The Zebrafish miR-183 Family Regulates Endoderm Convergence and Heart Development via S1Pr2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Breeding and Ethical Considerations
2.2. miRNA Mimic Microinjection
2.3. Whole-Mount In Situ Hybridization (WISH)
2.4. Real-Time Quantitative PCR (RT-qPCR)
2.5. Dual Luciferase Reporter Assay
2.6. Statistical Analysis
3. Results
3.1. The miR-183 Family Is Highly Conserved in Vertebrates
3.2. Overexpression of miR-183 Affects Early Heart Development
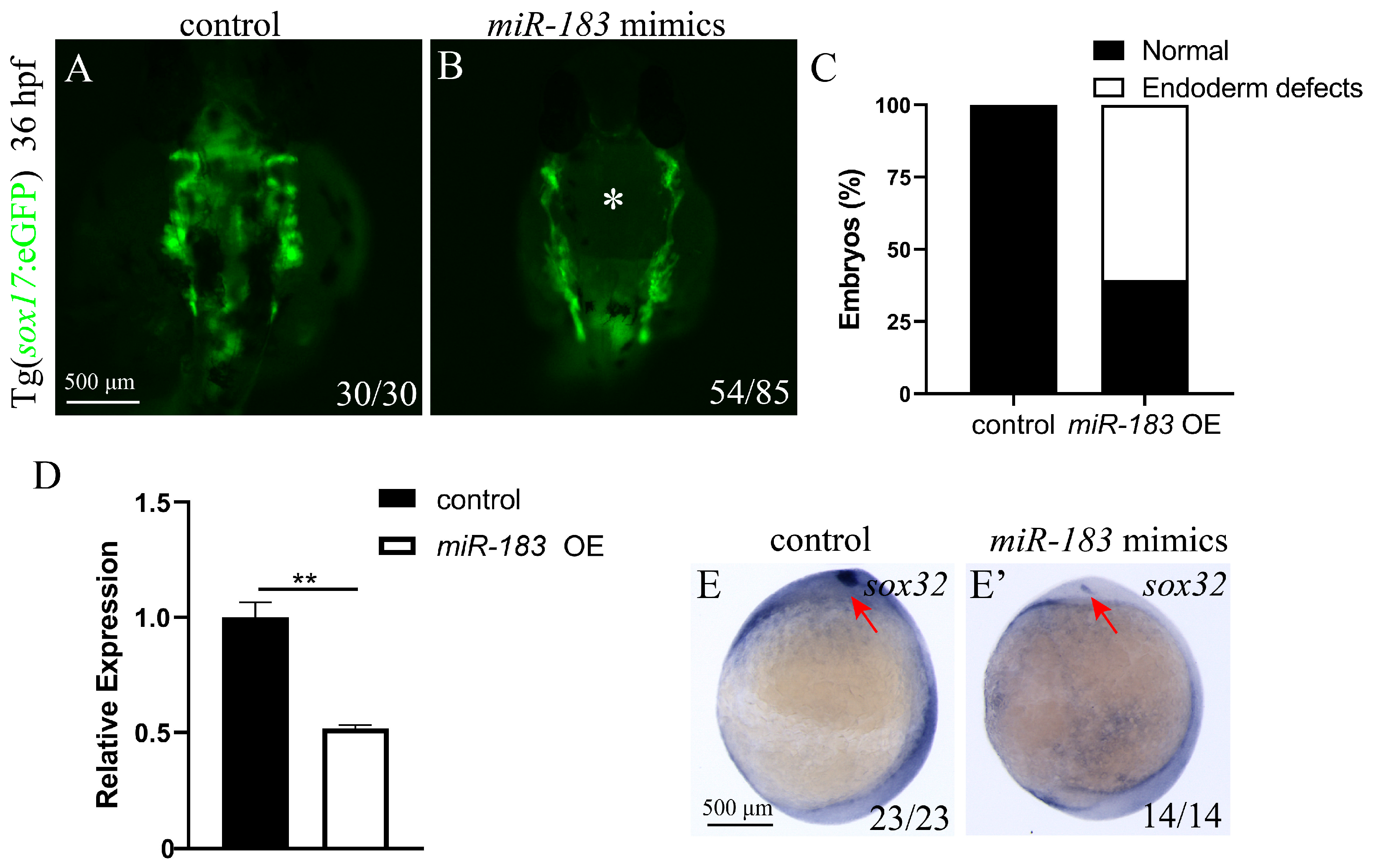
3.3. miR-183 Overexpression Affects Endoderm Convergence
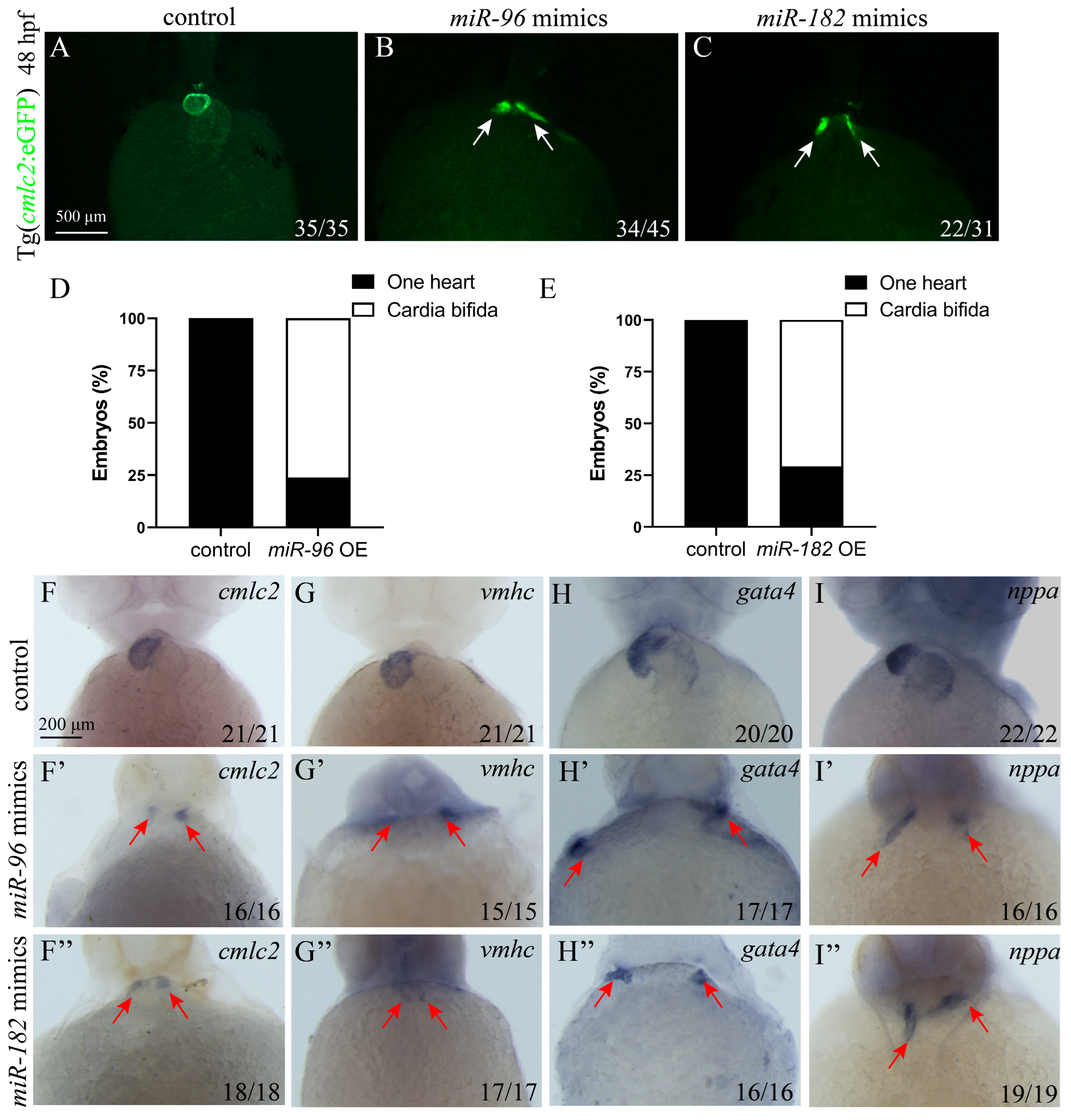
3.4. Overexpression of miR-96 and miR-182 Leads to Cardia Bifida
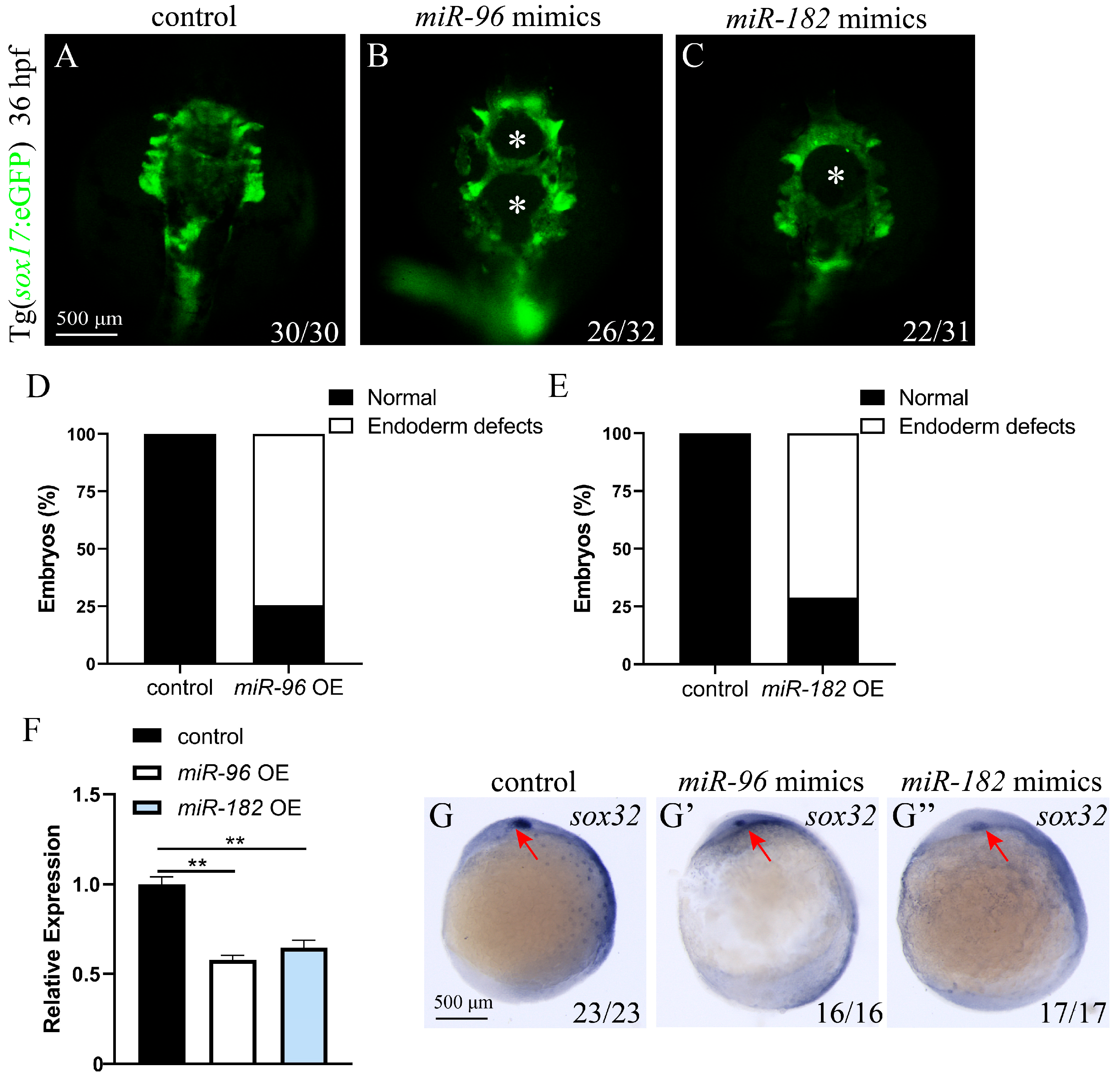
3.5. miR-96 and miR-182 Overexpression Resulted in Endodermal Defects
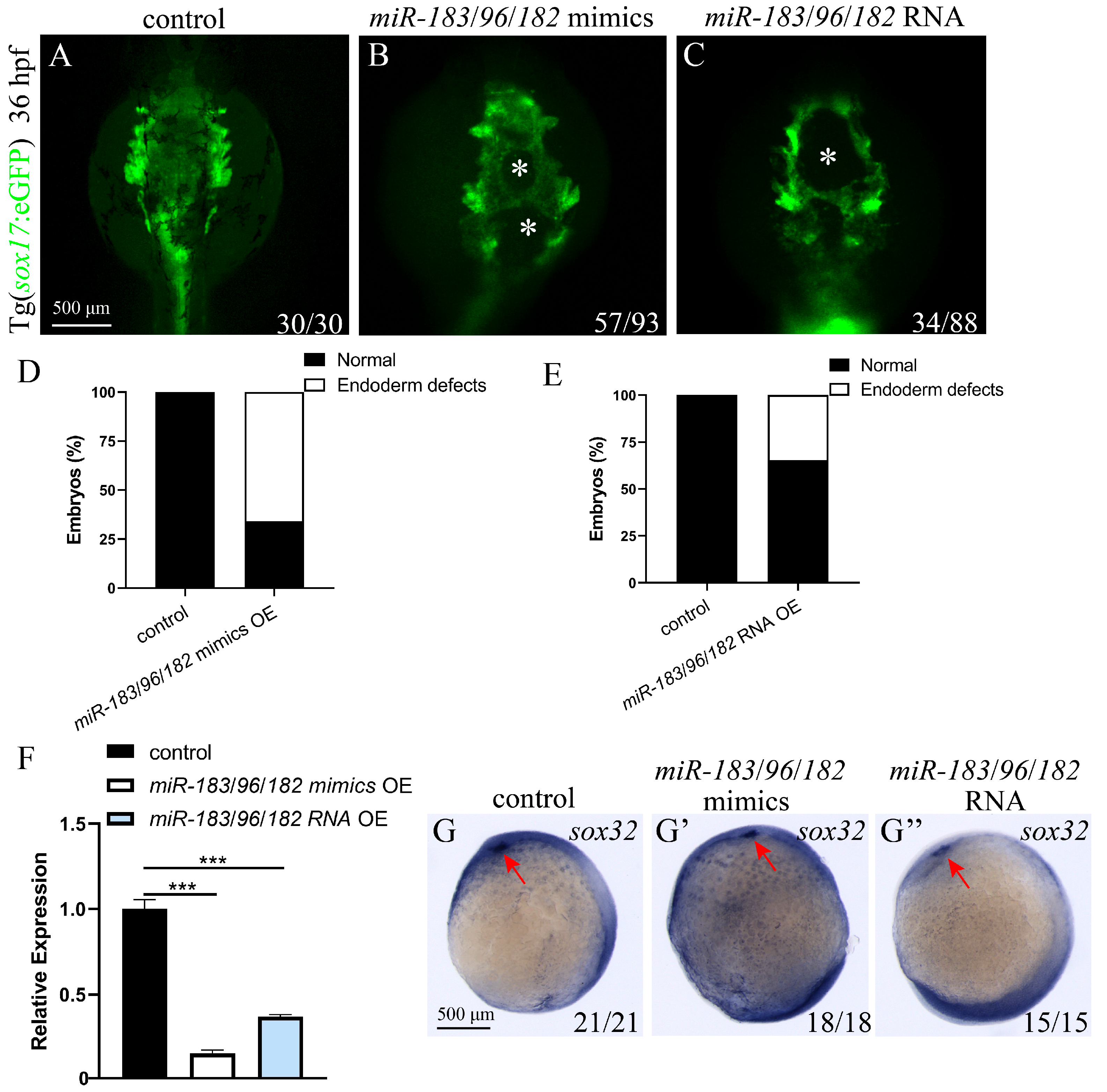
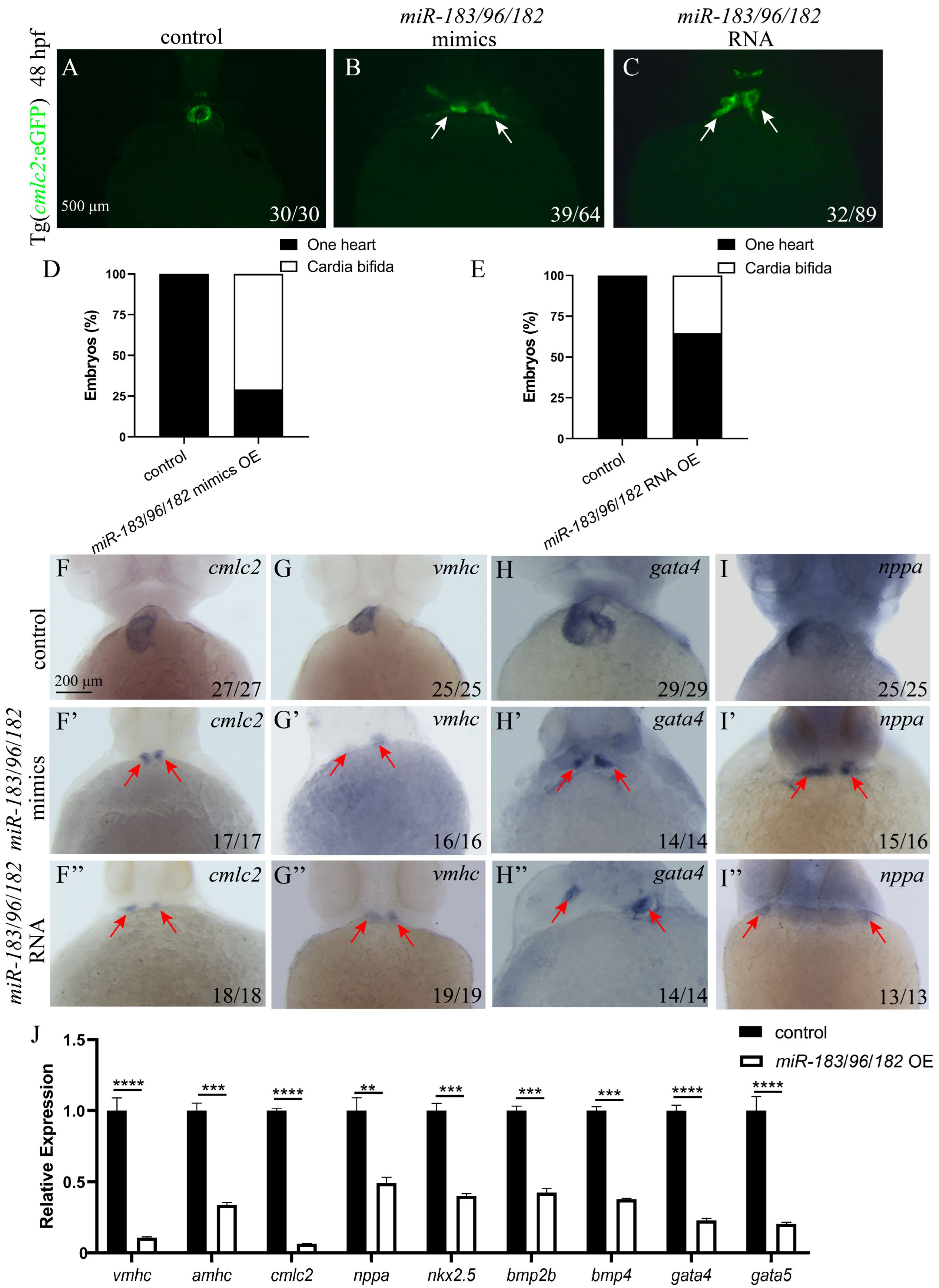
3.6. Co-Expression of the miR-183 Family Affects Endoderm Convergence and Cardiac Precursor Cell Migration
3.7. miR-183 Family Regulates Endodermal and Cardiac Precursor Cell Migration via the S1pr2 Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowotschin, S.; Hadjantonakis, A.K.; Campbell, K. The endoderm: A divergent cell lineage with many commonalities. Development 2019, 146, dev150920. [Google Scholar] [CrossRef]
- Brennan, J.; Lu, C.C.; Norris, D.P.; Rodriguez, T.A.; Beddington, R.S.; Robertson, E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 2001, 411, 965–969. [Google Scholar] [CrossRef]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef]
- Stainier, D.Y.; Fouquet, B.; Chen, J.N.; Warren, K.S.; Weinstein, B.M.; Meiler, S.E.; Mohideen, M.A.; Neuhauss, S.C.; Solnica-Krezel, L.; Schier, A.F.; et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996, 123, 285–292. [Google Scholar] [CrossRef]
- Li, S.; Zhou, D.; Lu, M.M.; Morrisey, E.E. Advanced cardiac morphogenesis does not require heart tube fusion. Science 2004, 305, 1619–1622. [Google Scholar] [CrossRef]
- Shan, J.P.; Wang, X.L.; Qiao, Y.G.; Wan Yan, H.X.; Huang, W.H.; Pang, S.C.; Yan, B. Novel and functional DNA sequence variants within the GATA5 gene promoter in ventricular septal defects. World J. Pediatr. WJP 2014, 10, 348–353. [Google Scholar] [CrossRef]
- Rosenquist, G.C. Cardia bifida in chick embryos: Anterior and posterior defects produced by transplanting tritiated thymidine-labeled grafts medial to the heart-forming regions. Teratology 1970, 3, 135–142. [Google Scholar] [CrossRef]
- Varner, V.D.; Taber, L.A. Not just inductive: A crucial mechanical role for the endoderm during heart tube assembly. Development 2012, 139, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Kupperman, E.; An, S.; Osborne, N.; Waldron, S.; Stainier, D.Y. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 2000, 406, 192–195. [Google Scholar] [CrossRef]
- Ye, D.; Lin, F. S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence. Development 2013, 140, 789–799. [Google Scholar] [CrossRef]
- Ye, D.; Xie, H.; Hu, B.; Lin, F. Endoderm convergence controls subduction of the myocardial precursors during heart-tube formation. Development 2015, 142, 2928–2940. [Google Scholar] [CrossRef]
- Xie, H.; Ye, D.; Sepich, D.; Lin, F. S1pr2/Gα13 signaling regulates the migration of endocardial precursors by controlling endoderm convergence. Dev. Biol. 2016, 414, 228–243. [Google Scholar] [CrossRef]
- Zhang, Q.; Peyruchaud, O.; French, K.J.; Magnusson, M.K.; Mosher, D.F. Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood 1999, 93, 2984–2990. [Google Scholar]
- Lagos-Quintana, M.; Rauhut, R.; Meyer, J.; Borkhardt, A.; Tuschl, T. New microRNAs from mouse and human. RNA 2003, 9, 175–179. [Google Scholar] [CrossRef]
- Cho, Y.J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef]
- Navarro-Calvo, J.; Esquiva, G.; Gómez-Vicente, V.; Valor, L.M. MicroRNAs in the Mouse Developing Retina. Int. J. Mol. Sci. 2023, 24, 2992. [Google Scholar] [CrossRef]
- Mahmoodian Sani, M.R.; Hashemzadeh-Chaleshtori, M.; Saidijam, M.; Jami, M.S.; Ghasemi-Dehkordi, P. MicroRNA-183 Family in Inner Ear: Hair Cell Development and Deafness. J. Audiol. Otol. 2016, 20, 131–138. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Zhang, Y.; Tang, X.; Gao, L.; Jian, H. MiR-183-5p inhibitor promotes mitoxantrone-induced immunogenic death of hepatoma cells by targeting STC1. Int. J. Clin. Exp. Pathol. 2025, 18, 351–363. [Google Scholar] [CrossRef]
- Li, S.; Meng, W.; Guo, Z.; Liu, M.; He, Y.; Li, Y.; Ma, Z. The miR-183 Cluster: Biogenesis, Functions, and Cell Communication via Exosomes in Cancer. Cells 2023, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, M.; Sievänen, T.; Korhonen, T.M.; Tuomikoski, J.; Pylvänäinen, K.; Äyrämö, S.; Seppälä, T.T.; Mecklin, J.P.; Laakkonen, E.K.; Jokela, T. Integrative omics approaches to uncover liquid-based cancer-predicting biomarkers in Lynch syndrome. Int. J. Cancer 2025, 1–14. [Google Scholar] [CrossRef]
- Borsos, B.N.; Páhi, Z.G.; Pankotai, T. Exploring potent miRNA combinations for detecting early-stage breast cancer. Mol. Ther. Nucleic Acids 2025, 36, 102621. [Google Scholar] [CrossRef]
- Mohammaddoust, S.; Sadeghizadeh, M. Mir-183 functions as an oncogene via decreasing PTEN in breast cancer cells. Sci. Rep. 2023, 13, 8086. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cai, J.; Kong, L.; Ying, L.; Liu, X.; Jiang, M.; Pan, D. Upregulation of miR-183 inhibits the invasion and migration of endometrial stromal cells in endometriosis patients by downregulating Ezrin. Front. Oncol. 2025, 15, 1537528. [Google Scholar] [CrossRef] [PubMed]
- Fresneda Alarcon, M.; Abdullah, G.A.; Beggs, J.A.; Kynoch, I.; Sellin, A.; Cross, A.; Haldenby, S.; Antczak, P.; Caamaño Gutiérrez, E.; Wright, H.L. Complexity of the neutrophil transcriptome in early and severe rheumatoid arthritis: A role for microRNAs? J. Leukoc. Biol. 2025, 117, qiaf090. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Huang, D.; Qin, Y.; Lou, X.; Gao, H.; Ye, Z.; Wang, F.; Wang, Y.; Jing, D.; et al. Small extracellular vesicle-miR-183-5p mediated crosstalk between tumor cells and macrophages in high-risk pancreatic neuroendocrine tumors. Oncogene 2025, 44, 2907–2923. [Google Scholar] [CrossRef]
- Karakuş, F.; Ece, A.; Kuzu, B. New targets and biomarkers for doxorubicin-induced cardiotoxicity in humans: Implications drawn from toxicogenomic data and molecular modelling. J. Biomol. Struct. Dyn. 2024, 1–13. [Google Scholar] [CrossRef]
- Alizadeh Saghati, A.; Sharifi, Z.; Hatamikhah, M.; Salimi, M.; Talkhabi, M. Unraveling the relevance of SARS-CoV-2 infection and ferroptosis within the heart of COVID-19 patients. Heliyon 2024, 10, e36567. [Google Scholar] [CrossRef]
- Castellan, R.F.; Vitiello, M.; Vidmar, M.; Johnstone, S.; Iacobazzi, D.; Mellis, D.; Cathcart, B.; Thomson, A.; Ruhrberg, C.; Caputo, M.; et al. miR-96 and miR-183 differentially regulate neonatal and adult postinfarct neovascularization. JCI Insight 2020, 5, e134888. [Google Scholar] [CrossRef]
- Lin, D.; Cui, B.; Ma, J.; Ren, J. MiR-183-5p protects rat hearts against myocardial ischemia/reperfusion injury through targeting VDAC1. BioFactors 2020, 46, 83–93. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Meese, E.; Abdul-Khaliq, H.; Raedle-Hurst, T. MicroRNA-183-3p Is a Predictor of Worsening Heart Failure in Adult Patients With Transposition of the Great Arteries and a Systemic Right Ventricle. Front. Cardiovasc. Med. 2021, 8, 730364. [Google Scholar] [CrossRef]
- Guzzolino, E.; Pellegrino, M.; Ahuja, N.; Garrity, D.; D’Aurizio, R.; Groth, M.; Baumgart, M.; Hatcher, C.J.; Mercatanti, A.; Evangelista, M.; et al. miR-182-5p is an evolutionarily conserved Tbx5 effector that impacts cardiac development and electrical activity in zebrafish. Cell. Mol. Life Sci. 2020, 77, 3215–3229. [Google Scholar] [CrossRef] [PubMed]
- Mourelatos, Z.; Dostie, J.; Paushkin, S.; Sharma, A.; Charroux, B.; Abel, L.; Rappsilber, J.; Mann, M.; Dreyfuss, G. miRNPs: A novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002, 16, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef]
- David, N.B.; Rosa, F.M. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development 2001, 128, 3937–3947. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Liu, N.; Olson, E.N. MicroRNA regulatory networks in cardiovascular development. Dev. Cell 2010, 18, 510–525. [Google Scholar] [CrossRef]
- Pierce, M.L.; Weston, M.D.; Fritzsch, B.; Gabel, H.W.; Ruvkun, G.; Soukup, G.A. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol. Dev. 2008, 10, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, X.J.; Wu, K.C.; Zhang, C.J.; Zhou, G.H.; Lv, J.N.; Sun, L.F.; Cheng, F.F.; Cai, X.B.; Jin, Z.B. miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance. Proc. Natl. Acad. Sci. USA 2017, 114, 6376–6381. [Google Scholar] [CrossRef]
- Wienholds, E.; Kloosterman, W.P.; Miska, E.; Alvarez-Saavedra, E.; Berezikov, E.; de Bruijn, E.; Horvitz, H.R.; Kauppinen, S.; Plasterk, R.H. MicroRNA expression in zebrafish embryonic development. Science 2005, 309, 310–311. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef]
- Xu, S.; Witmer, P.D.; Lumayag, S.; Kovacs, B.; Valle, D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J. Biol. Chem. 2007, 282, 25053–25066. [Google Scholar] [CrossRef]
- Busskamp, V.; Krol, J.; Nelidova, D.; Daum, J.; Szikra, T.; Tsuda, B.; Jüttner, J.; Farrow, K.; Scherf, B.G.; Alvarez, C.P.; et al. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 2014, 83, 586–600. [Google Scholar] [CrossRef]
- Kuhn, S.; Johnson, S.L.; Furness, D.N.; Chen, J.; Ingham, N.; Hilton, J.M.; Steffes, G.; Lewis, M.A.; Zampini, V.; Hackney, C.M.; et al. miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2355–2360. [Google Scholar] [CrossRef]
- Mao, S.; Zhao, J.; Zhang, Z.-J.; Zhao, Q. MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology 2022, 227, 152204. [Google Scholar] [CrossRef]
- Gu, H.; Duan, Y.; Li, S.; Wang, Q.; Zhen, W.; Zhang, W.; Zhang, Y.; Jiang, M.; Wang, C. miR-96-5p regulates myocardial infarction-induced cardiac fibrosis via Smad7/Smad3 pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1874–1888. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-H.; Capeling, M.; Villanueva, J.W.; Kanke, M.; Shanahan, M.T.; Huang, S.; Cubitt, R.; Rinaldi, V.D.; Schimenti, J.C.; Spence, J.R.; et al. Integrative genome-scale analyses reveal post-transcriptional signatures of early human small intestinal development in a directed differentiation organoid model. BMC Genom. 2023, 24, 641. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.C.; Chien, K.R. Fashioning the vertebrate heart: Earliest embryonic decisions. Development 1997, 124, 2099–2117. [Google Scholar] [CrossRef]
- Nascone, N.; Mercola, M. An inductive role for the endoderm in Xenopus cardiogenesis. Development 1995, 121, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.J.; Wayman, G.A.; Christian, J.L. Bone morphogenetic protein function is required for terminal differentiation of the heart but not for early expression of cardiac marker genes. Mech. Dev. 2001, 100, 263–273. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Geiger, E.; Blinder, J.; Benson, D.W.; Goldmuntz, E. NKX2.5 mutations in patients with congenital heart disease. J. Am. Coll. Cardiol. 2003, 42, 1650–1655. [Google Scholar] [CrossRef]
- Azpiazu, N.; Frasch, M. tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef]
- Schmitt, M.; Cockcroft, J.R.; Frenneaux, M.P. Modulation of the natriuretic peptide system in heart failure: From bench to bedside? Clin. Sci. 2003, 105, 141–160. [Google Scholar] [CrossRef]
- Grassini, D.R.; Lagendijk, A.K.; De Angelis, J.E.; Da Silva, J.; Jeanes, A.; Zettler, N.; Bower, N.I.; Hogan, B.M.; Smith, K.A. Nppa and Nppb act redundantly during zebrafish cardiac development to confine AVC marker expression and reduce cardiac jelly volume. Development 2018, 145, dev160739. [Google Scholar] [CrossRef]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Tremblay, M.; Sanchez-Ferras, O.; Bouchard, M. GATA transcription factors in development and disease. Development 2018, 145, dev164384. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Ma, Q.; Juraszek, A.L.; Moses, K.; Schwartz, R.J.; Izumo, S.; Pu, W.T. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J. Clin. Investig. 2005, 115, 1522–1531. [Google Scholar] [CrossRef]
- Reiter, J.F.; Alexander, J.; Rodaway, A.; Yelon, D.; Patient, R.; Holder, N.; Stainier, D.Y. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999, 13, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Agathon, A.; Alexander, J.; Thisse, C.; Waldron, S.; Yelon, D.; Thisse, B.; Stainier, D.Y. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Liu, L.; Lv, J.; Xie, H.; Shi, Q.; Tao, G.; Zheng, X.; Zhu, L.; Xiong, L.; Xie, H. The Zebrafish miR-183 Family Regulates Endoderm Convergence and Heart Development via S1Pr2 Signaling Pathway. Biomolecules 2025, 15, 1434. https://doi.org/10.3390/biom15101434
Zeng T, Liu L, Lv J, Xie H, Shi Q, Tao G, Zheng X, Zhu L, Xiong L, Xie H. The Zebrafish miR-183 Family Regulates Endoderm Convergence and Heart Development via S1Pr2 Signaling Pathway. Biomolecules. 2025; 15(10):1434. https://doi.org/10.3390/biom15101434
Chicago/Turabian StyleZeng, Ting, Ling Liu, Jinrui Lv, Hao Xie, Qingying Shi, Guifang Tao, Xiaoying Zheng, Lin Zhu, Lei Xiong, and Huaping Xie. 2025. "The Zebrafish miR-183 Family Regulates Endoderm Convergence and Heart Development via S1Pr2 Signaling Pathway" Biomolecules 15, no. 10: 1434. https://doi.org/10.3390/biom15101434
APA StyleZeng, T., Liu, L., Lv, J., Xie, H., Shi, Q., Tao, G., Zheng, X., Zhu, L., Xiong, L., & Xie, H. (2025). The Zebrafish miR-183 Family Regulates Endoderm Convergence and Heart Development via S1Pr2 Signaling Pathway. Biomolecules, 15(10), 1434. https://doi.org/10.3390/biom15101434




