27, gaab013. [Google Scholar] [CrossRef]
- Lau, S.L.; Yuen, L.Y.; Ho, C.S.; Chan, M.H.M.; Ma, R.C.W.; Tam, W.H. Gestational Age-specific Reference Intervals for Androgens in Pregnancy. J. Clin. Endocrinol. Metab. 2024, 110, 176–184. [Google Scholar] [CrossRef]
- Othman, M.A.; Badawy, E.R.; Sobh, A.M.A.; Shaltout, A.S.; Mostafa, S.A. Effect of 17-Hydroxyprogesterone Caproate on Interleukin-6 and Tumor necrosis factor-alpha in expectantly managed early-onset preeclampsia. Egypt J. Immunol. 2023, 30, 109–118. [Google Scholar] [CrossRef]
- Amaral, L.M.; Faulkner, J.L.; Elfarra, J.; Cornelius, D.C.; Cunningham, M.W.; Ibrahim, T.; Vaka, V.R.; McKenzie, J.; LaMarca, B. Continued Investigation Into 17-OHPC: Results From the Preclinical RUPP Rat Model of Preeclampsia. Hypertension 2017, 70, 1250–1255. [Google Scholar] [CrossRef]
- Elfarra, J.T.; Cottrell, J.N.; Cornelius, D.C.; Cunningham, M.W., Jr.; Faulkner, J.L.; Ibrahim, T.; Lamarca, B.; Amaral, L.M. 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Pregnancy Hypertens. 2020, 19, 226–232. [Google Scholar] [CrossRef]
- Kiprono, L.V.; Wallace, K.; Moseley, J.; Martin, J., Jr.; LaMarca, B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am. J. Obstet. Gynecol. 2013, 209, 44.e1–44.e6. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.M.; Cottrell, J.N.; Comley, K.M.; Cunningham, M.W., Jr.; Witcher, A.; Vaka, V.R.; Ibrahim, T.; LaMarca, B. 17-Hydroxyprogesterone caproate improves hypertension and renal endothelin-1 in response to sFlt-1 induced hypertension in pregnant rats. Pregnancy Hypertens. 2020, 22, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Keiser, S.D.; Veillon, E.W.; Parrish, M.R.; Bennett, W.; Cockrell, K.; Fournier, L.; Granger, J.P.; Martin, J.N.; LaMarca, B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am. J. Hypertens. 2009, 22, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, L.; Ding, Y.; Peng, M.; Deng, Y. Progesterone Enhances the Invasion of Trophoblast Cells by Activating PI3K/AKT Signaling Pathway to Prevent Preeclampsia. Cell Transplant. 2023, 32, 09636897221145682. [Google Scholar] [CrossRef]
- Uddin, M.N.; Horvat, D.; Jones, R.O.; Beeram, M.R.; Zawieja, D.C.; Perger, L.; Sprague, D.C.C.; Kuehl, T.J. Suppression of aldosterone and progesterone in preeclampsia. J. Matern. Fetal Neonatal Med. 2015, 28, 1296–1301. [Google Scholar] [CrossRef]
- Pei, J.; Liu, Z.; Wang, C.; Chu, N.; Liu, L.; Tang, Y.; Liu, H.; Xiang, Q.; Cheng, H.; Li, M.; et al. Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia. Biomolecules 2022, 12, 422. [Google Scholar] [CrossRef]
- Cottrell, J.N.; Witcher, A.C.; Comley, K.; Cunningham, M.W., Jr.; Ibrahim, T.; Cornelius, D.C.; LaMarca, B.; Amaral, L.M. Progesterone-induced blocking factor improves blood pressure, inflammation, and pup weight in response to reduced uterine perfusion pressure (RUPP). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R719–R727. [Google Scholar] [CrossRef]
- Meadors, A.; Comley, K.; Cottrell, J.N.; Ibhahim, T.; Cunningham, M.W., Jr.; Amaral, L.M. Progesterone-induced blocking factor blockade causes hypertension in pregnant rats. Am. J. Reprod. Immunol. 2024, 91, e13805. [Google Scholar] [CrossRef]
- Green, E.S.; Moldenhauer, L.M.; Groome, H.M.; Sharkey, D.J.; Chin, P.Y.; Care, A.S.; Robker, R.L.; McColl, S.R.; Robertson, S.A. Regulatory T cells are paramount effectors in progesterone regulation of embryo implantation and fetal growth. JCI Insight 2023, 8, e162995. [Google Scholar] [CrossRef]
- Kale, K.; Vishwekar, P.; Balsarkar, G.; Jassawalla, M.J.; Sawant, G.; Madan, T. Differential levels of surfactant protein A, surfactant protein D, and progesterone to estradiol ratio in maternal serum before and after the onset of severe early-onset preeclampsia. Am. J. Reprod. Immunol. 2020, 83, e13208. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, Y.; Peng, W.; Zhang, N.; Ye, Y. Progesterone inhibited endoplasmic reticulum stress associated apoptosis induced by interleukin-1beta via the GRP78/PERK/CHOP pathway in BeWo cells. J. Obstet. Gynaecol. Res. 2018, 44, 463–473. [Google Scholar] [CrossRef]
- Wan, J.; Hu, Z.; Zeng, K.; Yin, Y.; Zhao, M.; Chen, M.; Chen, Q. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2018, 11, 18–25. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, A.; Lu, X.; Chen, B.; Hua, Y.; Ma, Y. Association of phthalates exposure and sex steroid hormones with late-onset preeclampsia: A case-control study. BMC Pregnancy Childbirth 2024, 24, 577. [Google Scholar] [CrossRef]
- Zheng, J.J.; Wang, H.O.; Huang, M.; Zheng, F.Y. Assessment of ADMA, estradiol, and progesterone in severe preeclampsia. Clin. Exp. Hypertens. 2016, 38, 347–351. [Google Scholar] [CrossRef]
- Yaghi, O.; Prasad, S.; Boorman, H.; Kalafat, E.; Khalil, A. Is micronized vaginal progesterone effective for the prevention of preeclampsia in twin pregnancies? Am. J. Obstet. Gynecol. 2024, 231, e72–e75. [Google Scholar] [CrossRef]
- Razi, Z.R.M.; Schindler, A.E. Review on role of progestogen (dydrogesterone) in the prevention of gestational hypertension. Horm. Mol. Biol. Clin. Investig. 2016, 27, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.E. Present and future aspects of dydrogesterone in prevention or treatment of pregnancy disorders: An outlook. Horm. Mol. Biol. Clin. Investig. 2016, 27, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.R.Z.; Lim, J.F.; Nawawi, N.H.M.; Luqman, M.; Zolkeplai, M.F.; Rangkuty, H.S.; Nor, N.A.M.; Tamil, A.; Shah, S.A.; Tham, S.W.; et al. A pilot study to determine whether progestogen supplementation using dydrogesterone during the first trimester will reduce the incidence of gestational hypertension in primigravidae. Gynecol. Endocrinol. 2014, 30, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.B.; Ahmad, M.F.B.; Kwang, N.B.; Shan, L.P.; Shafie, N.M.; Omar, M.H. Dydrogesterone support following assisted reproductive technique (ART) reduces the risk of pre-eclampsia. Horm. Mol. Biol. Clin. Investig. 2016, 27, 93–96. [Google Scholar] [CrossRef]
- Elenis, E.; Joelsson, L.S.; Stavreus-Evers, A.; Wånggren, K. Perinatal outcomes of progesterone in natural frozen-thawed embryo transfer pregnancies: Insights from 2 randomized controlled trials. Fertil. Steril. 2024, 124, 104–112. [Google Scholar] [CrossRef]
- Conrad, K.P.; von Versen-Höynck, F.; Baker, V.L. Potential role of the corpus luteum in maternal cardiovascular adaptation to pregnancy and preeclampsia risk. Am. J. Obstet. Gynecol. 2022, 226, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94 (Suppl. S161), 8–16. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Ng, S.S.M.; Baig, K.M.; Driggers, P.; Segars, J. Progesterone-Mediated Non-Classical Signaling. Trends Endocrinol. Metab. 2017, 28, 656–668. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, C.; Zhao, S.; Wang, B.; Wang, H.; Zhang, J.; Wang, Y.; Cheng, H.; Zhu, L.; Shen, R.; et al. Exposure to DEHP or its metabolite MEHP promotes progesterone secretion and inhibits proliferation in mouse placenta or JEG-3 cells. Environ. Pollut. 2020, 257, 113593. [Google Scholar] [CrossRef]
- De Guzman, R.M.; Jacobskind, J.S.; Rosinger, Z.J.; Rybka, K.A.; Parra, K.E.; Caballero, A.L.; Sharif, M.S.; Justice, N.J.; Zuloaga, D.G. Hormone Regulation of Corticotropin-Releasing Factor Receptor 1 in the Female Mouse Brain. Neuroendocrinology 2024, 114, 1139–1157. [Google Scholar] [CrossRef]
- Chiang, S. Recent advances in smooth muscle tumors with PGR and PLAG1 gene fusions and myofibroblastic uterine neoplasms. Genes Chromosomes Cancer 2021, 60, 138–146. [Google Scholar] [CrossRef]
- Goldman, S.; Shalev, E. Difference in progesterone-receptor isoforms ratio between early and late first-trimester human trophoblast is associated with differential cell invasion and matrix metalloproteinase 2 expression. Biol. Reprod. 2006, 74, 13–22. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, J.H.; Park, T.C.; Lee, H.J. Relationship Between Gonadotropin-Releasing Hormone/Gonadotropin-Releasing Hormone Receptor Signaling and Progesterone Receptors in Human Trophoblasts. J. Reprod. Med. 2016, 61, 275–281. [Google Scholar]
- Park, M.; Park, K.; Lee, J.; Shin, Y.Y.; An, S.; Kang, S.S.; Cho, W.; An, B.; Kim, S.C. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int. J. Mol. Med. 2018, 41, 2943–2951. [Google Scholar] [CrossRef]
- Ferreira, L.M.R.; Meissner, T.B.; Mikkelsen, T.S.; Mallard, W.; O’dOnnell, C.W.; Tilburgs, T.; Gomes, H.A.B.; Camahort, R.; Sherwood, R.I.; Gifford, D.K.; et al. A distant trophoblast-specific enhancer controls HLA-G expression at the maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2016, 113, 5364–5369. [Google Scholar] [CrossRef]
- Pavličev, M.; Wagner, G.P.; Chavan, A.R.; Owens, K.; Maziarz, J.; Dunn-Fletcher, C.; Kallapur, S.G.; Muglia, L.; Jones, H. Single-cell transcriptomics of the human placenta: Inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017, 27, 349–361. [Google Scholar] [CrossRef]
- Tilburgs, T.; Crespo, Â.C.; van der Zwan, A.; Rybalov, B.; Raj, T.; Stranger, B.; Gardner, L.; Moffett, A.; Strominger, J.L. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 7219–7224. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ferreira, L.M.; Mazzanti, A.; Yu, H.; Gu, B.; Meissner, T.B.; Li, Q.; Strominger, J.L. Progesterone-mediated remodeling of the maternal-fetal interface by a PGRMC1-dependent mechanism. J. Reprod. Immunol. 2024, 163, 104244. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, A.; Pereira, M.T.; Aslan, S.; Payan-Carreira, R.; Reichler, I.M.; Agaoglu, R.A.; Kowalewski, M.P. Membrane-bound progesterone receptors in the canine uterus and placenta; possible targets in the maintenance of pregnancy. Theriogenology 2023, 210, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, S.; Grazul-Bilska, A.T.; Borowicz, P.P.; Reyaz, A.; Valkov, V.; Reynolds, L.P. Placental development during early pregnancy in sheep: Progesterone and estrogen receptor protein expression. Theriogenology 2018, 114, 273–284. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Bairagi, S.; Kraisoon, A.; Dorsam, S.T.; Reyaz, A.; Navanukraw, C.; Borowicz, P.P.; Reynolds, L.P. Placental development during early pregnancy in sheep: Nuclear estrogen and progesterone receptor mRNA expression in the utero-placental compartments. Domest. Anim. Endocrinol. 2019, 66, 27–34. [Google Scholar] [CrossRef]
- Steinhauser, C.; Bazer, F.; Burghardt, R.; Johnson, G. Expression of progesterone receptor in the porcine uterus and placenta throughout gestation: Correlation with expression of uteroferrin and osteopontin. Domest. Anim. Endocrinol. 2017, 58, 19–29. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Castillo-Marco, N.; Clemente-Ciscar, M.; Cordero, T.; Muñoz-Blat, I.; Amadoz, A.; Jimenez-Almazan, J.; Monfort-Ortiz, R.; Climent, R.; Perales-Marin, A.; et al. Disrupted PGR-B and ESR1 signaling underlies defective decidualization linked to severe preeclampsia. Elife 2021, 10, e70753. [Google Scholar] [CrossRef]
- Zhou, P.; Ouyang, L.; Jiang, T.; Tian, Y.; Deng, W.; Wang, H.; Kong, S.; Lu, Z. Progesterone and cAMP synergistically induce SHP2 expression via PGR and CREB1 during uterine stromal decidualization. FEBS J. 2024, 291, 142–157. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Suzuki, M.; Mochizuki, H.; Kametaka, S.; Ohmaru-Nakanishi, T.; Azumi, M.; Kusama, K.; Kato, K.; Tamura, K. Downregulation of PGRMC1 accelerates differentiation and fusion of a human trophoblast cell line. J. Endocrinol. 2024, 260, e230163. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Kojima, J.; Yonekawa, R.; Azumi, M.; Kusama, K.; Nishi, H.; Tamura, K. PGRMC1 Regulates Cellular Senescence via Modulating FOXO1 Expression in Decidualizing Endometrial Stromal Cells. Biomolecules 2022, 12, 1046. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Negishi, R.; Mukoyama, T.; Yonekawa, R.; Kojima, J.; Azumi, M.; Kusama, K.; Nishi, H.; Tamura, K. Regulatory action of PGRMC1 on cyclic AMP-mediated COX2 expression in human endometrial cells. J. Pharmacol. Sci. 2023, 153, 188–196. [Google Scholar] [CrossRef]
- Yokouchi-Konishi, T.; Liu, Y.; Feng, L. Progesterone receptor membrane component 2 is critical for human placental extravillous trophoblast invasion. Biol. Reprod. 2023, 109, 759–771. [Google Scholar] [CrossRef]
- Medina-Laver, Y.; Gonzalez-Martin, R.; de Castro, P.; Diaz-Hernandez, I.; Alama, P.; Quiñonero, A.; Palomar, A.; Dominguez, F. Deciphering the role of PGRMC2 in the human endometrium during the menstrual cycle and in vitro decidualization using an in vitro approach. Hum. Reprod. 2024, 39, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.S.; Pierron, V.; Michalovich, D.; Astle, S.; Thornton, S.; Peltoketo, H.; Lam, E.W.-F.; Gellersen, B.; Huhtaniemi, I.; Allen, J.; et al. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J. Endocrinol. 2005, 187, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Dong, J.; Thomas, P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-alpha. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E899–E911. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J.; Groenen, P.; Kelder, J.; de Vlieg, J.; Zhu, Y.; Tubbs, C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology 2007, 148, 705–718. [Google Scholar] [CrossRef]
- Lu, J.; Reese, J.; Zhou, Y.; Hirsch, E. Progesterone-induced activation of membrane-bound progesterone receptors in murine macrophage cells. J. Endocrinol. 2015, 224, 183–194. [Google Scholar] [CrossRef]
- Intlekofer, K.A.; Petersen, S.L. 17beta-estradiol and progesterone regulate multiple progestin signaling molecules in the anteroventral periventricular nucleus, ventromedial nucleus and sexually dimorphic nucleus of the preoptic area in female rats. Neuroscience 2011, 176, 86–92. [Google Scholar] [CrossRef]
- Peluso, J.J. Progesterone Signaling and Mammalian Ovarian Follicle Growth Mediated by Progesterone Receptor Membrane Component Family Members. Cells 2022, 11, 1632. [Google Scholar] [CrossRef]
- Pru, J.K. Pleiotropic Actions of PGRMC Proteins in Cancer. Endocrinology 2022, 163, bqac078. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Willibald, M.; Bayer, G.; Stahlhut, V.; Poschmann, G.; Stühler, K.; Gierke, B.; Pawlak, M.; Seeger, H.; Mueck, A.O.; Niederacher, D.; et al. Progesterone receptor membrane component 1 is phosphorylated upon progestin treatment in breast cancer cells. Oncotarget 2017, 8, 72480–72493. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Zhang, Y.; Mueck, A.O.; Willibald, M.; Seeger, H.; Fehm, T.; Brucker, S.; Neubauer, H. Increased expression of progesterone receptor membrane component 1 is associated with aggressive phenotype and poor prognosis in ER-positive and negative breast cancer. Menopause 2017, 24, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Schneck, H.; Ruan, X.; Seeger, H.; Cahill, M.A.; Fehm, T.; Mueck, A.O.; Neubauer, H. Membrane-receptor initiated proliferative effects of dienogest in human breast cancer cells. Gynecol. Endocrinol. 2013, 29, 160–163. [Google Scholar] [CrossRef]
- Lintao, R.C.V.; Richardson, L.S.; Kammala, A.K.; Chapa, J.; Yunque-Yap, D.A.; Khanipov, K.; Golovko, G.; Dalmacio, L.M.M.; Menon, R. PGRMC2 and HLA-G regulate immune homeostasis in a microphysiological model of human maternal-fetal membrane interface. Commun. Biol. 2024, 7, 1041. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Remus, E.W.; Sayeed, I.; Won, S.; Lyle, A.N.; Stein, D.G. Progesterone protects endothelial cells after cerebrovascular occlusion by decreasing MCP-1- and CXCL1-mediated macrophage infiltration. Exp. Neurol. 2015, 271, 401–408. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Kennard, S.; Huby, A.-C.; Antonova, G.; Lu, Q.; Jaffe, I.Z.; Patel, V.S.; Fulton, D.J.R.; de Chantemèle, E.J.B. Progesterone Predisposes Females to Obesity-Associated Leptin-Mediated Endothelial Dysfunction via Upregulating Endothelial MR (Mineralocorticoid Receptor) Expression. Hypertension 2019, 74, 678–686. [Google Scholar] [CrossRef]
- Park, Y.G.; Choi, J.; Seol, J.W. Angiopoietin-2 regulated by progesterone induces uterine vascular remodeling during pregnancy. Mol. Med. Rep. 2020, 22, 1235–1242. [Google Scholar] [CrossRef]
- Mallawarachchi, S.; Cebecioglu, R.E.; Althumayri, M.; Beker, L.; Fernando, S.; Koydemir, H.C. Systematic design and evaluation of aptamers for VEGF and PlGF biomarkers of Preeclampsia. BMC Biotechnol. 2024, 24, 64. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Mannaerts, D.; Ratajczak, J.; Boeren, E.; Faes, E.; Van Craenenbroeck, A.H.; Jacquemyn, Y.; Van Craenenbroeck, E.M. MicroRNAs targeting VEGF are related to vascular dysfunction in preeclampsia. Biosci. Rep. 2021, 41, BSR20210874. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, L.; Man, C.W.; Huang, J.; Wang, C.C.; Zhang, S.; Li, T.C. Differential expression of vascular endothelial growth factor angiogenic factors in different endometrial compartments in women who have an elevated progesterone level before oocyte retrieval, during in vitro fertilization-embryo transfer treatment. Fertil. Steril. 2015, 104, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.; Sharifi, M.; Rashidi, B. Ovarian stimulation and exogenous progesterone affect the endometrial miR-16-5p, VEGF protein expression, and angiogenesis. Microvasc. Res. 2021, 133, 104074. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.; Sharifi, M.; Rashidi, B. Evaluating the effect of ovarian stimulation and exogenous progesterone on CD31-positive cell density, VEGF protein, and miR-17-5p expression of endometrium immediately before implantation. Biomed. Pharmacother. 2021, 133, 110922. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, H.J.; Seol, J.W.; Jang, J.Y.; Cho, Y.; Kim, K.R.; Choi, Y.; Lydon, J.P.; DeMayo, F.J.; Shibuya, M.; et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med. 2013, 5, 1415–1430. [Google Scholar] [CrossRef]
- Canumil, V.A.; Bogetti, E.; de la Cruz Borthiry, F.L.; Ribeiro, M.L.; Beltrame, J.S. Steroid hormones and first trimester vascular remodeling. Vitam. Horm. 2021, 116, 363–387. [Google Scholar] [CrossRef]
- Cutini, P.H.; Massheimer, V.L. In vitro effects of progesterone and the synthetic progestin medroxyprogesterone acetate on vascular remodeling. Mol. Cell Endocrinol. 2019, 498, 110543. [Google Scholar] [CrossRef]
- Alawadhi, M.; Kilarkaje, N.; Mouihate, A.; Al-Bader, M.D. Role of progesterone on dexamethasone-induced alterations in placental vascularization and progesterone receptors in ratsdagger. Biol. Reprod. 2023, 108, 133–149. [Google Scholar] [CrossRef]
- Yu, P.; Li, S.; Zhang, Z.; Wen, X.; Quan, W.; Tian, Q.; Gao, C.; Su, W.; Zhang, J.; Jiang, R. Progesterone-mediated angiogenic activity of endothelial progenitor cell and angiogenesis in traumatic brain injury rats were antagonized by progesterone receptor antagonist. Cell Prolif. 2017, 50, e12362. [Google Scholar] [CrossRef]
- Xu, X.; Ruan, X.; Ju, R.; Wang, Z.; Yang, Y.; Cheng, J.; Gu, M.; Mueck, A.O. Progesterone Receptor Membrane Component-1 May Promote Survival of Human Brain Microvascular Endothelial Cells in Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Dement. 2022, 37, 15333175221109749. [Google Scholar] [CrossRef]
- Xia, Z.; Xiao, J.; Dai, Z.; Chen, Q. Membrane progesterone receptor alpha (mPRalpha) enhances hypoxia-induced vascular endothelial growth factor secretion and angiogenesis in lung adenocarcinoma through STAT3 signaling. J. Transl. Med. 2022, 20, 72. [Google Scholar] [CrossRef]
- Kim, S.; Shim, S.; Kwon, J.; Ryoo, S.; Byeon, J.; Hong, J.; Lee, J.H.; Kwon, Y.G.; Kim, J.Y.; Kim, Y.M. Alleviation of preeclampsia-like symptoms through PlGF and eNOS regulation by hypoxia- and NF-kappaB-responsive miR-214-3p deletion. Exp. Mol. Med. 2024, 56, 1388–1400. [Google Scholar] [CrossRef]
- Ssengonzi, R.; Wang, Y.; Maeda-Smithies, N.; Li, F. Endothelial Nitric Oxide synthase (eNOS) in Preeclampsia: An Update. J. Pregnancy Child Health 2024, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Fan, Y.Y.; Yang, C.R.; Gao, X.R.; Zhang, L.L.; Hu, Y.; Wang, Y.Q.; Jun, H. Progesterone amplifies oxidative stress signal and promotes NO production via H2O2 in mouse kidney arterial endothelial cells. J. Steroid Biochem. Mol. Biol. 2016, 155, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Thomas, P. Additive effects of low concentrations of estradiol-17beta and progesterone on nitric oxide production by human vascular endothelial cells through shared signaling pathways. J. Steroid Biochem. Mol. Biol. 2017, 165, 258–267. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tan, W.; Guo, Y.; Luo, M.; Shang, F.-F.; Xia, Y.; Luo, S. Progesterone promotes endothelial nitric oxide synthase expression through enhancing nuclear progesterone receptor-SP-1 formation. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H341–H348. [Google Scholar] [CrossRef]
- Khorram, O.; Han, G. Influence of progesterone on endometrial nitric oxide synthase expression. Fertil. Steril. 2009, 91, 2157–2162. [Google Scholar] [CrossRef]
- Ellmann, S.; Sticht, H.; Thiel, F.; Beckmann, M.W.; Strick, R.; Strissel, P.L. Estrogen and progesterone receptors: From molecular structures to clinical targets. Cell Mol. Life Sci. 2009, 66, 2405–2426. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Protective actions of progesterone in the cardiovascular system: Potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids 2013, 78, 583–588. [Google Scholar] [CrossRef]
- Collier, A.Y.; Smith, L.A.; Karumanchi, S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 2021, 82, 362–370. [Google Scholar] [CrossRef]
- Pianta, S.; Magatti, M.; Vertua, E.; Signoroni, P.B.; Muradore, I.; Nuzzo, A.M.; Rolfo, A.; Silini, A.; Quaglia, F.; Todros, T.; et al. Amniotic mesenchymal cells from pre-eclamptic placentae maintain immunomodulatory features as healthy controls. J. Cell. Mol. Med. 2016, 20, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Mobley, Y.; Henson, J.; Davies, M.; Skariah, A.; Dambaeva, S.; Gilman-Sachs, A.; Beaman, K.; Lampley, C.; Kwak-Kim, J. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J. Reprod. Immunol. 2018, 125, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Leavey, K.; Grynspan, D.; Cox, B.J. Both “canonical” and “immunological” preeclampsia subtypes demonstrate changes in placental immune cell composition. Placenta 2019, 83, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Milosevic-Stevanovic, J.; Krstic, M.; Radovic-Janosevic, D.; Popovic, J.; Tasic, M.; Stojnev, S. Number of decidual natural killer cells & macrophages in pre-eclampsia. Indian J. Med. Res. 2016, 144, 823–830. [Google Scholar] [CrossRef]
- Buckley, R.; Whitley, G.; Dumitriu, I.; Cartwright, J. Macrophage polarisation affects their regulation of trophoblast behaviour. Placenta 2016, 47, 73–80. [Google Scholar] [CrossRef]
- ento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Du, M.; Wang, W.; Huang, L.; Guan, X.; Lin, W.; Yao, J.; Li, L. Natural killer cells in the pathogenesis of preeclampsia: A double-edged sword. J. Matern. Fetal Neonatal Med. 2022, 35, 1028–1035. [Google Scholar] [CrossRef]
- Fukui, A.; Yokota, M.; Funamizu, A.; Nakamua, R.; Fukuhara, R.; Yamada, K.; Kimura, H.; Fukuyama, A.; Kamoi, M.; Tanaka, K.; et al. Changes of NK cells in preeclampsia. Am. J. Reprod. Immunol. 2012, 67, 278–286. [Google Scholar] [CrossRef]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: Role of decidual T regulatory cells. J. Reprod. Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef]
- Steinborn, A.; Schmitt, E.; Kisielewicz, A.; Rechenberg, S.; Seissler, N.; Mahnke, K.; Schaier, M.; Zeier, M.; Sohn, C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol. 2012, 167, 84–98. [Google Scholar] [CrossRef]
- Solano, M.E. Decidual immune cells: Guardians of human pregnancies. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Hecher, K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 2013, 19, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, G.; Tournaye, H.; Macklon, N.; Petraglia, F.; Arck, P.; Blockeel, C.; van Amsterdam, P.; Pexman-Fieth, C.; Fauser, B.C. Dydrogesterone: Pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod. Biomed. Online 2019, 38, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.; Stæhr, C.S.; Klok, F.S.; Lebech, M.; Hviid, T.V.F. Evidence for a shift in placental HLA-G allelic dominance and the HLA-G isoform profile during a healthy pregnancy and preeclampsiadagger. Biol. Reprod. 2021, 105, 846–858. [Google Scholar] [CrossRef]
- Johnsen, G.M.; Fjeldstad, H.E.S.; Drabbels, J.J.M.; Haasnoot, G.W.; Eikmans, M.; Størvold, G.L.; Alnaes-Katjavivi, P.; Jacobsen, D.P.; Scherjon, S.A.; Redman, C.W.G.; et al. A possible role for HLA-G in development of uteroplacental acute atherosis in preeclampsia. J. Reprod. Immunol. 2021, 144, 103284. [Google Scholar] [CrossRef]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Schindler, A.E. Progestogens and immunology. Best. Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 17–23. [Google Scholar] [CrossRef]
- Raghupathy, R.; Szekeres-Bartho, J. Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. Int. J. Mol. Sci. 2022, 23, 1333. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al Mutawa, E.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage. Int. J. Obstet. Gynaecol. 2005, 112, 1096–1101. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al-Mutawa, E.; Al-Azemi, M.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J. Reprod. Immunol. 2009, 80, 91–99. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Wegmann, T.G. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J. Reprod. Immunol. 1996, 31, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Csabai, T.; Pallinger, E.; Kovacs, A.F.; Miko, E.; Bognar, Z.; Szekeres-Bartho, J. Altered Immune Response and Implantation Failure in Progesterone-Induced Blocking Factor-Deficient Mice. Front. Immunol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Hudic, I.; Szekeres-Bartho, J.; Vrtacnik, E.B.; Klun, I.V.; Brkic, S.; Frangez, H.B.; Jancar, N.; Mesalic, L.; Bogdan, A.; Hudic, L.D. Progesterone induced blocking factor (PIBF) taken in early pregnancy predicts the pregnancy outcome in women undergoing in vitro fertilization procedure. J. Reprod. Immunol. 2020, 140, 103150. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Dauven, D.; Zenclussen, A.C. Progesterone-driven local regulatory T cell induction does not prevent fetal loss in the CBA/JxDBA/2J abortion-prone model. Am. J. Reprod. Immunol. 2017, 77, e12626. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Zhao, M.; Lu, F.; Yu, X.; Li, L.; Gu, Z.; Deng, Y.; Guan, R. Progesterone modulates CD4(+) CD25(+) FoxP3(+) regulatory T Cells and TGF-beta1 in the maternal-fetal interface of the late pregnant mouse. Am. J. Reprod. Immunol. 2022, 88, e13541. [Google Scholar] [CrossRef]
- Severance, A.L.; Kinder, J.M.; Xin, L.; Burg, A.R.; Shao, T.-Y.; Pham, G.; Tilburgs, T.; Goodman, W.A.; Mesiano, S.; Way, S.S. Maternal-fetal conflict averted by progesterone- induced FOXP3+ regulatory T cells. iScience 2022, 25, 104400. [Google Scholar] [CrossRef]
- Thiele, K.; Hierweger, A.M.; Riquelme, J.I.A.; Solano, M.E.; Lydon, J.P.; Arck, P.C. Impaired Progesterone-Responsiveness of CD11c(+) Dendritic Cells Affects the Generation of CD4(+) Regulatory T Cells and Is Associated With Intrauterine Growth Restriction in Mice. Front. Endocrinol. 2019, 10, 96. [Google Scholar] [CrossRef]
- Furcron, A.E.; Romero, R.; Plazyo, O.; Unkel, R.; Xu, Y.; Hassan, S.S.; Chaemsaithong, P.; Mahajan, A.; Gomez-Lopez, N. Vaginal progesterone, but not 17alpha-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am. J. Obstet. Gynecol. 2015, 213, 846.e1–846.e19. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tseng, J.T.; Wang, C.Y.; Su, M.T.; Huang, J.Y.; Kuo, P.L. Medroxyprogesterone acetate drives M2 macrophage differentiation toward a phenotype of decidual macrophage. Mol. Cell. Endocrinol. 2017, 452, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Solano, M.E.; Arck, P.C. Steroids, Pregnancy and Fetal Development. Front. Immunol. 2019, 10, 3017. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.; Anipindi, V.C.; Nguyen, P.V.; Boudreau, J.; Liang, H.; Wan, Y.; Snider, D.P.; Kaushic, C. High Physiological Concentrations of Progesterone Reverse Estradiol-Mediated Changes in Differentiation and Functions of Bone Marrow Derived Dendritic Cells. PLoS ONE 2016, 11, e0153304. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J. The Role of Progesterone in Feto-Maternal Immunological Cross Talk. Med. Princ. Pract. 2018, 27, 301–307. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Kinsky, R.; Chaouat, G. The effect of a progesterone-induced immunologic blocking factor on NK-mediated resorption. Am. J. Reprod. Immunol. 1990, 24, 105–107. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Par, G.; Dombay, G.; Smart, Y.C.; Volgyi, Z. The antiabortive effect of progesterone-induced blocking factor in mice is manifested by modulating NK activity. Cell. Immunol. 1997, 177, 194–199. [Google Scholar] [CrossRef]
- Klossner, R.; Groessl, M.; Schumacher, N.; Fux, M.; Escher, G.; Verouti, S.; Jamin, H.; Vogt, B.; Mohaupt, M.G.; Gennari-Moser, C. Steroid hormone bioavailability is controlled by the lymphatic system. Sci. Rep. 2021, 11, 9666. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Li, B.; Liu, M.; Liu, D.; Deng, M.; Wang, Y.; Xia, X.; Jiang, Q.; Chen, D. Effects of human chorionic gonadotropin, estradiol, and progesterone on interleukin-18 expression in human decidual tissues. Gynecol. Endocrinol. 2017, 33, 265–269. [Google Scholar] [CrossRef]
- Ren, J.; Hou, H.; Zhao, W.; Wang, J.; Peng, Q. Administration of Exogenous Progesterone Protects Against Brucella abortus Infection-Induced Inflammation in Pregnant Mice. J. Infect. Dis. 2021, 224, 532–543. [Google Scholar] [CrossRef]
- Sanchon-Sanchez, P.; Herraez, E.; Macias, R.I.; Estiu, M.C.; Fortes, P.; Monte, M.J.; Marin, J.J.; Romero, M.R. Relationship between cholestasis and altered progesterone metabolism in the placenta-maternal liver tandem. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166926. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, Y.; Li, S.; Xu, Y.; Parolia, A.; Rizvi, S.; Wang, W.; Zhai, Y.; Xiao, R.; Li, X.; et al. Progestogen-driven B7-H4 contributes to onco-fetal immune tolerance. Cell 2024, 187, 4713–4732e19. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Espino-Y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Hayder, H.; Shan, Y.; Chen, Y.; O’bRien, J.A.; Peng, C. Role of microRNAs in trophoblast invasion and spiral artery remodeling: Implications for preeclampsia. Front. Cell Dev. Biol. 2022, 10, 995462. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Y.; Zhang, X.; Wang, C.; Pei, J.; Gu, W. Transcriptomic profiling in hypoxia-induced trophoblast cells for preeclampsia. Placenta 2023, 136, 8–17. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef]
- Qin, J.; Lv, B.; Yao, Y.; Han, X.; Xue, Z.; Lin, C.-P.; Xue, J.; Ji, Y. CTNND1 affects trophoblast proliferation and specification during human embryo implantation. Biol. Reprod. 2025, 112, 46–53. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Wang, L.; Yu, H.; Yu, X.; Zou, Y. PUM1 modulates trophoblast cell proliferation and migration through LRP6. Biochem. Cell Biol. 2021, 99, 735–740. [Google Scholar] [CrossRef]
- Cui, X.; Sun, J.; Liang, C.; Zheng, Q.; Yang, X.; Liu, S.; Yan, Q. Progesterone promotes embryo adhesion by upregulating c-Fos/c-Jun transcription factor-mediated poFUT1 expressiondagger. Biol. Reprod. 2019, 101, 675–685. [Google Scholar] [CrossRef]
- Morimoto, H.; Ueno, M.; Tanabe, H.; Kono, T.; Ogawa, H. Progesterone depletion results in Lamin B1 loss and induction of cell death in mouse trophoblast giant cells. PLoS ONE 2021, 16, e0254674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).